Abstract
Objective
Oral cancer constitutes a major health issue in developing countries, representing the leading cause of death. Quantitative assessment by sophisticated diagnostic techniques is becoming increasingly important. Hence, a histochemical staining procedure and morphometric evaluation are used to obtain optimal information on the cellular events. The objective of present study is to assess the variation in cellular area, nuclear area, cellular diameter, nuclear diameter and nuclear/cytoplasmic ratio respectively in normal subjects, smokeless tobacco users, (smokers, combination and oral squamous cell carcinoma patients.
Methods
Total 125 number of subjects were divided into five groups, each comprising 25 subjects of more than 40 years of age. These groups were: a. Normal, b. smokeless tobacco users, c. smokers d. combination and e. oral squamous cell carcinoma. Oral smears were obtained, stained with Feulgen stain and the cells were measured cytomorphometrically using Nikon imaging software.
Results
Our study showed a significant reduction in the cellular diameter, cellular area and increase in the nuclear diameter, nuclear area and nuclear/cytoplasmic ratio in oral squamous cell carcinoma patients as compared to tobacco users and normal patients. Significant changes were found in group I, II, III and IV when compared with group V but as such no significant intergroup variation was found in cellular and nuclear dimensions in smokers, smokeless tobacco users, combination and control group.
Conclusion
Quantitative parameters could be assessed by cytomorphometry. Cytomorphological changes in exfoliated squames could serve as a useful adjunct in the early diagnosis of oral squamous cell carcinomas.
Keywords: Cancer, Cytomorphometry, Exfoliative cytology, Feulgen reaction, Imaging software, Papanicolaou stain, Premalignant lesion, Tobacco
Introduction
Cellular proliferation is a biological process of vital importance for living organisms and abnormal cell proliferation appears to be a precursor of cancer development.(1) Numerous advances have been made in prevention, diagnosis and treatment of the cancer but it still continues to torment mankind. (2) Available data suggests that by 2020 there would be 15 million new cancer cases every year and 10 million cancer deaths worldwide. (3)
Majority of oral cancers arise from long standing potentially malignant lesions. The absence of classical clinical features of advanced oral cancer including ulceration, in duration, elevation and cervical lymphadenopathy delays the detection of early stage oral cancer. (4) The 5-year survival rate of oral squamous cell carcinoma (OSCC) patients has remained approximately 50% despite advances in the field of oncology. (5) So detection of potentially malignant lesions at their earliest stage is required to tackle this problem. Exfoliative oral cytology has emerged as a painless, non-invasive procedure for obtaining cell samples that can be analyzed by sophisticated diagnostic techniques such as cytomorphometry. (6) It is well accepted by the patient, simple to perform and is therefore an attractive option for the early diagnosis of oral cancer.
The literature review has substantiated the fact that malignant cells show a significant variation in morphology and dimensions. The enlarged nuclear size is due to an increase in the nuclear contents required for replication and the mean value for the cellular size decreases. Thus the quantitative methods of smear assessment can specify the prospective behaviour of oral dysplasias. (7)
Quantitative techniques have been applied to cytology to increase its diagnostic reliability above and beyond subjective cytopathological interpretation. In the present study we aimed to establish these techniques in routine clinical practice for early diagnosis of oral cancer. This could result in better treatment outcome and prognosis. Thus, the objective of our study is to correlate the variations in cellular area(CA), nuclear area(NA), cellular diameter(CD), nuclear diameter and nuclear/cytoplasmic ratio respectively in normal subjects, smokeless tobacco users, smokers, combination (both smokers and smokeless tobacco users) and OSCC patients using cytomorphometry. This work may help us in determining the possibility of using it as a diagnostic tool which could assist as screening modality (replace with - of oral cancer.
Subjects and Methods
The present study was submitted to the ethical committee of Genesis Institute of Dental Sciences and Research, Ferozepur for evaluation and ethical clearance was granted to carry out this non-invasive study. The study comprised of cytomorphometric analysis of oral smears obtained from 125 individuals, which were further categorized into subgroups (Table 1) of 25 each (healthy subjects, smokeless tobacco users, smokers, combination and OSCC patients). Healthy subjects (non – tobacco users and non – alcoholics) were considered as control. Subjects more than 40 years of age (males) using tobacco for 10 years or more were included. Those using smokeless tobacco forms (≥5 gutka sachets/zarda/betel quids per day) and smoking tobacco (≥5 cigarettes or bidis per day) were considered. Anaemic patients, chronic alcoholics, patients with history of any debilitating systemic disease or medication and patients undergoing radiotherapy were excluded to avoid associated cellular and nuclear alterations.
Table 1.
Distribution of Subjects
| Group | Subjects | Number |
|---|---|---|
| I | Normal patients (N) | 25 |
| II | Smokeless tobacco users (ST) | 25 |
| III | Smokers (S) | 25 |
| IV | Combination(C) [smokeless tobacco + smoking] | 25 |
| V | OSCC patients (Ca) | 25 |
Group distribution of subjects
The written consent was obtained from the subjects to carry out the procedure and thorough history and physical examination was done. The cells were collected by using a standard sterilized wooden spatula over the mucosal surface. The smear was obtained on the labelled glass slide; fixed with 95% alcohol spray fixative for one hour and stained with Feulgen stain which was specific for nucleus and counterstained with cytoplasmic stain (Rapid PAP kit/OG-6, EA-50). The stained smears had red-purple nuclei/micronuclei and orange to pink-red to blue-green cytoplasm depending on the degree of maturation.
The morphometric analysis of stained smears was done under research light microscope (Nikon E200). The slides were screened under X10 magnification, images of the cells were captured under objective of X40 magnification with digital camera and subjected to computer analysis. The sampling was done in a zigzag manner in order to avoid measuring the same cells again. Fifty cells with well-defined borders were randomly selected. Only cells that were fully included in the field of vision and had clearly defined cellular and nuclear outlines were considered. Clumped or folded and cells with unusually distorted outline or nuclei were not considered for the analysis. The morphometric analysis was done using Nikon imaging software (NIS) ELEMENTS D 3.1. The perimeter of the individual cells and nuclei was traced (Figure 1) and the software automatically calculated the cellular and nuclear area and corresponding diameter by converting number of boundary pixels detected to micrometer square(μm2) in different groups (Figures 2,3). The measurement was recorded in μm/μm2 respectively. By using these values NA/CA and ND/CD were calculated. Since this study involved multiple groups, one-way ANOVA (Analysis of Variance) was used for comparing the parameters. Comparison of the mean NA, CA, ND, CD and N/C values in between the groups was done using multiple comparison test by Tukey-HSD procedure. Groups I, II, III and IV were compared with group V. Intergroup variations were also observed between group I, II, III and IV.
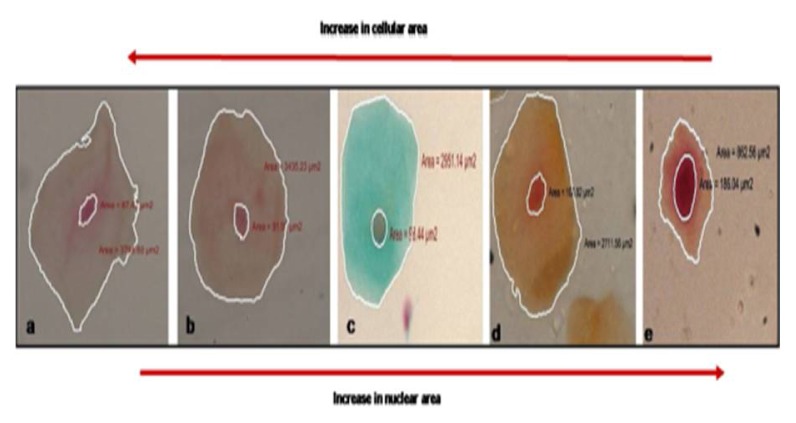
Figure 1.
Depicting progressive increase in nuclear area and reduction in cellular area in a) normal b) smokeless tobacco users c) combination d) smokers and e) oral cancer patients.
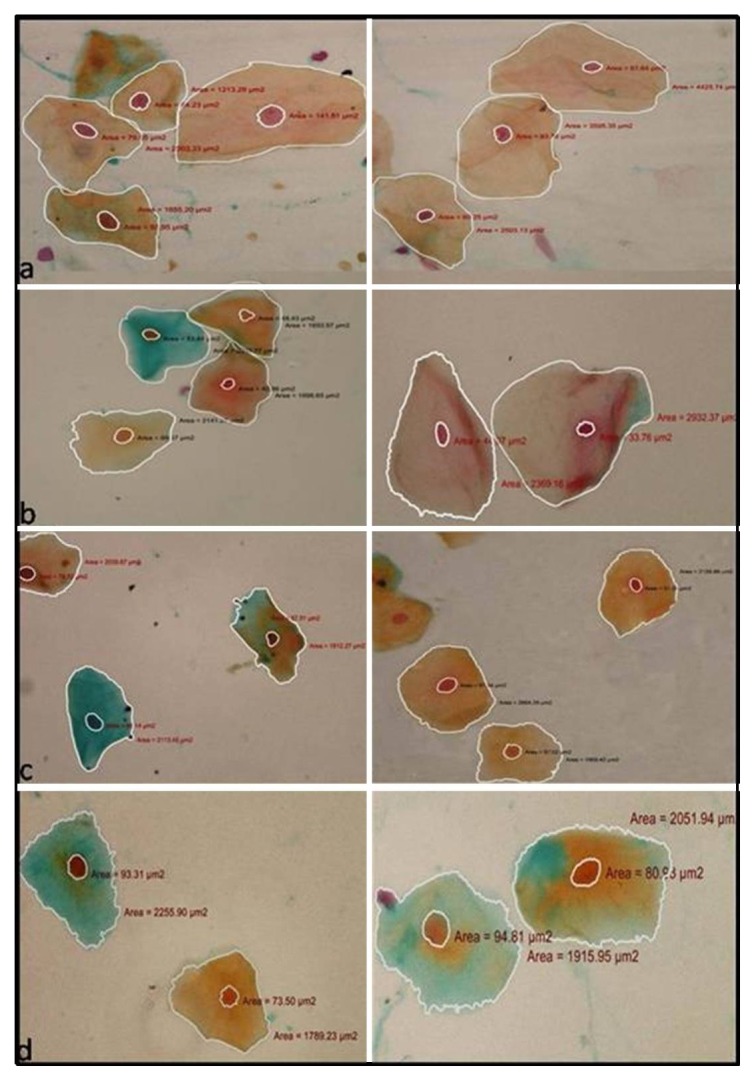
Figure 2.
Cytological smears from a) normal patients b) smokeless tobacco users c) smokers and d) combination group depicting exfoliated epithelial squames with nuclear and cellular outline tracing and corresponding measurements (a–d ×40)
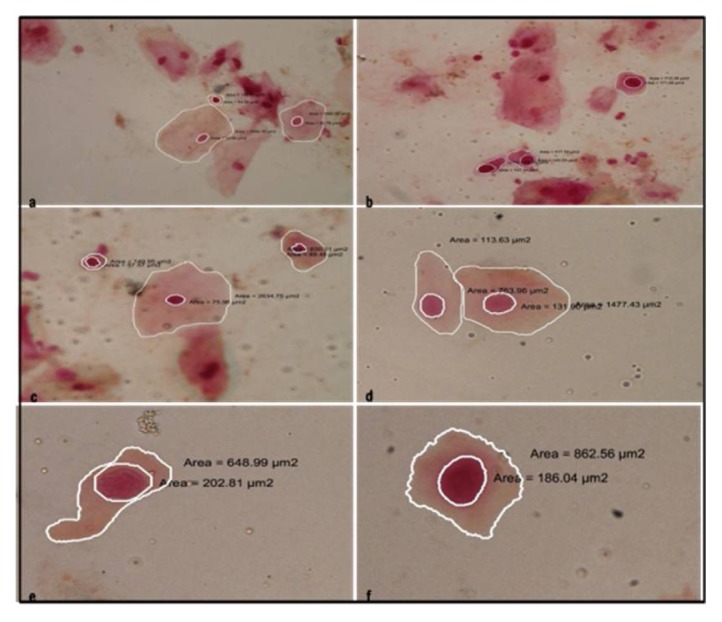
Figure 3.
Cytological smears from oral cancer group depicting exfoliated epithelial cells with nuclear and cellular outline tracing and corresponding measurements. Features of dysplasia like nuclear and cellular pleomorphism, hyperchromatism and altered N/C ratio are also observed (a–f ×40)
Results
Analysis of variance (ANOVA) showed a significant group effect for CD, ND and ratio of ND/CD (Table 2), when group I, II, III and IV were compared with group V. The mean value of ND of the keratinocytes in group V smears was significantly higher (10.25 ± 0.90) as compared to group I, group II, group III, group IV (p= 0.000 i.e highly significant value). Lowest value was observed in group I (8.42 ± 0.74). The highest value of CD of the keratinocytes was seen in group I (54.04±3.33) and lowest in group V (46.32 ± 5.80). The decrease in CD and increase in ND in group V was highly significant as compared to group I, II, III and IV (p = 0.000). Significant increase in ND/CD ratio was also observed in group V (p= 0.000). The mean values of CD, ND and ND/CD were found to be highly significant (p=0.000) by Tukey HSD multiple comparison test when these parameters of group I, II, III, IV were compared with group V. ANOVA also showed a significant group effect for CA, NA and ratio of NA/CA (Table 3). There was a significant increase in mean NA in group V patients (84.17± 14.59) as compared to other groups (p=0.000). Lowest value was observed in group I (64.85±9.87). Mean CA value showed significant reduction in group V patients as compared to group I, II, III and IV (p=0.000) and a significant increase in NA/CA ratio was observed in group V (p=0.000) as compared to other groups.
Table 2.
Mean Values of Cellular Diameter, Nuclear Diameter and Ratio of ND/CD Diameter(μm)
| Groups | Celluar Diameter | Nuclear Diameter | Ratio ND:CD | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Group I | 54.04 | 3.33 | 8.42 | 0.74 | 0.1558 | 0.0068 |
| Group II | 53.91 | 4.96 | 8.62 | 0.83 | 0.1599 | 0.0054 |
| Group III | 52.63 | 6.01 | 9.10 | 0.92 | 0.1729 | 0.0080 |
| Group IV | 53.16 | 9.38 | 8.89 | 0.75 | 0.1672 | 0.0060 |
| Group V | 46.32 | 5.80 | 10.25 | 0.90 | 0.2213 | 0.0134 |
| p-value | 0.000(S) | 0.000(S) | 0.000(S) | |||
SD-standard deviation, S- significant p-value.
Significant decrease in cell diameter and increase in nuclear diameter and ND/CD ratio in squamous cell carcinoma patients when compared to controls and tobacco users (Group II, III, IV and V are compared with Group I).
Table 3.
Mean Values of Cellular Area, Nuclear Area and Ratio of NA/CA(μm2)
| Group | Cellular area | Nuclear Area | Ratio NA:CA | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Group I | 2450.33 | 280.17 | 64.85 | 9.87 | 0.0265 | 0.0042 |
| Group II | 2365.78 | 412.25 | 64.95 | 10.94 | 0.0275 | 0.0059 |
| Group III | 2349.92 | 502.81 | 69.60 | 13.18 | 0.0296 | 0.0067 |
| Group IV | 2360.42 | 1022.36 | 65.01 | 10.58 | 0.0279 | 0.0069 |
| Group V | 1795.48 | 424.67 | 84.17 | 14.59 | 0.0469 | 0.0141 |
| p-value | 0.000(S) | 0.000(S) | 0.000(S) | |||
SD- standard deviation, S- significant p-value
Significant decrease in cellular area and increase in nuclear area and NA/CA ratio in squamous cell carcinoma patients when compared to controls and tobacco users (Group II, III, V and V are compared with Group I).
Tukey HSD formula for pair wise comparison revealed that differences in mean values of CA were significant when group I, II, III, IV were compared with group V respectively. Differences in mean values of NA and NA/CA were significant when comparing group I, II, III, IV with V (p=0.000). Individual smears from different groups showed a wide variation in the range CD, ND, CA and NA values.
Difference in mean values of CD, ND, ND/CD, CA, NA and NA/CA were highly significant when comparing group I and group V (p= 0.000, 0.000, 0.000,0.001, 0.000, 0.000), group II and group V (p= 0.000, 0.000, 0.000,0.007, 0.000, 0.000), group III and group V(p=0.001, 0.000, 0.000,0.010, 0.000, 0.000) and group IV and group V (p=0.002, 0.000, 0.000, 0.008, 0.000, 0.000) respectively.(The p-value <0.05 is significant, the p-value <0.000 is highly significant).
Discussion
Oral squamous cell carcinoma constitutes approximately 3% of all malignancies and more than 90% of cancers of the oral cavity and oropharynx.(8) The high morbidity and mortality rates of OSCC necessitate its early detection and treatment.(7) Clinical examination along with biopsy has been considered as the standard technique for diagnosing precancerous and cancerous oral lesions. However, biopsy is an invasive procedure associated with potential morbidity and psychological implications for some patients. (9) In recent times, the continuing development of automated cytomorphometric techniques, DNA cytophotometry and quantitative methods of smear assessment has enhanced the diagnostic reliability of oral exfoliative cytology. (6)
Cowpe, Longmore and Green (1985) demonstrated that malignant changes could be detected by exfoliative cytology through estimation of ratio of nuclear size to cellular size. Since then a lot of studies had been carried out to evaluate the influence of diverse systemic and external factors on cellular size, nuclear size and ratio of nuclear size to cellular size using the quantitative techniques. But the results are varied and controversial. (10)
In our study we found that mean nuclear and cellular diameter ranged from 7.02 to 9.95μm and 48.39 to 60.13μm respectively in normal subjects. These values were well within the range given by Goldsby et al (1964) who in their computations revealed that the extent of ND ranged from 4.5 to 13.5 μm and CD ranged from 29.7 to 98 μm. (11) However, Nayar and Sivapathasundram (2003) in their study found the range of nuclear and cellular diameter to vary from 7.8 to 8.28 μm and 46.19 to 48.18μm respectively. (12) Cowpe (1984) applied quantitative techniques to exfoliative cytology of normal and abnormal human oral mucosal squames and found it difficult to set a normal baseline criteria for exfoliated normal oral squames, related to age and site. There was a wide variation in the size of the cells and nuclei in individual smears from normal subjects which could be explained on the basis of differences in development and maturation of individual cells. (13)
Our study showed quantitative alterations in the form of reduced cellular (CD, CA) and increased nuclear (ND, NA, and N/C) measurements in tobacco users. However significant variations in size were observed only when these groups were compared with the carcinoma group. Few other authors have also found significant differences in OSCC patients when compared with the mucosa of tobacco users.(14,5) However no significant differences were observed in reduced cellular and increased nuclear size and N/C ratio in our study when smokeless tobacco users, smokers and combination groups were compared with normal patients and with each other. Einstein and Sivapathasundharam (2005) also found no significant difference in the mean cellular and nuclear diameter in chewing group as compared to normal. But combination group showed significant differences in above parameters in their study. (14) However Hande and Chaudhary (2010) in their study found significant reduction in CD and increase in ND in tobacco chewers. (13)
Observations of Silverman, Becks and Farber (1958) were in agreement with our study. They found that in “benign oral lesions, no significant qualitative and quantitative alteration in cellular and nuclear morphology could be appreciated”. Cowpe, Longmore and Green (1985) found no significant variation in cytomorphological values for smokers. (10)
Our findings were in contrast to the study conducted in Sri Lankan population which revealed a significant reduction in cellular dimensions in tobacco chewers and combined habit group and no significant decrease in cell size of smokers. (17) But the nuclear diameter was significantly higher in smokers, chewers and combination group. Einstein and Sivapathasundharam (2005) reported a “statistically significant reduction in CD and increase in ND in smokers and those with combined habit in the South Indian population”. (14) Ogden, Cowpe and Green (1990) assessed the effect of cigarette smoking on the oral mucosa and their study revealed a significant increase in NA for smokers but no significant variation in CA between the smokers and non-smokers. (18)
Variations in our findings from other studies could be attributed to certain factors. The regional variations in tobacco usage could be one of the contributing cause. In the Northern states, smoking of tobacco is relatively higher as compared to tobacco chewing except in Punjab where tobacco prevalence is one of the lowest as majority of its population (58%) practice Sikh religion, which prohibits tobacco consumption. This interstate level variation reflects distinct regional and cultural influences or impact of tobacco related public policies in different states. (19) Cytomorphological changes could also depend on the duration, frequency and quality (obnoxious nature) of tobacco products. (15) It could also be because normal cells exfoliating in enormous numbers outnumber the total number of abnormal cells available for cytologic sampling or dysplastic or cancerous cells are obscured by keratin formation thus impeding recognition of cellular and nuclear abnormalities. (20)
We found no significant alterations in cellular and nuclear dimensions in tobacco users but it did not imply that tobacco was slower in producing cellular alterations as only quantitative parameters were considered for comparison of the values. So it could be hypothesized that tobacco use also resulted in qualitative alterations that were not analyzed in our study. Einstein and Sivapathasundaram (2005) stated that the “combustion end products and heat produced by smoking is more harmful to mucosa than local effects of tobacco products like tobacco specific nitrosamines (TSNA) which are weak carcinogens in tobacco chewers”. (14) However as considered earlier Ogden, Cowpe and Green (1990) refuted the smoking tobacco as primary etiological factor in the development of oral cancer. (18) The incidence of oral cancer unlike lung cancer did not show an appreciable increase with the increased consumption of cigarette(s). (21) Combined use of alcohol with tobacco appeared to be a significant risk factor for oral cancer with distinct cytomorphological changes. (22) This was one of the reasons we excluded alcoholic patients from our study groups.
The malignant cells from cancerous lesions of the mouth freely exfoliate and smears prepared could serve as essential diagnostic aids. However cellular appearance of benign oral lesion did not show appreciable or consistent differences from each other to permit diagnosis of lesions by cytological examination alone. (23) In our study, we found that the mean ND (10.25 μm) and NA (84.17 μm2) values of keratinocytes were significantly higher in OSCC lesions (p=0.000) when compared with those from the tobacco users and normal patients (Figure. 2, 3). This was supported by Cowpe (1984) who suggested that malignant cells display a significant increase in mean NA. (13) Johnston (1952) supported the view that malignant cells showed smaller cytoplasmic/nuclear area ratios than the normal cells. (24) Camilleri and Smith (1965) also found increased N/C ratio with progression from benign to malignant state. (25) Franklin and Smith (1980) reported that the N/C ratio relates nuclear and cytoplasmic volume (space) thus representing the significant cellular and nuclear alterations at a morphological level. (26)
Our study was in agreement with the findings of Khandelwal and Solomon (2010) who stated that “Cytomorphometric analysis of keratinocytes could serve as a useful adjunct in the early diagnosis of OSCC”. (5) In accordance with our study Ramesh et al (1998), Hande and Chaudhary (2010) also found significant cytomorphometric changes in malignant lesions. (27, 15) In contrast to our findings Cowpe, Longmore and Green (1988) concluded that nuclear size is not always a clear cytologic indication of dysplastic change. They found nuclear size to vary with advancing age in normal oral squames and did not find an increase in size in abnormal lesions. (28) Hegde V(2011) suggested that decrease in the mean cytoplasmic diameter of exfoliated buccal mucosal cells could serve as an early indicator of dysplastic change especially in lesions which appear histologically benign. (29)
Different studies have been carried out establishing the role of oral exfoliative cytology as a non-invasive alternative diagnostic tool for oral cancer, potentially malignant disorders, iron deficiency anemia and diabetes. Sumanthi et al (2012) found a significant increase in the average nuclear diameter (ND) and N/C ratio of the anemic group when compared to the control group without any significant changes in cell diameter (CD) (30)) Joshi PS and Kaijkar MS (2013) used Feulgen stain and morphometric analysis to differentiate dysplastic and malignant cells from normal ones and concluded that brush cytology could be of great value for monitoring and follow up of suspicious lesions. (31) Patel PV and Gujjari SK (2013) found significant alterations in the cellular pattern of gingival mucosa cells in a non-smoker diabetic, but the alteration was to a greater extent in smoker diabetics demonstrating a synergistic effect of smoking and diabetes on gingival mucosa. (32) Acharya S, Tayaar SA, Khwaja T (2013) found that gutkha chewing influences the cellular alterations and mean of nuclear dimensions of keratinocytes in gutkha chewers was much greater than that of healthy, but was lesser than the mean NA and ND of OSCC. Sankhla B (2014) suggested that diabetes can produce morphological and functional alterations in the oral epithelial cells, detectable by cytomorphometric assessment. (33)
On the basis of our findings and above mentioned studies we support the view that quantitative assessment of exfoliative squames could be an adjunctive technique for diagnosing oral cancer in early stages. Advanced computer assisted image analysis systems have surpassed subjective assessment based on clinical characteristics. (4)
Conclusion
Quantitative metric changes in cellular and nuclear morphology could be the early indicators of malignant alterations. Increase in nuclear and reduction in cellular dimension is an early morphometric alteration in squamous cell carcinoma patients. Following the anticipated increase in automation and the increase in number of lesions to be investigated in the future, it is hoped that cytology could be sufficiently well standardized as a diagnostic tool for early detection of oral malignancy. Measuring Nuclear DNA content with nuclear and cell morphometry would have been more discriminating. Evaluation of a greater number of cases is essential to establish the cut-off criterias of various quantitative parameters and to improve the sensitivity and specificity.
References
- 1.Tumuluri V, Thomas GA, Fraser IS. Analysis of the Ki67 antigen at the invasive tumour front of human squamous cell carcinoma. J OralPathol Med. 2002;31:598–604. doi: 10.1034/j.1600-0714.2002.00042.x. [DOI] [PubMed] [Google Scholar]
- 2.Kotnis A, Sarin Mulherkar R. Genotype, phenotype and cancer: role of low penetrance genes and environment in tumour susceptibility. J Biosci. 2005;30:93–102. doi: 10.1007/BF02705154. [DOI] [PubMed] [Google Scholar]
- 3.Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2009;45:454–60. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Pektas ZO, Keskin A, Gunhan O, Karslioglu Y. Evaluation of nuclear morphometery and DNA ploidy status for detection of malignant and premalignant oral lesions: quantitative cytologic assessment and review of methods for cytomorphometric measurements. J Oral Maxillofac Surg. 2006;64:628–35. doi: 10.1016/j.joms.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Khandelwal S, Solomon MC. Cytomorphological analysis of keratinocytes in oral smears from tobacco users and oral squamous cell carcinoma lesions - a histochemical approach. Int J Oral Sci. 2010;2:45–52. doi: 10.4248/IJOS10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diniz-Freitas M, Garcia-Garcia A, Crespo-Abelleira A, Martins-Carneiro JL, Gandara-Rey JM. Applications of exfoliative cytology in the diagnosis of oral cancer. Med Oral Patol Oral Cir Bucal. 2004;9:355–61. [PubMed] [Google Scholar]
- 7.Maraki D, Becker J, Boecking A. Cytologic and DNA cytometric very early diagnosis of oral cancer. J Oral Pathol Med. 2004;33:398–404. doi: 10.1111/j.1600-0714.2004.0235.x. [DOI] [PubMed] [Google Scholar]
- 8.Akbulut N, Oztas B, Kursun S, Evirgen S. Delayed diagnosis of oral squamous cell carcinoma: a case series. J Med Case Rep. 2011;5:291–4. doi: 10.1186/1752-1947-5-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acha A, Ruesga MT, Rodríguez MJ, Martínez de Pancorb MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Med Oral Patol Oral Cir Bucal. 2005;10:95–102. [PubMed] [Google Scholar]
- 10.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of normal oral squames: an age, site and sex-related survey. J R Soc Med. 1985;78:995–1004. doi: 10.1177/014107688507801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldsby JW, Newton GL, Staats OJ. Nuclear and cellular size variations in clinically normal exfoliated buccal mucosal cells. Acta Cytol. 1964;8:80–4. [PubMed] [Google Scholar]
- 12.Nayar AK, Sivapathasundaram B. Cytomorphometric analysis of exfoliated normal buccal mucosal cell. Ind J Dent Res. 2003;14:88–92. [PubMed] [Google Scholar]
- 13.Cowpe JG. Quantitative exfoliative cytology of normal and abnormal oral mucosal squames: preliminary communication. J R Soc Med. 1984;77:928–31. doi: 10.1177/014107688407701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Einstein TB, Sivapathasundaram B. Cytomorphometric analysis of buccalmucosa of tobacco users. Ind J Dent Res. 2005;16:4–26. [PubMed] [Google Scholar]
- 15.Hande AH, Chaudhary MS. Cytomorphometric analysis of buccal mucosa of tobacco chewers. Rom J Morphol Embryo. 2010;51:527–32. [PubMed] [Google Scholar]
- 16.Silverman S, Becks H, Farber SM. The diagnostic value of intraoral cytology. J Dent Res. 1958;37:195–205. doi: 10.1177/00220345580370020301. [DOI] [PubMed] [Google Scholar]
- 17.Ramesh T, Mendis BRRN, Ratnatunga N, Thattil RO. The effect of tobacco smoking and of betel chewing with tobacco on the buccal mucosa: a cytomorphometric analysis. J Oral Pathol Med. 1999;28:385–8. doi: 10.1111/j.1600-0714.1999.tb02108.x. [DOI] [PubMed] [Google Scholar]
- 18.Ogden GR, Cowpe JG, Green MW. Quantitative exfoliative cytology of normal buccal mucosa: effect of smoking. J Oral Pathol Med. 1990;19:53–5. doi: 10.1111/j.1600-0714.1990.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 19.Rani M, Bonu S, Jha P, Nguyen SN, Jamjoum L. Tobacco use in India: prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tobacco Control. 2003;12:1–8. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sciubba JJ. Improving detection of precancerous and cancerous oral lesions. Computer-assisted analysis of the oral brush biopsy. U.S. Collaborative Oral CDx Study Group. J Am Dent Assoc. 1999;130:1445–57. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 21.Ali I, Wani WA, Saleem K. Cancer scenario in India with future perspectives. Cancer Therapy. 2011;8:56–70. [Google Scholar]
- 22.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head & Neck. 2007;29:779–92. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 23.Singh A. Role of exfoliative cytology in oral lesions: with special reference to rule out malignancy. J Coll Med Sci Nepal. 2010;6(2):29–37. [Google Scholar]
- 24.Johnston DG. Cytoplasmic: nuclear ratios in the cytological diagnosis of cancer. Cancer. 1952;5:945–9. doi: 10.1002/1097-0142(195209)5:5<945::aid-cncr2820050510>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri GE, Smith CJ. Exfoliative cytology of early lesions of experimental oral cancer in the hamster. Arch Oral Biol. 1965;10:465–70. doi: 10.1016/0003-9969(65)90112-3. [DOI] [PubMed] [Google Scholar]
- 26.Franklin CD, Smith CJ. Stereological analysis of histological parameters in experimental premalignant hamster cheek pouch epithelium. J Pathol. 1980;130:201–15. doi: 10.1002/path.1711300309. [DOI] [PubMed] [Google Scholar]
- 27.Ramesh T, Mendis BRRN, Ratnatunga N, Thattil RO. Cytomorphometric analysis of squames obtained from normal oral mucosa and lesions of oral leukoplakia and squamous cell carcinoma. J Oral Pathol Med. 1998;27(2):83–6. doi: 10.1111/j.1600-0714.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 28.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of abnormal oral mucosal smears. J R SocMed. 1988;81(9):509–13. doi: 10.1177/014107688808100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde V. Cytomorphometric analysis of squames from oral premalignant and malignant lesions. J ClinExp Dent. 2011;3(5):e441–4. [Google Scholar]
- 30.Sumanthi J, Reddy GS, Anuradha CH, Chandrasekhar P, Prasad LK, Ramana Reddy BV. A study on cytomorphometric analysis of exfoliative buccal cells in iron deficiency anemic patients. Contemp Clin Dent. 2012;3(Suppl 2):S156–S159. doi: 10.4103/0976-237X.101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joshi PS, Kaijkar MS. Cytomorphometric Analysis of Oral Premalignant and Malignant Lesions using Feulgen stain and exfoliative brush cytology [Google Scholar]
- 32.Patel PV, Gujjari SK. Cytomorphometric analysis of the gingival epithelium in type 2 diabetic patients with and without smoking habit. J Cytol. 2013;30(2):109–115. doi: 10.4103/0970-9371.112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya S, Tayaar SA, Khwaja T. Cytomorphometric analysis of the keratinocytes obtained from clinically normal buccal mucosa in chronic gutkha chewers. J Cranio Max Dis. 2013;2:134. [Google Scholar]
- 34.Sankhla B, Sharma A, Shetty RS, Bolla SC, Gantha NS, Reddy PJ. Exfoliative cytology of buccal squames: A quantitative cytomorphometric analysis of patients with diabetes. Int Soc Prev Community Dent. 2014 Sep;4(3):182–7. doi: 10.4103/2231-0762.142024. (underlines not required) [DOI] [PMC free article] [PubMed] [Google Scholar]