Abstract
Objective
The primary purpose of this study was to clarify the influence of the early response to surgery on brain structure and cognitive function in patients with breast cancer. It was hypothesized that the structure of the thalamus would change during the early response after surgery due to the effects of anesthesia and would represent one aspect of an intermediate phenotype of postoperative cognitive dysfunction (POCD).
Methods
We examined 32 postmenopausal females with breast cancer and 20 age-matched controls. We assessed their cognitive function (attention, memory, and executive function), and performed brain structural MRI 1.5 ± 0.5 days before and 5.6 ± 1.2 days after surgery.
Results
We found a significant interaction between regional grey matter volume (rGMV) in the thalamus (P < 0.05, familywise error (FWE), small volume correction (SVC)) and one attention domain subtest (P = 0.001, Bonferroni correction) after surgery in the patient group compared with the control group. Furthermore, the changes in attention were significantly associated with sevoflurane anesthetic dose (r 2 = 0.247, β = ‒0.471, P = 0.032) and marginally associated with rGMV changes in the thalamus (P = 0.07, FWE, SVC) in the Pt group.
Conclusion
Our findings suggest that alterations in brain structure, particularly in the thalamus, may occur shortly after surgery and may be associated with attentional dysfunction. This early postoperative response to anesthesia may represent an intermediate phenotype of POCD. It was assumed that patients experiencing other risk factors of POCD, such as the severity of surgery, the occurrence of complications, and pre-existing cognitive impairments, would develop clinical POCD with broad and multiple types of cognitive dysfunction.
Introduction
As a result of the long-term survival of patients with breast cancer due to improved screening and treatment, the quality of life (QOL) of such patients has received increased attention. Maintaining QOL is now critical, particularly for patients in industrialized countries, because the number of patients with breast cancer worldwide is increasing by >1,000,000 annually [1,2]. Cognitive dysfunction as a result of cancer therapy affects the QOL of breast cancer patients [3,4]. Although the influence of chemotherapy on cognitive dysfunction has been well characterized [5], patients often experience cognitive dysfunction even before starting chemotherapy [6,7]. In the current study, we focused on cognitive dysfunction in patients with breast cancer after surgery, which is known as postoperative cognitive dysfunction (POCD), and assessed its potential impact on QOL [8,9].
The clinical features of POCD, such as the rates of complication and risk factors, have been elucidated, but its neuropathology remains unclear due to the gap between clinical and pre-clinical observations [10]. The clinical features of POCD encompass a variety of cognitive impairments [11], and several risk factors including advanced age, long duration of anesthesia, the level of surgical severity, the occurrence of complications, and pre-existing cognitive impairments [12–14]. POCD has been defined by the International Study of Post-Operative Cognitive Dysfunction (ISPOCD) as the deterioration of two or more cognitive functions from multiple cognitive domains [12,15]. Thus, in clinical terms, POCD comprises dysfunction in a variety of cognitive domains, and it seems to be associated with multiple risk factors [10]. In contrast, pre-clinical studies have demonstrated that the fundamental neuropathology underlying postoperative cognitive decline can be caused by a single risk factor, such as anesthesia or inflammation [16–18]. Thus, well-controlled clinical studies are required to better characterize the roles that individual risk factors play in clinical situations.
To address this issue, the present study used neuroimaging methods to assess the early responses of patients to general anesthesia following a low invasive surgery. To assess the responses to general anesthesia as a single risk factor for POCD, other risk factors were controlled using three criteria: 1) patients underwent surgery for breast cancer, which was selected because this procedure is unlikely to induce systemic inflammation; 2) clinical assessments for the study occurred prior to the initiation of adjuvant therapy; and 3) no patients had pre-existing cognitive impairments and no complications after surgery. Also, neuroimaging is a powerful tool for assessing common brain structural changes which could be identified as an intermediate phenotype, even if the cognitive changes are not apparent enough to be classed as a syndrome [19]. However, few POCD neuroimaging studies have been reported. Chen et al. reported that a smaller regional grey matter volume (rGMV) in the hippocampus before surgery was a risk factor for POCD at postoperative day 4 [20]. Kline et al. performed a longitudinal study demonstrating reductions in the total gray matter and regional hippocampus volumes 5–9 months after surgery [21]. Because of the high incidence of POCD soon after surgery [13], assessment of cognitive decline and structural brain changes in the early postoperative period may allow the detection of one neuropathological aspect of POCD.
The aim of the present study was to clarify the influence of the early response to surgical operations on brain structure and cognitive function in patients with breast cancer. We recruited 32 postmenopausal females with early-stage breast cancer (Pt), and 20 age-matched postmenopausal healthy controls (HC). We assessed cognitive functions (attention, memory, and executive functions), and collected brain structural magnetic resonance (MR) images 1.5 ± 0.5 days before (pre) and 5.6 ± 1.2 days after (post) surgery in both the Pt and HC groups.
Pre-clinical studies suggest that general anesthesia plays a key role in the development of POCD symptoms [10]. Although general anesthesia acts on various regions in brain, the thalamus seems to be a major target. For example, the volatile and intravenous anesthetics sevoflurane [22], propofol [23], and fentanyl [24] reduced cerebral blood flow, and sevoflurane [25] reduced regional glucose metabolism in the thalamus. The thalamus plays an important role in coordinating the flow of information in the brain, and integrating broad cognitive processes [26,27], including incoming sensory impulses of pain [28], the regulation of arousal and sleep [29,30], and anesthetic-induced loss of consciousness [31]. Therefore, we hypothesized that a reduction in thalamic rGMV shortly after surgery and cognitive dysfunction associated with a reduction in thalamic rGMV would be observed.
Materials and Methods
Participants
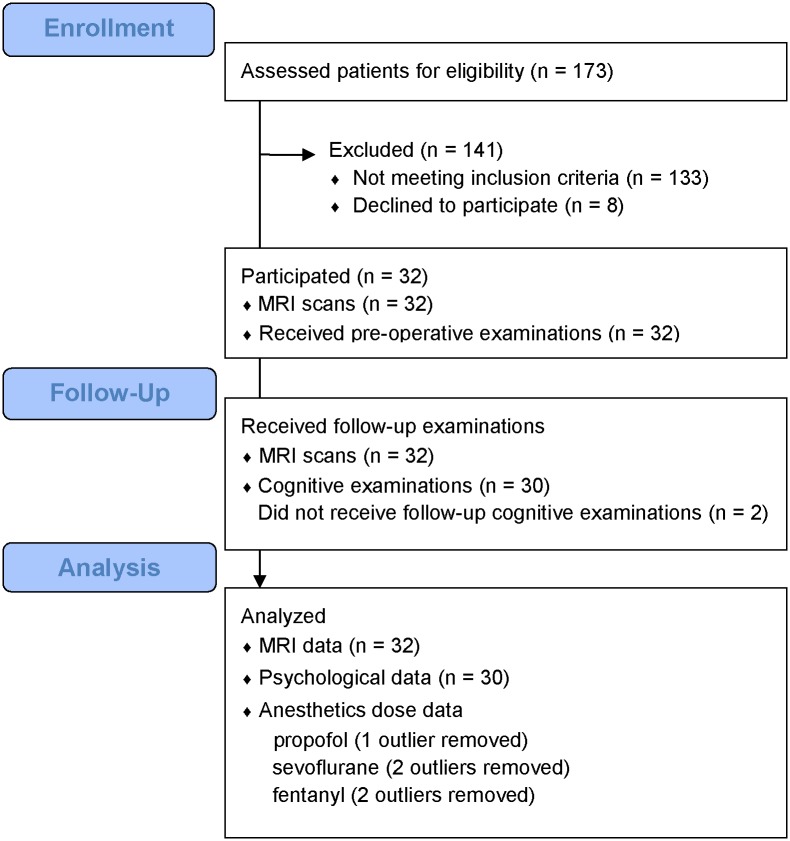
Patients with breast cancer who were planned to receive surgical operation were recruited from Tohoku University hospital between February 2012 and April 2013. The inclusion criteria were as follows: 1) female gender to minimize gender-based brain differences, and 2) post-menopausal females aged ≤ 80 years to reduce the influence of hormonal status on cognition. The following exclusion criteria were applied: 1) any history of cancer therapy, including chemotherapy and hormonal therapy; 2) any history of neurological disorders, traumatic brain injury, or psychiatric disorders; 3) any history of substance abuse and dependence; 4) diabetes, uncontrolled hypertension, or any organ failure; and 5) any contraindication to undergoing an MRI scan. One hundred and seventy-three women who were newly diagnosed with breast cancer were screened for eligibility. One hundred and thirty-three patients did not meet the study’s eligibility criteria. The most common reasons for ineligibility were prememopausal status and receiving neoadjuvant therapy. Eight patients declined to participate because they were either uninterested, too busy, or lived too far away. Thirty two patients were recruited and completed MRI scans. Thirty patients completed the cognitive examinations and 2 patients did not undergo a post-operative examination, even in the absence of physical complications after surgery. Thirty two MRI data and 30 psychological data were analyzed in patients (Fig 1). All clinical information was collected from medical records. We also recruited 20 healthy subjects who resided in the same geographical areas as the patients using advertisements in a local free newspaper in January 2013. The inclusion and exclusion criteria were identical to those for cancer patients, except for the requirement of a history of breast cancer surgery. Nineteen of the 20 healthy controls completed the study protocol, and one control participant could not undergo MRI scanning due to a metal finger ring that could not be removed at time on the scan. All subjects reported their height, weight, education history, and lifetime alcohol intake using self-administered questionnaires at the time of entry to the study. The questionnaires for alcohol intake included the duration of alcohol intake, the frequency of alcohol consumption per week, and the amount and types of alcohol consumed per day. Written informed consent was obtained from all subjects. The Ethics Committee of the Tohoku University Graduate School of Medicine approved this study. This study was conducted between February 2012 and May 2013 in Sendai city, Miyagi prefecture, Japan.
Fig 1. Enrollment, Participation, Follow-up, and Analysis of the patient group.
Protocol
Assessments were performed during hospitalization. Preoperative assessment was performed 1 or 2 days before surgery (pre), and postoperative assessment was performed within 1 week of surgery (post). The healthy group underwent two assessments, with a 1-week interval. Because the present study was observational, there were no restrictions on the types of anesthesia used. The study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN 000007287).
Psychological measurements
To evaluate the influence of surgery, we assessed a broad range of cognitive functions in five categories (attention, processing speed, memory, executive function, and working memory).
Attention was assessed using a Digit Cancellation Task (D-CAT) [32]. The test sheet consisted of 12 rows of 50 digits, which each contained five sets of the numbers 0–9 arranged in a random order. Participants were instructed to search for target number(s) with a slash mark as quickly and as accurately as possible for 1 min. Three trials were used: first with a single target number (6), second with two target numbers (9 and 4), and third with three (8, 3, and 7).
Processing speed was assessed using digit symbol coding, which is a subtest within the Wechsler Adult Intelligence Scale, third edition (WAIS-III) [33]. This test used a key, which shows a series of symbols paired with numbers; participants were instructed to draw each symbol under its corresponding number for 2 min.
Memory was measured by Logical Memory I (immediate) and II (30-min delay) tests, which are subtests of the Japanese version of the Wechsler Memory Scale-Revised (WMS-R) test [34–36]. Participants memorized two short, paragraph-length stories (stories A and B). The stories were scored in terms of the number of story units recalled, as specified in the WMS-R scoring protocol.
Executive function and processing speed were measured using the Stroop test [37,38], including response inhibition and impulsivity; we used Stroop 2 (the reverse Stroop task) and Stroop 4 (the Stroop task). To measure processing speed, we used Stroop 1 and 3.
Working memory was measured using digital span—backwards, which is a subtest of WAIS-III [33]. Participants were instructed to memorize numbers, and repeat them in reverse order.
We also evaluated quality of life using the Japanese version of the World Health Organization Quality of Life Instrument—Short Version; WHOQOL-BREF (WHO/QOL-26) [39].
Image acquisition
All MRI data were acquired using a 3-T Philips Intera Achieva scanner (Best, Netherlands). A magnetization-prepared rapid gradient echo (MPRAGE) sequence was used to collect high-resolution T1-weighted structural images (240 × 240 matrix; 6.5-ms repetition time; 3-ms echo time; 24-cm field of view; 162 slices; 1.0-mm slice thickness).
Voxel-based morphometry analysis (VBM)
VBM was performed to investigate the structural changes due to surgery. The structural data were pre-processed using statistical parametric mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Mathworks Inc., Natick, MA, USA). The present study employed diffeomorphic anatomical registration through exponentiated lie (DARTEL) algebra, which is an improved VBM method for registration that can more accurately achieve inter-subject brain image registration [40]. This procedure is advantageous for longitudinal data because rigid inter-subject registration data across different time points can be explored in a linear way for local differences.
To begin with, T1-weighted structural images of each individual were segmented into six tissue sections using the new segmentation algorithm implemented in SPM8. Default parameters were used for this new segmentation process, except for affine regularization, which was performed using the International Consortium for Brain Mapping (ICBM) template for East Asian brains. We then proceeded to the DARTEL registration process implemented in SPM8. During this process, we used DARTEL-imported images of the two-tissue probability map (TPM) of gray and white matter created using the abovementioned segmentation process. First, the template for the DARTEL procedures was created using T1WI data from the pre-scan of all participants in the study. Next, we used this template to perform DARTEL (using default parameters) for the pre- and post-scan T1WI of all subjects. The resulting images were then normalized spatially to the Montreal Neurological Institute (MNI) space to obtain images with 1.5 × 1.5 × 1.5-mm3 voxels. In addition, we performed a volume change correction (modulation) by modulating each voxel with the Jacobian determinants derived from the spatial normalization, which enabled regional differences in the absolute amount of brain tissue to be determined [41]. Subsequently, all images were smoothed by convolution with an isotropic Gaussian kernel of 8-mm full-width at half-maximum (FWHM).
Statistical analysis
Demographic differences for continuous variables (such as age) between the Pt and HC groups were calculated using two sample t-tests. To examine the general deterioration of cognitive function and QOL soon after surgery, we compared Pt with HC using analysis of covariance (ANCOVA) models. We used the post score as the dependent variable, the group (Pt, HC) as the categorical variable, and the pre score and age as covariates. Statistical significance was assessed at P < 0.0045 using Bonferroni corrections for comparison of multiple psychological measurements (P < 0.05/11). The results that differed significantly between groups were subsequently subjected to the correlation analyses described below.
To determine whether anesthesia was associated with the cognitive functions, which deteriorated in the Pt group compared to the HC group after surgery, we performed multiple regression analysis in the Pt group. Because the sample size of this analysis was relatively small, we used the change ratio of cognitive scores, which was obtained by dividing the post—pre scores by the pre scores, as dependent variables to keep the number of covariates appropriately and include pre-surgery cognitive performance in the analyses. We analyzed using the change ratio of differential cognitive measures as dependent variables, the doses of sevoflurane and fentanyl as independent variables, and age, height, and weight as covariates. Although we also used propofol for general anesthesia, we examined the correlation only with sevoflurane and fentanyl, because there were small individual, body-size-dependent differences in the propofol dose due to bolus usage. The anesthetics dose data that were 1.5 SD more or less than the mean were excluded from the analysis as outliers. The data from propofol, sevoflurane and fentanyl doses had one, two, and two patients as outliers, respectively, who were all different individuals. In addition, as described before, two patients did not undergo a post-operative examination. Therefore, we performed correlation analyses between psychological and anesthetics data, including sevoflurane and fentanyl doses from 28 patients, respectively. The threshold for statistical significance was set at P < 0.05 (one-tailed), with Bonferroni corrections for multiple comparisons for the amount of anesthetics (sevoflurane and fentanyl), with a strong priori hypothesis that the cognitive function would deteriorate due to the use of anesthetics. All statistical analyses were performed using SPSS 20 (SPSS, Chicago, IL).
Differences in rGMV were assessed as a group (Pt/HC) by time (pre/post) interaction using an analysis of covariance (ANCOVA) model on SPM8. The analyses were performed using age as a covariate. Small volume correction (SVC) was applied to one ROI based on the hypothesis (thalamus) using anatomical masks from the “Human aal atlas” within the Wake Forest University PickAtlas 3.04 (http://fmri.wfubmc.edu/software/PickAtlas) [42,43]. The ROI was defined from bilateral hemispheres. A significance level was set at P = 0.05 corrected for multiple comparisons (voxel-level family-wise error). In the analyses, we included only voxels that showed GMV values >0.10 to avoid possible partial volume effects around the borders between grey and white matter, as well as between grey matter and cerebrospinal fluid. Next, multiple regression analyses were performed for the patient group within the ROI (thalamus) to determine whether the reductions in rGMV were associated with the cognitive decline, and with anesthesia. To verify the relationship between the reduced rGMV and cognitive decline, the change ratios of the differential cognitive measures, which differed significantly between the Pt and HC groups, were included as dependent variables. The post-pre rGMV was used independent variables and age was treated nuisance covariates. To verify the relationship between the reduced rGMV and anesthesia, the post—pre rGMV was included as a dependent variable, the doses of the differential anesthetics were used as independent variables, and age were treated as nuisance covariates. Again, we excluded all voxels with GMV values >0.10. The significance level was set at P = 0.05 corrected for multiple comparisons (voxel-level family-wise error).
Results
Participant demographics and clinical data
No significant differences (P < 0.05) were found between the Pt and HC groups in terms of demographic characteristics (Table 1). The clinical data describing surgery are presented in Table 2. All surgeries were performed under general anesthesia. Although there were no restrictions on the type of anesthesia used in the current protocol, the same combination of anesthetics were used (propofol, sevoflurane, and fentanyl), except for in two individuals (remifentanil or nitrous oxide was added to the above combination of anesthetics). No patients experienced clinical complications, including delirium.
Table 1. Demographic characteristics of patients and controls subjects.
| Pt; n = 30 | HC; n = 19 | P value | |
|---|---|---|---|
| Age (years) | 60 ± 7 | 59 ± 5 | 0.69 |
| Height (cm) | 158 ± 4 | 157 ± 6 | 0.34 |
| Weight (kg) | 57 ± 9 | 52 ± 6 | 0.08 |
| Education (years) | 13 ± 2 | 14 ± 2 | 0.14 |
| Alcohol consumption (years × g/week) | 135 ± 263 | 83 ± 221 | 0.47 |
| Left-handed (%) | 1 (3) | 0 (0) |
Values are expressed as means ± SD, or numbers (%).
P values are derived from t-tests for continuous variables.
Abbreviations: Pt, Patients; HC, healthy controls.
Table 2. Surgery and postoperative data in the patient group.
| Pt; n = 32 | |
|---|---|
| Breast cancer stage 0 or 1 (%) | 22 (69) |
| Total mastectomy (%) | 11 (34) |
| Duration of operation (min) | 136 ± 37 |
| Duration of anesthesia (min) | 180 ± 38 |
| Dose of propofol (mg) | 106 ± 17 a |
| Dose of sevoflurane (mL) | 59 ± 19 b |
| Dose of fentanyl (mg) | 0.28 ± 0.05 b |
| Post-operative hospital stay (day) | 8.9 ± 1.4 |
Values are expressed as mean ± SD, or numbers (%).
Abbreviations: Pt, Patients.
a One outlier was excluded.
b Two outliers were excluded.
Psychological assessment
A significant group (Pt/HC) by time (pre/post) interaction was observed in the DCAT1 scores, indicating poor attentional performance in the patient group after surgery (Table 3). Although the attentional scores in the Pt group showed no significant decline. However, the significant group by time interaction provides sufficient evidence of cognitive decline as a lack of learning effect [44,45].
Table 3. Summary of neuropsychological assessment.
| Pt; n = 30 | HC; n = 19 | P value | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre (base line) | Post (1 week) | |||
| Attention | ||||||
| D-CAT1 | 29.2 ± 5.2 | 31.4 ± 5.1 | 29.5 ± 5.4 | 35.5 ± 5.8 | 0.001 * | |
| D-CAT2 | 45.5 ± 8.1 | 48.0 ± 6.3 | 52.4 ± 9.9 | 48.8 ± 7.9 | 0.024 | |
| D-CAT3 | 50.3 ± 11.0 | 53.3 ± 9.7 | 59.0 ± 14.6 | 61.6 ± 12.2 | 0.28 | |
| Processing speed | ||||||
| Stroop 1 | 56.4 ± 10.3 | 57.9 ± 9.9 | 58.3 ± 9.7 | 63.2 ± 9.1 | 0.01 | |
| Stroop 3 | 39.9 ± 7.5 | 42.0 ± 7.2 | 46.1 ± 7.9 | 42.8 ± 6.3 | 0.005 | |
| Digit symbol cording | 68.8 ± 18.1 | 74.5 ± 18.2 | 77.5 ± 12.3 | 87.3 ± 14.1 | 0.06 | |
| Memory | ||||||
| Immediate story recall | 20.9 ± 6.9 | 29.8 ± 7.2 | 24.2 ± 4.7 | 29.5 ± 5.1 | 0.033 | |
| Delayed story recall | 16.3 ± 6.7 | 26.3 ± 8.5 | 20.1 ± 5.3 | 27.4 ± 6.0 | 0.27 | |
| Executive function | ||||||
| Stroop 2 | 48.6 ± 9.3 | 50.8 ± 9.6 | 53.0 ± 9.1 | 53.8 ± 8.2 | 0.60 | |
| Stroop 4 | 33.8 ± 8.7 | 34.8 ± 9.8 | 40.0 ± 11.4 | 41.2 ± 10.3 | 0.27 | |
| Working memory | ||||||
| Digit span (backward) | 5.9 ± 2.1 | 6.3 ± 2.4 | 7.0 ± 1.6 | 7.7 ± 1.8 | 0.17 | |
| Quality of life | ||||||
| WHO-QOL 26 (average overall) | 6.2 ± 0.8 | 6.3 ± 1.4 | 7.2 ± 1.2 | 7.2 ± 1.6 | 0.84 | |
Values are expressed as means ± SD.
P values express the between-group significance after 1 week, and were derived from ANCOVA models.
*Significant at P <0.0045 using Bonferroni correction in cognitive tests.
Abbreviations: Pt, Patients; HC, healthy controls; Pre, before surgery or baseline; Post, after surgery or 1 week after baseline; D-CAT, digit cancellation task; WHO QOL, World Health Organization quality of life.
Psychological test scores and anesthesia doses
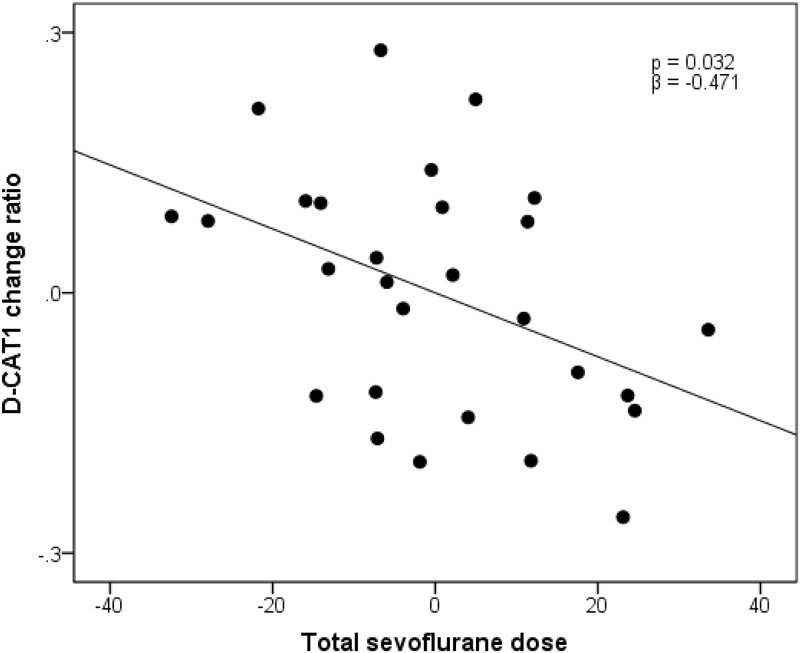
After controlling for age, weight, and height, sevoflurane dose was significantly associated with the change ratio of attention score (DCAT1) in the multiple regression analysis (r2 = 0.247, β = −0.471, P = 0.032; Fig 2). In contrast, there was no significant association between the change ratio of attention score (DCAT1) and fentanyl dose (r 2 = 0.186, P = 0.111).
Fig 2. Association between attention (D-CAT1) and anesthesia (sevoflurane) in the patient group.
Residual plots with trend lines depicting the correlations between residuals in the multiple regression analyses, using the change ratio of D-CAT1 scores as the dependent variable, and the total dose of sevoflurane and other confounding factors as independent variables. Two patients who did not receive post-operative examination and 2 patients who were assigned as outliers of the sevoflurane dose were removed from the analysis. Therefore the data from 28 patients were analyzed. Abbreviations: D-CAT, digit cancellation task.
rGMV differences
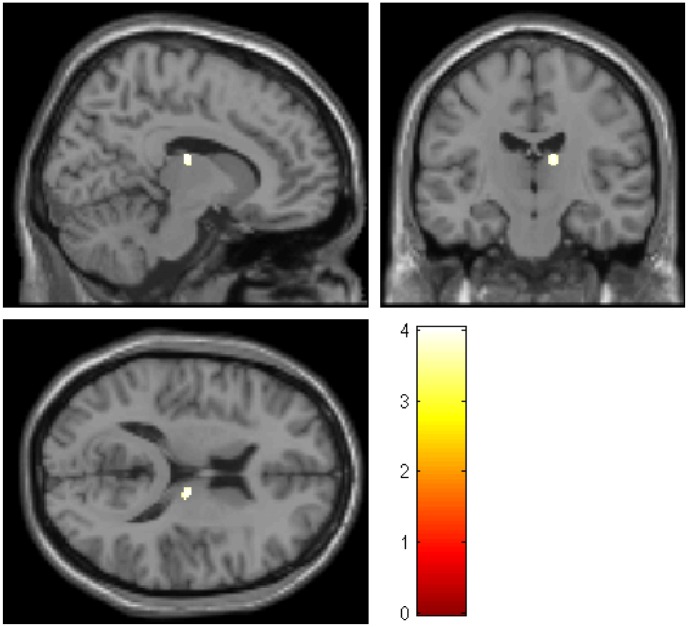
Among the areas with a priori hypothesis related to anesthesia, a significant group (Pt/HC) by time (pre/post) interaction was found in the right thalamus (x = 12, y = −16, z = 15; P < 0.05, SVC; Fig 3). The mean pre-rGMV at the peak voxel in each cluster was 0.178 ± 0.028, and the mean post-rGMV was 0.156 ± 0.029 in the Pt group. The mean pre-rGMV at the peak voxel was 0.156 ± 0.033, and the mean post-rGMV was 0.191 ± 0.035 in the HC group.
Fig 3. Regions showing the significant group (Pt/HC) by time (pre/post) interactions (right thalamus; p < 0.05, SVC).
The colored bar shows the scale of the t-value. Abbreviations: Rt, right; Pt, patients; HC, healthy controls; Pre, before surgery; Post, after surgery.
Psychological test scores and rGMV in the Pt
We found a marginally significant correlation between the change ratio of one attention domain subtest (D-CAT1) and post−pre thalamic rGMV (x = 8, y = −12, z = 7; P = 0.07, SVC).
Anesthesia and rGMV in the Pt
There was no significant association between the anesthesia (sevoflurane) and the reduction in rGMV in the thalamus.
Discussion
This was the first study to investigate brain structural change during the early phase responses to a low invasive surgery. Consistent with our hypothesis, we found rGMV reduction in the thalamus, which was a priori ROI given its association with anesthesia, soon after breast cancer surgery. These responses were thought to be caused by general anesthesia because other POCD risk factors, such as pre-existing cognitive impairments, systemic inflammation, and the occurrence of complications, were controlled. In addition, the Pt group showed a significant decrease in the learning effect on one subtest of the attention domain compared with the HC group. Furthermore, sevoflurane dose was significantly associated with changes in the attention score in the Pt group. Although the association between changes in thalamic rGMV and Pt attention scores did not reach the expected threshold, the correlation was marginally significant.
Our findings suggest that changes in attention and brain structures, particularly in the thalamus, may occur after surgery and may be associated with attentional dysfunction. This finding is consistent with those from previous longitudinal studies [10,46]. Attention is a fundamental cognitive function, and is associated with many other cognitive functions, including memory [47], processing speed [48], and working memory [49]. Among the subscales of the D-CAT battery, the D-CAT1 requires only sustained attention, whereas the D-CAT2 and D-CAT3 require the additional ability of selective attention and working memory [50]. Therefore, it was assumed that the purest attentional change would be detected using this subscale. In addition, the thalamus plays a fundamental role in cognitive functions, such as coordinating information flow in the brain, integrating broad cognitive processes [26,27] including incoming sensory impulses of pain [28], and regulating arousal and sleep [29,30]. As for attentional domain, the thalamus is associated with top-down modulation of attention with the frontal and parietal (usually right-lateralized) cortices [51,52]. Also, positron emission tomography (PET) study of healthy volunteers revealed activation of brain networks, including the thalamus, during an attentional orientation task [53]. With regard to specific thalamic regions, we observed a reduction in the volume of the lateral dorsal thalamus, and volume changes in the medial dorsal thalamus were correlated with changes in attention. The lateral dorsal thalamus exhibits connectivity with the precuneus [54], which plays a central part in the default mode network (DMN) [55]. Because the activity of DMN competes with those associated with attention for external stimuli [55], it is possible that the lateral dorsal part contributes to attentional dysfunction via the precuneus. On the other hand, the medial dorsal thalamus is strongly connected with the prefrontal cortex [56], which plays a critical role in attentional control [57] and may be involved in attentional dysfunction.
Our findings, together with those from previous studies, indicate that the attentional dysfunction and reduced thalamic volume observed in patients may represent an intermediate phenotype of POCD that manifest without broad cognitive dysfunction. Moreover, we believe that patients with suchl changes are at risk for POCD. Previous clinical studies have observed a high incidence of POCD soon after surgery, but rapid attenuation of these symptoms as time progressed [13], and a review of the risk factors for POCD determined that they result in multiple types of cognitive dysfunction [10]. In contrast, pre-clinical studies have demonstrated that each individual risk factor, including general anesthesia and systemic inflammation, can cause cognitive decline in particular domains under well-controlled experimental settings [16,17]. Based on these findings, it was assumed that all of the patients who had undergone surgery with general anesthesia were similarly affected by the anesthesia and that a majority of these patients would fully recover after surgery in the absence of exposure to other POCD risk factors. However, if other risk factors such as advanced age, long duration of anesthesia, high severity of the surgery, post-surgical complications, and pre-existing cognitive impairments were present, then some of the patients would develop clinical POCD with multiple and broad types of cognitive dysfunction [12–14].
A lot of knowledge about a relationship between general anesthesia and thalamus support our assumption about the short-term effect of anesthesia on POCD. A number of studies demonstrated the impact of anesthesia on the brain. Neurotoxicity of anesthesia, including sevoflurane, has been reported in animal studies to induce neuronal cell death [18,58,59]. In human studies, it was demonstrated that the thalamus is a key target for the actions of anesthetics [60]. In addition, anesthetics (including sevoflurane) reduced regional cerebral blood flow [22] and regional glucose metabolism [25] in the thalamus. Additionally, the potential involvement of anesthesia in the development of POCD is a much-debated topic. Some studies have indicated that the risk of POCD increases when general anesthesia is used [61], but a meta-analysis examining the relationship between type of anesthesia (regional or general) and POCD concluded that general anesthesia does not contribute to long-term POCD [62]. Our findings indicate that general anesthesia is a risk factor for POCD in the early postoperative period. However, it is likely that additional risk factors are necessary for the development of POCD. Thus, compared with other risk factors, the effect of general anesthesia may be too subtle to contribute to long-term POCD.
Our study had several strengths and limitations that should be discussed. The strengths of this study included controlling a number of risk factors for POCD, the prospective cohort design, a uniform protocol of MRI scanning at two time points, and the homogeneity of lower surgical stress. According to these strengths, we successfully detected thalamic volume reduction and attentional dysfunction as an intermediate phenotype of POCD. Limitations of this study included the relatively small sample size, which might have prevented the detection of more subtle effects on cognitive function. Another limitation of the present study is that the significant group-by-time interaction in the right thalamus was based not only on decreased thalamic volume in the patient group but also on increased thalamic volume in the control group. We conclude that the short-term intervention with psychological tests was responsible for the increased rGMV in the HCs. A previous study found significant increases in rGMV following a very short-term intervention (90 min at most) [63]. Thus, it is possible that a short-term intervention of several psychological tests (about 2 h) relevant to the learning effects observed in the psychological measures could have caused structural changes in the brains of HCs. Thus, we believe that our finding of altered thalamic volume after surgery was reliable. Moreover, we could not completely exclude the effects of inflammation. We did not measure inflammatory factors to estimate effects of inflammation. However, a previous animal study found that low invasive surgery did not impair hippocampal-dependent cognitive function [64], which is thought to be highly vulnerable to inflammation [65]. Thus, inflammation-mediated changes were unlikely to have contributed to cognitive dysfunction in patients after low invasive surgery, such as that for breast cancer. Finally, because there was no direct relationship between the amount of anesthetic used and the change in thalamic volume, it was not possible to conclude whether the anesthesia could have contributed to this reduction based on the present findings. However, a significant amount of previous evidence supports this relationship [22–25].
Despite these limitations, we detected significant changes in cognitive function and brain structure in the early postoperative stage. In general, the clinical symptoms of POCD are too heterogeneous to accurately clarify its neuropathology, but the present findings can provide new insights for future studies aiming to improve the QOL of patients who receive surgery.
Supporting Information
(DOCX)
(DOCX)
(PDF)
(PDF)
Acknowledgments
We thank our study participants, the psychological test examiners, and all of our colleagues at the Institute of Development, Aging, and Cancer and at Tohoku University Hospital for their support.
Financial Disclosure
Financial support for this study was provided by a Grant for Special Project Research from the International Research Institute of Disaster Science, a Grant-in-Aid for Young Scientists (B) (KAKENHI 24790653) from the Ministry of Education, Culture, Sports, Science and Technology in Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
Our study was conducted using a grant (A Grant-in-Aid for Scientists (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology etc.). Ryuta Kawashima, the principal investigator, receives royalty income from Nintendo Inc. "Nintendo 3DS", "ONI training", "Tetris" which are products of Nintendo Inc., which were planned to be used in an intervention study involved in the whole protocol of “Neurological underpinnings of cognitive dysfunction in breast cancer patients.” However, the current study did not employ intervention using these games. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Data Availability
All imaging files and cognitive data are available from the Harvard Dataverse Network (doi: 10.7910/DVN/C1NI4T).
Funding Statement
Financial support for this study was provided by a Grant for Special Project Research from the International Research Institute of Disaster Science, a Grant-in-Aid for Young Scientists (B) (KAKENHI 24790653) from the Ministry of Education, Culture, Sports, Science and Technology in Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. Epub 2011/02/26. 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. Epub 2011/02/08. 10.3322/caac.20107 . [DOI] [PubMed] [Google Scholar]
- 3. Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K. Mild Cognitive Impairment after Adjuvant Chemotherapy in Breast Cancer Patients—Evaluation of Appropriate Research Design and Methodology to Measure Symptoms. Breast Cancer. 2005;12. Epub 287. [DOI] [PubMed] [Google Scholar]
- 4. Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Titus-Ernstoff L, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23(19):4399–405. Epub 2005/07/05. 10.1200/JCO.2005.03.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–86. Epub 2012/09/26. 10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Correa DD, Ahles TA. Neurocognitive changes in cancer survivors. Cancer J. 2008;14(6):396–400. Epub 2008/12/09. . [DOI] [PubMed] [Google Scholar]
- 7. Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109(9):1905–13. Epub 2007/03/14. 10.1002/cncr.22610 . [DOI] [PubMed] [Google Scholar]
- 8. Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, et al. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68(3):369–75. Epub 2006/06/02. . [DOI] [PubMed] [Google Scholar]
- 9. Newman MF, Grocott HP, Mathew JP, White WD, Landolfo K, Reves JG, et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke; a journal of cerebral circulation. 2001;32(12):2874–81. Epub 2001/12/12. . [DOI] [PubMed] [Google Scholar]
- 10. Hovens IB, Schoemaker RG, van der Zee EA, Heineman E, Izaks GJ, van Leeuwen BL. Thinking through postoperative cognitive dysfunction: How to bridge the gap between clinical and pre-clinical perspectives. Brain Behav Immun. 2012;26(7):1169–79. Epub 2012/06/26. 10.1016/j.bbi.2012.06.004 . [DOI] [PubMed] [Google Scholar]
- 11. Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106(3):572–90. Epub 2007/02/28. . [DOI] [PubMed] [Google Scholar]
- 12. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–61. Epub 1998/04/03. . [DOI] [PubMed] [Google Scholar]
- 13. Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. Epub 2007/12/25. 10.1097/01.anes.0000296071.19434.1e . [DOI] [PubMed] [Google Scholar]
- 14. Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108(1):8–17. Epub 2007/12/25. 10.1097/01.anes.0000296072.02527.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275–89. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 16. Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518–22. Epub 2010/11/03. 10.1073/pnas.1014557107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, et al. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol B ol Psychiatry. 2010;34(8):1426–32. Epub 2010/08/10. 10.1016/j.pnpbp.2010.07.027 . [DOI] [PubMed] [Google Scholar]
- 18. Hofacer RD, Deng M, Ward CG, Joseph B, Hughes EA, Jiang C, et al. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 2013;73(6):695–704. Epub 2013/03/26. 10.1002/ana.23892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet neurology. 2010;9(1):119–28. Epub 2010/01/20. 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen MH, Liao Y, Rong PF, Hu R, Lin GX, Ouyang W. Hippocampal volume reduction in elderly patients at risk for postoperative cognitive dysfunction. Journal of anesthesia. 2013;27(4):487–92. Epub 2013/02/02. 10.1007/s00540-012-1548-6 . [DOI] [PubMed] [Google Scholar]
- 21. Kline RP, Pirraglia E, Cheng H, De Santi S, Li Y, Haile M, et al. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116(3):603–12. Epub 2012/02/02. 10.1097/ALN.0b013e318246ec0b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlunzen L, Vafaee MS, Cold GE, Rasmussen M, Nielsen JF, Gjedde A. Effects of subanaesthetic and anaesthetic doses of sevoflurane on regional cerebral blood flow in healthy volunteers. A positron emission tomographic study. Acta Anaesthesiol Scand. 2004;48(10):1268–76. Epub 2004/10/27. 10.1111/j.1399-6576.2004.00505.x . [DOI] [PubMed] [Google Scholar]
- 23. Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19(13):5506–13. Epub 1999/06/23. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg. 1997;84(1):120–6. Epub 1997/01/01. . [DOI] [PubMed] [Google Scholar]
- 25. Schlunzen L, Juul N, Hansen KV, Gjedde A, Cold GE. Regional cerebral glucose metabolism during sevoflurane anaesthesia in healthy subjects studied with positron emission tomography. Acta Anaesthesiol Scand. 2010;54(5):603–9. Epub 2010/01/21. 10.1111/j.1399-6576.2010.02208.x . [DOI] [PubMed] [Google Scholar]
- 26. Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102(34):12212–7. Epub 2005/08/17. 10.1073/pnas.0407259102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sherman SM, Guillery RW. Exploring the Thalamus and Its Role in Cortical Function, 2nd ed Cambrige: The MIT Press; 2009. [Google Scholar]
- 28. Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–72. Epub 2000/06/10. . [DOI] [PubMed] [Google Scholar]
- 29. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–85. Epub 1993/10/29. . [DOI] [PubMed] [Google Scholar]
- 30. Franks NP, Zecharia AY. Sleep and general anesthesia. Can J Anaesth. 2011;58(2):139–48. Epub 2010/12/21. 10.1007/s12630-010-9420-3 . [DOI] [PubMed] [Google Scholar]
- 31. White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage. 2003;19(2 Pt 1):402–11. Epub 2003/06/20. . [DOI] [PubMed] [Google Scholar]
- 32. Hatta T, Ito Y, Yoshizaki K. D-CAT manual (Screening test for attention). Osaka: Union Press; 2006. [Google Scholar]
- 33. Wechsler D. Wechsler Adult Intelligence Scale Third edition San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 34. Wechsler D. Memory scale-Revised. New York: The Psychological Corporation; 1987. [Google Scholar]
- 35. Sugishita M. Wechsler Memory Scale-Revised (Japanese). Tokyo: Nihonbunkakagakusha; 2001. [Google Scholar]
- 36. Omura K. Simultaneous confirmatory factor analysis of the Wechsler memory scale—revised for two standardization samples: a comparison of group from Japan and the United States In: Sugishita M, editor. J Clin Exp Neuropsychol; 2004. p. 645–52. [DOI] [PubMed] [Google Scholar]
- 37. Stroop RJ. Studies of interference in serial verbal reactions. Journal of Experimental Psychology 1935. p. 643–62. [Google Scholar]
- 38. Hakoda Y. Group version of the Stroop and reverrse-Stroop test: the effects of reaction mode, order and In: Sasaki M editor. Kyoikushinrigakukenkyu (Educ.Psychol.Res); 1990. p. 389–94. [Google Scholar]
- 39. Tazaki M, Nakane Y. WHOQOL-26 information. Tokyo: Kaneko Shobou; 1997. [Google Scholar]
- 40. Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. Epub 2009/02/07. 10.1016/j.neuroimage.2008.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaster C. VBM Toolbox for SPM8 2011. Available: http://dbm.neuro.uni-jena.de/vbm8/vbm8-r361.zip.
- 42. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–31. Epub 2000/07/27. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–9. Epub 2003/07/26. . [DOI] [PubMed] [Google Scholar]
- 44. Zehnder AE, Blasi S, Berres M, Spiegel R, Monsch AU. Lack of practice effects on neuropsychological tests as early cognitive markers of Alzheimer disease? Am J Alzheimers Dis Other Demen. 2007;22(5):416–26. Epub 2007/10/26. 10.1177/1533317507302448 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol. 2008;19(4):623–9. Epub 2007/11/03. 10.1093/annonc/mdm500 . [DOI] [PubMed] [Google Scholar]
- 46. Hedayati E, Schedin A, Nyman H, Alinaghizadeh H, Albertsson M. The effects of breast cancer diagnosis and surgery on cognitive functions. Acta Oncol. 2011;50(7):1027–36. Epub 2011/05/11. 10.3109/0284186X.2011.572911 . [DOI] [PubMed] [Google Scholar]
- 47. Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr Opin Neurobiol. 2007;17(2):177–84. Epub 2007/03/24. 10.1016/j.conb.2007.03.005 . [DOI] [PubMed] [Google Scholar]
- 48. Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proc Natl Acad Sci U S A. 2001;98(9):5363–7. Epub 2001/04/20. 10.1073/pnas.081074098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fougnie D. The Relationship between Attention and Working Memory: Nova Science Publishers; 2008.
- 50. Hatta T, Masui T, Ito Y, Ito E, Hasegawa Y, Matsuyama Y. Relation between the prefrontal cortex and cerebro-cerebellar functions: evidence from the results of stabilometrical indexes. Applied neuropsychology. 2004;11(3):153–60. Epub 2004/12/14. 10.1207/s15324826an1103_3 . [DOI] [PubMed] [Google Scholar]
- 51. Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. Epub 1990/01/01. 10.1146/annurev.ne.13.030190.000325 . [DOI] [PubMed] [Google Scholar]
- 52. Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(4):343–61. Epub 1998/07/08. . [DOI] [PubMed] [Google Scholar]
- 53. Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain: a journal of neurology. 1997;120 (Pt 3):515–33. Epub 1997/03/01. . [DOI] [PubMed] [Google Scholar]
- 54. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain: a journal of neurology. 2006;129(Pt 3):564–83. Epub 2006/01/10. 10.1093/brain/awl004 . [DOI] [PubMed] [Google Scholar]
- 55. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. Epub 2008/04/11. 10.1196/annals.1440.011 . [DOI] [PubMed] [Google Scholar]
- 56. Ketz NA, Jensen O, O'Reilly RC. Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends in neurosciences. 2015;38(1):3–12. Epub 2014/12/03. 10.1016/j.tins.2014.09.007 . [DOI] [PubMed] [Google Scholar]
- 57. Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2009;192(3):489–97. Epub 2008/11/26. 10.1007/s00221-008-1642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135(3):815–27. Epub 2005/09/13. 10.1016/j.neuroscience.2005.03.064 . [DOI] [PubMed] [Google Scholar]
- 59. Bercker S, Bert B, Bittigau P, Felderhoff-Muser U, Buhrer C, Ikonomidou C, et al. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotoxicity research. 2009;16(2):140–7. Epub 2009/06/16. 10.1007/s12640-009-9063-8 . [DOI] [PubMed] [Google Scholar]
- 60. Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn. 2000;9(3):370–86. Epub 2000/09/20. 10.1006/ccog.1999.0423 . [DOI] [PubMed] [Google Scholar]
- 61. Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta anaesthesiologica Scandinavica. 1980;24(4):279–87. Epub 1980/08/01. . [DOI] [PubMed] [Google Scholar]
- 62. Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: A meta-analysis. Indian journal of anaesthesia. 2011;55(4):358–63. Epub 2011/10/21. 10.4103/0019-5049.84850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taubert M, Draganski B, Anwander A, Muller K, Horstmann A, Villringer A, et al. Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(35):11670–7. Epub 2010/09/03. 10.1523/JNEUROSCI.2567-10.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental gerontology. 2008;43(9):840–6. Epub 2008/07/08. 10.1016/j.exger.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fidalgo AR, Cibelli M, White JP, Nagy I, Noormohamed F, Benzonana L, et al. Peripheral orthopaedic surgery down-regulates hippocampal brain-derived neurotrophic factor and impairs remote memory in mouse. Neuroscience. 2011;190:194–9. Epub 2011/06/28. 10.1016/j.neuroscience.2011.05.073 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
(PDF)
Data Availability Statement
All imaging files and cognitive data are available from the Harvard Dataverse Network (doi: 10.7910/DVN/C1NI4T).