Abstract
Saprophagous (feeding on decaying matter) insects often use carbon dioxide (CO2) as a cue for finding food. Humus-feeding larvae of the giant rhinoceros beetle Trypoxylus dichotomus exhibit a clumped distribution in natural microhabitats, but the mechanisms driving the distribution were unknown. Herein, I examined whether larvae use CO2 as a cue for fermented humus and aggregate in the vicinity of the food. I found that (i) larvae of T. dichotomus are strongly attracted to CO2, (ii) larvae orient toward highly fermented humus when given a choice between highly and poorly fermented humus, (iii) the highly fermented humus emits more CO2 than the poorly fermented humus, and (iv) larvae grow larger when fed highly fermented humus rather than poorly fermented humus. The clumped distribution of larvae is probably formed along the concentration gradient of CO2 induced by heterogeneity of fermented organic materials in soil. My laboratory experiments also revealed that larvae are chemically attracted to each other. Moreover, CO2 concentrations in soil were increased by the larval respiration, and small amounts of CO2 (much less than emitted during respiration by a single larva) were sufficient for larval attraction. These results suggest that not only response to fermented food resources, but also respiratory CO2 from conspecifics may lead to aggregation. Enhanced densities resulted in reduced weight gain under experimental conditions. However, exploiting a high-value resource at enhanced densities still led to greater body weight compared to individually exploiting a low-value resource. This demonstrates the adaptive value of the response to CO2 sources in this species.
Introduction
Within a highly complex environment, insects need to locate their potential food resources. Chemical cues play a crucial role in finding distant resources. Especially for soil-living insects that cannot rely on visual cues, a broad range of chemical cues is used [1–3]. Among them, carbon dioxide (CO2) elicits a strong behavioural response in the majority of soil-living insects including phytophagous [1–6] and saprophagous species [7], [8]. Because CO2 is emitted from various sources other than food, the response of insects to CO2 is sometimes modulated by additional specific cues [1–3]. For example, larvae of the root-feeding weevil Sitona lepidus are not attracted to CO2, but initiate intensive searching behaviour to locate more specific chemical cues to their host in the presence of CO2 [4]. On the other hand, CO2 has been reported to represent a sufficient orientation cue for many species [9], [10]. Thus, they may be attracted to CO2 sources other than their food under natural conditions, which in turn may affect insect feeding and performance.
Larvae of the giant rhinoceros beetle Trypoxylus dichotomus inhabit the soil humus layer and feed on decaying organic matter. The larvae are known to exhibit a clumped distribution throughout their larval periods in natural humus microhabitats (Fig 1A) [11]. This clumped distribution is likely because of clumped deposition of eggs and heterogeneity of humus [11], but the mechanism driving the formation of the distribution remains unclear. I hypothesized that the larvae aggregate in a zone of high saprophytic activity using chemical cues, such as CO2 from microbial respiration. Furthermore, it has been suggested that the larvae of T. dichotomus are attracted to chemicals from conspecifics [11]. The chemicals have not been identified, but respiratory CO2 may be involved in conspecific attraction if larvae use CO2 to find food resources. Herein, I first examined whether the larvae were attracted to CO2 from food resources and conspecifics. Second, I examined whether the aggregation leads to decreased or increased larval performance in terms of weight gain. Although some group-living insects may gain net benefits by group formation [12–14], aggregation often increases competition for resources, negatively affecting performance [15], [16].
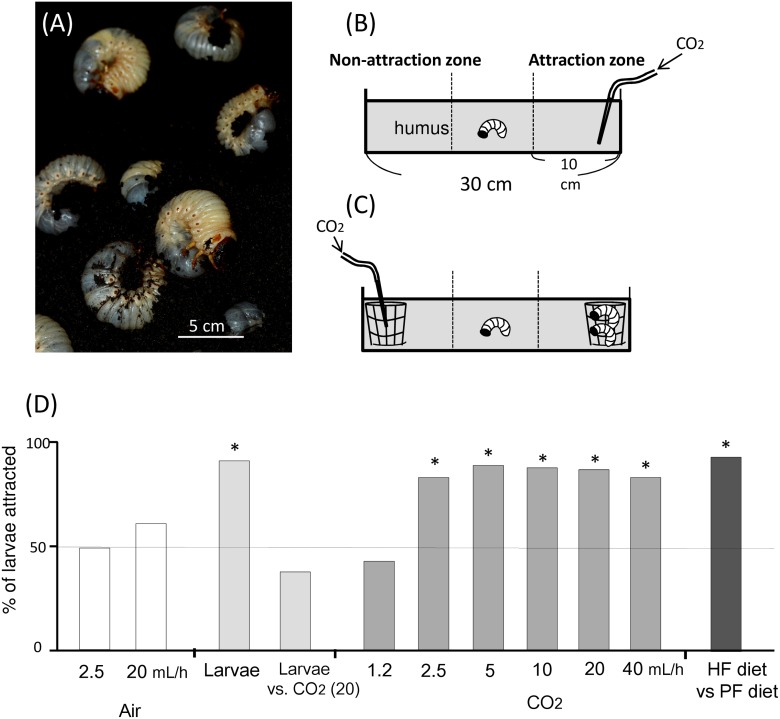
Fig 1. Trypoxylus dichotomus larval response to humus, conspecifics and synthetic CO2 .
(A) A group of 3rd instar larvae in a microhabitat. Soil above the larvae was removed. (B and C) Schematic representation of the behavioural arena established in a previous study [11]. In (B), the larval response to various amounts of CO2 (or air for the control) was tested. CO2 was pumped through a pipette into the soil using a syringe driver. In (C), larvae were given the choice of 20 mL/h of CO2 or two conspecific larvae in a mesh cage. In both systems, ‘attraction zones’ and ‘non-attraction zones’ were set within 10 cm from each end. (D) The percentage of larvae attracted to air, CO2, highly fermented (HF) humus [against poorly fermented (PF) humus] or to two larvae in a mesh cage. The total number of larvae found in the attraction zone was compared with that of larvae in the non-attraction zone. Asterisks indicate a significant difference (P < 0.05) from 50% (dashed line); analysed using the binomial test. The sample sizes (the sum of the larvae in the attraction and non-attraction zones) were 12, 17, 22, 20, 20, 22, 19, 16, 20, 15, and 16 from left to right.
Materials and Methods
All the experiments and insect rearing were conducted at 24.5–25.0°C and 60–70% relative humidity. The air in the laboratory was constantly exchanged using an air fan, and the concentration of CO2 was maintained at 420–450 ppm. The humus used was Sanyo-Bark (Sanyo Chip Kogyo Co. Ltd., Yamaguchi, Japan). However, in rearing experiments and the choice test between two types of humus, less-fermented bark that was sold for stag beetle rearing (Dorcus Owner’s Shop, Osaka, Japan) and fermented bark commercially sold for rearing of beetles belonging to the family Dynastinae (Dorcus Owner’s Shop) was used.
Insects
The Trypoxylus dichotomus larvae used in all experiments, except for the rearing experiments, were in the middle of their 3rd (final) instar (70–110 days old). These were individuals from a colony established from adults (n = 13) that had been collected from several fields in Tokyo during the summers of 2012 and 2013. T. dichotomus is not an endangered or protected species, and collection of unprotected insects in this area does not require special permission. Further details of the egg collection and larval rearing methods are available in Kojima et al. [11]. Briefly, females were individually reared in cages containing humus. Their eggs were collected once a week and individually introduced into 450-mL plastic cups filled with humus. The humus was renewed every 20–30 days. The body weight of the larvae used in the experiments was between 18–25 g. For rearing experiments, I used early-stage 2nd instar larvae (1.0–1.5 g).
Measurement of larval and soil respiration
I measured the emission rate of respiratory CO2 of T. dichotomus larvae and two types of humus used in the choice test and rearing experiments using a Vaisala CARBOCAP Hand-Held Carbon Dioxide Meter GM70 with a GMP222 infrared CO2 probe (Vaisala Oyj, Helsinki, Finland). The accuracy of the probes was ±2% of the reading. The system was equilibrated for 5 min prior to measuring the next sample.
A 3rd instar larva and a small electric fan were placed inside an 860-mL plastic cup, and a CO2 probe was inserted through a hole in the lid of the cup. To measure humus respiration, 80 g of humus soil was placed into a sealed plastic chamber (1.7 L) with the fan. I filled any gaps with paper clay to avoid exposure to air. The CO2 concentration in the cup or chamber linearly increased immediately after the introduction of a larva or humus. Immediately after and for 5 min after the introduction of a larva or humus, the CO2 concentration in the cup or chamber was recorded. CO2 production rate (nmol min-1 g-1) of a lava or humus was calculated from the concentration increments during 5 min. The measurement was replicated using 6 different samples or larvae.
Larval response to synthetic CO2 and humus
I investigated the responses of T. dichotomus larvae to conspecifics, synthetic CO2 and humus to clarify the the mechanism driving the formation of the larval clumped distribution. The experimental system, following the method described by Kojima et al. [11], is shown in Fig 1B and 1C.
Firstly, I tested whether T. dichotomus larvae were attracted to conspecifics. I buried two steel-wire screen cages (6 cm diameter × 7 cm height; mesh size, 3 mm), one containing only humus (Sanyo-Bark) and the other containing humus and two 3rd instar T. dichotomus larvae, at the ends of a rectangular plastic container (30 cm × 7 cm × 7 cm) filled with humus. I then placed the experimental larva at the centre of the container, and allowed it burrow into the humus for 1 h. The position of the larva’s head was recorded at the end of each experiment. Larvae found within 5 cm of the release point were excluded from data analysis (<20% in all the behavioural assays). The total number of larvae found in the attraction zone (n1) was compared with that in the non-attraction zone (n2). The null hypothesis, n1/(n1 + n2) = 0.5, was tested using the binomial test.
I tested whether T. dichotomus larvae were attracted to synthetic CO2. A 25-mL syringe was filled with CO2 (>99.5% purity) and connected to a silicon tube that was attached to a Pasteur pipette. CO2 was constantly pumped through the pipette using a syringe pump (Sanyo Chip Kogyo Co. Ltd., Kyoto, Japan), at a rate of 1.2, 2.5, 5, 10, 20, or 40 mL h-1. The pipette was buried in humus at the end of the rectangular cage, as shown in Fig 1B. I placed the experimental larva at the centre of the container and recorded the position of the larva’s head 1 h later. As a control, air instead of CO2 was provided at a rate of 2.5 or 20 mL h-1.
I tested whether T. dichotomus larvae prefer conspecifics to CO2, using the experimental system shown in Fig 1C. Two steel-wire screen cages containing only humus or humus and two 3rd instar T. dichotomus larvae were buried at opposite ends of the rectangular container. In the screen cage without insects, CO2 was provided through a Pasteur pipette at a rate of 20 mL h-1 (corresponding to the respiration of two larvae), as described above.
Furthermore, I tested if larvae preferred highly fermented humus to poorly fermented humus. Two steel-wire screen cages containing high-quality humus or low-quality humus (ca. 80 g) were buried at the opposite ends of the rectangular container. In this test, black dirt (Ratec, Gunma, Japan) was used as a substrate instead of humus. My preliminary experiment showed that black dirt emits only a small amount of CO2 (< 0.5 nmol min-1 g-1).
The humus soil in the rectangular container was stirred and the two ends were exchanged after each trial. The soil was renewed after 5 successive trials. Sample sizes are presented in the Fig 1D legends.
Effect of larval density on CO2 concentration
I determined if larvae increased CO2 concentration in humus. I randomly placed 24 T. dichotomus larvae into a plastic cage (44 cm × 66 cm × 40 cm in height) that contained humus to a depth of 30 cm. The larval density corresponded with that observed within natural microhabitats [11]. Another cage containing the same amount of humus was used as a control. After five days, the two cages were evenly divided into six squares (22 cm × 22 cm), and the soil CO2 concentration at a depth of 20 cm was recorded at the centre of each square using the aforementioned CO2 meter, the probe of which was covered with an in-soil adapter (Vaisala). I also measured soil temperature at a depth of 20 cm using a digital thermometer (Sato Keiryoki MFG. Co. Ltd., Tokyo, Japan) because larvae might increase soil temperature by activation of soil microbes. Immediately after measuring the CO2 and temperature in all six squares, I removed the humus and counted the number of larvae in each square of the treatment cage (i.e., the cage containing larvae). If larvae were on the border between squares, the position of the head was recorded. Furthermore, soil respiration apart from larval respiration was examined as an index of microbial activity because CO2 concentration in humus may be affected by the fermentation level of the humus. Eighty grams of humus soil was collected from the bottom of each square and immediately placed into a sealed plastic chamber (1.7 L). The CO2 concentration in the chamber linearly increased. Increments of CO2 concentration were measured using the CO2 meter for 5 min, and the rate of CO2 emission (nmol min-1 g-1) was calculated as described above. The mean CO2 concentration, soil respiration, and temperature for the six squares were calculated for each cage. All humus in the two cages (control and treatment) was renewed prior to the next trial. The trials were replicated eight times in total. A paired t-test was used for the comparison between the control and treatment.
I examined the relationship between larval density (i.e., the number of larvae in a square) and the CO2 concentration, soil respiration or soil temperature in the treatment cage using a linear mixed model (LMM). The CO2 concentration, soil respiration or soil temperature was used as an objective variable, and larval density was used as the explanatory variable. The baseline of CO2 concentrations were different among the eight trials (see Results), and thus, trial identity was included as a random factor in order to account for the variance. These analyses were performed using the statistical platform R v. 3.0.1 [17].
Effects of larval density and humus quality on body weight gain
I investigated whether (i) larvae grow larger when fed highly fermented rather than poorly fermented humus and (ii) larvae incur a fitness cost or benefit in terms of body weight gain when they aggregate. For this experiment, the larvae had been individually reared after hatching in fermented bark (highly-quality food). The 2nd instar larvae (1.0–1.5 g) were reared under solitary, medium-density (three larvae), and high-density (nine larvae) conditions for 5 days in 860 mL plastic cups. Larval heads were painted with an oil-based ink for identification. The cup was filled with highly fermented or poorly fermented humus. Larval body weight was measured before and after 5 days of treatment. The larvae were individually reared for a month after the treatment and their sex was determined at the 3rd instar [18]. The effects of food quality and density on larval weight after 5 days of treatment were determined using a LMM. The identity of the mother of each larva was included as a random factor because a previous study indicated that offspring body size varies depending on maternal identity [19]. Body weight after treatment was a response variable, and density, food quality, and the interaction between density and food quality were included as explanatory variables. The initial body weight and sex were also included as covariates. Since the interaction term was significant (see Results), post-hoc analyses were conducted under each nutrient condition using a LMM in which density, initial body weight and sex were entered as explanatory variables. Pairwise comparisons between treatments were subsequently conducted using the LMM with Tukey’s tests. The number of larvae used for each treatment was 26–41.
Results
Measurement of larval and soil respiration
The larvae of T. dichotomus emitted 356 ± 15 nmol min-1 g-1 (mean ± SE, n = 6) of CO2. The highly fermented and poorly fermented humus emitted 9.00 ± 0.23 (n = 6) and 2.09 ± 0.10 nmol min-1 g-1 (n = 6) of CO2, respectively.
Larval response to synthetic CO2 and humus
In the behavioural assays using two caged larvae, the frequency of larvae found was significantly greater in the attraction zone than in the non-attraction zone (P < 0.01 by the binomial test, Fig 1D). When various amounts of CO2 (1.2, 2.5, 5, 10, 20, or 40 mL h-1) were provided, larvae of T. dichotomus were attracted to 2.5–40 mL/h of CO2 within 1 h (Fig 1D). The larvae were not attracted to air (Fig 1D). When two caged larvae were tested against 20 mL h-1 of CO2, no significant difference was observed between the two choices (larvae vs. CO2; Fig 1D). In the choice between highly and poorly fermented humus, larvae were attracted to highly fermented humus (Fig 1D).
Effect of larval density on CO2 concentration
The soil CO2 concentration was 32 ± 8.1% (mean ± SE, n = 8 cages) higher in cages with insects than in those without insects (Fig 2A; t 7 = 3.82, P = 0.0065, paired t-test). In cages containing insects, the number of larvae within a square was positively correlated with the soil CO2 concentration (Fig 2B; t = 4.16, P < 0.001, LMM). Soil respiration in cages with insects was 27 ± 6.3% (mean ± SE, n = 8 cages) higher than that in cages without insects (Fig 2C; t 7 = 4.13, P = 0.0044, paired t-test). In cages containing insects, soil respiration rate was higher in the quadrats containing more larvae (Fig 2D; t = 3.22, P = 0.0018, LMM). Soil temperature was not higher in the squares containing more larvae (t = −0.16, P = 0.55, LMM), and it was almost significantly higher in cages containing larvae [24.63 ± 0.13°C (mean ± SE, n = 8 cages)] than in those without larvae [24.48 ± 0.13°C (mean ± SE, n = 8 cages), t 7 = 2.32, P = 0.053, paired t-test].
Fig 2. CO2 concentration and Trypoxylus dichotomus larval density/presence.
(A) Comparison of soil CO2 concentrations between containers (triangles) with and without insects (circles). The data from eight pairs of replicates are presented in different colours. (B) The relationship between larval density (i.e., the number of larvae in a quadrat) and CO2 concentration in that quadrat. (C) Comparison of soil respiration between containers with (triangles) and without insects (circles). (D) The relationship between larval density and soil respiration.
Effect of larval density and humus quality on body weight gain
LMM analysis using pooled data showed that density, food quality, their interaction, sex and initial weight were significantly correlated to larval body weight after treatment (Table 1).
Table 1. Larval weight gain under high and low nutrient conditions is density dependent.
The effects of density (1, 3, or 9 larvae/cage) and food quality (highly fermented or poorly fermented humus) on larval weight gain were tested using the LMM. Sex and initial weight were included as covariates, and identity of the mother of each larva was included as a random factor. Following an analysis of pooled data, additional analyses under each nutrition condition were conducted because the interaction term between density and food quality was significant.
| Explanatory variable | Pooled data | Highly fermented | Poorly fermented | |||
|---|---|---|---|---|---|---|
| χ 2 | P | χ 2 | P | χ 2 | P | |
| Density | 47.8 | <0.001 | 29.9 | <0.001 | 28.1 | <0.001 |
| Initial weight | 89.2 | <0.001 | 40.1 | <0.001 | 35.0 | <0.001 |
| Sex | 5.06 | 0.0244 | 1.8 | 0.19 | 10.7 | 0.001 |
| Food quality | 53.1 | <0.001 | - | - | - | - |
| Density × Food quality | 14.0 | <0.001 | - | - | - | - |
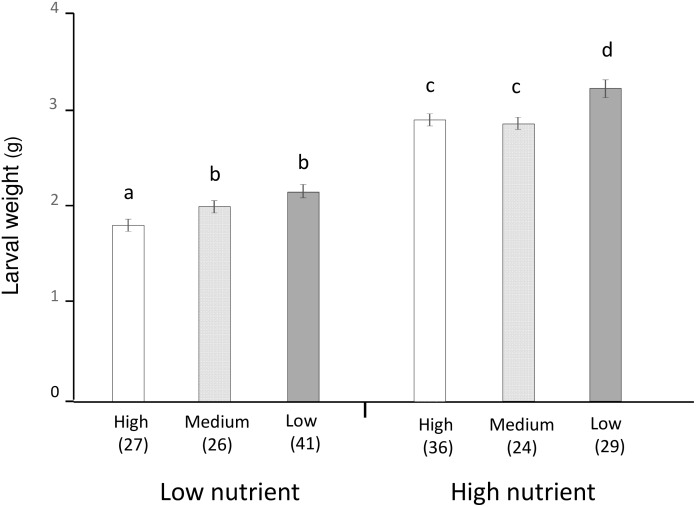
When larvae were provided poorly fermented food, significant effects of density and initial weight were detected, but sex did not affect body weight after treatment [males: 1.96 ± 0.045 g (mean ± SE, n = 51), females: 2.03 ± 0.043 g (n = 44)] (Table 1). However, enhancing larval density reduces weight gain under both high and low humus quality. Body weight of larvae reared alone [2.11 ± 0.047 g (n = 41)] was 5% and 17% higher than that of larvae reared at medium [2.01 ± 0.049 g (n = 26)] or high densities [1.81 ± 0.051 g (n = 27)], respectively (Fig 3). Body weight of larvae reared alone and at medium density was significantly greater than that of larvae reared at high densities, but there was no difference between that of larvae reared alone and at medium density (Fig 3).
Fig 3. Effect of density on Trypoxylus dichotomus larval growth.
Body weights of 2nd instar larvae reared at low (1 larva/cage), medium (3 larvae/cage), and high (9 larvae/cage) densities when provided poorly fermented (left) or highly fermented (right) humus. Error bars, SE. Letters indicate P < 0.05 in pairwise comparisons analysed by a linear mixed model with Tukey test. Detailed statistical values are presented in Table 1. Sample sizes are shown in parentheses. Error bars, SE.
Under high-nutrient conditions, significant effects of density and initial weight were detected (Table 1). In addition, male larvae were significantly heavier than females [males: 3.02 ± 0.065 g (n = 45), females: 2.78 ± 0.045 g (n = 44)] (Table 1). The body weight of larvae reared alone [3.14 ± 0.070 g (n = 29)] was 15% and 11% higher than that of larvae reared at medium [2.74 ± 0.070 g (n = 24)] or high densities [2.82 ± 0.061 g (n = 36)], respectively (Fig 3B). The body weight of larvae reared alone was significantly heavier than that of larvae reared at medium and high densities, but there was no difference between that of larvae reared at medium and high densities (Fig 3).
Discussion
I found that (i) larvae of T. dichotomus are strongly attracted to CO2, (ii) larvae orient toward highly fermented humus when given a choice between highly fermented humus and poorly fermented humus, (iii) highly fermented humus emits more CO2 than the poorly fermented humus, and (iv) larvae grow larger when reared in highly fermented rather than poorly fermented humus. Saprophagous insects feed on bacteria or fungi as a main source of protein [20]. CO2 is a cue for fermented organic substances in the saprophagous species [7], [8]. The orientation behaviour toward CO2 is also widespread in phytophagous [1–6] and blood-sucking species [21], [22], and this behaviour is thought to be an adaptive response for finding food [5].
The larvae of T. dichotomus are reported to exhibit a clumped distribution within microhabitats [11]. Larval distribution may occur along the concentration gradient of CO2. Under natural conditions, the concentration of CO2 in soil is probably heterogeneous because of the clumped distribution of fermented resources, and larvae likely aggregate in the vicinity of preferred food. The body weight of larvae reared alone was higher than that of larvae reared at medium or high densities. The high larval density probably leads to intra-specific competition for food and space. This is in contrast to results observed in group-living species in which aggregated individuals show a higher growth rate than solitary individuals [23], [24]. However, the body weight of T. dichotomus larvae reared at high density in the highly fermented humus was greater than that of larvae reared at low density in the poorly fermented humus. Thus, finding highly fermented humus using CO2 is beneficial for larvae even though conspecific neighbours aggregate at the high-CO2 site and compete with each other.
Male larvae were heavier than female larvae under high nutrition conditions only. Thus, at this stage a sexual size dimorphism becomes only apparent if high quality food is available. Such sexual difference in plasticity of body size has been reported in other species [25], [26]. Male body size of T. dichotomus is known to be strongly related to the individual’s abilities in intrasexual competition and male body size may be under positive directional selection [27]. Males may invest as many resources as possible into increasing body growth, which increases the condition dependency (i.e., plasticity) of the trait (see [25]).
Although the heterogeneity of organic materials mainly generates the gradients of CO2 concentration in soil, larvae themselves may also partly create the gradients. Consistent with a previous finding [11], larvae were found to be attracted to conspecifics. My laboratory experiments also showed that CO2 concentration in soil is increased by the larvae. Moreover, the results of behavioural assays indicated that only a small amount of respiratory CO2 (i.e., much less than the CO2 emitted during respiration by a single larva) is sufficient for larval attraction. The CO2 emission rate of larvae per unit mass was ca. 40- to 100-fold higher than that of substrates. The effect of respiratory CO2 on the orientation behaviour of neighbours may not be negligible when heterogeneity of substrates of microhabitats is low.
A previous study showed that conspecific chemical attraction has been observed in 2nd- and 3rd-instars, but not in 1st-instar larvae [11]. Although 2nd and 3rd-instar larvae of this species are large enough to generate steep CO2 gradients by respiration within homogeneous soil, small 1st-instar larvae may emit smaller amount of CO2 than neighbours are able to detect. The previous study also reported that T. dichotomus larvae with ablated maxillary palps were not attracted to conspecifics [11]. However, ablating the antennae did not affect the larval aggregation behaviour [11]. Thus, the sensors of CO2 in T. dichotomus appear to be present on maxillary palps but not antennae. The sensory organ for CO2 in insects is generally located on maxillary palps (e.g., mosquitoes [28]), labial palps (e.g., moths [29]), or antennae (e.g., fruit flies [30]). In the phytophagous scarab larvae Melolontha melolontha, antennae are the only head appendage to physiologically respond to CO2, and labial and maxillary palps did not respond to CO2 [31], which is opposite to my finding in T. dichotomus. The sensory organ of CO2 is likely different even among species belonging to the same family (e.g., Scarabaeidae).
In some insect species, individuals aggregate through pheromone or other signals to gain benefits such as predation avoidance, thermoregulation and efficient access to food [16]. In contrast, results of the choice test between synthetic CO2 and larvae indicate that cues other than respiratory CO2 play no or only a negligible role in conspecific attraction in T. dichotomus. Furthermore, the aggregation is unlikely to enhance larval growth. Conspecific attraction through respiratory CO2 in this species may be a by-product of the response to CO2 as a cue for food.
Larval respiration is not the only factor responsible for higher concentrations of CO2 near the vicinity of larvae. I showed that the presence of larvae led to an increase in soil respiration and soil temperature. This suggests that soil microbes were activated near the vicinity of larvae. Soil microbes have been reported to be activated through the feeding and excretion processes of various kinds of soil-living invertebrates [32–34]. Although the mechanism underlying the stimulation of microbes by T. dichotomus larvae is unclear, the larvae probably generate a CO2 gradient both by their own respiration and by activating soil microbes.
In summary, larvae of T. dichotomus are attracted to CO2. Because their food emits CO2, it is probably a critical cue for food in the soil-living larvae. The clumped distribution of larvae within microhabitats reported in a previous study [11] is likely formed along the concentration gradient of CO2 created by heterogeneous saprophytic activities in soil. In addition, I found that larvae are attracted to respiratory CO2 of neighbours. Not only response to fermented food resources, but also conspecific attraction may result in aggregation. When larvae aggregate at a zone of high saprophytic activities, they compete for food and/or space, reducing larval growth gain. However, the cost of competition was outweighed by the benefit for exploiting high-quality food resources, and thus the response to CO2 is adaptive in this species.
Acknowledgments
I thank S. Mori, T. Takanashi, T. Fujii, R. Nakano, and Y. Ishikawa for advice on measuring CO2, S. Hoshizaki for advice on rearing experiments, M. Shimada for his help and suggestions throughout the study, and two anonymous reviewers for their constructive critique and suggestions on improving the manuscript.
Data Availability
Data are available on Dryad (doi:10.5061/dryad.52473).
Funding Statement
This study is funded by Japan Society for the Promotion of Science (https://www.jsps.go.jp/english/index.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Johnson SN, Nielsen UN (2012) Foraging in the dark—Chemically mediated host plant location by belowground insect herbivores. J Chem Ecol 38: 604–614. 10.1007/s10886-012-0106-x [DOI] [PubMed] [Google Scholar]
- 2. Erb M, Huber M, Robert CAM, Ferrieri AP, Machado RAR, Arce CCM (2013) The role of plant primary and secondary metabolites in root-herbivore behaviour, nutrition and physiology. Adv Insect Physiol 45: 53–95. [Google Scholar]
- 3. Johnson SN, Gregory PJ (2006) Chemically-mediated host-plant location and selection by root-feeding insects. Physiol Entomol 31: 1–13. 10.1111/j.1365-3032.2005.00487.x [DOI] [Google Scholar]
- 4. Johnson SN, Zhang XX, Crawford JW, Gregory PJ, Hix NJ, Jarvis SJ, et al. (2006) Effects of CO2 on the searching behaviour of the root-feeding clover weevil. Bull Entomol Res 96: 361–366. [PubMed] [Google Scholar]
- 5. Guerenstein PG, Hildebrand JG (2008) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53: 161–178. 10.1146/annurev.ento.53.103106.093402 [DOI] [PubMed] [Google Scholar]
- 6. Rogers CD, Evans KA, Parker J, Pappa VA (2013) Behavioural response of wheat bulb fly (Delia coarctata, Diptera: Anthomyiidae) larvae to the primary plant metabolite carbon dioxide. Bull Entomol Res 103: 675–682. 10.1017/S0007485313000382 [DOI] [PubMed] [Google Scholar]
- 7. Jones OT, Coaker TH (1978) A basis for host plant finding in phytophagous larvae. Entomol Exp Appl 24: 472–484. 10.1111/j.1570-7458.1978.tb02807.x [DOI] [Google Scholar]
- 8. Bernklau EJ, Fromm EA, Judd TM, Bjostad LB (2005) Attraction of subterranean termites (Isoptera) to carbon dioxide. J Econ Entomol 98: 476–484. [DOI] [PubMed] [Google Scholar]
- 9. Doane JF, Lee YW, Klingler J, Westcott ND (1975) The orientation response of Ctenicera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and to carbon dioxide. Can Entomol 107: 1233–1251. [Google Scholar]
- 10. Bernklau EJ, Bjostad LB (1998) Behavioral responses of first-instar western corn root worm (Coleoptera: Chrysomelidae) to carbon dioxide in a glass bead bioassay. J Econ Entomol 91: 444–456. 10.1093/jee/91.2.444 [DOI] [Google Scholar]
- 11. Kojima W, Ishikawa Y, Takanashi T (2014) Chemically-mediated group formation in soil-dwelling larvae and pupae of the beetle Trypoxylus dichotomus . Naturwissenschaften 101: 687–695. 10.1007/s00114-014-1199-6 [DOI] [PubMed] [Google Scholar]
- 12. Krause J, Ruxton GD (2002) Living in groups. Oxford Univ Press, Oxford. [Google Scholar]
- 13. Wertheim B, van Baalen EJA, Dicke M, Vet LEM (2005) Pheromone-mediated aggregation in nonsocial arthropods: an evolutionary ecological perspective. Annu Rev Entomol 50: 321–346. 10.1146/annurev.ento.49.061802.123329 [DOI] [PubMed] [Google Scholar]
- 14. Averill AL, Prokopy RJ (1987) Intraspecific competition in the tephritid fruit fly Rhagoletis pomonella. Ecology 68: 878–886. 10.2307/1938359 [DOI] [Google Scholar]
- 15. Lyimo EO, Takken W, Koella JC (1992) Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae . Entomol Exp Appl 63: 265–271. 10.1111/j.1570-7458.1992.tb01583.x [DOI] [Google Scholar]
- 16. Alexander RD (1974) The evolution of social behavior. Annu Rev Ecol Syst 5: 325–383. [Google Scholar]
- 17. R Development Core Team (2013) R: A language and environment for statistical computing. R Foundation for statistical computing; Vienna, Austria: [Google Scholar]
- 18. Tsurumaki H (1987) Collecting and breeding of the Japanese rhinoceros beetle (in Japanese). Saishu To Shiiku 49: 254–257. [Google Scholar]
- 19. Kojima W (2015) Variation in body size in the giant rhinoceros beetle Trypoxylus dichotomus is mediated by maternal effects on egg size. Ecol Entomol 40: 420–427. 10.1111/een.12205 [DOI] [Google Scholar]
- 20. Tanahashi M, Matsushita N, Togashi K (2009) Are stag beetles fungivorous? J Insect Physiol 55: 983–988. 10.1016/j.jinsphys.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 21. Barrozo RB, Lazzari CR (2004) The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odours. Chem sens 29: 319–329. 10.1093/chemse/bjh035 [DOI] [PubMed] [Google Scholar]
- 22. Wang C, Gibb T, Bennett GW, McKnight S (2009) Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J Econ Entomol 102: 1580–1585. [DOI] [PubMed] [Google Scholar]
- 23. Denno R, Benrey B (1997) Aggregation facilitates larval growth in the neotropical nymphalid butterfly Chlosyne janais . Ecological Entomology 22: 133–141. 10.1046/j.1365-2311.1997.t01-1-00063.x [DOI] [Google Scholar]
- 24. Fordyce JA (2003) Aggregative feeding of pipevine swallowtail larvae enhances hostplant suitability. Oecologia 135: 250–257. 10.1007/s00442-003-1177-8 [DOI] [PubMed] [Google Scholar]
- 25. Stillwell RC, Blanckenhorn WU, Teder T, Davidowitz G, Fox CW (2010) Sex differences in phenotypic plasticity affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu Rev Entomol 55: 227–245. 10.1146/annurev-ento-112408-085500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walzer A, Schausberger P (2011) Sex-specific developmental plasticity of generalist and specialist predatory mites (Acari: Phytoseiidae) in response to food stress. Biol J Linn Soc 102: 650–660. 10.1111/j.1095-8312.2010.01593.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hongo Y (2007) Evolution of male dimorphic allometry in a population of the Japanese horned beetle Trypoxylus dichotomus septentrionalis . Behav Ecol Sociobiol 62: 245–253. 10.1007/s00265-007-0459-2 [DOI] [Google Scholar]
- 28. Grant AJ, Aghajanian JG, O'Connell RJ, Wigton BE (1995) Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A 177: 389–396. 10.1007/BF00187475 [DOI] [PubMed] [Google Scholar]
- 29. Bogner F, Boppré M, Ernst KD, Boeckh J (1986) CO2 sensitive receptors on labial palps of Rhodogastria moths (Lepidoptera: Arctiidae): physiology, fine structure and central projection. J Comp Physiol A 158: 741–749. 10.1007/BF01324818 [DOI] [PubMed] [Google Scholar]
- 30. Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB (2007) Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445: 86–90. 10.1038/nature05466 [DOI] [PubMed] [Google Scholar]
- 31. Eilers EJ, Talarico G, Hansson B, Hilker M, Reinecke A (2012) Sensing the underground—ultrastructure and function of sensory organs in root-feeding Melolontha melolontha (Coleoptera: Scarabaeinae) larvae. PLoS One 7, e41357 10.1371/journal.pone.0041357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lavelle P, Bignell D, Lepage M, Wolters V, Roger P, Ineson P, et al. (1997) Soil function in a changing world: the role of invertebrate ecosystem engineers. Eur J Soil Biol 33: 159–193. [Google Scholar]
- 33. Binet F, Fayolle L, Pussard M (1998) Significance of earthworms in stimulating soil microbial activity. Biol Fert Soils 27: 79–84. 10.1007/s003740050403 [DOI] [Google Scholar]
- 34. Brown GG, Barois I, Lavelle P (2000) Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur J Soil Biol 36: 177–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on Dryad (doi:10.5061/dryad.52473).