Abstract
Retinoids and alpha hydroxy acids differ in mechanism of action for treatment of photodamage, but concurrent use may produce a synergistic effect by combining retinoid-induced normalization of cellular differentiation with alpha hydroxy acid-induced exfoliation (in hydrophilic areas) and enhanced dermal and epidermal hydration. A recent bioengineered molecule, ethyl lactyl retinoate (alpha hydroxy acid retinoid conjugate), is the first to deliver alpha hydroxy acids and retinoids together in a hydrolysis-based time-released fashion. This could improve efficacy while minimizing irritation commonly associated with retinoid use. An eight-week clinical study was conducted to examine the efficacy and tolerability of this formulation; 25 women aged 54.1 ±8.9 years (mean ± SD) with moderate-to-severe photodamage (as determined by physician investigators using the Glogau Wrinkle Scale) employed a twice-daily regimen of cleanser (7.8% 1-lactic acid, 2% salicylic acid) and anti-aging serum (0.1% alpha hydroxy acids-retinoids, 6.5% 1-lactic acid) with concurrent use of sun protection factor 50+ sunscreen as needed. Longitudinal analysis of study data revealed statistically significant improvement in photodamage, dryness/flaking, dyschromia, and global appearance at eight weeks. All study products were well-tolerated throughout. Investigators concluded that the alpha hydroxy acid retinoid conjugate is a safe and effective topical therapy for moderate-to-severe photodamage, warranting further study, (clinicaltrials.gov, NCT02422836, https://clinicaltrials.gov/ct2/show/NCT02422836?term=NCT02422836)
Photodamage is characterized by a variety of aspects that, whole, show themselves as accelerated aging of the skin. Fine lines and wrinkles, dyschromia, flakiness, leathery appearance, sallow complexion, and other changes in tone and texture of skin are common.1 At the cellular level, photodamage is characterized2 by irregular thickening of the stratum corneum, thinning epidermis, reduced levels of glycosaminoglycans (GAGs) resulting in decreased moisture retention in the epidermis and dermis, reduced and fragmented collagen and elastin, collapsed fibroblasts, and reduced basal cell division. The causes are theorized to be the result of extrinsic stress from ultraviolet (UV) radiation and other sources of environmentally induced reactive oxygen species (ROS, or free radicals, e.g., infrared radiation, ozone, pollution)3-4 as compared to intrinsic oxidative stress causing normal aging of skin. Topical treatment is an ideal frontline therapeutic choice for many patients, especially those suffering from mild-to-moderate photodamage, because of the simplicity and relatively low associated cost, staving off the need for more powerful device-based options. More severe photodamage is resistant to treatment via topical formulation due to issues of tolerability when using actives at therapeutically relevant strengths.
Although heavily studied, retinoids (RC; vitamin A and its derivatives) are not well-understood, seeming to have an inexplicably wide variety of biological effects, including immunomodulation, increased collagen production, regulation of skin cell metabolism and cellular turnover, thickening of the epidermis, an increase in the height of rete ridges and the number of dermal papillae, normalization of melanocyte function, and an increase in dermal fibroblast production and activity.5-8 They have been vigorously investigated for the treatment of photodamage and though often harnessed for dermatologic use, associated skin irritation limits their utility as a topical somewhat, and a variety of strategies have been employed to mitigate irritation and maximize adherence.9
Alpha hydroxy acids (AHA) are nontoxic fruit- or food-based organic acids (e.g., glycolic acid, lactic acid, malic acid, etc.); lactic acid is a natural component of human body tissue.10 Chemically, AHAs consist of a carboxylic acid functional group with a hydroxyl group (alcohol) on the adjacent (alpha) carbon atom. Used for skin moisturization and exfoliation of dead skin cells, AHAs have been applied for a variety of dermatologic indications involving abnormal keratinization, including dry skin, dandruff, wrinkles, and acne among others. Clinical research has demonstrated their mechanism of action primarily involving reduced corneocyte cohesion (exfoliation) and upregulation of dermal and epidermal hyaluronan production (moisturization) with subsequent visual improvements in skin texture, tone, and radiance without inflammation when properly formulated.10-16 These factors make AHA ideal for dermatologic applications.17
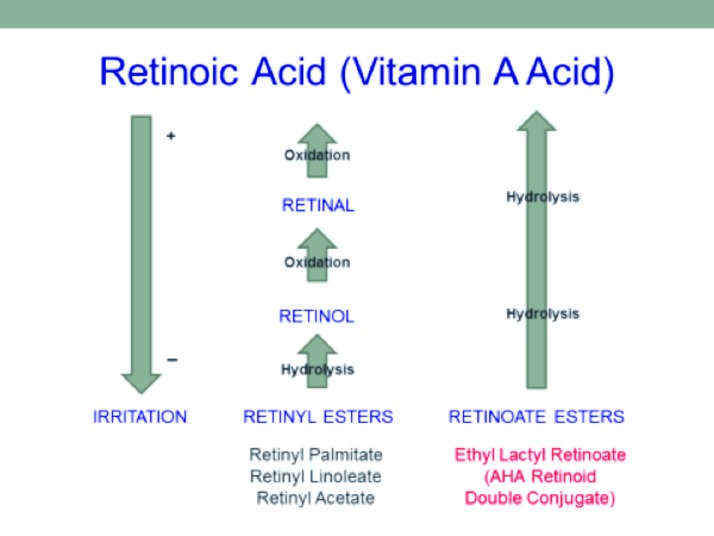
Therapeutic doses of topically applied retinoids frequently cause skin irritations that interfere with treatment. Figure 1 shows the relationship between commonly used retinoids and retinoic acid and the general irritation level and the process by which they are converted from one form to another via oxidation or hydrolysis. Esters are molecules made by joining an organic carboxylic acid with an alcohol through a condensation reaction; these typically provide increased stability and reduced irritation versus the parent compounds. Attempts to reduce retinoid irritation by esterifying vitamin A with fatty acids or other common organic acids, such as palmitic acid or acetic acid to produce retinyl esters (e.g., retinyl palmitate and retinyl acetate) negatively impact efficacy and thus, therapeutic potential. Chemically, AHAs are both carboxylic acids and alcohols, but reactions where AHAs are reacted as “alcohols” are uncommon. Retinoate esters of vitamin A can be made by combining AHA (as the alcohol) with vitamin A acid.
Figure 1.
Vitamin A derivatives, method of conversion and irritation potential
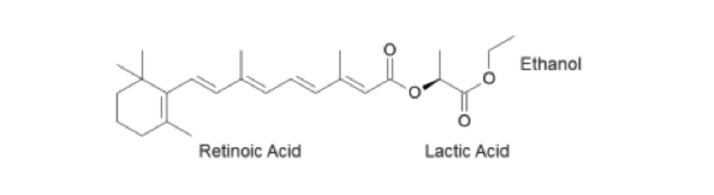
A novel retinoid ester may provide a topical alternative for moderate-to-severe cases of photodamage. This bioengineered molecule, known as ethyl lactyl retinoate (AHA retinoid conjugate, or AHA-RC), is the first double conjugate retinoid to deliver both AHA and RC to skin on a hydrolysis-based time-released mechanism biologically designed to maximize efficiency and minimize potential irritation from the retinoid component. The molecular structure for AHA-RC is shown in Figure 2. Conjugation of AHA with the larger, more lipophilic retinoid and again with ethanol improves deliverability of the resulting compound. Theoretically, such a molecule would exhibit the beneficial properties of both AHA and RC while minimizing RC-associated irritation, thereby providing an effective, yet gentler, topical therapy for photodamage that may be effective for moderate-to-severe cases otherwise resistant to treatment with topical sera.
Figure 2.
Molecular diagram of AHA retinoid conjugate (AHA-RC).
The purpose of this eight-week, full-face clinical study was to evaluate efficacy and tolerability of a twice-daily, three product, skin care regimen using a bioengineered AHA retinoid conjugate ester in patients with moderate-to-severe photodamage.
PATIENTS AND METHODS
Subjects. Women (n=25) aged 54.1±8.9 years (mean ± SD, age range 36-65) with moderate-to-severe lines/wrinkles (grade 3 or higher on the Glogau Scale, as shown in Figure 3) were enrolled in the study. Exclusion criteria included hypersensitivity to constituents of any of the study products; concurrent use of any topical or oral medication or therapy deemed by the investigator as potentially interfering with the study; use of anti-aging skincare products (cosmetic and drug products with AHA, salicylic acid, vitamin A, retin-A, vitamin C, growth factors or peptides, antioxidants such as idebenone, coffeeberry extract, CE ferulic, or phloretin) within the previous three months and during the course of the study; use of medical treatments for the skin for anti-aging (such as laser, chemical peels, microdermabrasion, or other therapies) within six months prior to or during the study period; pregnancy or breastfeeding during the study period; lack of willingness to avoid prolonged exposure to sunlight, sunlamps, or tanning beds; participation in another study concurrently or within 30 days of enrollment; and presence of any condition or disorder (dermatologic or otherwise), which the investigator decided may interfere with successful participation in the study or accurate evaluation of endpoints. The study was conducted under current Good Clinical Practices guidelines using an independent investigational review board-approved protocol.
Figure 3.

Glogau Photodamage Scale
Procedure. The study was conducted at the JUVA Skin & Laser Center/MediSpa (New York, New York) between February 2013 and May 2013. After an initial screening visit (Days -10 to -7) during which informed consent, medical history, inclusion/exclusion criteria review, initial endpoint assessment, and other pre-screening activities were performed, enrolled subjects were instructed to discontinue use of any and all facial products except dry mineral foundation and eye makeup for 7 to 10 days prior to beginning the study (“washout” period). A baseline (Day 0) pre-screening was reviewed, baseline endpoint evaluations were performed, baseline digital photographs were taken, and product was dispensed with clear instructions as to study guidelines and product use protocol.
The three-product regimen included cream skin cleanser (7.8% 1-lactic acid, 2% salicylic acid), anti-aging serum (0.1% AHA-RC, 6.5% 1-lactic acid), and sunscreen SPF 50+. Subjects were instructed to apply anti-aging serum after use of cream skin cleanser in the morning and evening. Sunscreen SPF 50+ was to be applied after morning application of anti-aging serum. For cleansing, study participants applied a small amount of product to be gently lathered and massaged over the face, followed by thorough water rinse and patting dry. Anti-aging serum was pumped (one or two pumps as needed) into the hands and thoroughly massaged into the face, neck, and décolletage. Liberal application of sunscreen SPF 50+ was expected 15 minutes prior to sun exposure and to be reapplied as needed. Subject diaries were included to promote adherence and obtain subject commentary or observations. Subjects were also instructed to notify the investigator immediately in the case of intolerable irritation or other adverse events within 48 hours of discovery.
At the Week 4 (Day 28) visit, subject diaries were collected (with new ones dispensed), digital photography and visual expert grading of endpoints were performed, and adverse events were recorded along with any concomitant medication information. At Week 8 (Day 56), subject diaries and remaining product were collected, digital photography and visual expert grading of endpoints was performed, and adverse events were recorded along with any concomitant medication information.
Physician evaluation and photography. Digital photography and evaluation of clinical endpoints was implemented at baseline as well as Weeks 4 and 8 of the study period. Dryness/Flaking, Dyschromia, Stinging/ Burning, and Erythema/Redness were evaluated by the investigator on a 0 to 5 scale (0=none, 1=minimal, 2=mild, 3=moderate, 4=moderately severe, 5=severe). Improvement in dyschromia was measured at Week 8 only. Global improvement was measured on a 0 to 4 scale (0=no improvement, 1=minimal improvement, 2=mild improvement, 3=moderate improvement, 4=marked improvement). Photographs were taken using the Canfield VISIA digital imaging system (Canfield Imaging Systems, Fairfield, New Jersey) with the camera mounted on a stereotactic head positioning device. Images were captured from three angles (full front at 0°, from left at 45°, and from right at -45°) at controlled distances under standard room lighting. Photographs were taken 20 minutes after onsite facial cleansing with no makeup or jewelry allowed.
Data analysis. The primary clinical endpoint was the change between photodamage grade (using the Glogau Scale) at the Week 4 visit and Week 8 visit versus the grade evaluation at the baseline visit (Day 0). Similar comparison was made for other clinical endpoints (Dryness/Flaking, Dyschromia, Stinging/Burning, and Erythema/Redness) as well. Aggregate data were analyzed via Mann-Whitney test using the cutoff of p<0.05. Subject reports of tolerability and efficacy were also recorded via post-study subject self-assessment questionnaire conducted at final Week 8 follow-up, with results tallied as percentages.
RESULTS
All subjects completed the study. No adverse events or tolerability issues were reported.
Physician evaluation. The Mann-Whitney test was used to analyze aggregate data with a cutoff of p<0.05. Percentages of subjects demonstrating grades of improvement for clinical endpoints at Weeks 4 and 8 are shown in Tables 1 and 2, respectively.
TABLE 1.
Percent change from baseline to Week 4
| PHOTODAMAGE‡ | DRYNESS | STINGING | ERYTHEMA | |
|---|---|---|---|---|
| Change (%) | 1.32 | 55.80 | 71.43 | 84.21 |
| p value*† | 0.322 | <0.001 | 0.002 | <0.001 |
| NS | S | S | S |
Statistical significance was defined as p<0.05
NS=not statistically significant, S=statistically significant
as measured using the Glogau Scale
TABLE 2.
Percent change from baseline to Week 8
| PHOTODAMAGE‡ | DRYNESS | DYSCHROMIA | STINGING | ERYTHEMA | |
|---|---|---|---|---|---|
| Change(%) | 17.11 | 46.51 | 56.52 | 76.19 | 52.63 |
| p value*† | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| S | S | S | S | S |
Statistical significance was defined as p<0.05
NS=not statistically significant, S=statistically significant
as measured using the Glogau Scale
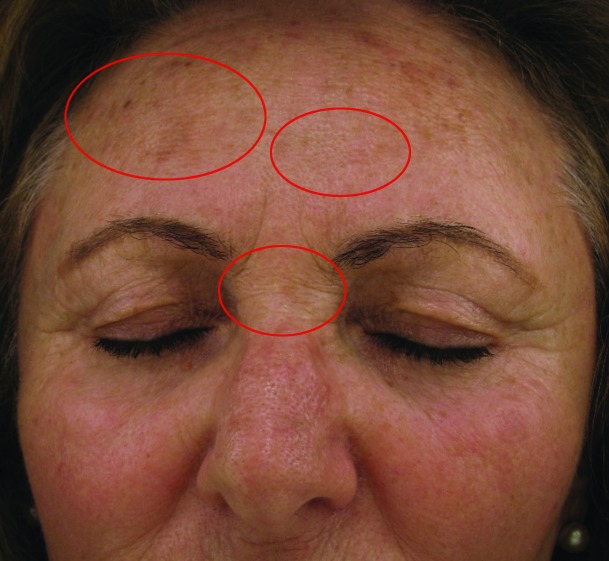
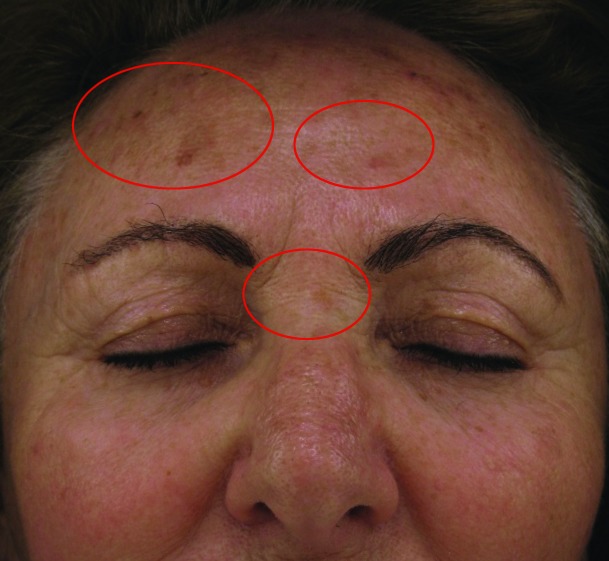
Improvement in fine lines and wrinkles did not manifest significantly by Week 4, but advanced to statistically significant levels by Week 8. There were also apparent reductions in level of improvement between Weeks 4 and 8 in dryness (55.8 vs. 46.51%, respectively) and erythema (84.21 vs. 52.63%, respectively). Stinging was improved slightly more at Week 8 than Week 4, but was highly improved on both cases. Before and after (baseline and Week 8, respectively) photographs of subject improvement are given in Figures 4 and 5.
Figure 4.
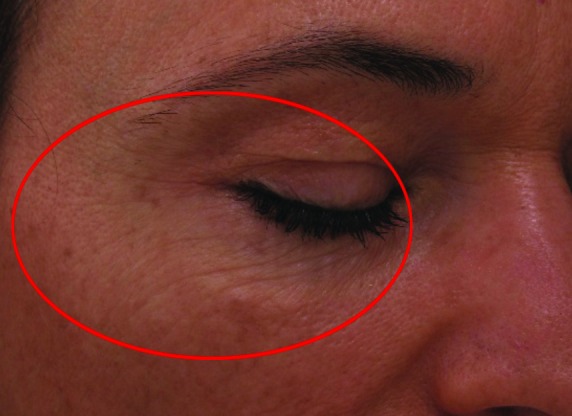
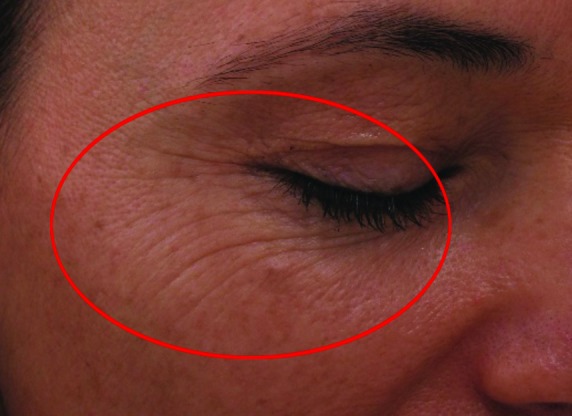
Before (baseline, at left) and after (at week 8 follow-up, right) photographs showing improvement in wrinkling at the right periorbital region of a Caucasian, non-Hispanic woman (age 52 years).
Figure 5.
Before (baseline, at left) and after (at week 8 follow-up, right) photographs showing improvement in dyschromia at the upper face of a Caucasian, non-Hispanic woman (age 64 years).
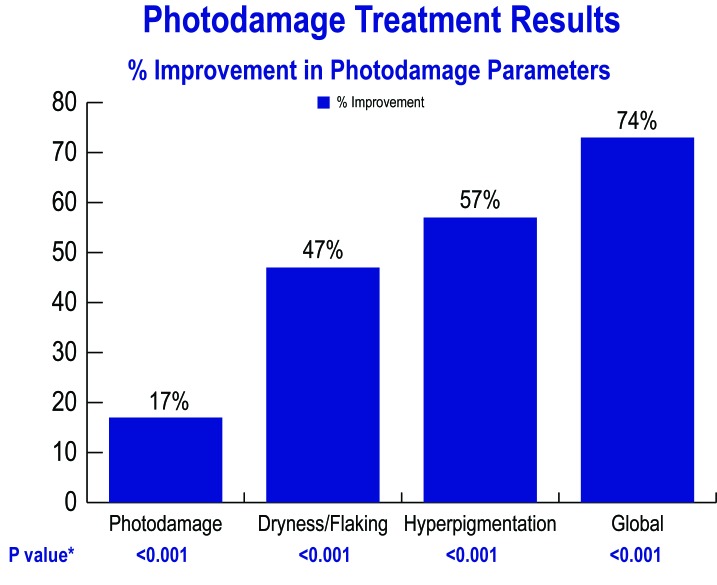
Percentage improvements at Week 8 with the endpoint showing the greatest improvement listed first are Stinging, Dyschromia, Erythema, Dryness, and Photodamage. Global improvement at Week 8 was 72.8 percent and was statistically significant (p<0.001). Some key results are graphically demonstrated in Figure 6.
Figure 6.
Photodamage treatment results; percent improvement in photodamage parameters
DISCUSSION
While photodamage in its various manifestations can be more aggressively treated with device-based modalities including ablative and nonablative lasers (fractional or nonfractional), microdermabrasion, intense pulsed light (IPL) with or without photodynamic therapy (PDT), and other energy-based methods, topical treatment is an excellent early intervention.1 Younger or less photodamaged patients may be averse to or simply not require aggressive therapy; treatment is relatively inexpensive and can be performed in the convenience of one’s home; risk is minimal with topical treatment in comparison to device-based modalities. Other advantages of topical therapy include lack of need for downtime or any type of recovery period and ease of use. Physicians can easily prescribe therapy rather than expend office time (theirs or that of ancillary staff) performing treatments. One major disadvantage to topical remedies is the need for strict adhrence because they require daily use. Patients may become bored with therapy, impatient with slower onset of results, or—as happens with less tolerable therapies— become discouraged and discontinue regular use due to discomfort or other issues.
Both AHA and RC have been applied as less aggressive topicals for treatment of photodamage, but individual utility is limited. The effect of AHA is limited to exfoliation and moisturization, and more aggressive concentrations of RC topicals cause irritation. By combining the two in a novel formulation, synergistic improvements in tolerability and efficacy are hypothesized. The novel bioengineered molecule is believed to facilitate delivery of the RC and mitigate potential irritation. Because the AHA component (lactic acid) of the molecule is limited in concentration due to the conjugated retinoid, additional AHA concentration is supplemented in the formulation to increase overall AHA to 6.5%. Combining effective concentrations of AHA and RC is useful for photodamage and extends the utility of topical therapies, perhaps allowing users to avoid or temporarily offset the need for inconvenient and relatively costly device-based alternatives.
The outcomes revealed in this clinical study do indeed demonstrate improvement in photodamage and tolerability of the treatment regimen. While improvement in fine lines and wrinkles (measured by the Glogau Scale) was less dramatic, not significantly manifesting until after assessment at Week 4, there were obvious and significant improvements in all other criteria across the board at both Week 4 and 8 follow-up visits. This may encourage adherence by providing noticeable results to patients without discomfort that might inhibit enthusiasm. Stinging sensation showed the strongest improvement, strengthening any suggestion of tolerability. It is also notable that the subject population consisted of those presenting with moderate-to-severe photodamage; this is somewhat profound given the topical nature of the investigated therapy.
To summarize, this study demonstrates the potential utility of this novel AHA-RC molecule when applied regularly as a treatment for photodamage. Study outcomes include significant improvement across all evaluated criteria and thus suggest that additional study, including vehicle controls, larger and more diverse study population, and extended study time period might more firmly delineate the actual duration of effect and ceiling of improvement. Specific focus of endpoint criteria designed for more thorough analysis may also be more revealing; further investigation would also provide additional proof of safety and tolerability.
CONCLUSION
The three product regimen used in this study was shown to be safe and effective for treatment of moderate-to-severe photodamage with statistically significant levels of correction exhibited for all clinical endpoints by Week 8 follow-up (study conclusion). Additional controlled study with larger, more diverse populations would further reveal the utility of this novel retinoid ester.
Footnotes
DISCLOSURE:Dr. Katz is a paid independent research consultant for US Cosmeceutechs, LLC. Joseph Lewis and Laura McHugh are employees of US Cosmeceutechs, LLC, owner of pending patents for technologies used in this study. Arthur Pellegrino and Lativia Popescu are employed by Elizabeth Arden, Inc., an investor in US CosmeceuTechs, LLC.
REFERENCES
- 1.Visscher MO, Pan BS, Kitzmiller WJ. Photodamage: treatments and topicals for facial skin. Facial Plast Surg Clin North Am. 2013;21(1):61–75. doi: 10.1016/j.fsc.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Rubin MG. Manual of Chemical Peels: Superficial and Medium Depth. Philadelphia, Pa: JB lippincott Co; 1995. Photoaged and photodamaged skin; pp. 1–2. [Google Scholar]
- 3.Pinnell SR. Photodamage and oxidative stress and protection provided by topical antioxidants. J Am Acad Dermatol. 2003;48(1):1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JA, II, DiNardo JC, McDaniel DH. Oxidative stress, the damage-accumulation theory of skin aging, and the role of antioxidants in the future of topical skin protection. Cosmet Dermatol. 2009;22(11):576–582. [Google Scholar]
- 5.Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- 6.Kang S. Photoaging and tretinoin. Clin Dermatol. 1998;16(2):357–364. doi: 10.1016/s0733-8635(05)70018-8. [DOI] [PubMed] [Google Scholar]
- 7.Kligman LH. Topical retinoic acid enhances repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- 8.Zelickson AS, Mortaz JH, Weiss JS, et al. Topical tretinoin in photoaging: an ultrastructural study. J Gutan Aging Gosmet Dermatol. 1988;1:41–47. [Google Scholar]
- 9.Rolewski SL. Clinical review: topical retinoids. Dermatol Nurs. 2003;15(5):447–450,459-465. [PubMed] [Google Scholar]
- 10.Yu RJ, Van Scott E. Alpha-hydroxy acids: science and therapeutic use. Cosmet Dermatol. 1994;7(10S):12–20. [Google Scholar]
- 11.Berardesca E, Maibach H. AHA mechanism of action. Cosmetics and Toiletries Magazine. 1995;110:30–31. [Google Scholar]
- 12.Neudecker BA, Stern R. Alpha hydroxy acids stimulate hyaluronan deposition: an in-vitro study using human dermal fibroblasts. J Drugs Dermatol. Pre-publication draft dated Nov 2014. [Google Scholar]
- 13.Bernstein EF, Lee J, Brown DB, et al. Glycolic acid treatment increases type I collagen mRNA and hyaluronic acid content of human skin. Dermatol Surg. 2001;27(5):429–433. doi: 10.1046/j.1524-4725.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AW, Nole GE, Rozen MG, et al. Skin tolerance of AHAs: a comparison of lactic and glycolic acids and the role of pH. Coscmet Dermatol. 1997;10(2):38–45. [Google Scholar]
- 15.Van Scott E, Yu RJ. Hyperkeratinization, corneocyte cohesion, and alpha hydroxyl acids. J Am Acad Dermatol. 1984;11:867–879. doi: 10.1016/s0190-9622(84)80466-1. [DOI] [PubMed] [Google Scholar]
- 16.DiNardo JC, Grove GL, Moy LS. Clinical and histological effects of glycolic acid at different concentrations and pH levels. Dermatol Surg. 1996;22(5):421–424. doi: 10.1111/j.1524-4725.1996.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Scott E, Yu RJ. Alpha hydroxyacids: therapeutic potentials. Can J Dermatol. 1989;1(5):408–112. [Google Scholar]