Abstract
Fungi are an exceptional source of diverse and novel cytochrome P450 monooxygenases (P450s), heme-thiolate proteins, with catalytic versatility. Agaricomycotina saprophytes have yielded most of the available information on basidiomycete P450s. This resulted in observing similar P450 family types in basidiomycetes with few differences in P450 families among Agaricomycotina saprophytes. The present study demonstrated the presence of unique P450 family patterns in basidiomycete biotrophic plant pathogens that could possibly have originated from the adaptation of these species to different ecological niches (host influence). Systematic analysis of P450s in basidiomycete biotrophic plant pathogens belonging to three different orders, Agaricomycotina (Armillaria mellea), Pucciniomycotina (Melampsora laricis-populina, M. lini, Mixia osmundae and Puccinia graminis) and Ustilaginomycotina (Ustilago maydis, Sporisorium reilianum and Tilletiaria anomala), revealed the presence of numerous putative P450s ranging from 267 (A. mellea) to 14 (M. osmundae). Analysis of P450 families revealed the presence of 41 new P450 families and 27 new P450 subfamilies in these biotrophic plant pathogens. Order-level comparison of P450 families between biotrophic plant pathogens revealed the presence of unique P450 family patterns in these organisms, possibly reflecting the characteristics of their order. Further comparison of P450 families with basidiomycete non-pathogens confirmed that biotrophic plant pathogens harbour the unique P450 families in their genomes. The CYP63, CYP5037, CYP5136, CYP5137 and CYP5341 P450 families were expanded in A. mellea when compared to other Agaricomycotina saprophytes and the CYP5221 and CYP5233 P450 families in P. graminis and M. laricis-populina. The present study revealed that expansion of these P450 families is due to paralogous evolution of member P450s. The presence of unique P450 families in these organisms serves as evidence of how a host/ecological niche can influence shaping the P450 content of an organism. The present study initiates our understanding of P450 family patterns in basidiomycete biotrophic plant pathogens.
Introduction
For the last five decades cytochrome P450 monooxygenases (P450s/CYPs), heme-thiolate proteins, have been in the spotlight [1] because of their catalytic versatility [2] and potential biotechnological value [3, 4]. These enzymes can be found in all living organisms belonging to different biological kingdoms [5] and are known to play a key role in organisms’ primary and secondary metabolism, including degradation of xenobiotic compounds [1]. Because of their stereo- and regio-specific oxidation of substrates, these enzymes have become critical in organisms’ survival [6–9]. Among biological kingdoms, the fungal kingdom and species in them present a large amount of information on different aspects of P450s. The aspects include P450 family diversity [10], catalytic versatility [11–13], P450 family enrichment [13], thermostable P450s [14], P450s as drug-target [4, 15] and provision of biotechnologically valuable P450s [4].
Analysis of P450s in fungi revealed the highest P450 family diversity in ascomycetes compared to basidiomycetes [16]. Species belonging to Ascomycota and Basidiomycota show different P450 family types in their genomes [17]. Basidiomycetes are one of the unique fungi capable of complete degradation of plant material including the most recalcitrant plant cell wall component, lignin [18]. The recent explosion in genome sequencing of basidiomycetes helped researchers to understand the P450 patterns, their distribution and evolution in these organisms [13, 14, 16, 17, 19–22]. However, the available knowledge on basidiomycete P450s mostly came from wood-degrading species belonging to the order Agaricomycotina. Analysis of P450s across the Agaricomycotina species, such as Phanerochaete chrysosporium [23, 24], P. carnosa [19], Postia placenta [25, 26], Ganoderma sp. [20]. G. lucidum [27], Agaricus bisporus [28, 23], Ceriporiopsis (Gelatoporia) subvermispora [29], Serpula lacrymans [13], Bjerkandera adusta and Phlebia brevispora [20], revealed almost the same P450 family patterns, despite the presence of a few different P450 families in these species. Furthermore, recent study revealed that certain P450 families in these organisms are enriched via paralogous evolution of member P450s to help the organism adapt to different ecological niches, such as colonization on plant material [13].
Apart from the dead wood degrading basidiomycetes described above, efforts were made regarding genome sequencing of basidiomycete biotrophic plant pathogens to understand the virulence factors responsible for pathogenesis [30–36]. The basidiomycete biotrophic plant pathogen genomes sequenced include the biotrophic ubiquitous parasite of maize and a model fungus for the study of microbe-plant pathogen interactions, Ustilago maydis [30], another maize biotrophic pathogen, Sporisorium reilianum [35], an intracellular rice pathogen, Tilletiaria anomala [34], obligate biotrophic pathogens of crop plants, Melampsora laricis-populina [31] and M. lini [33], Puccinia graminis f. sp. tritici [31], an intracellular pathogen of ferns, Mixia osmundae [36] and the ubiquitous plant pathogen Armillaria mellea [32]. Contrary to the basidiomycete plant pathogens, their counterparts, ascomycete plant pathogen P450s, have been extensively characterized through profiling of P450s in these organisms and also functional analysis of some P450s [16,17,21,22].
To date, systematic and comparative analysis of P450s in basidiomycete plant pathogens, especially biotrophs, has not been carried out. Previous studies on comparative analysis of P450s in fungi [16, 17, 21, 22] focused on different aspects of fungal P450s. A thorough analysis of P450s in the basidiomycete biotrophic plant pathogens has not been reported. In this study, we performed genome-wide identification, annotation and evolutionary analysis of P450s in basidiomycete plant pathogens, especially biotrophs belonging to three different orders: Agaricomycotina (A. mellea), Pucciniomycotina (M. laricis-populina, M. lini, M. osmundae and P. graminis) and Ustilaginomycotina (U. maydis, S. reilianum and T. anomala). Furthermore, we performed comparative analysis of biotrophic plant pathogen P450s with non-pathogen fungi and focused on the analysis of P450 patterns between different orders and P450 family expansion, if any, in these biotrophic plant pathogens.
Materials and Methods
Species selection and P450 mining
Basidiomycete biotrophic plant pathogens and non-pathogens belonging to three different orders were selected for analysis of P450s (Table 1). These species’ genome sequencing data have been published and are available for public use (Table 1). P450 mining in these species was carried out following the methodology that has been described meticulously in the literature [13, 14, 16, 19–21, 26, 37, 38]. Briefly, putative proteomes of each species were downloaded from the respective species’ databases listed in Table 1. The putative proteome was subjected to the NCBI Batch Web-search tool [37] to separate proteins into different functional categories. The proteins that were grouped under the P450 superfamily were selected and checked for the presence of two P450 signature motifs, FXXGXRXCXG (also known as CXG) in the heme-binding domain and the EXXR motif in the K-helix [39–41]. Identification of P450s in organisms depend solely on identification of these two P450 signature motifs and this method is well documented in the literature, particularly any P450 showing one of the motifs considered pseudo-P450 [13,14,16, 19–21, 26, 37, 38]. The sequences that showed both motifs were selected for naming. The length of selected P450s is >400 amino acids, indicating that these P450s are full-length. For this reason assigning the family and subfamily can be done without any errors. The P450s that showed one of the motifs represent pseudo-P450s, hence they were not annotated. The P450s of Malassezia globosa that have been annotated and are available for public use at Cytochrome P450 Homepage [42] are used in this study.
Table 1. Information on databases used to download the whole proteomes of basidiomycete biotrophic plant pathogens and non-pathogens.
All databases were located at the MycoCosm portal of the Joint Genome Institute (JGI), United States Department of Energy (US-DOE) [43].
Assigning family and subfamilies
The above selected P450s were assigned to different P450 families and P450 subfamilies using the methodology that is described meticulously in the literature [13, 14, 16, 19–21, 26, 37, 38]. Briefly, the above selected P450s were blasted at the Cytochrome P450 Homepage [38] against all named fungal P450s and P450s of Postia placenta, Bjerkandera adusta, Phlebia brevispora and Ganoderma sp. [26, 20]. Homolog P450s (henceforth referred to as reference P450s) that showed the highest percentage identity to putative P450s were noted. P450s were grouped into families and subfamilies based on the International Cytochrome P450 Nomenclature criteria, i.e. P450s showing >40% identity were assigned to the same P450 family and P450s that showed >55% identity were grouped under the same P450 subfamily [48–50]. P450s that showed less than 40% identity to annotated P450s were assigned to new P450 families with the kind help of Prof David R Nelson, University of Tennessee Health Science Centre, Memphis, Tennessee, USA. Furthermore, alignment of P450s on the phylogenetic tree was taken into consideration while assigning the family and subfamily to the putative P450s. The P450s of P. graminis, U. maydis and M. globosa have been annotated and are available for public use [42]. In this case, protein IDs for P450s in these organisms were assigned from their respective genomic databases (Table 1) if the machine-annotated proteins available on the respective species’ genomic databases (Table 1) showed 100% identity to the annotated P450s on the Cytochrome P450 Homepage [42].
Construction of P450 phylogenetic tree
The P450 phylogenetic tree was constructed following the methodology previously described [22]. Briefly, the annotated basidiomycete P450s were aligned by adjusting them to the P450 profile hidden Markov model (PF00067, the Pfam protein families database, http://pfam.xfam.org/) with the HMMER package 3.1 (http://hmmer.janelia.org/) [51,52]. Then, the phylogenetic trees based on the above alignments were inferred by FastTree version 2.1.4 using the maximum-likelihood method (http://www.microbesonline.org/fasttree/) [53]. The generated tree data were submitted to iTOL (http://itol.embl.de/upload.cgi) for making phylogenetic trees [54].
Agaricomycotina saprophytes P450s
P450s belonging to the Agaricomycotina saprophytes P. chrysosporium, P. carnosa, A. bisporus, Ganoderma sp., P. placenta, S. lacrymans, B. adusta and P. brevispora were resourced from published literature [13, 19, 10, 16] and used for comparison with A. mellea P450s.
Gene-structure and gene tandem-duplication analysis
Analysis of the gene structure for selected P450 family members was carried out following the methodology described elsewhere [13, 14]. Briefly, selected P450 family members’ intron-exon arrangements were analysed. The length of exons was noted as an indication of possible gene duplication, if P450s showed conservation in the size of exons. Genomic localization of member P450s was also carried out to assess the tandem arrangement of P450s. Localization of P450s on a scaffold/node and their DNA region from start to end were noted and used for identification of tandemly duplicated P450s.
Results and Discussion
Basidiomycete biotrophic plant pathogens P450ome
Genome-wide data mining for P450s in basidiomycete biotrophic plant pathogens revealed the presence of a large number of P450s in A. mellea (267) (Table 2 and S1 Table). In comparison to A. mellea, the other seven plant pathogens showed the lowest number of P450s in their genomes (Table 2 and S1 Table). Among the seven plant pathogens M. laricis-populina showed the highest number of P450s (27) in its genome, whereas M. osmundae showed the lowest number of P450s (14) in its genome. The number of P450s in other plant pathogens is highest compared to basidiomycetes, the human pathogen Cryptococcus neoformans and mycoparasite Tremella mesenterica that show eight P450s in their genome [42, 55].
Table 2. Genome-wide annotation and comparative analysis P450s in basidiomycete biotrophic plant pathogens.
| Order | Species name | No. of P450s | No. of P450 families | No. of P450 subfamilies |
|---|---|---|---|---|
| Agaricomycotina | A. mellea | 267 | 30 | 65 |
| Pucciniomycotina | M. laricis-populina | 27 | 14 | 16 |
| M. lini | 22 | 13 | 17 | |
| M. osmundae | 14 | 14 | 14 | |
| P. graminis | 17 | 9 | 10 | |
| Ustilaginomycotina | U. maydis | 23 | 19 | 22 |
| S. reilianum | 16 | 15 | 16 | |
| T. anomala | 17 | 11 | 15 |
New P450 families and subfamilies in basidiomycete biotrophic plant pathogens
Annotation of P450 families (assigning the P450 families and subfamilies to the putative P450s) revealed the presence of new P450 families in basidiomycete biotrophic pathogens. Based on the International Cytochrome P450 Nomenclature criteria [48–50], A. mellea P450s can be grouped into 30 P450 families and 65 P450 subfamilies (Table 2). Among the remaining seven biotrophic plant pathogens, U. maydis and P. graminis showed the highest and lowest number of P450 families and subfamilies in their genomes (Table 2). Some P450s of A. mellea contain one of the two P450 signature motifs, hence these P450s are regarded as pseudo-P450s and not annotated. Future availability of good genomic DNA sequence and better gene prediction methods will facilitate the annotation of these P450s. For the same reason a single P450 is omitted from the annotation for M. laricis-populina and M. lini. The number of P450 families and subfamilies in each species is listed in Table 2. A detailed analysis of reference P450s used for annotation of basidiomycete biotrophic pathogen P450s is given in S2 Table.
Analysis of P450 families revealed the presence of 41 new P450 families and 27 new P450 subfamilies in these biotrophic plant pathogens (Table 3). U. maydis and M. lini showed the highest (12) and lowest (1) number of new P450 families in their genomes. Among new subfamilies, M. lini showed the highest number of new P450 subfamilies in its genome (8) and a single new P450 family was observed in M. laricis-populina and S. reilianum (Table 3). Detailed analysis of the number and name of the new P450 families and subfamilies identified in each biotrophic plant pathogen is presented in Table 3.
Table 3. Information on new families and new subfamilies found in basidiomycete biotrophic plant pathogens.
| Total number | Name | |||
|---|---|---|---|---|
| New families | New subfa-milies | New families | New subfamilies | |
| A. mellea | 5 | 4 | CYP5417, CYP5431, CYP5622, CYP5623, CYP6006 | CYP5366B1, CYP5154F1, CYP5142M1, CYP5340D1 |
| M. laricis-populina | 4 | 1 | CYP5395-CYP5398 | CYP5139J1 |
| M. lini | 1 | 8 | CYP5399 | CYP5152NSF, CYP5221NSF1, CYP5230NSF, CYP5232NSF, CYP5233NSF, CYP52233NSF 1 & NSF2, CYP5396NSF |
| M. osmundae | 8 | 3 | CYP5662-CYP5669 | CYP5139S1, CYP5141M1, CYP522E1 |
| P. graminis | 4 | 3 | CYP5230-CYP5233 | CYP5152B1, CYP5221B1, CYP5221C1 |
| U. maydis | 12 | 3 | CYP5025-CYP5034, CYP5643, CYP5644 | CYP53C1, CYP504C1, CYP504D1, |
| S. reilianum | 3 | 1 | CYP5032, CYP5636, CYP6007 | CYP5640B1 |
| T. anomala | 4 | 4 | CYP5026, CYP5367, CYP5639, CYP5641 | CYP5028B1, CYP5031B1, CYP5031NSF, CYP5076D1 |
Phylogenetic analysis of P450s and their clade features
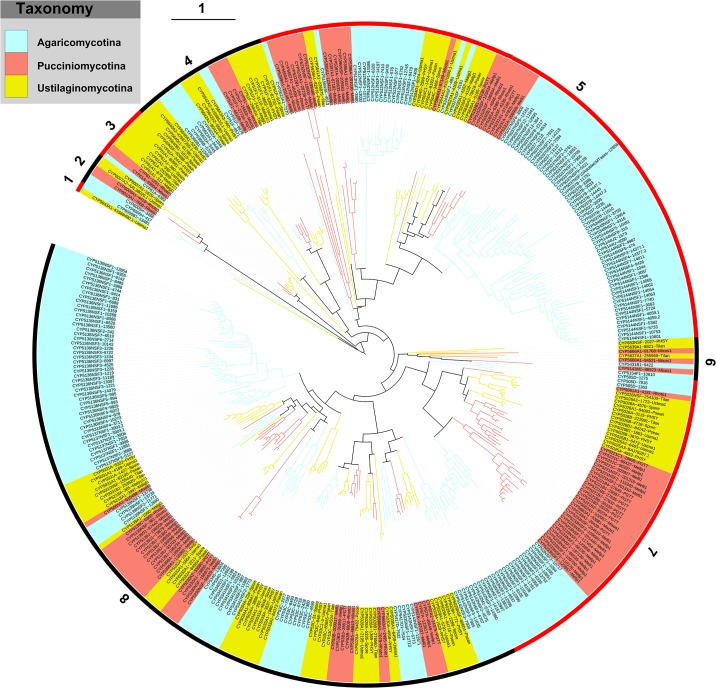
The phylogenetic tree of basidiomycete biotrophic plant pathogens was constructed based on their protein sequences (Fig 1). The P450s clustered together on the tree, indicating that they possibly belonged to the same family. The phylogenetic tree played a key role in assigning the family to putative P450s, where the percentage of identity criteria with annotated P450s becomes equal to 40% identity. The alignment helped to find the closest neighbor and thus its percentage identity to its neighbor. Phylogenetic analysis of biotrophic plant pathogen P450s showed numerous branches of P450s in the phylogenetic tree, indicating their highly evolved divergence. This is also reflected in their low percentage identity with homolog P450s belonging to Agaricomycotina species (S2 Table) and the presence of new P450 families and subfamilies (Table 3).
Fig 1. Phylogenetic tree of P450s belonging to basidiomycete biotrophic plant pathogens and non-pathogens used in this study as listed in Table 1.
The branches with different colours show their taxonomy, as indicated in the legend. Ancestral branches with children that had identical colours were assigned the same colour as the children. The outer numbers indicate the eight clades, and their ranges are marked by alternating red and black. Each P450 is presented with its family and subfamily following its protein ID (parenthesis). A high-resolution phylogenetic tree is provided as S1 Fig.
In order to understand the evolution of P450s, a higher level classification of P450s has been proposed [56], indicating their divergence from a common ancestor. In this study, the basidiomycete biotrophic plant pathogens and non-pathogen putative P450s that were annotated in this study were grouped into eight clades based on their phylogenetic relationships (Table 4). Clades 5 and 8 are large branches. Among them, CYP5136, CYP5144, CYP5037 are the very frequently occurring P450s in A. mellea. The CYP5221 and CYP5231 families were expanded in P. graminis and M. laricis-populina (Fig 1). The explosion of these P450 families suggests their specific role in plant pathogens (discussed in the coming sections).
Table 4. Clade level classification of P450 families.
| Clade | CYP family |
|---|---|
| 1 | CYP5643, CYP6006 |
| 2 | CYP6005, CYP6010, CYP6009, CYP6007 |
| 3 | CYP61, CYP540 |
| 4 | CYP5640, CYP5366, CYP5622, CYP5642, CYP51 |
| 5 | CYP5623, CYP5399, CYP5669, CYP5667, CYP5222, CYP5232, CYP5638, CYP5641, CYP5156, CYP5395, CYP5397, CYP5396, CYP512, CYP504, CYP5664, CYP5644, CYP5343, CYP5027, CYP5220, CYP5636, CYP5152, CYP5093, CYP5065, CYP5231, CYP5037, CYP5348, CYP5144 |
| 6 | CYP683, CYP5639, CYP5666, CYP5637, CYP5668, CYP5431, CYP5141, CYP5154 |
| 7 | CYP505, CYP5661, CYP5026, CYP5025, CYP5221, CYP5662, CYP5663, CYP5233, CYP63 |
| 8 | CYP5142, CYP5029, CYP67, CYP5143, CYP5035, CYP5417, CYP5030, CYP5660, CYP5028, CYP53, CYP5033, CYP5034, CYP5341, CYP5340, CYP5230, CYP5032, CYP5398, CYP5139, CYP5665, CYP5218, CYP5031, CYP5137, CYP5316, CYP5136 |
Basidiomycete biotrophic plant pathogens contain unique P450 families
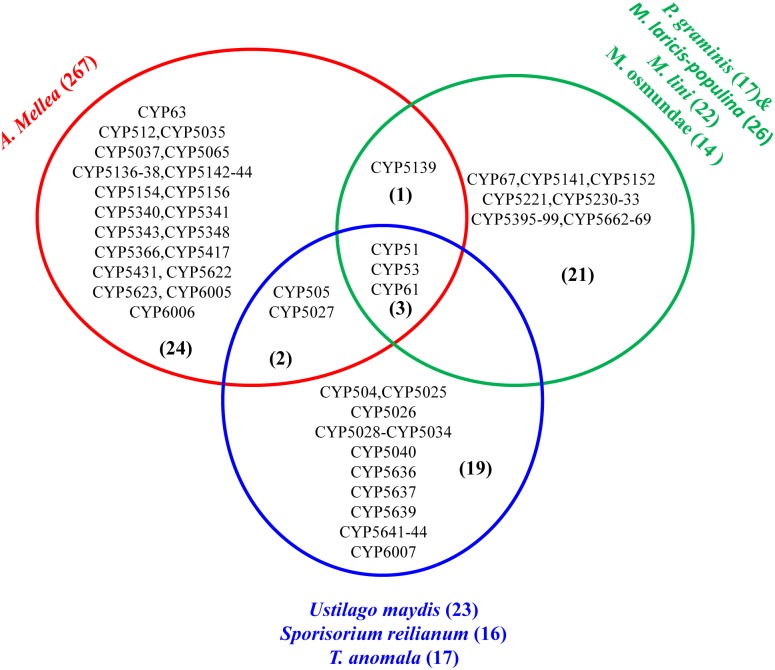
Comparison of P450 families between basidiomycete biotrophic plant pathogens revealed the presence of unique P450 families in these species, possibly reflecting the characteristics of their order (Fig 2). As shown in Fig 3, only three P450 families, CYP51, CYP53 and CYP61, are conserved across biotrophic plant pathogens. These P450 families are known to be highly conserved in fungi. A. mellea shares a single P450 family (CYP5139) with biotrophs belonging to Pucciniomycotina and two P450 families (CYP505 and CYP5027) with biotrophs belong to Ustilaginomycotina. Interestingly, the CYP61 family that is conserved across fungi [16, 17] is missing from P. graminis and M. laricis-populina. In a previous study the absence of this P450 family was observed in these organisms [17]. A. mellea belonging to the Agaricomycotina contains 24 unique P450 families, whereas 21 unique P450 families were found in the Pucciniomycotina species used in this study. Ustilaginomycotina species contain 19 unique P450 families (Fig 2). This clearly suggests that basidiomycete biotrophs belonging to different orders harbour unique P450 families in their genomes.
Fig 2. Family level comparative analysis of putative cytochrome P450 monooxygenases between fungal orders represented by A. mellea (Agaricomycotina), P. graminis, M. laricis-populina, M. lini and M. osmundae (Pucciniomycotina) and U. maydis, S. reilianum and T. anomala (Ustilaginomycotina).
The number in parenthesis indicates P450 family numbers. The number in parenthesis next to each species indicates the total P450 count in the particular species.
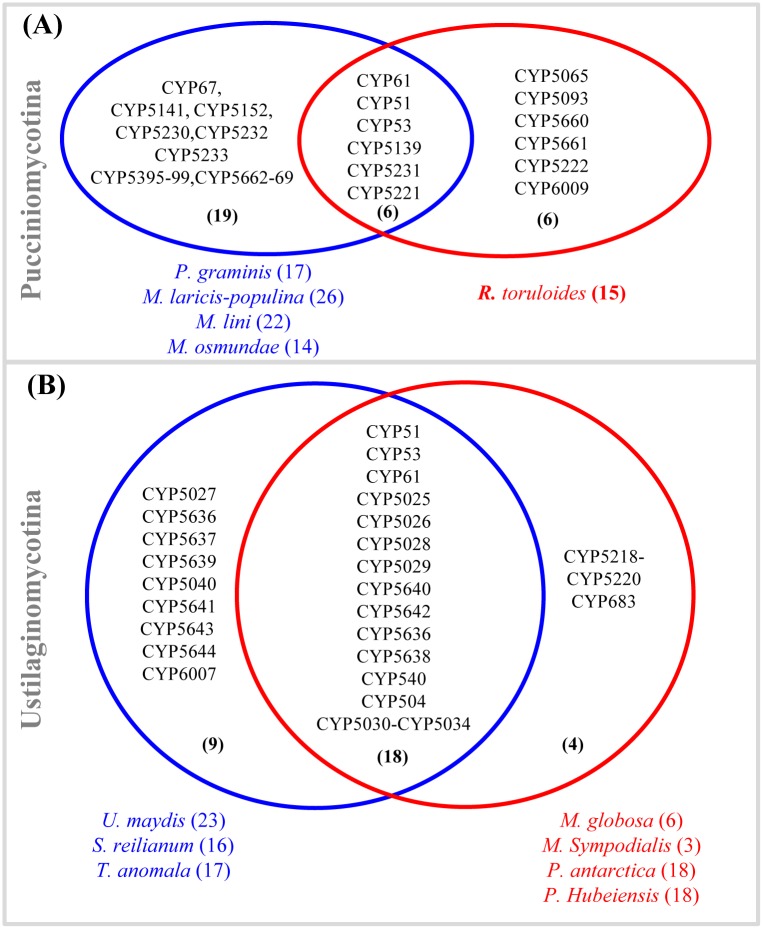
Fig 3. Comparative analysis of member P450s between biotrophic and non-biotrophic basidiomycetes belonging to Pucciniomycotina (A) and Ustilaginomycotina (B).
The number in parenthesis indicates P450 family numbers. The number in parenthesis next to each species indicates the total P450 count in the particular species.
In order to gain more insight on the unique nature of the biotrophic plant pathogen P450 family contingent, we performed comparative analysis of P450 families between biotrophic plant pathogens and non-biotrophs. Comparison of A. mellea P450 families with Agaricomycotina saprophytes revealed the presence of five unique P450 families (CYP5417, CYP5431, CYP5622, CYP5623 and CYP6006) in the A. mellea genome (S3 Table). Interestingly, some of the P450 families were expanded in A. mellea compared to P450 family members in Agaricomycotina saprophytes. A detailed analysis on A. mellea P450 families that were expanded is presented in the next section. Comparative analysis revealed the presence of 19 and 9 unique P450 families in biotrophic plant pathogens of Pucciniomycotina and Ustilaginomycotina (Fig 3). The presence of unique P450 families in biotrophs suggests that these P450 families play a role in their adaptation to the biotrophic nature. Future genome sequencing of a greater number of Pucciniomycotina non-pathogens may provide conclusive evidence on unique P450 families in this order of biotrophs, as currently only one species genome is available (Fig 3).
P450 family expansion in basidiomycete biotrophic plant pathogens
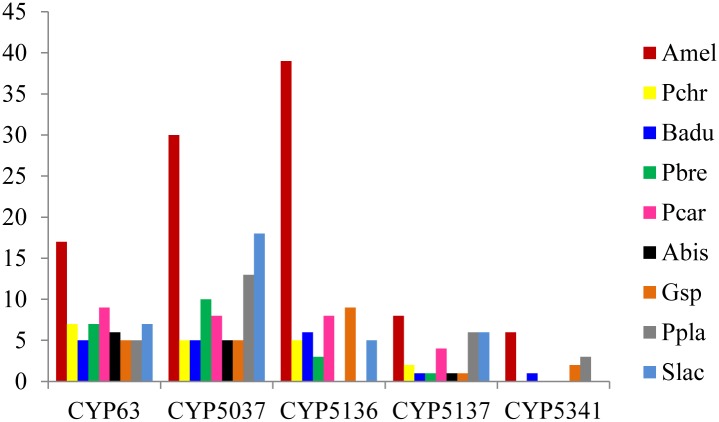
Basidiomycetes, especially species belonging to the Agaricomycotina, are well characterized in terms of their P450 annotation and evolutionary analysis [13, 19, 20, 26]. This will give us an advantage when performing a detailed comparison of P450s between saprophytes and biotrophic plant pathogens. Comparative analysis of A. mellea P450s with P450s of Agaricomycotina saprophytes revealed that the P450 contingent of A. mellea is unique in terms of P450 family expansion, where certain P450 families were expanded with a very high number of member P450s (Fig 4 and S3 Table).
Fig 4. Comparative analysis of putative representatives of enriched P450 families in Agaricomycotina species.
The member count in the P450 families that are expanded in A. mellea is compared with the member count of the same families present in Agaricomycotina saprophytes. The X-axis represents the P450 count and the Y-axis represents P450 families.
The P450 families CYP63, CYP5037, CYP5136, CYP5137 and CYP5341 are expanded in A. mellea. The number of members in these P450 families is as follows: CYP63–17 members; CYP5037–30 members; CYP5136–39 members; CYP5137—eight members and CYP5341- six members. A recent study showed the expansion nature of these P450 families in different Agaricomycotina species [13]. The authors suggested that expansion of these P450 families was due to member P450s’ duplications (paralogous evolution) in order to serve organism adaptation such as colonization of plant material. Comparison of the number of members in these P450 families showed that A. mellea harbours the largest number of members in these P450 families across Agaricomycotina species used in this study (Fig 4 and S3 Table). Analysis of member P450s suggested that the CYP5221 and CYP5233 P450 families are expanded in P. graminis and M. laricis-populina (Fig 1).
Paralogous evolution of expanded P450 families
It is well known that P450 family expansion is possible because of the duplication of member P450s (paralogous evolution) in an organism [13, 16, 57]. Previous studies on animal (arthropods) [57], fungal [13] and oomycetes [16] P450s revealed the expansion of a large number of P450 families and this expanding nature is attained owing to members’ duplication. In order to understand the mechanism behind the expansion of a large number of P450 families in biotrophic plant pathogens, we proceeded to analyze the paralogous evolution of member P450s, if any.
Paralogous evolution of member P450s can be assessed by analysing the percentage identity among member P450s or the gene structure where duplicated member P450s show conservation in the size of exons vis à vis the location of introns. Considering the high percentage identity among the member P450s of expanded P450 families we looked into the gene structure of member P450s and also assessed tandem duplications. Analysis of gene-structure data revealed the conservation of exon size across the members of expanded P450 families (S4 Table). Gene-structure analysis suggested that the CYP63 family in A. mellea initially contained only two members (orthologs) and one of the members is duplicated 14 times (S4 Table). CYP5136 and CYP5317 family members showed high conservation in exon size and based on the size of the exons it is clear that all the members are paralogs. Four orthologs were observed for the CYP5037 family, where one of the orthologs was duplicated and generated 16 paralogs (S4 Table). Two orthologs were observed for the CYP5341 family, where one ortholog was duplicated twice. The CYP5221 and CYP5233 families contained members that were paralogs, as all of the members showed high conservation in exon size (S4 Table).
From the above data it is clear that members of expanded P450 families of basidiomycete biotrophic plant pathogens were duplicated after speciation (paralogous evolution). Analysis of P450 members of expanded families revealed the presence of a large number of tandemly arranged P450s (S5 Table). Ten members were tandemly arranged in the CYP5136 family (S5 Table). The number of P450s that are tandemly arranged is as follows: CYP5211—seven members; CYP5037—five members; CYP63—four members; CYP5233—three members and CYP5137—two members. Overall, based on conservation in exon size and tandem arrangement of member P450s in the expanded P450 families, we conclude that paralogous evolution of member P450s in these families resulted in expansion of these P450 families.
Functional role of P450s in basidiomycete biotrophic plant pathogens
The presence of numerous P450s in A. mellea, expansion of certain P450 families and the presence of distinct P450 families in basidiomycete biotrophic plant pathogens suggest that P450s in these organisms play a key role. Based on homologous P450s characterized in other fungal species, functional analysis for some P450s can be predicted. CYP51, a conserved P450 family across the fungi and also conserved in the species analysed in this study, is involved in the biosynthesis of membrane ergosterol by performing 14α-demethylation of lanosterol [58]. The CYP61 family, which is missing from M. laricis-populina and P. graminis (Fig 1), is also involved in membrane ergosterol biosynthesis where it catalyzes C-22 sterol desaturase activity [59]. The absence of CYP61 in M. laricis-populina and P. graminis is possibly due to their lifestyle; they are obligate biotrophs that extract essential sterols from plants, as previously suggested [17]. The CYP53 family, also known as benzoate-p-hydroxylase, is conserved in biotrophic plant pathogens used in this study (Fig 2). This family is well known for its involvement in detoxification of anti-fungal agents [60]. Study showed that CYP53 family members oxidize benzoate and its derived compounds [61, 62] and plant material stilbene and its derivatives [26]. A recent study suggested that CYP53 family members play a key role in fungal colonization of plant material by detoxification of anti-fungal compounds released by plants or generated during plant material degradation [15]. Also, this study suggested that CYP53 family members play a role in the generation of a secondary metabolite, veratryl alcohol, which is crucial in the degradation of the plant cell wall component, lignin [15]. Based on CYP53 function and the presence of CYP53 members in all four plant pathogens, we conclude that CYP53 members possibly play a key role in colonization of these species on plants through involvement in detoxification of anti-fungal agents and degradation of wood.
Special focus on functional analysis of expanded P450 families in A. mellea revealed the requirement for expansion of these families in this species. Particularly the CYP63, CYP5037 and CYP5136 families are well known for their catalytic versatility [13]. The CYP512 family, which is expanded in other Agaricomycotina members, also shows catalytic versatility [13]. These families are involved in not only oxidation of xenobiotic compounds, but also in oxidation of key metabolic intermediates in fungi [13]. It is evident that these P450 families possibly play a key role in A. mellea towards successful colonization on plants (infection), hence these P450 families are expanded in this species. A putative ortholog of the CYP504 family is present only in U. maydis involved in oxidation of phenylacetate and its derived compounds [63, 64]. Phenylacetate is a plant growth hormone [65] and oxidation of this and its derivatives by CYP504 clearly suggests that after infection this P450 may be involved in interfering with the growth of plants by oxidizing the plant growth hormone by U. maydis. It is noteworthy that the CYP504 family usually presents in most of the plant pathogens [17]. CYP505 family members are involved in oxidation of fatty acids [66] and their role in the generation of mycotoxin fumonisin has also been elucidated [67]. The CYP5138, CYP5139 and CYP5144 families were shown to oxidize xenobiotic compounds [13, 37]. The fused P450s belonging to CYP6000 series contain the N-terminal heme-peroxidase motif and C-terminal heme-domain characteristic of P450s [16]. As CYP6005-CYP6007 family members also contain the same motifs as CYP6001 [68], it is possible that P450s belong to CYP6005-CYP6007 families are involved in oxidation of fatty acids. Overall, based on the above available homologous P450s functions, we conclude that P450s in these plant pathogens possibly play a key role not only in their primary metabolism, but also in successful colonization on living plants by degradation of plant material, detoxification of plant defence chemicals and oxidation of xenobiotic compounds. Functional characterization of P450s in these organisms will provide more insight into their role.
Conclusions
It is well known that ecological niches including the host (a parasite or a symbiont or a commensal) play a key role in shaping the genome content of an organism. Fungi, especially saprophytic species belonging to Agaricomycotina, play a key role in the carbon cycle by degradation of one of the most abundant photosynthetically fixed carbon sources, i.e. plant material. Because of their adaptation to the same ecological niche, similar P450 family types were observed, despite a few differences in P450 families among Agaricomycotina saprophytes. In this study, we present a good example of the influence of ecological niches on the P450 patterns of an organism. Analysis of putative P450s in basidiomycete biotrophic plant pathogens revealed the presence of unique P450 families, possibly reflecting the characteristics of their order. The presence of unique P450 families in these biotrophic plant pathogens serves as good evidence of how a host can influence shaping the P450 content of an organism. These unique P450 family members might play a key role in successful infection of the host. It is noteworthy that P450 patterns in basidiomycete plant pathogens are poorly studied compared to their counterpart ascomycete plant pathogens. This study is the first report on comparative analysis of P450s in basidiomycete biotrophic plant pathogens at order level.
Supporting Information
(PDF)
Each species P450s was presented with its protein IDs that were identified in our analysis at species individual databases listed in Table 1. The number in parenthesis next to the species name is the total P450 count in the species.
(DOCX)
P450 sequences for U. maydis, M. globosa and P. graminis were retrieved from the Cytochrome P450 Homepage [42] and corresponding protein IDs were assigned as per their databases at the Joint Genome Institute (Table 1). Protein IDs for reference P450s (homolog P450s with highest percent identity) from the Cytochrome P450 Homepage [42] are not shown in the table, considering their availability on the webpages listed in Table 1.
(DOCX)
(DOCX)
Gene-structure analysis was carried out by analysis of conservation of exon size across the member P450s. The size of each exon in member P450s is arranged in a way that reflects the conservation pattern. Possible orthologs in each family are also shown in the table.
(XLSX)
The member P450s’ genomic localization such as scaffold/node and the DNA region (start and end) are shown in the table. P450s are presented with their protein IDs. Member P450s that are tandemly duplicated are highlighted in red font.
(DOCX)
Acknowledgments
The authors are deeply grateful to Prof David R Nelson, University of Tennessee Health Science Centre, Memphis, Tennessee, USA for naming the P450 families and providing Ustilago maydis P450s. They are grateful to Dr Mary Catherine Aime, Department of Botany and Plant Pathology, Purdue University, USA and Dr Igor Grigoriev, US Department of Energy Joint Genome Institute, USA for granting permission to use Tilletiaria anomala genome for P450 analysis. The authors also want to extend their thanks to Ms Barbara Bradley, Pretoria, South Africa for English language editing.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
KS and SSM express their sincere gratitude to the Central University of Technology (CUT), Bloemfontein, Free State, South Africa, for a grant from the University Research and Innovation Fund and Emerging Researcher Award (to KS). KS and SSM thank the Department of Higher Education and Training (DHET) and Technology Innovation Agency (TIA), South Africa for a research grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yamazaki H. Fifty years of cytochrome P450 research. 1st ed Springer; Japan; 2014. 10.1007/978-4-431-54992-5 [DOI] [Google Scholar]
- 2. Bernhardt R. Cytochromes P450 as versatile biocatalysts. J Biotechnol. 2006; 24: 128–145. [DOI] [PubMed] [Google Scholar]
- 3. Urlacher VB, Eiben S. Cytochrome P450 monooxygenases: Perspectives for synthetic application. Trends Biotechnol. 2006; 24: 324–330. [DOI] [PubMed] [Google Scholar]
- 4. Kelly SL, Kelly DE. Microbial cytochrome P450 biodiversity and biotechnology, where do cytochrome P450 come from, what do they do and what can they do for us? Phil. Trans. R. Soc. B. Biol. Sci. 2013; 368: 20120476 10.1098/rstb.2012.0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson DR. A world of cytochrome P450s. Phil. Trans. R. Soc. B. Biol. Sci. 2013; 368: 20120430 10.1098/rstb.2012.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fraser JA, Davis MA, Hynes MJ. The gene gmdA, encoding an amidase and bzuA, encoding a cytochrome P450, are required for benzamide utilization in Aspergillus nidulans . Fungal Genet Biol. 2002; 35: 135–146. [DOI] [PubMed] [Google Scholar]
- 7. Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003; 48: 77–84. [DOI] [PubMed] [Google Scholar]
- 8. Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA. 2003; 100: 12989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLean KJ, Carroll P, Lewis DG, Dunford AJ, Seward HE, Neeli R, et al. Characterization of active site structure in CYP121. A cytochrome P450 essential for viability of Mycobacterium tuberculosis H37Rv. J Biol Chem. 2008; 283: 33406–16. 10.1074/jbc.M802115200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson DR. Progress in tracing the evolutionary paths of cytochrome P450. Biochim Biophys Acta. 2011; 1814: 14–18. 10.1016/j.bbapap.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 11. Syed K, Porollo A, Lam YW, Grimmett PW, Yadav JS. CYP63A2, a catalytically versatile fungal P450 monooxygenase capable of oxidizing higher-molecular-weight polycyclic aromatic hydrocarbons, alkylphenols, and alkanes. Appl Environ Microbiol. 2013; 79: 2692–2702. 10.1128/AEM.03767-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hlavica P. Evaluation of structural features in fungal cytochromes P450 predicted to rule catalytic diversification. Biochim Biophys Acta. 2013; 1834: 205–220. 10.1016/j.bbapap.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 13. Syed K, Shale K, Pagadala NS, Tuszynski J. Systematic identification and evolutionary analysis of catalytically versatile cytochrome P450 monooxygenase families enriched in model basidiomycete fungi. PLoS ONE 2014; 9(1): e86683 10.1371/journal.pone.0086683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Syed K, Shale K, Nazir KHMNZ, Krasevec N, Mashele SS, Pagadala NS. Genome-wide identification, annotation and characterization of novel thermostable cytochrome P450 monooxygenases from the thermophilic biomass-degrading fungi Thielavia terrestris and Myceliophthora thermophila . Genes Genom. 2014; 36: 321–333. [Google Scholar]
- 15. Jawallapersand P, Mashele SS, Kovačič L, Stojan J, Komel R, Pakala SB, et al. Cytochrome P450 monooxygenase CYP53 family in fungi: comparative structural and evolutionary analysis and its role as a common alternative anti-fungal drug target. PLoS ONE 2014; 9(9): e107209 10.1371/journal.pone.0107209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sello MM, Jafta M, Nelson DR, Chen W, Yu J-H, Parvez M, et al. Diversity and evolution of cytochrome P450 monooxygenases in Oomycetes. Sci Rep. 2015; 5: 11572 10.1038/srep11572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moktali V, Park J, Fedorova-Abrams ND, Park B, Choi J, Lee YH, et al. Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genomics 2012; 13: 525 10.1186/1471-2164-13-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martínez AT, Speranza M, Ruiz-Dueñas FJ, Ferreira P, Camarero S, Guillén F, et al. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol. 2005; 8: 195–204. [PubMed] [Google Scholar]
- 19. Suzuki H, MacDonald J, Syed K, Salamov A, Hori C, Aerts A, et al. Comparative genomics of the white-rot fungi, Phanerochaete carnosa and P. chrysosporium, to elucidate the genetic basis of the distinct wood types they colonize. BMC Genomics. 2012; 13: 444 10.1186/1471-2164-13-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Syed K, Nelson DR, Riley R, Yadav JS. Genome-wide annotation and comparative genomics of cytochrome P450 monooxygenases (P450s) in the Polyporale species Bjerkandera adusta, Ganoderma sp. and Phlebia brevispora . Mycologia 2013; 105: 1445–1455. 10.3852/13-002 [DOI] [PubMed] [Google Scholar]
- 21. Kgosiemang IKR, Mashele SS, Syed K. Comparative genomics and evolutionary analysis of cytochrome P450 monooxygenases in fungal subphylum Saccharomycotina. J Pure Appl Microbiol. 2014; 8: 291–302. [Google Scholar]
- 22. Chen W, Lee MK, Jefcoate C, Kim SC, Chen F, Yu JH. Fungal cytochrome P450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol Evol. 2014; 6: 1620–34. 10.1093/gbe/evu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martinez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J, et al. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol. 2004; 22: 695–700. [DOI] [PubMed] [Google Scholar]
- 24. Syed K, Yadav JS. P450 monooxygenases (P450ome) of the model white rot fungus Phanerochaete chrysosporium . Crit Rev Microbiol. 2012; 38: 339–363. 10.3109/1040841X.2012.682050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martinez D, Challacombe J, Morgenstern I, Hibbett D, Schmoll M, Kubicek CP, et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA 2009; 106: 1954–1959. 10.1073/pnas.0809575106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ide M, Ichinose H, Wariishi H. Molecular identification and functional characterization of cytochrome P450 monooxygenases from the brown-rot basidiomycete Postia placenta . Arch Microbiol. 2012; 194: 243–53. 10.1007/s00203-011-0753-2 [DOI] [PubMed] [Google Scholar]
- 27. Chen S, Xu J, Liu C, Zhu Y, Nelson DR, Zhou S, et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum . Nature Commun. 2012; 3: 913 10.1038/ncomms1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morin E, Kohler A, Baker AR, Foulongne-Oriol M, Lombard V, Nagy LG, et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc Natl Acad Sci USA 2012; 109: 17501–6. 10.1073/pnas.1206847109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandez-Fueyo E, Ruiz-Dueñas FJ, Ferreira P, Floudas D, Hibbett DS, Canessa P, et al. Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc Natl Acad Sci USA 2012; 109: 5458–5463. 10.1073/pnas.1119912109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamper J, Kahmann R, Bolker M, Ma LJ, Brefort T, Saville BJ, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis . Nature 2006; 444: 97–101. [DOI] [PubMed] [Google Scholar]
- 31. Duplessis S, Cuomo CA, Lin YC, Aerts A, Tisserant E, Veneault-Fourrey C, et al. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Natl Acad Sci USA 2011; 108: 9166–71. 10.1073/pnas.1019315108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins C, Keane TM, Turner DJ, O'Keeffe G, Fitzpatrick DA, Doyle S, et al. Genomic and proteomic dissection of the ubiquitous plant pathogen, Armillaria mellea: Toward a new infection model system. J Proteome Res. 2013; 12: 2552–70. 10.1021/pr301131t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nemri A, Saunders DG, Anderson C, Upadhyaya NM, Win J, Lawrence GJ, et al. The genome sequence and effector complement of the flax rust pathogen Melampsora lini . Front Plant Sci. 2014; 5: 98 10.3389/fpls.2014.00098 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toome M, Ohm RA, Riley RW, James TY, Lazarus KL, Henrissat B, et al. Genome sequencing provides insight into the reproductive biology, nutritional mode and ploidy of the fern pathogen Mixia osmundae . New Phytol. 2014; 202: 554–64. 10.1111/nph.12653 [DOI] [PubMed] [Google Scholar]
- 35. Schirawski J, Mannhaupt G, Munch K, Brefort T, Schipper K, Doehlemann G, et al. Pathogenicity determinants in smut fungi revealed by genome comparison. Science 2010; 330: 1546–8. 10.1126/science.1195330 [DOI] [PubMed] [Google Scholar]
- 36. Toome M, Kuo A, Henrissat B, Lipzen A, Tritt A, Yoshinaga Y, et al. Genome sequence of a rare smut relative, Tilletiaria anomala UBC 951. Genome Announc. 2014; 2(3). pii: e00539–14. 10.1128/genomeA.00539-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hirosue S, Tazaki M, Hiratsuka N, Yanai S, Kabumoto H, Shinkyo R, et al. Insight into functional diversity of cytochrome P450 in the white-rot basidiomycete Phanerochaete chrysosporium: involvement of versatile monooxygenase. Biochem Biophys Res Commun. 2011; 407: 118–123. 10.1016/j.bbrc.2011.02.121 [DOI] [PubMed] [Google Scholar]
- 38. Mthakathi NT, Kgosiemang IKR, Chen W, Mohlatsane ME, Mojahi TJ, Yu J-H, et al. Cytochrome P450 monooxygenase analysis in free-living and symbiotic microalgae Coccomyxa sp. C-169 and Chlorella sp. NC64A. Algae 2015; 30: 233–239. [Google Scholar]
- 39. Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem. 1992; 267: 83–90. [PubMed] [Google Scholar]
- 40. Sirim D, Widmann M, Wagner F, Pleiss J. Prediction and analysis of the modular structure of cytochrome P450 monooxygenases. BMC Struct Biol. 2010. 10: 34 10.1186/1472-6807-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Syed K, Mashele SS. Comparative Analysis of P450 Signature Motifs EXXR and CXG in the Large and Diverse Kingdom of Fungi: Identification of Evolutionarily Conserved Amino Acid Patterns Characteristic of P450 Family. PLoS ONE 2014; 9(4): e95616 10.1371/journal.pone.0095616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nelson DR. The cytochrome P450 homepage. Hum Genomics 2009; 4: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grigoriev IV, Nikitin R, Haridas S, Kuo A, Ohm R, Otillar R, et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucleic Acids Res. 2014; 42: D699–704. 10.1093/nar/gkt1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu Z, Zhang S, Liu H, Shen H, Lin X, Yang F, et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides . Nat Commun. 2012; 3: 1112 10.1038/ncomms2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gioti A, Nystedt B, Li W, Xu J, Andersson A, Averette AF, et al. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis . MBio 2013; 4(1):e00572–12. 10.1128/mBio.00572-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morita T, Koike H, Koyama Y, Hagiwara H, Ito E, Fukuoka T, et al. Genome sequence of the basidiomycetous yeast Pseudozyma antarctica T-34, a producer of the glycolipid biosurfactants mannosylerythritol lipids. Genome Announc. 2013; 4: e0006413 10.1128/genomeA.00064-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Konishi M, Hatada Y, Horiuchi J. Draft genome sequence of the basidiomycetous yeast-like fungus Pseudozyma hubeiensis SY62, which produces an abundant amount of the biosurfactant mannosylerythritol lipids. Genome Announc. 2013; 27: pii: e00409–13. 10.1128/genomeA.00409-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, et al. The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993; 12: 1–51. 10.1089/dna.1993.12.1 [DOI] [PubMed] [Google Scholar]
- 49. Nelson DR. Cytochrome P450 nomenclature. Methods Mol Biol. 1998; 107: 15–24. 10.1385/0-89603-519-0:15 [DOI] [PubMed] [Google Scholar]
- 50. Nelson DR. Cytochrome P450 nomenclature, 2004. Methods Mol Biol (Clifton, NJ). 2006; 320: 1–10. [DOI] [PubMed] [Google Scholar]
- 51. Eddy SR. Accelerated profile HMM searches. PLoS Comp Biol. 2011; 7:e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. The Pfam protein families database. Nucleic Acids Research 2014; 42: D222–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Price MN, Dehal PS, Arkin AP. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009; 26: 1641–1650. 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Letunic I, Bork P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007; 23: 127–128. [DOI] [PubMed] [Google Scholar]
- 55. Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012; 336: 1715–1719. 10.1126/science.1221748 [DOI] [PubMed] [Google Scholar]
- 56. Nelson DR. Cytochrome P450 and the individuality of species. Arch Biochem Biophys. 1999; 369: 1–10. [DOI] [PubMed] [Google Scholar]
- 57. Feyereisen R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. Biochim Biophys Acta. 2011; 1814: 19–28. 10.1016/j.bbapap.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 58. Lepesheva GI, Waterman MR.CYP51- the omnipotent P450. Mol Cell Endocrinol. 2004; 215: 165–170. 10.1016/j.mce.2003.11.016 [DOI] [PubMed] [Google Scholar]
- 59. Kelly SL, Lamb DC, Baldwin BC, Corran AJ, Kelly DE. Characterization of Saccharomyces cerevisiae CYP61, sterol delta22-desaturase, and inhibition by azole antifungal agents. J Biol Chem. 1997; 272: 9986–9988. [DOI] [PubMed] [Google Scholar]
- 60. Faber BW, van Gorcom RFM, Duine JA. Purification and characterization of benzoate-para-hydroxylase, a cytochrome P450 (CYP53A1), from Aspergillus niger . Arch Biochem Biophys. 2001; 394: 245–254. [DOI] [PubMed] [Google Scholar]
- 61. Matsuzaki F, Wariishi H. Molecular characterization of cytochrome P450 catalyzing hydroxylation of benzoates from the white-rot fungus Phanerochaete chrysosporium . Biochem Biophys Res Commun. 2005; 334: 1184–90. [DOI] [PubMed] [Google Scholar]
- 62. Durairaj P, Jung E, Park HH, Kim B-G, Yun H. Comparative functional characterization of a novel benzoate hydroxylase cytochrome P450 of Fusarium oxysporum . Enzyme Microb Tech. 2015; 70: 58–65. [DOI] [PubMed] [Google Scholar]
- 63. Mingot JM, Penalva MA, Fernandez-Canon JM.Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 monooxygenase catalysing phenylacetate 2-hydroxylation, results in penicillin over production. J Biol Chem. 1999; 274: 14545–14550. [DOI] [PubMed] [Google Scholar]
- 64. Ferrer-Sevillano F, Fernandez-Canon JM. Novel phacB-encoded cytochrome P450 monooxygenase from Aspergillus nidulans with 3-hydroxyphenylacetate 6-hydroxylase and 3, 4-dihydroxyphenylacetate 6-hydroxylase activities. Eukaryotic Cell 2007; 6: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wightman F, Lighty DC. Identification of phenylacetic acid as a natural auxin in the shoots of higher plants. Physiol Plant 1982; 55: 17–24. [Google Scholar]
- 66. Nakayama N, Takemae A, Shoun H. Cytochrome P450foxy, a catalytically self-sufficient fatty acid hydroxylase of the fungus Fusarium oxysporum . J Biochem. 1996; 119: 435–440. 10.1093/oxfordjournals.jbchem.a021260 [DOI] [PubMed] [Google Scholar]
- 67. Proctor RH, Brown DW, Plattner RD, Desjardins AE. Coexpression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis . Fungal Genet Biol. 2003; 38: 237–249. [DOI] [PubMed] [Google Scholar]
- 68. Brodhun F, Göbel C, Hornung E, Feussner I. Identification of PpoA from Aspergillus nidulans as a fusion protein of a fatty acid heme dioxygenase/peroxidase and a cytochrome P450. J Biol Chem. 2009; 284: 11792–11805. 10.1074/jbc.M809152200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Each species P450s was presented with its protein IDs that were identified in our analysis at species individual databases listed in Table 1. The number in parenthesis next to the species name is the total P450 count in the species.
(DOCX)
P450 sequences for U. maydis, M. globosa and P. graminis were retrieved from the Cytochrome P450 Homepage [42] and corresponding protein IDs were assigned as per their databases at the Joint Genome Institute (Table 1). Protein IDs for reference P450s (homolog P450s with highest percent identity) from the Cytochrome P450 Homepage [42] are not shown in the table, considering their availability on the webpages listed in Table 1.
(DOCX)
(DOCX)
Gene-structure analysis was carried out by analysis of conservation of exon size across the member P450s. The size of each exon in member P450s is arranged in a way that reflects the conservation pattern. Possible orthologs in each family are also shown in the table.
(XLSX)
The member P450s’ genomic localization such as scaffold/node and the DNA region (start and end) are shown in the table. P450s are presented with their protein IDs. Member P450s that are tandemly duplicated are highlighted in red font.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.