Abstract
Efficient processing of incoming sensory information is critical for an organism’s survival. It has been widely observed across systems and species that the representation of sensory information changes across successive brain areas. Indeed, peripheral sensory neurons tend to respond densely to a broad range of sensory stimuli while more central neurons tend to instead respond sparsely to a narrow range of stimuli. Such a transition might be advantageous as sparse neural codes are thought to be metabolically efficient and optimize coding efficiency. Here we investigated whether the neural code transitions from dense to sparse within the midbrain Torus semicircularis (TS) of weakly electric fish. Confirming previous results, we found both dense and sparse coding neurons. However, subsequent histological classification revealed that most dense neurons projected to higher brain areas. Our results thus provide strong evidence against the hypothesis that the neural code transitions from dense to sparse in the electrosensory system. Rather, they support the alternative hypothesis that higher brain areas receive parallel streams of dense and sparse coded information from the electrosensory midbrain. We discuss the implications and possible advantages of such a coding strategy and argue that it is a general feature of sensory processing.
Keywords: Weakly electric fish, Midbrain, Sparseness, Neural code
1. Introduction
Understanding the neural code remains a central problem in neuroscience. Such understanding is in part complicated by the fact that the representation of sensory information changes across brain areas. Indeed, peripheral sensory neurons tend to respond to a wide range of stimuli. Such a dense coding strategy is in contrast to the sparse coding strategy exhibited in more central brain areas where neurons respond sparse to behaviorally relevant stimulus features [29]. Studies across systems and species have revealed that such a transition from dense to sparse neural codes when moving more centrally is observed ubiquitously [29]. However, the mechanisms mediating the transition from dense to sparse representations are not well understood in general.
Weakly electric fish offer an attractive model system for studying neural coding strategies because their anatomy and natural stimuli are both well characterized [5,6,20]. These fish generate an electric field around their body through the electric organ discharge (EOD) and sense perturbations of this field through an array of electroreceptor organs scatted on their skin surface [8]. These project to pyramidal neurons within the electrosensory lateral line lobe (ELL) that respond densely to electrosensory stimuli [8]. ELL pyramidal neurons project to the midbrain Torus semicircularis (TS): this brain area consists of 11 layers and comprises approximately 50 cells types [5,6].
Previous studies performed in TS have shown that some TS neurons respond very selectively (i.e., sparse), whereas other TS neurons instead respond non-selectively (i.e., dense) to electrosensory stimuli in a manner that was similar to that of ELL pyramidal neurons [22,36,37]. This result appears to be at odds with the current dogma that the neural code transitions from dense to sparse when moving more centrally. One hypothesis is that the transition from dense to sparse coding occurs within the TS. If correct, then this would imply that neurons that respond less selectively receive direct input from ELL and project either within TS or to lower brain areas, whereas more selective TS neurons project to higher brain areas. An alternative hypothesis is that both groups of neurons project to higher brain areas. However, since sparse and dense neurons were not labeled in previous studies, it is currently not known whether the representation of sensory information transitions from dense to sparse in TS or, alternatively, whether sparse and dense information streams are both sent to higher brain areas.
2. Methods
2.1. Animals
All surgical procedures were approved by and in accordance with McGill University’s animal care committee. Adult specimens of the weakly electric fish Apteronotus leptorhynchus were obtained from local tropical fish suppliers. They were acclimated and housed in accordance with previously published guidelines [13]. 62 animals of either sex were used in this study.
2.2. Surgery and recordings
The surgical and electrophysiological recording techniques have been previously described [7,9,22,30]. Intracellular electrodes were filled with (in mM: 100 KAc, 43 neurobiotin, 20 KOH, 10HEPES, 2 KCl, 1 MgCl2 anhydrous, pH 7–7.3). Amplification of recordings was achieved with A-M systems 1700 (for extracellular) and Axoclamp 2B (0.1 headstage for extracellular and intracellular) amplifiers. Recorded signals were then digitized at a sampling rate of 10-kHz by a Power1401 using Spike2 software (Cambridge Electronic Design, Cambridge, UK), and stored on a PC computer for offline analysis.
2.3. Stimulation
2.3.1. Stimulus generation
Detailed descriptions of the stimulus protocol, generation and the rationale behind stimulus design have been presented previously [2,33,36]. Stimulus contrast was 15%, similar to that used previously [11]. It is important to realize here that the animal’s EOD is a carrier and that the meaningful stimulus here is the EOD amplitude modulation (AM), as AM sensitive cells constitute the main input to the Torus. Our stimuli consisted of both first and second-order features: we note that these correspond to the second- and third-order features of the full signal received by the animal, respectively. The first and second order components of the electrical stimulus we will henceforth be referred to as AM stimuli (AMs) and envelope stimuli (ES), respectively, and are described below [22–24,26,31].
2.3.2. Stimulus classification
AMs consisted of steps, sinusoids across a range of frequencies (0.1, 1, 2, 4, 8, 16, 32, 64, 128, 256 Hz) which are referred to as beat frequencies, agonistic communication signals known as type II or small chirps, and courtship communication signals known as type I or big chirps that typically display a concurrent reduction in EOD amplitude, as done previously [36]. Chirps were played on top of sinusoids with frequencies (type II: 2, 5, 10 and 25 Hz; type I: 100, 150, 250 and 350 Hz). ES consisted of all combinations of sinusoidal first order (1, 10, 50, and 200 Hz) and second order (0.1, 1, 10 and 50 Hz) waveforms with the second order frequency lower than the first order, as well as the following combination: 32 Hz first order with 0.1 Hz second order. Objects similar to those used in previous studies were moved back and forth lateral to and along the animals rostro-caudal axis at velocities 1, 10 and 20 cm/s [7,9,15,16,36].
2.4. Analysis
2.4.1. Preprocessing
All offline analysis was performed in Matlab using custom written analysis scripts (MathWorks, Natick, MA). Peristimulus time histograms (PSTHs) were generated for anywhere between 10 and 20 trials and for step, beat, small and big chirp stimuli and smoothed with a 6-ms-wide box filter.
2.4.2. Lifetime sparseness
Lifetime sparseness of individual neurons was characterized using the lifetime sparseness index (SI) [34]:
The activity fraction (AI) was computed for each neuron (n = 134) as follows.
For the step stimulus and all chirp stimuli the response Ri for a given stimulus i was taken as the mean cross trial firing rate modulation during a response window of 250 ms that encompassed the firing rate modulations following the onset of both step and chirps as well as the offset of the step. For beat frequency, envelope and moving object stimuli, Ri for a given stimulus i was taken as the mean firing rate throughout the duration of the stimulus’ presentation.
2.4.3. Response significance
Response significance was calculated as done previously [17,36]. PSTHs were generated using 6 ms bins during a 250 ms time window beginning at the stimulus onset. For the step stimulus, entropy was additionally calculated for the stimulus offset for the same time window length. The entropy E is defined by Kajikawa and Hackett [14]:
2.4.4. Designating sparse and dense populations
Neurons were designated as either sparse or dense in a similar fashion to previous studies [36]. Observations falling into bins below 0.3 corresponding to the trough of the bimodal distribution were defined as dense whereas values above 0.3 were designated as sparse, corresponding to the large and readily apparent trough dividing the distribution (see Fig. 1D). We note that this threshold is actually lower than that used previously [36] but that this does not affect the qualitative nature of our results showing that a significant fraction of dense coding neurons project to higher brain areas (data not shown). We also note that previous studies have shown that SI values of ELL and dense TS neurons are similarly distributed [36].
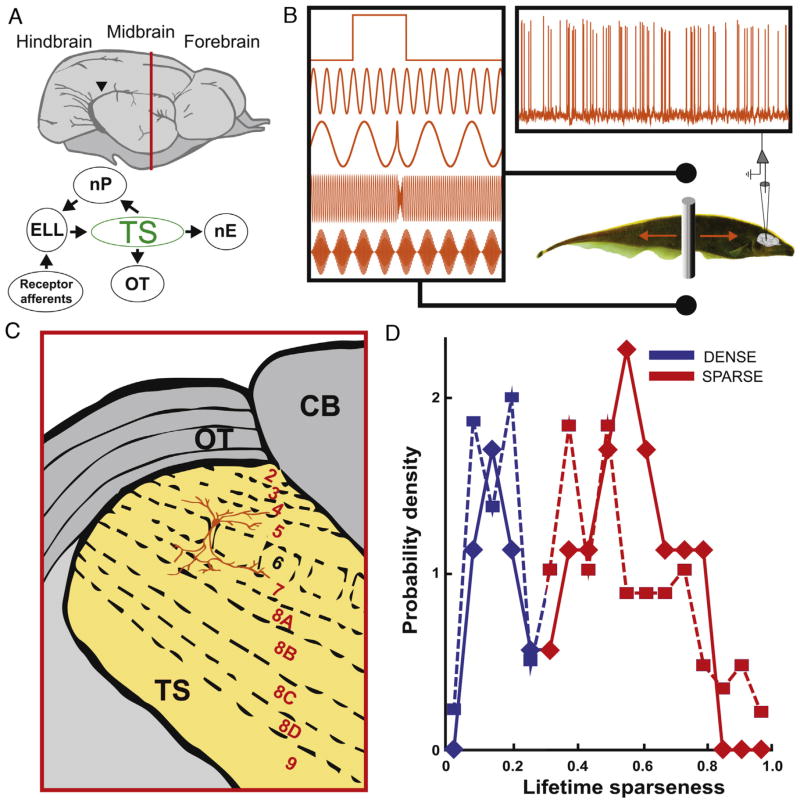
Fig. 1. Recording setup and cell identification.
(A) Top: schematic side view of the Apteronotus leptorhynchus brain adapted from [20]. Bottom: anatomical connections. OT: optic tectum; nP: nucleus praeminentialis; ELL: electrosensory lateral line lobe; TS: Torus semicircularis; nE: nucleus electrosensorius. (B) Recording setup: the fish is presented with multiple stimuli while TS neurons are being recorded from intracellularly and extracellularly. The stimuli (from top to bottom) shown are: step, beat, small chirp, big chirp, envelope. The grey bar shows the moving object. (C) Coronal section taken at the vertical red line in (A) showing TS layers. CB: cerebellum. (D) Lifetime sparseness index SI distribution for 134 (squares, dashed line) and 29 filled (diamonds, solid line) TS neurons. The SI value at the trough (~0.3) was used as a threshold to separate sparse (red) and dense (blue) coding neurons. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.5. Histology
Following electrophysiological recordings animals were perfused through the heart with paraformaldehyde. The brain was removed from the animal and stored in a fixative until twenty-four hours before sectioning at which point it was cryofixed in a solution of paraformaldehyde and sucrose. 80 μm parasagittal sections from the recorded hemisphere where made on a cryostat (Leica CM3050 S) with a carbon fiber blade. Sections were subsequently stained with streptavidin CY3, thereby labeling filled cells. A green fluorescent Nissl stain was used to aid in the visualization of TS layers. Filled cells were then identified according to a Golgi staining study of the TS [5]. Cell identity was based on comparison between morphological features (e.g., cell body size, the absence, presence or density of spines, dendritic arbor shape and size as well as the presence or absence of processes and the extent to which they might project to other layers within TS) and previously published classification [5,6]. At most three neurons were filled per animal and the identity of the filled cells could easily be reconciled with recorded activities based on the position of the electrode relative to landmarks on the brain’s surface as well as the recording depth.
3. Results
3.1. Classification of sparse and dense coding neurons
In vivo patch recordings allowed us to fill neurons with neurobiotin, while eliciting responses to multiple stimuli (Fig. 1A–C). We first used both intracellular and extracellular recordings from n = 134 TS neurons and computed lifetime sparseness index SI values. Consistent with previous results, the resulting distribution (Fig. 1D) was characterized by a prominent trough at SI = ~0.3 (Hartigan’s dip test, p = 0.02). Neurons whose SI value was lower than this threshold were classified as “dense” (Fig. 1D, blue), whereas neurons whose SI value was higher than this threshold were classified as “sparse” (Fig. 1D, red).
3.2. Response profiles of dense coding and sparse coding neurons
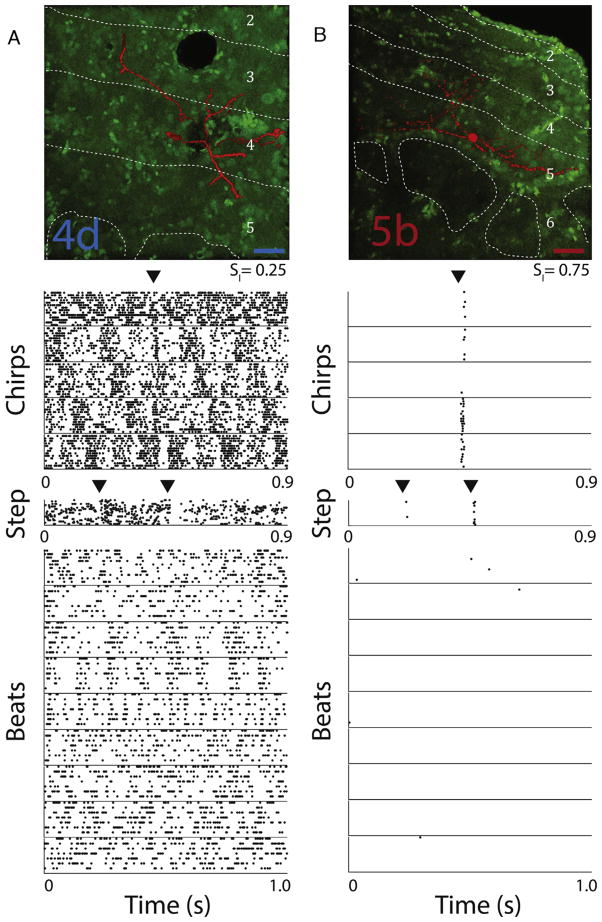
We recorded from n = 29 TS neurons that were also filled with neurobiotin and subsequently identified according to previously published classification [5,6]. 9 (~30%) of these neurons were classified as dense whereas the remainder (~70%) was classified as sparse. These percentages were comparable to those obtained using the full (n = 134) dataset (dense: 35%, sparse 65%) and the distributions of SI values obtained from either dataset were not significantly different from one another (Kolmogorov–Smirnov test, p = 0.26). An example dense coding cell (SI = 0.25) was identified as type 4d and responded to most stimuli through modulations in spiking activity (Fig. 2A). In contrast, an example sparse coding cell (SI = 0.75) was identified as type 5b and responded with a few spikes only to some stimuli (Fig. 2B).
Fig. 2. Responses to electrosensory stimuli from example dense and sparse TS neurons.
(A) Top: confocal image of a dense coding TS neuron identified as a layer 4d neuron on the basis of a previous Golgi classification [5]. Dashed white lines demarcate boundaries between layers, labeled accordingly. The scale bar indicates 25 μm. Bottom: raster plots showing this neuron’s response to repeated presentations (10–20 trials) of 15 different stimuli, 5 chirps (1 courtship and 4 agonistic), 1 artificial step stimulus and 9 different beat frequencies (1, 2, 4, 8, 16, 32, 64, 128, and 256 Hz). Arrowheads indicate chirp or step onset as well as step offset. We obtained SI = 0.25 for this neuron. (B) Same as A, but for a sparse coding TS neuron identified as a layer 5b neuron. We obtained SI = 0.75 for this neuron. The scale bar indicates 25 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Investigating the distribution of dense coding and sparse coding neurons in the TS
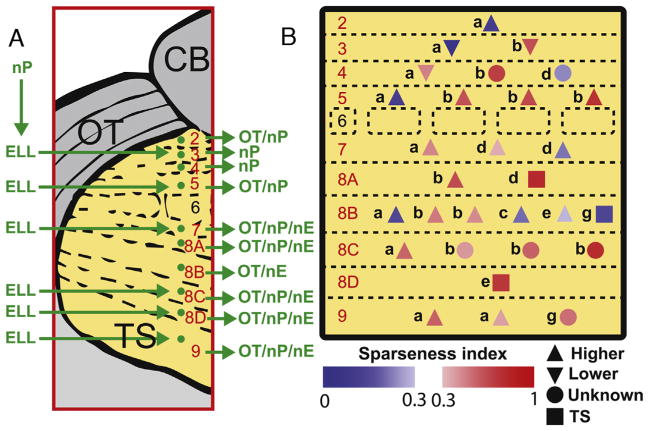
Previous studies [5,6] have identified the afferent and efferent projections of most TS neuron types and these are summarised in Fig. 3A. Fig. 3B shows the identity and location, and efferent projection of all n = 29 TS neurons that were recorded from and subsequently identified. Sparse and dense coding neurons were found across most if not all TS layers and their distributions were not significantly different from one another (Komolgorov–Smirnov test, p = 0.35). Surprisingly, most dense coding (6/9 or 66%) neurons projected to higher brain areas. Furthermore, 15/20 or 75% of sparse coding neurons projected to higher brain areas.
Fig. 3. Distribution and identification of sparse and dense coding TS neurons.
(A) Summary of inputs to and outputs from TS layers as per [5,6]. Note that TS layer 6 receives only input from the frequency modulation (FM) pathway that is not considered in the current study. (B) Cell type as per [5,6] and location within TS layers of sparse (red) and dense (blue) coding neurons. The colormaps indicate each neuron’s SI. Neurons marked with up triangles, squares, down triangles, or circles projected directly to higher brain areas, exclusively within TS, exclusively to lower brain areas, or to unknown brain areas, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
4.1. Summary of results
The goal of this study was to investigate the coding strategy used by the midbrain TS of the weakly electric fish A. leptorhynchus. One hypothesis was that the neural code transitions from dense to sparse within TS. An alternative hypothesis was that parallel streams of dense and sparse coded information are sent from TS to higher brain areas. We found that most dense neurons (66%) projected to higher brain areas, strongly supporting the latter hypothesis.
4.2. Coding of electrosensory information by midbrain neurons
The TS is thought to transmit electrosensory information to other midbrain structures including the Optic tectum (OT) as well as associative areas within the forebrain [6]. The OT is homologous to the mammalian superior colliculus and is a region of sensory motor integration. Importantly, topographic electrosensory maps within TS are transformed across these stations as electrosensory maps are found to be in spatial register with visual maps of the OT. TS also provides descending feedback to ELL via the nucleus praeminentialis (nP) [6]. Only the lower layers of TS (8,9) project to the forebrain [6].
Our results have shown that sparse and dense coding neurons were found across most TS layers. In particular, we found most dense and sparse coding neurons project to OT. Our results therefore suggest that OT receives parallel sparse and dense coded information streams. It is possible that the sparse coded information transmitted from TS to OT could serve a “where” detection function since topographic information is retained there, which would then be used to initiate proper motor responses. Such information could be used to enable the animal to successfully detect prey [28] or to maintain position within a refuge [10]. Additionally, the descending feedback from TS to nP could potentially help refine ELL neural responses and enable the animal to better locate objects in its environment through a sensory searchlight mechanism [3]. Further studies are needed to uncover the functional role of sparse and dense coded information in initiating motor responses and modifying ELL neural responses to sensory input.
Our results have also shown that sparse and dense coding neurons co-exist within the deeper layers of TS and project to the nucleus electrosensorius (nE). It is therefore likely that neurons within nE also receive parallel sparse and dense coded streams of information. Sparse coding neurons could serve to detect the occurrence of natural electrocommunication stimuli [36] that occur during agonistic and courtship behavior [38] independently of the conspecific’s location. In contrast, dense coding neurons could instead convey information about critical stimulus attributes such as conspecific identity that would be useful for behaviors such as avoidance responses [12,32] or envelope tracking [25]. Further studies are needed to understand how neurons in higher brain areas decode information that is transmitted by both sparse and dense neurons and whether these parallel streams are actually retained.
4.3. Functional advantages of parallel dense and sparse neural codes
What is the advantage of retaining dense neural codes more centrally? This question is particularly important since previous studies have argued that the transition from a dense to a sparse neural code is advantageous as they minimize energetic requirements while maximizing the efficiency of information transmission [1,29]. However, a candidate neural code should account for the high fidelity propagation of information across processing modules [18]. A dense coding stream might be more advantageous for propagating information with high temporal precision as well as for discrimination and decoding [35]. These observations agree with previous studies showing that dense coding neurons in TS are more advantageous for stimulus estimation while sparse coding neurons are instead more advantageous for stimulus detection [36]. If true, then sparse coding TS neurons would signal the time of occurrence of the stimulus (i.e., “when”) while the dense coding neurons would instead signal the stimulus’ identity (i.e., “what”).
4.4. Implications for other systems
It is likely that parallel sparse and dense coding streams constitute a general feature of sensory processing. Indeed, recent evidence from the olfactory systems of both drosophila and honey bees indicate that broad and narrowly tuned neurons can be found within the antennal lobe [39] and are thought to mediate adaptive behavior and perception respectively [4]. These dense and sparse neurons are thought to encode information as to the stimulus’ identity (“what”) and time of occurrence (“when”) [40]. The presence of these parallel dense and sparse streams is reminiscent of the “what” and “where” pathways of the mammalian visual system [21]. Moreover, dense coding neurons have been observed in the primate inferior temporal cortex: a highly centralized and specialized area in the visual system [19]. It is furthermore possible that sparse and dense coding neurons are found in both the Inferior colliculus and auditory cortex as neurons in these areas mark the occurrence of novel acoustic events with precisely timed spikes while conveying detailed stimulus information through changes in firing rate [27]. Parallel coding of information by which separate neural circuits are devoted to coding different stimulus attributes is furthermore seen ubiquitously [21].
HIGHLIGHTS.
The neural code is thought to transition from dense to sparse when moving centrally.
We labeled neurons in the electrosensory midbrain Torus semicircularis.
Our results show that both dense and sparse neurons project to higher brain areas.
Parallel streams of dense and sparse coded information co-exist in midbrain.
This has important implications for understanding information processing.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council and the Canada Research Chairs (M.J.C.).
References
- 1.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bastian J, Chacron MJ, Maler L. Receptive field organization determines pyramidal cell stimulus-encoding capability and spatial stimulus selectivity. J Neurosci. 2002;22:4577–4590. doi: 10.1523/JNEUROSCI.22-11-04577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman NJ, Maler L. Interaction of GABAB -mediated inhibition with voltage-gated currents of pyramidal cells: computational mechanism of a sensory searchlight. J Neurophysiol. 1998;80:3197–3213. doi: 10.1152/jn.1998.80.6.3197. [DOI] [PubMed] [Google Scholar]
- 4.Brill MF, Rosenbaum T, Reus I, Kleineidam CJ, Nawrot MP, Rossler W. Parallel processing via a dual olfactory pathway in the honeybee. J Neurosci. 2013;33:2443–2456. doi: 10.1523/JNEUROSCI.4268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr CE, Maler L. A Golgi study of the cell types of the dorsal torus semicircularis of the electric fish Eigenmannia: functional and morphological diversity in the midbrain. J Comp Neurol. 1985;235:207–240. doi: 10.1002/cne.902350206. [DOI] [PubMed] [Google Scholar]
- 6.Carr CE, Maler L, Heiligenberg W, Sas E. Laminar organization of the afferent and efferent systems of the torus semicircularis of gymnotiform fish: morphological substrates for parallel processing in the electrosensory system. J Comp Neurol. 1981;203:649–670. doi: 10.1002/cne.902030406. [DOI] [PubMed] [Google Scholar]
- 7.Chacron MJ, Fortune ES. Subthreshold membrane conductances enhance directional selectivity in vertebrate sensory neurons. J Neurophysiol. 2010;104:449–462. doi: 10.1152/jn.01113.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chacron MJ, Longtin A, Maler L. Efficient computation via sparse coding in electrosensory neural networks. Curr Opin Neurobiol. 2011;21:752–760. doi: 10.1016/j.conb.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chacron MJ, Toporikova N, Fortune ES. Differences in the time course of short-term depression across receptive fields are correlated with directional selectivity in electrosensory neurons. J Neurophysiol. 2009;102:3270–3279. doi: 10.1152/jn.00645.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan NJ, Fortune ES. The critical role of locomotion mechanics in decoding sensory systems. J Neurosci. 2007;27:1123–1128. doi: 10.1523/JNEUROSCI.4198-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deemyad T, Metzen MG, Pan Y, Chacron MJ. Serotonin selectively enhances perception and sensory neural responses to stimuli generated by same-sex conspecifics. PNAS. 2013;110:19609–19614. doi: 10.1073/pnas.1314008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiligenberg W. Neural Nets in Electric Fish. MIT Press; Cambridge MA: 1991. [Google Scholar]
- 13.Hitschfeld EM, Stamper SA, Vonderschen K, Fortune ES, Chacron MJ. Effects of restraint and immobilization on electrosensory behaviors of weakly electric fish. ILAR J. 2009;50:361–372. doi: 10.1093/ilar.50.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajikawa Y, Hackett TA. Entropy analysis of neuronal spike train synchrony. J Neurosci Methods. 2005;149:90–93. doi: 10.1016/j.jneumeth.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Khosravi-Hashemi N, Chacron MJ. Bursts and isolated spikes code for opposite movement directions in midbrain electrosensory neurons. PLoS One. 2012;7:e40339. doi: 10.1371/journal.pone.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khosravi-Hashemi N, Fortune ES, Chacron MJ. Coding movement direction by burst firing in electrosensory neurons. J Neurophysiol. 2011;106:1954–1968. doi: 10.1152/jn.00116.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krahe R, Bastian J, Chacron MJ. Temporal processing across multiple topographic maps in the electrosensory system. J Neurophysiol. 2008;100:852–867. doi: 10.1152/jn.90300.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Rotter S, Aertsen A. Spiking activity propagation in neuronal networks: reconciling different perspectives on neural coding. Nat Rev Neurosci. 2010;11:615–627. doi: 10.1038/nrn2886. [DOI] [PubMed] [Google Scholar]
- 19.Lin CP, Chen YP, Hung CP. Tuning and spontaneous spike time synchrony share a common structure in macaque inferior temporal cortex. J Neurophysiol. 2014;112:856–869. doi: 10.1152/jn.00485.2013. [DOI] [PubMed] [Google Scholar]
- 20.Maler L, Sas E, Johnston S, Ellis W. An atlas of the brain of the weakly electric fish Apteronotus leptorhynchus. J Chem Neuroanat. 1991;4:1–38. doi: 10.1016/0891-0618(91)90030-g. [DOI] [PubMed] [Google Scholar]
- 21.Marr D. Vision. Freeman; New York: 1982. [Google Scholar]
- 22.McGillivray P, Vonderschen K, Fortune ES, Chacron MJ. Parallel coding of first- and second-order stimulus attributes by midbrain electrosensory neurons. J Neurosci. 2012;32:5510–5524. doi: 10.1523/JNEUROSCI.0478-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzen MG, Avila-Akerberg O, Chacron MJ. Coding stimulus amplitude by correlated neural activity. Phys Rev E Stat Nonlin Soft Matter Phys. 2015;91:042717. doi: 10.1103/PhysRevE.91.042717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzen MG, Chacron MJ. Neural heterogeneities determine response characteristics to second—but not first-order stimulus features. J Neurosci. 2015;35:3124–3138. doi: 10.1523/JNEUROSCI.3946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzen MG, Chacron MJ. Weakly electric fish display behavioral responses to envelopes naturally occurring during movement: implications for neural processing. J Exp Biol. 2014;217:1381–1391. doi: 10.1242/jeb.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzen MG, Jamali M, Carriot J, Avila-Akerberg O, Cullen KE, Chacron MJ. Coding of envelopes by correlated but not single-neuron activity requires neural variability. PNAS. 2015;112:4791–4796. doi: 10.1073/pnas.1418224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 28.Nelson ME, MacIver MA. Prey capture in the weakly electric fish Apteronotus albifrons: sensory acquisition strategies and electrosensory consequences. J Exp Biol. 1999;202:1195–1203. doi: 10.1242/jeb.202.10.1195. [DOI] [PubMed] [Google Scholar]
- 29.Olshausen BA, Field DJ. Sparse coding of sensory inputs. Curr Opin Neurobiol. 2004;14:481–487. doi: 10.1016/j.conb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Rose GJ, Fortune ES. New techniques for making whole-cell recordings from CNS neurons in vivo. Neurosci Res. 1996;26:89–94. doi: 10.1016/0168-0102(96)01074-7. [DOI] [PubMed] [Google Scholar]
- 31.Savard M, Krahe R, Chacron MJ. Neural heterogeneities influence envelope and temporal coding at the sensory periphery. Neuroscience. 2011;172:270–284. doi: 10.1016/j.neuroscience.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamper SA, Fortune ES, Chacron MJ. Perception and coding of envelopes in weakly electric fishes. J Exp Biol. 2013;216:2393–2402. doi: 10.1242/jeb.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toporikova N, Chacron MJ. Dendritic SK channels gate information processing in vivo by regulating an intrinsic bursting mechanism seen in vitro. J Neurophysiol. 2009;102:2273–2287. doi: 10.1152/jn.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinje WE, Gallant JL. Natural stimulation of the nonclassical receptive field increases information transmission efficiency in V1. J Neurosci. 2002;22:2904–2915. doi: 10.1523/JNEUROSCI.22-07-02904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogels TP, Abbott LF. Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci. 2009;12:483–491. doi: 10.1038/nn.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonderschen K, Chacron MJ. Sparse and dense coding of natural stimuli by distinct midbrain neuron subpopulations in weakly electric fish. J Neurophysiol. 2011;106:3102–3118. doi: 10.1152/jn.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vonderschen K, Chacron MJ. Sparse coding of natural communication signals in midbrain neurons. Biomed Cent Neurosci. 2009;10:O3. [Google Scholar]
- 38.Walz H, Hupe GJ, Benda J, Lewis JE. The neuroethology of electrocommunication: how signal background influences sensory encoding and behavior in Apteronotus leptorhynchus. J Physiol Paris. 2013;107:13–25. doi: 10.1016/j.jphysparis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Gong J, Wang Q, Li H, Cheng Q, Liu Y, Zeng S, Wang Z. Parallel pathways convey olfactory information with opposite polarities in Drosophila. PNAS. 2014;111:3164–3169. doi: 10.1073/pnas.1317911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci. 2001;5:487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]