Abstract
Purpose
To assess whether calcium intake and common genetic variants of the calcium-sensing receptor (CASR) are associated with either aggressive prostate cancer (PCa) or disease recurrence after prostatectomy.
Methods
Calcium intake at diagnosis was assessed, and 65 common single-nucleotide polymorphisms (SNPs) in CASR were genotyped in 886 prostatectomy patients. We investigated the association between calcium intake and CASR variants with both PCa recurrence and aggressiveness (defined as Gleason score ≥4 + 3, stage ≥pT3, or nodal-positive disease).
Results
A total of 285 men had aggressive disease and 91 experienced recurrence. A U-shaped relationship between calcium intake and both disease recurrence and aggressiveness was observed. Compared to the middle quintile, the HR for disease recurrence was 3.07 (95 % CI 1.41–6.69) for the lowest quintile and 3.21 (95 % CI 1.47–7.00) and 2.97 (95 % CI 1.37–6.45) for the two upper quintiles, respectively. Compared to the middle quintile, the OR for aggressive disease was 1.80 (95 % CI 1.11–2.91) for the lowest quintile and 1.75 (95 % CI 1.08–2.85) for the highest quintile of calcium intake. The main effects of CASR variants were not associated with PCa recurrence or aggressiveness. In the subgroup of patients with moderate calcium intake, 31 SNPs in four distinct blocks of high linkage disequilibrium were associated with PCa recurrence.
Conclusions
We observed a protective effect of moderate calcium intake for PCa aggressiveness and recurrence. While CASR variants were not associated with these outcomes in the entire cohort, they may be associated with disease recurrence in men with moderate calcium intakes.
Keywords: Prostate cancer, Calcium-sensing receptor, Single-nucleotide polymorphism, Calcium intake, Clinical outcomes
Introduction
There is ample evidence to suggest an association between calcium homeostasis and the risk of prostate cancer (PCa). Increased intake of dairy products (the main source of dietary calcium) and supplemental calcium has been associated with increased risk of PCa [1–5], advanced disease [6, 7], and disease-specific mortality [5, 8–10]. The results of a meta-analysis [4] were consistent with a significant association between both higher intakes of dairy products (RR 1.11, 95 % CI 1.00–1.22) and higher intakes of calcium (RR 1.39, 95 % CI 1.09–1.77) with the development of PCa.
Mechanistically, the link between the association of calcium homeostasis and risk of prostate cancer is incompletely understood. Calcium homeostasis is critical to bone health, and bone metastases are a common manifestation of PCa with up to 3 % having metastasis at time of diagnosis, 11.5 % developing bone metastasis during follow-up, and 6.4 % having a skeletal-related event [11]. Skeletal involvement of PCa is associated with poor clinical outcomes and has been linked to altered bone metabolism in general and states of high bone turnover in particular [12].
The calcium-sensing receptor (CaSR) provides a possible link between aggressive PCa and calcium homeostasis. CaSR is a member of the G protein-coupled receptor superfamily, which is physiologically expressed in many tissues, including prostate, and plays an essential role in calcium homeostasis [13]. It has also been shown to exert pro-proliferative, anti-apoptotic, and pro-migratory effects on tumor cell lines including those of PCa [14–18]. Specifically, experimental data suggest that elevated extracellular calcium enhances the proliferation of skeletal metastatic prostate cell lines through increased expression of the CASR [18]. Furthermore, common genetic variants of CASR have been linked to PCa risk and disease progression [19, 20].
In the present study, we aimed to determine whether calcium intake and common genetic variation across the CASR gene are associated with PCa aggressiveness by two methods. We conducted (1) survival analysis for PCa recurrence among all cases and (2) logistic regression analysis comparing cases with a higher aggressive potential (defined as Gleason score ≥4 + 3 or stage ≥pT3) to cases with a lesser potential. We further examined potential interactions between calcium intake and CASR polymorphisms with PCa recurrence and aggressiveness.
Patients and methods
Study population
The Washington University Genetic Study (WUGS) cohort is comprised of men who were treated for PCa at Washington University in St. Louis between 2004 and 2010 [21]. All men were enrolled at the time of diagnosis and treated with radical prostatectomy. At the time of enrollment, men completed a questionnaire with demographic, smoking, and health information along with a food frequency questionnaire (FFQ). Germline DNA was obtained and purified at the time of enrollment. The population for this analysis was restricted to men in the cohort with both genetic and dietary data. To reduce the potential for population stratification, only white men of European descent were included (96 % of the original population). Three patients with missing information on stage or grade were excluded from the analysis.
Dietary data
The FFQ was initially developed for the National Cancer Institute—Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial and assessed frequency of consumption of 137 individual food items, 77 with questions on usual portion size and frequency. Use of supplements including calcium supplements was also assessed. Daily intake of total calcium (including supplementary calcium) and food calcium, along with intakes of phosphorus and vitamin D, was calculated by multiplying the frequency of consumption by the portion size of the food (in grams) and the nutrient intake per gram, and summing across all items on the questionnaire.
Genetic data
As part of the iCOGS/PRACTICAL study [21] genotyping information on 211,155 single-nucleotide polymorphisms (SNPs) measured using the Illumina Custom Infinium array (Illumina Incorporated, San Diego, USA) was available for the WUGS cohort. The iCOGS platform provided 95 % coverage of the common genetic variation (minor allele frequency ≥0.05) across the CASR gene (chromosome 3, base pairs 122,183,683–122,286,503), including two well-studied non-synonymous SNPs with potential functionality (rs1801725 and rs1042636). The MACH imputation program [22] was used to carry out multiple genotype imputation using the HapMap Phase II release #22 CEU reference panel [23]. SNPs that were out of Hardy–Weinberg equilibrium (p < 0.000001) were removed before genotype imputation. There were 65 markers genotyped between 30 kb down- and 15 kb upstream of the CASR gene. Five SNPs (rs1048213, rs1965357, rs7630625, rs937627, and rs9883981) were not available for imputation in the phased haplotypes of the reference panel population. The marker rs6438718 was out of Hardy–Weinberg equilibrium (p < 0.000001) and therefore removed before imputation. After restriction to common genetic variants (MAF ≥ 0.05) and the non-synonymous marker rs1801726 (MAF = 0.047), there were 176 markers with a mean imputation R2 of 0.99 (176 markers >0.30, 174 markers >0.80) left. In order to correct for multiple testing in the presence of linkage disequilibrium, a modified effective number of independent tests (Meff) approach as described by Gao et al. [24–26] was employed. Based on these remaining 176 markers, the inferred Meff was 27 (adjusted α level = 0.002).
Outcomes
Recurrent disease was defined as new onset bone metastasis, rising serum prostate-specific antigen (PSA) concentrations of ≥0.2 ng/mL during follow-up, or initiation of non-adjuvant treatment (defined as radiotherapy or androgen deprivation therapy ≥6 months following surgery for rising PSA even if it did not reach the threshold of 0.2 ng/mL). Time to event was defined as time from surgery to disease recurrence or censored at death from another cause or date of last follow-up visit. If more than one of the aforementioned events occurred, the time to the first event was considered. Twenty-nine patients were found to have nodal-positive disease at the time of surgery (disease recurrence); therefore, these patients were not considered in survival analysis but were included in the analysis of aggressive disease. Aggressive disease was defined as Gleason score ≥4 + 3, stage ≥pT3, or nodal-positive disease in the surgical specimen.
Statistical analysis
Calcium intake and PCa aggressiveness
All reported nutrient intakes were adjusted for total caloric intake using the residual method [27]. Cox proportional hazards models [28] were used to calculate the hazard ratios and 95 % confidence intervals for total calcium intake for the risk of disease recurrence. All models were evaluated for violations of the proportional hazards assumption by including interaction terms between the predictors and survival time (p > 0.05 for all comparisons). Logistic regression was used to assess whether total calcium intake was associated with greater risk of highly aggressive versus less aggressive disease. The energy-adjusted intakes were divided into quintiles. Tests for linear trend across total calcium quintiles were based on the p value from using the median intake in each quintile as a continuous variable. All models were adjusted for age at diagnosis. Models were additionally adjusted for smoking status (never, ever) and body mass index at the time of diagnosis [kg/m2] (<25.0, 25.0 – 30.0, >30.0). Models for disease recurrence were also adjusted for pathological stage (pT2, pT3a, ≥pT3b), Gleason score (5–6, 3 + 4, 4 + 3, 8–10), and PSA at diagnosis [ng/dL] (<4, 4–10, >10), while models for highly aggressive versus less aggressive disease were additionally adjusted for PSA at diagnosis. Additional adjustment for dietary factors including red meat, vegetables, fruits, vitamin D, and phosphorous did not significantly change the results. The distribution of patient and disease characteristics as well as nutrient intakes was evaluated using the Chi-square or Kruskal–Wallis test.
Genetic variation of the CASR gene and PCa aggressiveness
The ProbABEL software [29] was used to analyze the association of each SNP (by allele dosage) and time to disease recurrence (using a Cox proportional hazards model) and disease aggressiveness at the time of diagnosis (using a logistic regression model). To assess potential gene–environment (GxE) interactions, both Cox and logistic regression models containing the SNP of interest, categories of total calcium intake, and the SNP x calcium cross-product term were fit. For these analyses, we made the a priori decision to dichotomize total calcium intake into the top tertile versus the two lower tertiles, based on prior evidence that the association of calcium and aggressive PCa was observed only at higher intakes [1–3, 6–9, 30–34]. For the post hoc analysis of GxE interactions, calcium intake was categorized into moderate (800–1100 mg/day) and extreme (<800 or >1100 mg/day) based on the observed quintiles of increased hazard in the main analysis. In order to assess potential associations with time to disease recurrence and disease aggressiveness at the gene level, kernel machine joint association tests were carried out using coxKM [35, 36] and SKAT [37–39], respectively. Data management and statistical analyses were performed using SAS (version 9.3, SAS Institute, Cary, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). R and Stata (version 13.1, StataCorp, College Station, USA) were used to create the graphs.
Results
Table 1 shows the characteristics of the PCa cases (n = 886) included in the study. The median age at diagnosis was 61 years, and the patients were followed for a median time of 3 years. About one-third of the men had a family history of PCa, and half of them had a history of using tobacco products. Ninety-one men (10 %) experienced disease recurrence: 75 men (83 %) had isolated PSA recurrence (defined as PSA ≥0.2 ng/mL) without radiographic evidence of metastasis; three men (3 %) progressed to bone metastases; and eleven men (14 %) did not reach the PSA cutoff of 0.2 ng/mL but required non-adjuvant treatment for rising PSA. At the time of diagnosis, 285 (32 %) had aggressive disease; 69 (24 %) were diagnosed with high-grade disease, 111 (39 %) with advanced stage, and 105 (37 %) with both. Table 2 shows characteristics by quintiles of calcium intake. High calcium intake was also associated with higher vitamin D and phosphorous intakes (test for trend p < 0.0001 for both nutrients).
Table 1.
Characteristics of 886 men with prostate cancer in the WUGS cohort
| Age at diagnosis [years] | 61 (39–79) |
| Body mass index [kg/m2] | 28 (17–55) |
| Prostate-specific antigen [ng/dL] | 5 (1–35) |
| Follow-up [years] | 3 (0–8) |
| Family history of PCa | |
| Yes | 276 (31) |
| No | 610 (69) |
| Smoking status | |
| Never | 442 (50) |
| Ever | 444 (50) |
| Stage | |
| T2 | 670 (76) |
| T3a | 138 (15) |
| T3b or higher | 78 (9) |
| Gleason grade | |
| 5–6 | 364 (41) |
| 3 + 4 | 348 (39) |
| 4 + 3 | 102 (12) |
| 8–10 | 72 (8) |
| Recurrent disease | |
| New bone metastasis | 3 (3) |
| Rising serum PSA | 75 (83) |
| Non-adjuvant treatment | 13 (14) |
| Aggressive disease | |
| Gleason score ≥4 + 3 | 69 (24) |
| Stage ≥pT3 | 111 (39) |
| Both | 105 (37) |
| Total calcium intake [mg/day] | 1011 (53–3265) |
| Total vitamin D intake [IU/day] | 486 (7–2198) |
| Total phosphorous intake [mg/day] | 1501 (294–2706) |
Data are given as median (range) or count (percent of the entire cohort)
Table 2.
Characteristics of the 886 men with prostate cancer in the WUGS cohort by quintile of total calcium intake
| Quintiles of total calcium intake | Q1 (n = 177) | Q2 (n = 177) | Q3 (n = 177) | Q4 (n = 177) | Q5 (n = 178) | p value |
|---|---|---|---|---|---|---|
| Age at diagnosis [years] | 61 (39–77) | 60 (45–78) | 61 (40–75) | 61 (43–78) | 62 (39–79) | 0.8089 |
| Body mass index [kg/m2] | 28 (21–41) | 28 (19–51) | 28 (20–43) | 28 (21–55) | 28 (17–46) | 0.9000 |
| Prostate-specific antigen [ng/dL] | 5 (1–35) | 5 (1–28) | 5 (1–16) | 5 (1–30) | 5 (1–31) | 0.1155 |
| Follow-up [years] | 3 (0–7) | 3 (0–7) | 3 (0–8) | 3 (0–8) | 3 (0–8) | 0.7117 |
| Family history of PCa | ||||||
| Yes | 120 (68) | 124 (70) | 126 (71) | 112 (63) | 128 (72) | 0.4298 |
| No | 57 (32) | 53 (30) | 51 (29) | 65 (37) | 50 (28) | |
| Smoking status | ||||||
| Never | 79 (45) | 91 (51) | 86 (49) | 82 (46) | 104 (58) | 0.0911 |
| Ever | 98 (55) | 86 (49) | 91 (51) | 95 (54) | 74 (42) | |
| Stage | ||||||
| T2 | 125 (70) | 132 (75) | 146 (82) | 135 (76) | 132 (74) | 0.4758 |
| T3a | 35 (20) | 25 (14) | 18 (10) | 31 (18) | 29 (16) | |
| T3b or higher | 17 (10) | 20 (11) | 13 (8) | 11 (6) | 17 (10) | |
| Gleason grade | ||||||
| 5–6 | 76 (43) | 71 (40) | 74 (42) | 83 (47) | 60 (34) | 0.0813 |
| 3 + 4 | 63 (36) | 65 (37) | 76 (43) | 68 (38) | 76 (43) | |
| 4 + 3 | 17 (10) | 26 (15) | 15 (8) | 16 (9) | 28 (16) | |
| 8–10 | 21 (11) | 15 (8) | 12 (7) | 10 (6) | 14 (7) | |
| Total calcium intake [mg/day] | 680 (53–768) | 862 (768–935) | 1011 (936–1087) | 1195 (1088–1325) | 1577 (1332–3265) | <0.0001 |
| Total vitamin D intake [IU/day] | 162 (7–1256) | 218 (63–2101) | 550 (87–2168) | 596 (89–1661) | 683 (156–2198) | <0.0001 |
| Total phosphorous intake [mg/day] | 1270 (294–1579) | 1402 (725–1860) | 1518 (1195–2013) | 1624 (952–2125) | 1773 (1117–2706) | <0.0001 |
Data are given as median (range) or count (percent of the respective quintile)
PCa recurrence
In the multivariable survival analysis (adjusted for age, smoking status, BMI, PSA, stage, and grade), both low (lowest quintile) and high calcium intakes (top two quin-tiles) compared to moderate intake (middle quintile) were significantly associated with approximately threefold increases in the hazard of disease recurrence (Table 3). Given this U-shaped relationship, there was no statistically significant linear trend across quintiles of calcium intake.
Table 3.
Quintiles of calorie-adjusted total calcium intake and (multivariable-) adjusted effect estimates for clinical outcomes
| Quintiles of total calcium intake | Q1 | Q2 | Q3 | Q4 | Q5 |
|---|---|---|---|---|---|
| Disease recurrence (91 events among 857 men with prostate cancer) | |||||
| Median total calcium intake [mg/day] | 680 | 861 | 1011 | 1195 | 1577 |
| Recurrent disease/all cases: n | 22/172 | 15/171 | 10/171 | 21/171 | 23/172 |
| Age-adj.a HR (95 % CI) | 2.33 (1.11–1.93) | 1.52 (0.68–3.38) | 1.00 | 2.18 (1.03–4.64) | 2.42 (1.15–5.09) |
| Age-Smk-BMI-adj.b HR (95 % CI) | 2.30 (1.09–4.86) | 1.51 (0.68–3.35) | 1.00 | 2.21 (1.04–4.70) | 2.49 (1.18–5.24) |
| Age-Smk-BMI-PSA-Dis-adj.c HR (95 % CI) | 3.07 (1.41–6.69) | 1.57 (0.69–3.58) | 1.00 | 3.21 (1.47–7.00) | 2.97 (1.37–6.45) |
| Disease aggressiveness (285 cases of aggressive disease among 886 men with prostate cancer) | |||||
| Median total calcium intake [mg/day] | 678 | 860 | 1009 | 1193 | 1582 |
| Aggressive disease/all cases: n | 68/177 | 58/177 | 43/178 | 52/177 | 64/177 |
| Age-adj.d OR (95 % CI) | 2.03 (1.28–3.21) | 1.56 (0.98–2.49) | 1.00 | 1.31 (0.81–2.10) | 1.79 (1.13–2.84) |
| Age-Smk-BMI-adj.e OR (95 % CI) | 2.00 (1.26–3.18) | 1.59 (0.99–2.54) | 1.00 | 1.31 (0.81–2.11) | 1.86 (1.17–2.96) |
| Age-Smk-BMI-PSA-adj.f OR (95 % CI) | 1.80 (1.11–2.91) | 1.54 (0.95–2.50) | 1.00 | 1.27 (0.78–2.08) | 1.75 (1.08–2.85) |
Effect estimates are either given as hazard ratio (HR) with 95 % confidence interval (CI)
Adjusted for age at diagnosis
Additionally adjusted for smoking status (never, ever) and body mass index [kg/m2] (<25.0, 25.0–30.0, >30.0)
Additionally adjusted for pathological stage (pT2, pT3a, ≥pT3b), Gleason score (5–6, 3 + 4, 4 + 3, 8–10), and prostate-specific antigen [ng/dL] (<4, 4–10, >10), or as odds ratio (OR) with 95 % CI
Adjusted for age at diagnosis
Additionally adjusted for smoking status (never, ever) and body mass index [kg/m2] (<25.0, 25.0–30.0, >30.0)
Additionally adjusted for prostate-specific antigen [ng/dL] (<4, 4–10, >10)
Aggressive PCa risk
The multivariable logistic regression analysis (adjusted for age, smoking status, BMI, and PSA) showed that low calcium intake (lowest quintile) compared to moderate intake (middle quintile) was significantly associated with an approximately twofold increase in the risk of having highly aggressive disease compared to less aggressive disease at the time of diagnosis. Higher intakes (upper quintile) were associated with a less pronounced increase (Table 3), and there was no statistically significant linear trend across quintiles of calcium intake.
Genetic variation of the CASR gene
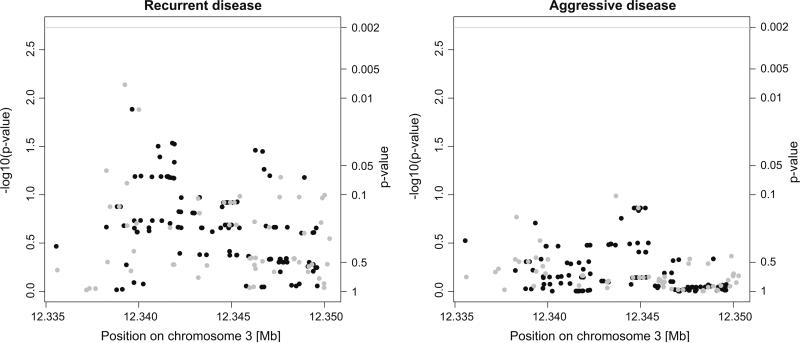
After correcting for multiple testing, no SNPs were significantly associated with disease recurrence or aggressiveness. Figure 1 shows the results of the tests for association between genetic variation of the genotyped and imputed markers and disease recurrence and aggressiveness. There was no evidence of a global association for common variation across the CASR gene for disease recurrence (p = 0.13) or aggressiveness (p = 0.46). There was no evidence of effect modification by calcium intake using the pre-specified approach with dichotomized turtles of calcium intake (p > 0.002 for the interaction term for all SNPs). A post hoc analysis taking into account the observed U-shaped relationship between calcium and recurrence suggested that calcium intake interacts with 31 SNPs (p < 0.002 for the interaction term, dichotomized into moderate and extreme intakes based on the main analysis) to modify the risk of PCa recurrence. These 31 variants were in four linkage disequilibrium blocks. Table 4 presents the associations of the most representative variant for each block (chosen using a haplotype-based tagging algorithm [40]) and prostate cancer recurrence stratified by moderate (800–1100 mg/day) and extreme calcium intakes (<800 or >1100 mg/day).
Fig. 1.
Manhattan plots showing the p values of the genotyped markers (gray dots) and imputed markers (black dots) for their association with recurrent (left) and aggressive disease (right), and the gene-wide significance level (gray line)
Table 4.
Post hoc analysis of GxE interactions for the association with disease recurrence, stratified by calcium intake
| SNP | MA | MAF | Moderate HR (95 % CI) | Extreme HR (95 % CI) | Interaction p value |
|---|---|---|---|---|---|
| Unadjusted model | |||||
| rs10222469 | A | 0.18 | 3.27 (1.80–5.93) | 0.63 (0.38–1.04) | 0.00003 |
| rs2036399 | T | 0.14 | 3.24 (1.76–5.97) | 0.55 (0.30–1.01) | 0.00005 |
| rs1393200 | G | 0.32 | 2.91 (1.69–4.99) | 0.80 (0.55–1.16) | 0.00011 |
| rs4678173 | A | 0.34 | 0.19 (0.08–0.48) | 1.17 (0.83–1.64) | 0.00044 |
| Multivariable-adjusted modela | |||||
| rs10222469 | A | 0.18 | 2.90 (1.66–5.04) | 0.68 (0.40–1.16) | 0.00008 |
| rs2036399 | T | 0.14 | 3.10 (1.70–5.67) | 0.57 (0.31–1.06) | 0.00006 |
| rs1393200 | G | 0.32 | 3.02 (1.74–5.26) | 0.92 (0.63–1.33) | 0.00013 |
| rs4678173 | A | 0.34 | 0.17 (0.07–0.44) | 1.14 (0.82–1.60) | 0.00012 |
Four SNPs remain associated with disease recurrence in men with moderate calcium intake (800–1100 mg/day, n = 336) but not in men with more extreme calcium intakes (<800 or >1100 mg/day, n = 521) after correcting for multiple testing (p < 0.002)
MA minor allele, MAF minor allele frequency, HR hazard ratio for a one-allele increase in the MA
Adjusted for pathological stage (pT2, pT3a, ≥pT3b), Gleason score (5–6, 3 + 4, 4 + 3, 8–10), and prostate-specific antigen [ng/dL] (<4, 4–10, >10)
Discussion
Our finding of increased risk of aggressive disease and recurrence with high calcium intake is consistent with prior work. To our knowledge, this is the first study investigating the effect of calcium intake on disease recurrence after surgical treatment of PCa. Several observational studies including large prospective cohort studies as well as a meta-analysis suggest an association between high calcium intake and increased risk of PCa, unfavorable disease characteristics, or poor clinical outcomes [1, 3–6, 9, 10, 41, 42]. The aforementioned studies reported increased risks with higher calcium intakes compared to lower intakes. Our data are consistent with not only an increased risk of being diagnosed with more aggressive disease but also an increased hazard of disease recurrence after surgical treatment, even when controlling for the more clinical and pathological characteristics of disease aggressiveness at diagnosis [5, 6, 41].
Our data also suggest an effect of similar magnitude in men with very low calcium intakes, showing a U-shaped relationship between calcium intake and the odds of aggressive disease at the time of diagnosis. Likewise, the hazard of disease recurrence after surgical treatment was increased in men with both very low and high calcium intakes. These results remained consistent after adjusting for potential confounders. These results are in line with a small number of studies suggesting a J- or U-shaped relationship between nutrient intakes (including calcium) and the risk of PCa [43, 44]. Other studies have observed approximately linear increases in the risk of PCa [2, 3, 6]; however, the employed methodology in these studies was not specifically geared toward assessing an underlying J- or U-shaped relationship. The association with low calcium intakes might be explained by differences in calcium intakes across cohorts. The intakes in the WUGS cohort were considerably higher per tertile (250–600 mg/dL) compared to the first National Health and Nutrition Examination Epidemiologic Follow-up Study [3], higher on average among cases (270 mg/L) compared to the men in King County described by Kristal et al. [2], and were less frequently categorized in the lowest category (<500 mg/dL) compared to the Health Professionals Follow-Up Study [6]. Low calcium intake could be confounded by poor nutrition in general, in this segment of the cohort, which could be associated with disease recurrence.
Moreover, the WUGS cohort consists entirely of surgical patients enrolled into a prospective study at a single academic center, which is likely associated with different demographic and geographic factors compared to the aforementioned populations.
A large meta-analysis demonstrated that serum calcium is regulated by the CASR gene and is influenced by its genetic variation [45]. However, the results linking serum calcium levels with PCa progression are mixed with one study showing no association [46] and two other studies reporting a positive association with PCa mortality [47, 48]. Three studies have assessed whether common genetic variation of the CASR gene is associated with PCa risk. A small study (458 cases and 248 controls) found a protective effect of the Q1011E minor allele (rs1801726) for PCa risk among African-American men [49]. A case–control study in Caucasian men (1193 cases/1244 controls) found a joint association of CASR polymorphisms with lethal PCa [20]. Finally, the Tromsø study (9404 genotyped men) found a twofold risk of PCa for rs17251221 homozygotes for the minor allele [19]. In the present study, we did not observe evidence that common genetic variations of the CASR gene were associated with recurrent or aggressive disease when controlling for multiple testing. In the pre-specified analysis, there was no evidence of GxE interactions. However, taking into account the observed U-shaped relationship, a post hoc analysis suggested that calcium intake significantly modified the association between 31 SNPs (that made up four distinct linkage disequilibrium blocks) and disease recurrence. This observation may be explained by extreme calcium states overwhelming any subtle biologic effect of the genetic variants. Both the effects of high levels of calcium in the setting of high intake and maximized calcium absorption in the setting of low intake may be more pronounced than the influence of the genetic background.
A limitation of our study is the reliance on a surrogate marker (PSA recurrence) as a measure of disease outcomes. While PSA recurrence is a relevant clinical event that is utilized in treatment decision making, it is less meaningful than studying PCa-specific mortality or metastasis and we cannot be certain that these results for recurrence would be the same as for mortality. Furthermore, the Gleason grading system is one important measure of disease aggressiveness but has its own limitations considering tissue heterogeneity and tumor multifocality. Strengths of the study include that all men were treated at a single center with prospective and consistent collection of clinical and outcome data. In addition, very complete diet questionnaire and genetic data were available for almost all men, allowing for an investigation of both lifestyle and genetic factors.
In summary, in the present study we observed a U-shaped association with high and low calcium intakes being associated with an increase in disease recurrence after radical prostatectomy as well as the risk of aggressive PCa at diagnosis. We found no evidence for an association of CASR polymorphisms and PCa progression or aggressiveness. A post hoc analysis suggested the presence of GxE interactions modifying the hazard of PCa recurrence.
Acknowledgments
The authors declare that they have no conflict of interest. Moritz Binder was supported by a German Academic Exchange Service Scholarship. Irene M. Shui was supported by a United States Army Department of Defense Prostate Cancer Postdoctoral Fellowship. Kathryn M. Wilson, Kathryn L. Penney, and Lorelei A. Mucci were supported by Prostate Cancer Foundation Young Investigator Awards. Biomarker work in the Division of Urology is supported by Anthony DiNovi and David McGraw.
References
- 1.Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the physicians’ health study. Am J Clin Nutr. 2001;74:549–554. doi: 10.1093/ajcn/74.4.549. [DOI] [PubMed] [Google Scholar]
- 2.Kristal AR, Cohen JH, Qu P, Stanford JL. Associations of energy, fat, calcium, and vitamin D with prostate cancer risk. Cancer Epidemiol Biomark Prev. 2002;11:719–725. [PubMed] [Google Scholar]
- 3.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the national health and nutrition examination epidemiologic follow-up study cohort. Am J Clin Nutr. 2005;81:1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 4.Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst. 2005;97:1768–1777. doi: 10.1093/jnci/dji402. doi:10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 5.Wright ME, Bowen P, Virtamo J, Albanes D, Gann PH. Estimated phytanic acid intake and prostate cancer risk: a prospective cohort study. Int J Cancer. 2012;131:1396–1406. doi: 10.1002/ijc.27372. doi:10.1002/ijc.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Rimm EB, Wolk A, Ascherio A, Stampfer MJ, Colditz GA, Willett WC. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58:442–447. [PubMed] [Google Scholar]
- 7.Michaud DS, Augustsson K, Rimm EB, Stampfer MJ, Willet WC, Giovannucci E. A prospective study on intake of animal products and risk of prostate cancer. Cancer Causes Control. 2001;12:557–567. doi: 10.1023/a:1011256201044. [DOI] [PubMed] [Google Scholar]
- 8.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Stampfer MJ, Willett WC. A prospective study of calcium intake and incident and fatal prostate cancer. Cancer Epidemiol Biomark Prev. 2006;15:203–210. doi: 10.1158/1055-9965.EPI-05-0586. doi:10.1158/1055-9965.EPI-05-0586. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. doi:10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nørgaard M, Jensen AØ, Jacobsen JB, Cetin K, Fryzek JP, Sørensen HT. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999–2007). J Urol. 2010;184:162–167. doi: 10.1016/j.juro.2010.03.034. doi:10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. doi:10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 13.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 14.Yano S, Macleod RJ, Chattopadhyay N, Tfelt-Hansen J, Kifor O, Butters RR, Brown EM. Calcium-sensing receptor activation stimulates parathyroid hormone-related protein secretion in prostate cancer cells: role of epidermal growth factor receptor transactivation. Bone. 2004;35:664–672. doi: 10.1016/j.bone.2004.04.014. doi:10.1016/j.bone.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Brown EM. Ca(2+)-sensing receptor expression and pthrp secretion in PC-3 human prostate cancer cells. Am J Physiol Endocrinol Metab. 2001;281:E1267–E1274. doi: 10.1152/ajpendo.2001.281.6.E1267. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Liu S, Miller RT. Role of p115rhogef in the regulation of extracellular ca(2+)-induced choline kinase activation and prostate cancer cell proliferation. Int J Cancer. 2011;128:2833–2842. doi: 10.1002/ijc.25633. doi:10.1002/ijc.25633. [DOI] [PubMed] [Google Scholar]
- 17.Lin KI, Chattopadhyay N, Bai M, Alvarez R, Dang CV, Baraban JM, Brown EM, Ratan RR. Elevated extracellular calcium can prevent apoptosis via the calcium-sensing receptor. Biochem Biophys Res Commun. 1998;249:325–331. doi: 10.1006/bbrc.1998.9124. doi:10.1006/bbrc.1998.9124. [DOI] [PubMed] [Google Scholar]
- 18.Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66:9065–9073. doi: 10.1158/0008-5472.CAN-06-0317. doi:10.1158/0008-5472.CAN-06-0317. [DOI] [PubMed] [Google Scholar]
- 19.Jorde R, Schirmer H, Njølstad I, Løchen ML, Bøgeberg Mathiesen E, Kamycheva E, Figenschau Y, Grimnes G. Serum calcium and the calcium-sensing receptor polymorphism rs17251221 in relation to coronary heart disease, type 2 diabetes, cancer and mortality: the tromsø study. Eur J Epidemiol. 2013;28:569–578. doi: 10.1007/s10654-013-9822-y. doi:10.1007/s10654-013-9822-y. [DOI] [PubMed] [Google Scholar]
- 20.Shui IM, Mucci LA, Wilson KM, Kraft P, Penney KL, Stampfer MJ, Giovannucci E. Common genetic variation of the calcium-sensing receptor and lethal prostate cancer risk. Cancer Epidemiol Biomark Prev. 2013;22:118–126. doi: 10.1158/1055-9965.EPI-12-0670-T. doi:10.1158/1055-9965.EPI-12-0670-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, Ghoussaini M, Luccarini C, Dennis J, Jugurnauth-Little S, Dadaev T, Neal DE, Hamdy FC, Donovan JL, Muir K, Giles GG, Severi G, Wiklund F, Gronberg H, Haiman CA, Schumacher F, Henderson BE, Le Marchand L, Lindstrom S, Kraft P, Hunter DJ, Gapstur S, Chanock SJ, Berndt SI, Albanes D, Andriole G, Schleutker J, Weischer M, Canzian F, Riboli E, Key TJ, Travis RC, Campa D, Ingles SA, John EM, Hayes RB, Pharoah PD, Pashayan N, Khaw KT, Stanford JL, Ostrander EA, Signorello LB, Thibodeau SN, Schaid D, Maier C, Vogel W, Kibel AS, Cybulski C, Lubinski J, Cannon-Albright L, Brenner H, Park JY, Kaneva R, Batra J, Spurdle AB, Clements JA, Teixeira MR, Dicks E, Lee A, Dunning AM, Baynes C, Conroy D, Maranian MJ, Ahmed S, Govindasami K, Guy M, Wilkinson RA, Sawyer EJ, Morgan A, Dearnaley DP, Horwich A, Huddart RA, Khoo VS, Parker CC, Van As NJ, Woodhouse CJ, Thompson A, Dudderidge T, Ogden C, Cooper CS, Lophatananon A, Cox A, Southey MC, Hopper JL, English DR, Aly M, Adolfsson J, Xu J, Zheng SL, Yeager M, Kaaks R, Diver WR, Gaudet MM, Stern MC, Corral R, Joshi AD, Shahabi A, Wahlfors T, Tammela TL, Auvinen A, Virtamo J, Klarskov P, Nordestgaard BG, Røder MA, Nielsen SF, Bojesen SE, Siddiq A, Fitzgerald LM, Kolb S, Kwon EM, Karyadi DM, Blot WJ, Zheng W, Cai Q, McDonnell SK, Rinckleb AE, Drake B, Colditz G, Wokolorczyk D, Stephenson RA, Teerlink C, Muller H, Rothenbacher D, Sellers TA, Lin HY, Slavov C, Mitev V, Lose F, Srinivasan S, Maia S, Paulo P, Lange E, Cooney KA, Antoniou AC, Vincent D, Bacot F, Tessier DC, Kote-Jarai Z, Easton DF. Identification of 23 new prostate cancer susceptibility loci using the icogs custom genotyping array. Nat Genet. 2013;45:385–391. 391, e1–2. doi: 10.1038/ng.2560. doi:10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. doi:10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International HapMap Consortium The international hapmap project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. doi:10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol. 2008;32:361–369. doi: 10.1002/gepi.20310. doi:10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Becker LC, Becker DM, Starmer JD, Province MA. Avoiding the high bonferroni penalty in genome-wide association studies. Genet Epidemiol. 2010;34:100–105. doi: 10.1002/gepi.20430. doi:10.1002/gepi.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao X. Multiple testing corrections for imputed snps. Genet Epidemiol. 2011;35:154–158. doi: 10.1002/gepi.20563. doi:10.1002/gepi.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life-tables. JR Stat Soc B. 1972;34:187–220. [Google Scholar]
- 29.Aulchenko YS, Struchalin MV, van Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinform. 2010;11:134. doi: 10.1186/1471-2105-11-134. doi:10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talamini R, La Vecchia C, Decarli A, Negri E, Franceschi S. Nutrition, social factors and prostatic cancer in a northern italian population. Br J Cancer. 1986;53:817–821. doi: 10.1038/bjc.1986.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mettlin C, Selenskas S, Natarajan N, Huben R. Beta-carotene and animal fats and their relationship to prostate cancer risk. A case–control study. Cancer. 1989;64:605–612. doi: 10.1002/1097-0142(19890801)64:3<605::aid-cncr2820640307>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case–control studies in canada. Nutr Cancer. 1999;34:173–184. doi: 10.1207/S15327914NC3402_8. doi:10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- 33.La Vecchia C, Negri E, D'Avanzo B, Franceschi S, Boyle P. Dairy products and the risk of prostatic cancer. Oncology. 1991;48:406–410. doi: 10.1159/000226970. [DOI] [PubMed] [Google Scholar]
- 34.De Stefani E, Fierro L, Barrios E, Ronco A. Tobacco, alcohol, diet and risk of prostate cancer. Tumori. 1995;81:315–320. doi: 10.1177/030089169508100503. [DOI] [PubMed] [Google Scholar]
- 35.Lin X, Cai T, Wu MC, Zhou Q, Liu G, Christiani DC, Lin X. Kernel machine snp-set analysis for censored survival outcomes in genome-wide association studies. Genet Epidemiol. 2011;35:620–631. doi: 10.1002/gepi.20610. doi:10.1002/gepi.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai T, Tonini G, Lin X. Kernel machine approach to testing the significance of multiple genetic markers for risk prediction. Biometrics. 2011;67:975–986. doi: 10.1111/j.1541-0420.2010.01544.x. doi:10.1111/j.1541-0420.2010. 01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. doi:10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nick-erson DA, Christiani DC, Wurfel MM, Lin X. Optimal unified approach for rare-variant association testing with application to small-sample case–control whole-exome sequencing studies. Am J Hum Genet. 2012;91:224–237. doi: 10.1016/j.ajhg.2012.06.007. doi:10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S, Wu MC, Lin X. Optimal tests for rare variant effects in sequencing association studies. Biostatistics. 2012;13:762–775. doi: 10.1093/biostatistics/kxs014. doi:10.1093/biostatistics/kxs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. doi:10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- 41.Rowland GW, Schwartz GG, John EM, Ingles SA. Calcium intake and prostate cancer among african americans: effect modification by vitamin D receptor calcium absorption genotype. J Bone Miner Res. 2012;27:187–194. doi: 10.1002/jbmr.505. doi:10.1002/jbmr.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson KM, Shui IM, Mucci LA, Giovannucci E. Calcium and phosphorus intake and prostate cancer risk: a 24-y follow-up study. Am J Clin Nutr. 2015;101:173–183. doi: 10.3945/ajcn.114.088716. doi:10.3945/ajcn.114.088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams CD, Whitley BM, Hoyo C, Grant DJ, Schwartz GG, Presti JC, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. Dietary calcium and risk for prostate cancer: a case–control study among US veterans. Prev Chronic Dis. 2012;9:E39. doi: 10.5888/pcd9.110125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case–control study in the nordic countries. Int J Cancer. 2004;108:104–108. doi: 10.1002/ijc.11375. doi:10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 45.Kapur K, Johnson T, Beckmann ND, Sehmi J, Tanaka T, Kutalik Z, Styrkarsdottir U, Zhang W, Marek D, Gudbjartsson DF, Milaneschi Y, Holm H, Diiorio A, Waterworth D, Li Y, Singleton AB, Bjornsdottir US, Sigurdsson G, Hernandez DG, Desilva R, Elliott P, Eyjolfsson GI, Guralnik JM, Scott J, Thorsteinsdottir U, Bandinelli S, Chambers J, Stefansson K, Waeber G, Ferrucci L, Kooner JS, Mooser V, Vollenweider P, Beckmann JS, Bochud M, Bergmann S. Genome-wide meta-analysis for serum calcium identifies significantly associated snps near the calcium-sensing receptor (CASR) gene. PLoS Genet. 2010;6:e1001035. doi: 10.1371/journal.pgen.1001035. doi:10.1371/journal.pgen.1001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson JL, Buskirk SJ, Heckman MG, Parker AS, Diehl NN, Tzou KS, Paryani NN, Ko SJ, Daugherty LC, Vallow LA, Pisansky TM. Evaluation of serum calcium as a predictor of biochemical recurrence following salvage radiation therapy for prostate cancer. ISRN Oncol. 2013;2013:239241. doi: 10.1155/2013/239241. doi:10.1155/2013/239241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner HG, Schwartz GG. Serum calcium and incident and fatal prostate cancer in the national health and nutrition examination survey. Cancer Epidemiol Biomark Prev. 2008;17:2302–2305. doi: 10.1158/1055-9965.EPI-08-0365. doi:10.1158/1055-9965.EPI-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skinner HG, Schwartz GG. A prospective study of total and ionized serum calcium and fatal prostate cancer. Cancer Epidemiol Biomark Prev. 2009;18:575–578. doi: 10.1158/1055-9965.EPI-08-0915. doi:10.1158/1055-9965.EPI-08-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz GG, John EM, Rowland G, Ingles SA. Prostate cancer in african-american men and polymorphism in the calcium-sensing receptor. Cancer Biol Ther. 2010;9:994–999. doi: 10.4161/cbt.9.12.11689. [DOI] [PubMed] [Google Scholar]