Abstract
Objective
Working memory impairment in individuals with chronic opioid dependence can play a major role in cognitive and treatment outcomes. Cognitive training targeting working memory shows promise for improved function in substance use disorders. To date, cognitive training has not been incorporated as an adjunctive treatment for opioid dependence.
Methods
Methadone maintenance patients were randomly assigned to experimental (n = 28) or active control (n = 28) 25-session computerized training and run in parallel. Cognitive and drug use outcomes were assessed before and after training.
Results
Participants in the experimental condition showed performance improvements on two of four working memory measures, and both groups improved on a third measure of working memory performance. Less frequent drug use was found in the experimental group than in the control group post-training. In contrast to previous findings with stimulant users, no significant effect of working memory training on delay discounting was found using either hypothetical or real rewards. There were no group differences on working memory outcome measures that were dissimilar from the training tasks, suggesting that another mechanism (e.g., increased distress tolerance) may have driven drug use results.
Conclusions
Working memory training improves performance on some measures of working memory in methadone maintenance patients, and may impact drug use outcomes. Working memory training shows promise in patients with substance use disorders; however, further research is needed to understand the mechanisms through which performance is improved and drug use outcomes are impacted.
Keywords: Methadone maintenance, Cognitive impairment, Memory training, Working memory, Delay discounting
1. Introduction
Opioid dependence is associated with impairment spanning multiple cognitive domains that may negatively affect daily psychosocial function, drug use behavior, and response to substance abuse treatment (Ersche and Sahakian, 2007; Gruber et al., 2007; Mintzer and Johnson, 2007; Rass et al., 2014). Deficits in working memory (WM) and in frontal brain regions thought to mediate WM have been observed in methadone maintenance patients, current opioid abusers not maintained on methadone, and abstinent opioid abusers (Darke et al., 2000; Ersche et al., 2006, 2005; Guerra et al., 1987; Lee et al., 2005; Mintzer and Stitzer, 2002; Ornstein et al., 2000; Papageorgiou et al., 2001; Pezawas et al., 2002; Piratsu et al., 2006; Prosser et al., 2006; Rapeli et al., 2007; Verdejo et al., 2005). WM, which is the ability to briefly hold on-line and manipulate small amounts of information to serve current goals, has important implications for daily functioning in healthy individuals and those with substance use disorders. WM is a core component of executive functions that select, initiate, monitor, and modulate other cognitive activities (Baddeley, 1992; Bechara and Martin, 2004; D’Esposito, 2007; Jurado and Rosselli, 2007; Repovs and Baddeley, 2006; Miyake et al., 2000). Substance abuse is associated with impaired performance on tasks involving working memory and executive function (e.g., inhibitory control, risk-taking, decision-making; Dretsch and Tipples, 2008; Finn et al., 1999; Hinson et al., 2003; Süß et al., 2002). Poor performance on WM and other tasks involving executive function predicts treatment outcomes in cognitively based substance use interventions, as reflected in higher dropout rates, less efficacious therapy, and greater susceptibility for relapse (Aharonovich et al., 2006, 2008; Dallery and Raiff, 2007; Grenard et al., 2008; Katz et al., 2005; Passetti et al., 2008; Schmitz et al., 2009; Teichner et al., 2001; Turner et al., 2009; Vocci, 2008).
Though few studies are available, recent work shows promise in using WM training to treat cognitive deficits that may limit the efficacy of behavioral and pharmacological interventions for substance use disorders (Houben et al., 2011; Bickel et al., 2011; Hinson et al., 2003; MacKillop et al., 2011; Shamosh et al., 2008). Significant and lasting improvement in WM following working memory training has been reported in several populations with poor working memory skills, and transfer to some non-trained cognitive tasks has been observed (Klingberg et al., 2005; McNab et al., 2009; Olesen et al., 2004; Thorell et al., 2009; Westerberg and Klingberg, 2007; Westerberg et al., 2007). For example, Bickel et al. (2011) found that working memory training decreased delay discounting in stimulant users, suggesting greater preferences for larger-later rewards relative to smaller-sooner ones. In contrast, increased delay discounting is typically associated with poor working memory function and substance use disorders (e.g., Ahn et al., 2011; Hinson et al., 2003; Kirby and Petry, 2004; MacKillop et al., 2011). To date, there have been no studies of WM training in opioid users. We sought to address this gap and evaluate the effects of WM training on cognitive and drug use outcomes in methadone maintenance patients.
2. Methods
2.1. Participants
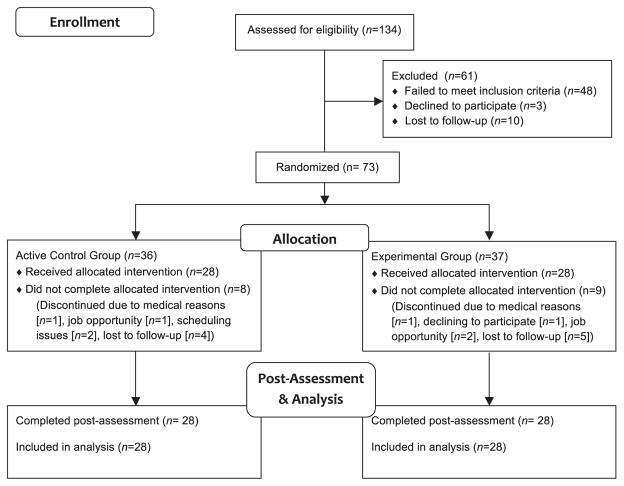
Individuals in methadone treatment for at least 2 months and on a stable methadone dose for at least 1 month were recruited from outpatient treatment programs in Baltimore, Maryland. Of 73 randomized participants, 56 completed the study (Fig. 1). Exclusion criteria were major untreated medical conditions (e.g., hypertension, diabetes), untreated Axis-I psychiatric disorders except substance abuse and dependence based on DSM-IV-TR criteria (Hudziak et al., 1993, 2004), use of any psychoactive drug other than caffeine and/or nicotine >4 times per week, daily alcohol consumption, or any condition associated with significant cognitive impairment (e.g., history of severe head trauma, HIV, ADHD). Participants without sufficient capacity to complete training and/or assessment (based on Shipley Institute of Living Scale estimated IQ and staff judgment) were also excluded (Zachary et al., 1985). Participants gave written informed consent and received compensation for participating. The Johns Hopkins University Institutional Review Board approved the study procedures.
Fig. 1.
CONSORT flow diagram. Experimental group participants who did not complete the study attended an average of 9.3 sessions (SD = 6.1, range = 3–19). Control group participants who did not complete the study attended an average of 9.1 sessions (SD = 5.5, range = 0–15).
2.2. Procedures
2.2.1. General procedures
Participants were randomly assigned to experimental or active control computerized training conditions using a minimization procedure (Pocock, 1983; Scott et al., 2002) to balance groups on age (18–34 and 35–55 years1), gender, estimated IQ (≤95 and >95), methadone treatment duration (2–6 months and >6 months), and methadone dose (≤90 mg and >90 mg). Participants completed 25 training sessions and pre- and post-training assessment sessions during trough methadone levels. Before every session, opioid withdrawal was assessed using a 20-item list of withdrawal-symptom adjectives (Strain et al., 2000). Acute alcohol intoxication was assessed using an Alco-Sensor IV (Intoximeters, Inc., St. Louis, MI) and behavioral tasks from the Brief Standardized Field Alcohol test (National Highway Traffic Safety Administration; Stuster and Burns, 1998). The session was rescheduled if the participant showed signs of opioid withdrawal, drug or alcohol intoxication, or fatigue, or had already taken his/her methadone before the session.
2.2.2. Training sessions
Training consisted of 25 45-min sessions 3–5 times per week at the study site. Research assistants monitored participants throughout each session. The training program (Cogmed QM; Cogmed Inc.) consisted of 12 exercises (8 per session) that require the maintenance and manipulation of sequences of verbal and/or visuo-spatial information in working memory (Klingberg et al., 2002, 2005). Three exercises were common across all sessions, whereas the other five varied. Training in the experimental condition involved an adaptive procedure in which difficulty (i.e., number of stimuli, or level) increased as proficiency was achieved, whereas training in the control condition consisted of a static procedure wherein the number of stimuli was always two. Experimental participants also received performance feedback. For the experimental condition, performance on training sessions was indexed using measures of improvement for the three training exercises completed at every session. Learning was calculated using a longitudinal linear model fit of the highest level obtained at each session, with session number as the covariate, and adjusted for highest level attained at baseline for each exercise. The performance variable learning slope was calculated as the average of the learning slopes for these three exercises that were completed at every session.
2.2.3. Pre- and post-training assessment sessions
Participants completed pre- and post-training assessment sessions that were administered by a research assistant blind to training condition (with one exception due to error). To avoid interference with methadone dosing schedules, both assessment sessions were split into two 1.5-h sessions over two separate days.
2.3. Cognitive outcomes
A detailed description of cognitive outcome measures can be found in Supplementary Materials.
2.3.1. Working memory
Working memory was assessed using verbal and nonverbal measures. Dependent measures included the following: (a) Digit Span (Wechsler, 1981): span length, forward and backward; (b) Operation Span (Turner and Engle, 1989; Engle et al., 1992): proportion of correctly recalled words; (c) N-back Task (Jonides et al., 1997; Mintzer and Griffiths, 2007): proportion of yes responses made to target letters (hit rate), proportion of yes responses made to non-target letters (false alarm rate), and signal detection measures of sensitivity in distinguishing between target and non-target letters (d′) and response bias (C) (Snodgrass and Corwin, 1988); and (d) Visuo-spatial Working Memory Task (Rapport et al., 2008): proportion of correctly replicated sequences.
2.3.2. Episodic memory and metamemory
Episodic memory was assessed using word recall and recognition. Dependent variables were the proportion of old responses made to old words (hit rate), proportion of old responses made to new words (false alarm rate), sensitivity in distinguishing between old and new words (d′), and response bias (C) (Snodgrass and Corwin, 1988). Metamemory was evaluated by calculating the Goodman–Kruskal gamma correlation between confidence ratings and recognition memory accuracy, collapsed across old and new words for sufficient power (Goodman and Kruskal, 1954).
2.3.3. Psychomotor speed and attention, trail making test A and B
Halstead (1947) and Reitan (1955) measured psychomotor speed and conceptual flexibility. Dependent measures were completion time (in seconds), number of errors, and the difference in time to complete B and A. Digit Symbol Substitution Task (DSST; McLeod et al., 1982; based on Wechsler, 1981) measured focused attention. Dependent measures were the number of trials attempted and the proportion of correct trials.
2.3.4. Reasoning, Raven’s standard progressive matrices
Raven (1939) assessed reasoning ability through the completion of patterns. The dependent measure was the proportion of correct trials.
2.3.5. Response inhibition and self-control, a continuous performance task
Epstein et al. (1998) measured response inhibition. Dependent measures were proportion of correct non-X trials (hit rate), proportion of incorrect X-trials (false alarm), sensitivity in distinguishing between non-X letters and X (d′), and response bias (C).
Two versions of the Delay Discounting Task measured self-control: (a) Hypothetical Delay Discounting Task (Baker et al., 2003; Johnson and Bickel, 2002; Johnson et al., 2007, 2010), and (b) Quick Discounting Operant Task (QDOT; Johnson, 2012), including actual contingencies in terms of reward delivery and the requirement to wait to receive rewards during the task.
A modified computerized version (Mintzer and Stitzer, 2002) of the Iowa Gambling Task (Bechara et al., 1994) measured risk-taking and self-control. The dependent measure was difference in number of cards selected from the advantageous versus disadvantageous decks, calculated separately for the decks associated with high frequency (i.e., C minus A) and low frequency (i.e., D minus B) of penalties, and collapsed across frequency of penalties [i.e. (C plus D) minus (A plus B)].
2.3.6. Substance use and functional outcomes
Self-reported drug use history and severity were assessed at baseline via a Drug History Questionnaire. Timeline Follow Back assessed number of days of recent use of cocaine, opiates, benzodiazepines, cannabis, and alcohol at pre- and post-assessment (Sobell and Sobell, 1996). Functional outcomes (i.e., composite measures for medical, employment, alcohol and other drug use, legal, family, and psychiatric status) were assessed via the Addiction Severity Index (ASI; McLellan et al., 1992) at pre- and post-assessment. Drug use during the study was assessed via urine specimens tested for cocaine, opiates, benzodiazepines, and cannabis and via breathalyzer for alcohol on training and assessment session days.
2.4. Statistical analyses
Data were analyzed with IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY), GraphPad Prism version 5 (GraphPad Software, Inc., La Jolla, CA), and Stata version 13 (StataCorp, L.P., College Station TX). Participant characteristics were analyzed using t-tests (age, years of education, estimated IQ, methadone treatment duration, and methadone dose) and chi-square analysis (gender, race). For assessment outcomes, a two (group: experimental vs. control) × two (session: pre- vs. post-training) mixed analysis of variance (ANOVA) was used to assess main effects and interactions for all variables except n-back, visuo-spatial working memory, and delay discounting. The n-back task was analyzed using a two (group: experimental vs. control) × two (session: pre- vs. post-training) mixed effects model with a random intercept and 0-back condition as covariate. The visuo-spatial working memory task was analyzed using a two (group: experimental vs. control) × two (session: pre-vs. post-training) × three (condition: 3, 5, or 7 dots, or items) mixed effects model with a random intercept. Fisher’s least significant difference (LSD) test was used to resolve significant interactions for post hoc analysis. All alpha levels were set at 0.05, and a Bonferroni correction for the working memory outcome measures set post hoc significance levels at p < .005.
For each of the two delay discounting tasks, delay discounting was quantified as the area under the curve metric (AUC; Myerson et al., 2001); AUC values can range from 0 to 1, with greater values indicating less delay discounting, or greater preference for later-later rewards. We assessed the orderliness of the discounting data by applying criterion 1 described by Johnson and Bickel (2008). Self-reported drug use history was analyzed using chi-square analysis for past month, year, and lifetime use for each drug. Heroin and cocaine use severity was analyzed using t-tests. Delay discounting AUC, Timeline follow back, and ASI composite measures were analyzed using a two (group: experimental vs. control) × two (session: pre- vs. post-training) mixed analysis of variance (ANOVA) for each functional outcome. Changes in odds of positive urine over time were compared between groups using a longitudinal logistic regression model that controlled for baseline urine status. The logistic regression model included baseline value, session, condition, and session × condition interaction, and the correlation matrix was exchangeable. Correlations between repeated urines within participants were handled using the method of generalized estimating equations (GEE; Zeger and Liang, 1986).
3. Results
3.1. Participant characteristics (Table 1)
Table 1.
Participant characteristics.
| Variable | Control (n = 28)
|
Experimental (n = 28)
|
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age in years | 43.5 | 7.1 | 43.3 | 8.8 |
| Years of education | 11.1 | 1.9 | 11.8 | 1.9 |
| Shipley IQ | 88.4 | 11.7 | 88.3 | 11.8 |
| Months in methadone treatment | 2.9 | 0.4 | 2.9 | 0.4 |
| Methadone dose (mg) | 99.3 | 25.3 | 96.5 | 19.0 |
| % | n | % | N | |
|
| ||||
| Race | ||||
| Black | 79 | 22 | 68 | 19 |
| White | 18 | 5 | 32 | 9 |
| Sex | ||||
| Female | 57 | 16 | 50 | 14 |
| Male | 43 | 12 | 50 | 14 |
Note: X2 and t-test analyses showed no significant group differences for these variables (all ps > 0.05). Although no group differences were found for methadone dose or duration in treatment, methadone dose is not well-correlated with methadone blood levels (Eap et al., 2000).
There were no significant group differences in age, gender, race, estimated IQ, years of education, days in methadone treatment, or methadone dose (Table 1). The experimental groups did not significantly differ on the number of days to complete the training (control group M = 44.3 [SD = 8.9] days; experimental group M = 45.2 [SD = 10.4] days; t[2,54] = −0.36; p = 0.72). For baseline measures of drug use, more participants in the experimental group reported using nicotine in the past year (X2[1] = 8.114, p = .004) and in their lifetime (X2[1] = 7.791, p = .005) (Table 2) compared to control group participants. Additionally, participants in the experimental group reported spending significantly more money per week on heroin than participants in the control group prior to study entry (t[54] = −2.207, p = .032).
Table 2.
(A) Self-reported prevalence of drug use. (B) Self-reported severity of drug use.
| A
| ||||||
|---|---|---|---|---|---|---|
| Drug | Control (n = 28)
|
Experimental (n = 28)
|
||||
| Lifetime | Past year | Past month | Lifetime | Past year | Past month | |
| Heroin | 100 (28) | 25 (7) | 11 (3) | 100 (28) | 50 (14) | 4 (1) |
| Alcohol | 96(27) | 61 (17) | 36 (10) | 96 (27) | 54 (15) | 43 (12) |
| Cocaine | 93 (26) | 43 (12) | 21 (6) | 89 (25) | 46 (13) | 21 (6) |
| Caffeine | 86 (24) | 86 (24) | 86 (24) | 93 (26) | 89 (25) | 89 (25) |
| Nicotine | 68 (19) | 61 (17) | 61 (17) | 96 (27) | 93 (26) | 82 (23) |
| Cannabis | 79 (22) | 21 (6) | 18 (5) | 89 (25) | 25 (7) | 18 (5) |
| Benzodiazepine | 32 (9) | 18 (5) | 11 (3) | 46 (13) | 14 (4) | 4 (1) |
| Hallucinogen | 7 (2) | 0 | 0 | 11 (3) | 0 | 0 |
| Amphetamine | 0 | 0 | 0 | 0 | 0 | 0 |
| B
| ||||||
|---|---|---|---|---|---|---|
| Drug | Control
|
Experimental
|
||||
| Years used | $ per day | $ per week | Years used | $ per day | $ per week | |
| Heroin | 18.3 (8.1) | 67 (78) | 419 (487) | 18.6 (10.0) | 108 (82) | 738 (590) |
| Cocaine | 17.4 (10.3) | 43 (62) | 216 (442) | 16.6 (11.1) | 52 (83) | 316 (603) |
Note: Values represent % (n) of participants reporting use. Data are missing for amphetamine use at all times for one control participant and for past month heroin use for one experimental participant. Groups did not differ on reported drug use history with the exception of nicotine in the past year (X2[1] = 8.114, p = .004] and lifetime (X2[1] = 7.791, p = .005).
Values represent Mean (Standard Deviation). Data are missing from two control and three experimental participants regarding cocaine spending. Groups did not differ on years of drug use or money spent on cocaine. The experimental group reported spending significantly more money per week on heroin than the control group (t[54] = −2.207, p = .032).
3.2. Cognitive performance (Tables 3 and 4)
Table 3.
Performance on working memory and episodic memory tasks at pre-training and post-training assessment.
| Measure | Control
|
Experimental
|
||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Digit span length | ||||
| Forward span | 6.14 (1.18) | 6.36 (1.22) | 6.04 (1.11) | 6.25 (1.18) |
| Backward spanb,c | 4.04 (1.04) | 4.07 (1.09) | 3.82 (0.98) | 4.68 (1.34) |
| Operation span | ||||
| Proportion correctb | 0.67 (0.15) | 0.72 (0.12) | 0.70 (0.13) | 0.72 (0.13) |
| N-back (2-back) | ||||
| Hit rate | 0.62 (0.21) | 0.63 (0.18) | 0.68 (0.19) | 0.68 (0.22) |
| False alarm rate | 0.21 (0.13) | 0.19 (0.14) | 0.17 (0.12) | 0.16 (0.13) |
| d′ (sensitivity) | 1.33 (0.93) | 1.36 (0.73) | 1.61 (0.92) | 1.69 (1.04) |
| C (response bias) | 0.26 (0.43) | 0.33 (0.38) | 0.23 (0.32) | 0.27 (0.37) |
| Visuo-spatial working memoryc | ||||
| Proportion correct (3 items) | 0.81 (0.14) | 0.79 (0.16) | 0.78 (0.22) | 0.87 (0.12) |
| Proportion correct (5 items) | 0.25 (0.22) | 0.34 (0.26) | 0.30 (0.27) | 0.48 (0.29) |
| Proportion correct (7 items) | 0.03 (0.07) | 0.06 (0.10) | 0.05 (0.12) | 0.11 (0.17) |
| Free recall | ||||
| Mean # correct responsesa | 4.46 (3.24) | 5.21 (3.51) | 6.93 (4.61) | 6.96 (5.12) |
| Recognition memory | ||||
| Hit rate | 0.75 (0.12) | 0.75 (0.13) | 0.76 (0.14) | 0.79 (0.13) |
| False alarm rateb | 0.24 (0.14) | 0.32 (0.21) | 0.19 (0.14) | 0.29 (0.19) |
| d′ (sensitivity)b | 1.50 (0.47) | 1.35 (0.70) | 1.75 (0.53) | 1.54 (0.66) |
| C (response bias)b | 0.02 (0.35) | −0.08 (0.47) | 0.11 (0.44) | −0.09 (0.45) |
| Metamemory | ||||
| Gamma | 0.31 (0.29) | 0.15 (0.39) | 0.35 (0.25) | 0.32 (0.34) |
Note: Values represent Mean (Standard Deviation). Superscript letters indicate significant main effect of group (a), main effect of session (b), or group × session interaction (c). Free recall and recognition memory data were missing from one of 28 participants in the experimental group. Metamemory data were missing from three of 28 participants in the control group and six of 28 participants in the experimental group.
Table 4.
Performance on measures of psychomotor speed, attention, reasoning, response inhibition, and self-control at pre-training and post-training assessment.
| Measure | Control
|
Experimental
|
||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Trail making test | ||||
| Trails A time (s)b | 44.43 (19.21) | 38.64 (13.23) | 38.96 (14.23) | 35.61 (12.11) |
| Trails B time (s) | 124.25 (69.56) | 113.46 (59.83) | 108.36 (59.42) | 107.46 (62.51) |
| Trails B-A time (s) | 79.82 (66.74) | 74.82 (54.48) | 69.39 (56.30) | 71.86 (58.92) |
| Trails B # errors | 0.75 (1.27) | 1.14 (1.43) | 0.71 (1.36) | 0.43 (0.92) |
| Digit symbol substitution test | ||||
| Number attemptedb | 18.25 (8.70) | 20.75 (8.82) | 21.52 (9.66) | 21.59 (10.42) |
| Proportion correct | 0.89 (0.21) | 0.94 (0.09) | 0.91 (0.13) | 0.92 (0.15) |
| Raven’s matrices | ||||
| Proportion correct (all sets)b | 0.62 (0.15) | 0.60 (0.16) | 0.68 (0.14) | 0.63 (0.15) |
| Continuous performance task | ||||
| Mean RT (s) | 0.45 (0.11) | 0.45 (0.09) | 0.44 (0.08) | 0.43 (0.07) |
| Standard error (s)b | 0.01 (0.00) | 0.01 (0.01) | 0.01 (0.00) | 0.01 (0.01) |
| Hit rateb | 0.98 (0.03) | 0.97 (0.04) | 0.98 (0.03) | 0.96 (0.06) |
| False alarm rate | 0.22 (0.16) | 0.23 (0.17) | 0.22 (0.16) | 0.25 (0.16) |
| d′ (sensitivity)b | 3.09 (0.82) | 3.02 (1.05) | 3.22 (0.93) | 2.83 (1.11) |
| C (response bias) | −0.69 (0.30) | −0.65 (0.28) | −0.73 (0.27) | −0.64 (0.30) |
| Delay discounting | ||||
| Hypothetical AUC | 0.22 (0.25) | 0.21 (0.25) | 0.29 (0.32) | 0.27 (0.30) |
| QDOT AUC | 0.60 (0.26) | 0.66 (0.29) | 0.63 (0.28) | 0.74 (0.28) |
| Iowa gambling task | ||||
| Net difference | 3.07 (28.16) | 3.86 (27.66) | −0.23 (14.58) | 1.46 (22.02) |
| Deck C-A | 5.14 (14.52) | 6.68 (15.13) | 1.31 (9.85) | 4.38 (12.43) |
| Deck D-B | −2.07 (15.96) | −2.82 (16.71) | −1.54 (10.75) | −2.92 (13.89) |
Note: Values represent Mean (Standard Deviation). Superscript letters indicate significant main effect of group (a), main effect of session (b), or group × session interaction (c). Delay discounting data for the QDOT and Digit Symbol Substitution Task data were missing from one of the 28 participants in the experimental group. Iowa Gambling Task data were missing from two of 28 participants in the experimental group.
3.2.1. Working memory
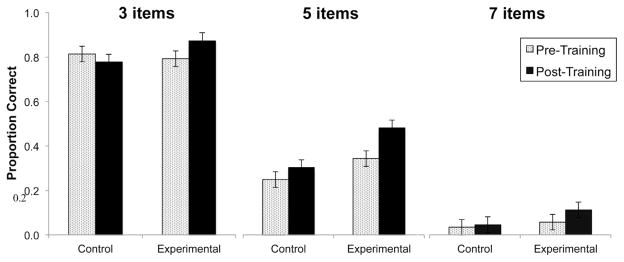
For the digit span task, a group × session interaction (F[1,54] = 8.176, p = .006) and main effect of session (F[1,54] = 9.659, p = .003) showed that experimental participants improved significantly on digit span backward following training compared to control participants. The interaction was driven by a significant improvement over time for the experimental group (LSD p < .001). There was no significant effect on digit span forward. For the operation span task, a main effect of session (F[1,54] = 7.672, p = .008) showed that both groups recalled a significantly higher proportion of words at the post-training assessment. For the visuo-spatial working memory task, a group × session interaction (β = 0.116, SE = 0.051, p = .023) showed improved performance on the visuo-spatial working memory task for the experimental group, but not for the control group (Fig. 2). There was a significant effect of condition (p < .001) and a non-significant attenuation of the improvement as task difficulty (i.e., number of items) increased. There were no significant effects on n-back task performance.
Fig. 2.
Visuo-spatial working memory performance as a function of group for pre-training assessment and post-training assessment. Data represent fitted (model-based) means ±1 standard error of the mean. Analysis showed a group × session interaction and a main effect of condition (ps < .05).
3.2.2. Episodic memory and metamemory
A main effect of group (F[1,53] = 4.143, p = .047) showed that the experimental group recalled more words overall. For the recognition memory task, main effects of session showed an increase in false alarm rate (F[1,53] = 13.427, p = .001), and decreases in d′ (F[1,53] = 6.024, p = .017) and C (F[1,53] = 8.428, p = .005) in the post-training session relative to pre-training in both conditions. There were no significant effects on metamemory.
3.2.3. Psychomotor speed and attention
A main effect of session (F[1,54] = 5.620, p = .021) showed that both groups completed Trail Making Test A faster at the post-training assessment relative to pre-training. Digit Symbol Substitution task performance showed a trend for more trials attempted at the post-training assessment (F[1,53] = 3.757, p = .058).
3.2.4. Reasoning
A main effect of session (F[1,54] = 7.696, p = .008) showed worse performance on Raven’s Matrices for both groups at the post-training assessment.
3.2.5. Response inhibition and self-control
For the continuous performance task, main effects of session showed decreased response time variability (F[1,54] = 7.391, p = .009), a decrease in hits (F[1,54] = 7.001, p = .011), and a decrease in d′ (F[1,54] = 4.361, p = .042) in the post-training session relative to pre-training in both conditions.
For the QDOT data, 3 and 1 participants, in the control and experimental groups, respectively, failed the orderliness criterion in either of the two assessments. For the hypothetical delay discount task data, 4 and 2 participants, in the control and experimental groups, respectively, failed the orderliness criterion in either of the two assessments. Eliminating these participants case-wise within each analysis did not affect whether any result met significance. Therefore, results are reported for the full data set. For the QDOT, which delivered real rewards, there was a significant main effect showing decreased discounting at post-training compared to pre-training (F(1,53) = 5.291, p = .03), however neither the main effect for group of the group × session interaction was significant. For the hypothetical discounting task, neither main effect nor their interaction was significant.
There were no significant effects on Iowa Gambling Task performance.
3.3. Substance use and functional outcomes
The timeline follow back measure of drug use showed a significant session × condition interaction for any substance use frequency across both 14-day (F[1,54] = 4.275, p = .043) and 30-day (F[1,54] = 5.726, p = .020) time periods (Table 5). Post hoc analysis showed that participants in the control condition reported significantly more drug use days at the post-training assessment compared to pre-training (14 day assessment, 2.93 vs. 1.29 days, LSD p = .002; 30 day assessment, 6.04 vs. 2.39 days, LSD p < .001), but no significant effect was found for experimental group participants. Participants in the experimental group reported fewer drug use days at the post-training assessment compared to control participants (14 day assessment, 1.32 vs. 2.93 days, LSD p = .045; 30 day assessment, 2.64 vs. 6.04 days, LSD p = .048). Addiction Severity Index composite scores revealed a significant main effect of session (F[1,53] = 4.111, p = .048) indicating improved employment status at the post-training assessment for both conditions, but no other significant differences.
Table 5.
Self-reported substance use and functional outcomes.
| Measure | Control
|
Experimental
|
||
|---|---|---|---|---|
| Pre-training | Post-training | Pre-training | Post-training | |
| Timeline follow back (days used) | ||||
| Any substance: 14 daysb,c | 1.29 (2.12) | 2.93 (3.54) | 1.18 (1.79) | 1.32 (2.16) |
| Any substance: 30 daysb,c | 2.39 (3.86) | 6.04 (7.54) | 2.32 (3.53) | 2.64 (4.68) |
| Addiction Severity Index composite measures | ||||
| Alcohol | 0.05 (0.09) | 0.07 (0.16) | 0.03 (0.06) | 0.03 (0.07) |
| Other drugs | 0.15 (0.08) | 0.15 (0.11) | 0.13 (0.07) | 0.12 (0.06) |
| Employment statusb | 0.87 (0.16) | 0.81 (0.23) | 0.87 (0.17) | 0.84 (0.20) |
| Family status | 0.08 (0.14) | 0.10 (0.20) | 0.04 (0.13) | 0.03 (0.08) |
| Legal status | 0.05 (0.11) | 0.07 (0.15) | 0.03 (0.08) | 0.03 (0.11) |
| Medical status | 0.24 (0.30) | 0.29 (0.37) | 0.22 (0.31) | 0.34 (0.33) |
| Psychiatric statusb | 0.06 (0.12) | 0.12 (0.19) | 0.02 (0.07) | 0.05 (0.12) |
Note: Values represent Mean (Standard Deviation). Superscript letters indicate significant main effect of group (a), main effect of session (b), or group × session interaction (c). Addiction Severity Index data were missing from one of the 28 participants in the experimental group.
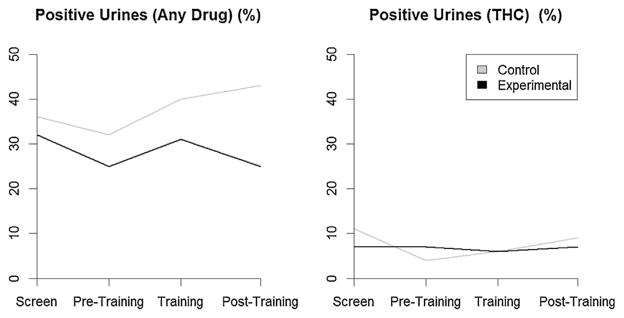
To increase power, analysis of positive urine screens was collapsed across drugs [i.e., cocaine, opiates, benzodiazepines, and cannabis (THC)] (Fig. 3). At baseline, participants in the experimental group were less likely to have positive urines (OR = 0.65, 95% CI = 0.25, 1.65), but the difference was not statistically significant. Among participants in the control group, the likelihood of a positive urine increased over time (OR = 1.01, 95% CI = 1.00, 1.02, p = 0.040), which can be interpreted as a 1% increase in odds of a positive urine with each session. There was no difference in slopes over time between the two groups (ratio of odds ratios = 1.00, 95% CI = 0.99, 1.01). These results were similar for each drug individually with the exception of THC. For THC, participants in the experimental group were more likely to have positive urines (OR = 2.35, 95% CI = 0.34, 16.28) at baseline, but the difference was not statistically significant. Among participants in the control group, the likelihood of a THC-positive urine increased over time (OR = 1.03, 95% CI = 1.00, 1.05, p = 0.030), which can be interpreted as a 3% increase in odds of a positive urine with each session. While rates of THC-positive urines were increasing over time in the control group, they were decreasing in the experimental group (ratio of odds ratios = 0.95, 95% CI = 0.92, 0.98, p = 0.002).
Fig. 3.
GEE model of the percent of positive urine rates across visits for any drug (left) and THC (right) for Experimental and Control groups.
3.4. Training data
Analysis of experimental group training performance (based on the three training exercises that were done at every session) showed a positive learning slope (M = 0.04, SD = 0.02), consistent with an overall average training improvement of one item in working memory across 25 training sessions.
4. Discussion
The aim of the present study was to evaluate the efficacy of a working memory training program to improve cognitive and substance use outcomes in methadone maintenance patients. Following training, participants in the experimental group showed improvement on working memory measures (Digit Span and Visuospatial Working Memory). Moreover, biological measures indicated that control participants’ drug use increased during the study, whereas experimental participants’ use remained constant. These results are promising and similar to past findings with working memory training in problem drinkers, who showed improved performance on working memory tasks and reduced drinking (Houben et al., 2011).
Despite improvement on working memory measures similar to training tasks, there was no improvement on dissimilar measures of working memory. Specifically, working memory improvements in the experimental group were limited to tasks similar to those participants were trained on (i.e., visuospatial working memory, digit span backward). Improvement on operation span, an un-trained working memory measure, was found for both groups and was therefore attributed to practice effects. These findings are consistent with Bickel et al. (2011), who did not find improvements in working memory following working memory training in stimulant users, Houben et al. (2011), whose outcome measures of WM performance consisted of the tasks participants had trained on, and other working memory training studies that found no generalization to working memory measures dissimilar from the training tasks (i.e., no “near transfer” occurred; see Shipstead et al., 2012 for review). Furthermore, no improvement was found in other cognitive domains (i.e., no “far transfer” occurred), similar to results reported by Bickel et al. (2011) with the exception of the null results for delay discounting. Whether this reflects a difference between stimulant and opioid users is unknown and may be addressed by future research. Thus, these findings contribute to the controversy regarding WM training effectiveness and generalization (e.g., Morrison and Chein, 2011; Klingberg, 2012; Melby-Lervag and Hulme, 2013; Shipstead et al., 2012).
Delay discounting was not differentially affected by working memory training as hypothesized. Our confidence in these results, however, is bolstered by the use of both a widely used hypothetical task as well as a task that delivers real rewards in real time, and by the fact that the discounting results were largely orderly (i.e., consistent with the expectation of stable or monastically decreasing values with increasing delays). Although our study did not show significant differences between groups, both groups showed significantly decreased discounting as measured by the real-rewards QDOT task, and both groups showed non-significant decreases in delay discounting in the hypothetical task, potentially consistent with the notion that working memory training and the control task challenged distress tolerance. Persistence in an energy-depleting task that may be challenging (e.g., adaptive training) or boring (e.g., control training) may evoke uncomfortable emotional states (e.g., negative affect, frustration, craving). In addition, both conditions required participants to sit quietly and focus for prolonged periods of time, an experience that they may not typically encounter in their daily lives. Evidence suggests that delay discounting is increased under conditions in which one is restricted from other sources of reward during delays (Johnson et al., 2015). As implied by the expression “a watched pot never boils,” such waiting conditions may be described as “frustrating,” and decisions for delayed outcomes may therefore be affected by distress tolerance. The ability to cope with feelings of frustration that may arise as task difficulty increases is important in working memory training, as is maintaining focus for prolonged periods. Thus, augmenting working memory training with training of coping skills, emotional regulation, or mindfulness may enhance both cognitive performance and psychosocial treatment outcomes (Bowen et al., 2009; Brewer et al., 2009; Desbordes et al., 2012; Garland et al., 2010; Sahdra et al., 2011; Witkiewitz et al., 2013). This tactic may be especially relevant for individuals with substance use disorders, who often exhibit deficits in emotion regulation (Gerra et al., 2003; Witkiewitz et al., 2012; Wong et al., 2013).
These findings suggest that cognitive training is feasible and potentially beneficial for individuals with opioid use disorders. Participants were able to learn and showed improvement on some working memory and drug use outcomes. However, further research is needed to determine the contribution of individual differences (e.g., cognitive abilities, psychiatric comorbidities, motivational/personality factors, distress intolerance) and the role of training regimen (e.g., modality, frequency, intensity, duration, feedback) on training efficacy and to assess the persistence of improved outcomes (Dunning and Holmes, 2014; Jaeggi et al., 2014; von Bastian and Oberauer, 2013).
Supplementary Material
Acknowledgments
We thank Daisy Hussein, Taylor Marcus, and Christina Bittar for protocol management and technical assistance, John Yingling for computer programming assistance and technical support, and Dr. Jeannie-Marie Leoutsakos for assistance with statistical analysis.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2015.08.012.
Footnotes
One participant was enrolled who exceeded the age limit due to staff error.
Conflict of interest
The authors have no conflicts of interest relevant to the present work to disclose.
Contributions
All authors contributed in a significant way to the manuscript and have read and approved the final manuscript. Dr. Mintzer provided overall scientific and administrative leadership to the project, and had supervisory responsibility for the design, conduct, analysis, and publication of the study. Drs. Johnson and Strain contributed to the design of the study. Dr. Johnson also analyzed delay discounting data and drafted discounting related manuscript content. Dr. Schacht contributed to the study design and supervised the conduct of the study, including training and supervising the research staff, assisting with patient recruitment, and data management. Dr. Schacht also managed the study analyses and drafted early versions of the manuscript. Katherine Buckheit contributed to the study design, trained and coordinated the research staff, recruited participants, managed data, and assisted in writing the methods section. Dr. Rass was the primary author, managed the literature searches and summaries of previous related work, and assisted in interpreting data analyses. All authors contributed to editing the manuscript.
Disclosures
Role of Funding Source: This work was supported by NIDA Grants R21 DA029708 (PI: Miriam Z. Mintzer, Ph.D.), K23 DA023186 (PI: Eric C. Strain, M.D.), R01 DA035277 (PI: Matthew W. Jonson), and T32 DA07209 (PI: George Bigelow, Ph.D.).
The research reported in this article was conducted while all authors were employed at Johns Hopkins University. Currently, Dr. Rass is an employee of the U.S. Food and Drug Administration (FDA), Dr. Rebecca Schacht is an employee of the University of Maryland, Baltimore County Katherine Buckheit is a student at Syracuse University, and Dr. Miriam Mintzer is an employee of the National Institutes of Health. The views in this article do not necessarily reflect those of the FDA, and no official support or endorsement of this article by the FDA is intended or should be inferred. The opinions expressed in this article are the authors’ own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government. The registered number for this clinical trial is NCT01271413.
Uncited references
Jaeggi et al. (2011), Kaiser et al. (2012), Morrison et al. (2014), Ochsner et al. (2012) and Paglieri (2013).
References
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Psychol Addict Behav. 2008;22:557–562. doi: 10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Hasin D, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O’Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory: the interface between memory and cognition. J Cogn Neurosci. 1992;4:281–288. doi: 10.1162/jocn.1992.4.3.281. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hil PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Clifasefi S, Garner M, Douglass A, Larimer ME, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abuse. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, Grier A, Bergquist KL, Reis DL, Potenza MN, Carroll KM, Rounsaville BJ. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Subst Abuse. 2009;30:306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology (Berl) 2007;190:485–496. doi: 10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95:687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B: Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes G, Negi LT, Pace TW, Wallace BA, Raison CL, Schwartz EL. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front Hum Neurosci. 2012;6:1–15. doi: 10.3389/fnhum.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretsch MN, Tipples J. Working memory involved in predicting future outcomes based on past experiences. Brain Cogn. 2008;66:83–90. doi: 10.1016/j.bandc.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Dunning DL, Holmes J. Does working memory training promote the use of strategies on untrained working memory tasks? Mem Cognit. 2014;42:854–862. doi: 10.3758/s13421-014-0410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap CB, Bourquin M, Martin J, Spagnoli J, Livoti S, Powell K, Bauman P, Deglon J. Plasma concentrations of the enantiomers of methadone and therapeutic response in methadone maintenance treatment. Drug Alcohol Depend. 2000;61:47–54. doi: 10.1016/s0376-8716(00)00121-6. [DOI] [PubMed] [Google Scholar]
- Engle RW, Cantor J, Carullo JJ. Individual differences in working memory and comprehension: a test of four hypotheses. J Exp Psychol Learn Mem Cogn. 1992;18:972–992. doi: 10.1037//0278-7393.18.5.972. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Conners CK, Sitarenios G, Erhardt D. Continuous performance test results of adults with attention deficit hyperactivity disorder. Clin Neuropsychol. 1998;12:155–168. [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian JB. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17:317–336. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology (Berl) 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: results of a randomized controlled pilot trial. J Psychoact Drugs. 2010;42:177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Baldaro B, Zaimovic A, Moi G, Bussandri M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug Alcohol Depend. 2003;71:25–35. doi: 10.1016/s0376-8716(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Goodman LA, Kruskal WH. Measures of association for cross classifications. J Am Stat Assoc. 1954;49:732–764. [Google Scholar]
- Grenard JL, Ames SL, Wiers RW, Thush C, Sussman S, Stacy AW. Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychol Addict Behav. 2008;22:426–432. doi: 10.1037/0893-164X.22.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA. Neuropsychological consequences of opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17:317–336. doi: 10.1007/s11065-007-9041-y. [DOI] [PubMed] [Google Scholar]
- Guerra D, Sole A, Cami J, Tobena A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20:261–270. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and Intelligence. University of Chicago Press; Chicago: 1947. [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, Jansen A. Getting a grip on drinking behavior training working memory to reduce alcohol abuse. Psychol Sci. 2011;22:968–975. doi: 10.1177/0956797611412392. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the child behavior checklist: a receiver-operating characteristic analysis. J Child Psychol Psychiatry. 2004;45:1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Helzer JE, Wetzel MW, Kessel KB, McGee B, Janca A, Przybeck T. The use of the DSM-III-R checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A. 2011;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, Jonides J. The role of individual differences in cognitive training and transfer. Mem Cognit. 2014;42:464–480. doi: 10.3758/s13421-013-0364-z. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic data in delay discounting research. Exp Clin Psychopharmacol. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol. 2007;15:187–194. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK, Baker F, Moore BA, Badger GJ, Budney AJ. Delay discounting in current and former marijuana-dependent individuals. Exp Clin Psychopharmacol. 2010;18:99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW. An efficient operant choice procedure for assessing delay discounting in humans: initial validation in cocaine-dependent and control individuals. Exp Clin Psychopharmacol. 2012;20:191–204. doi: 10.1037/a0027088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Herrmann ES, Johnson MW. Opportunity costs of reward delays and the discounting of hypothetical money and cigarettes. J Exp Anal Behav. 2015;103:87–107. doi: 10.1002/jeab.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, Koeppe RA. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kaiser AJ, Milich R, Lynam DR, Charnigo RJ. Negative urgency, distress tolerance, and substance abuse among college students. Addict Behav. 2012;37:1075–1083. doi: 10.1016/j.addbeh.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EC, King SD, Schwartz RP, Weintrub E, Barksdale W, Robinson R, Brown BS. Cognitive ability as a factor in engagement in drug abuse treatment. Am J Drug Alcohol Abuse. 2005;31:359–369. doi: 10.1081/ada-200056767. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Is working memory capacity fixed? J Appl Res Mem Cogn. 2012;1:194–196. [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, Gillberg CG, Forssberg H, Westerberg H. Computerized training of working memory in children with ADHD – a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: an fMRI study. Neurosci Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–466. [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M, Hulme C. Is working memory training effective? A meta-analytic review Dev Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. A triazolam/amphetamine dose-effect interaction study: dissociation of effects on memory versus arousal. Psychopharmacology (Berl) 2007;192:425–440. doi: 10.1007/s00213-007-0726-y. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Johnson MW. Opioids. In: Kalechstein A, Van Gorp W, editors. The Neuropsychological Consequences of Substance Abuse. Taylor & Francis; New York: 2007. pp. 263–319. [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morrison KL, Madden GJ, Odum AL, Friedel JE, Twohig MP. Altering impulsive decision making with an acceptance-based procedure. Behav Ther. 2014;45:630–639. doi: 10.1016/j.beth.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paglieri F. The costs of delay: waiting versus postponing in intertemporal choice. J Exp Anal Behav. 2013;99:362–377. doi: 10.1002/jeab.18. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Liappas I, Asvestas P, Vasios C, Matsopoulos GK, Nikolaou C, Nikita KS, Uzunoglu N, Rabavilas A. Abnormal P600 in heroin addicts with prolonged abstinence elicited during a working memory test. Neuroreport. 2001;12:1773–1778. doi: 10.1097/00001756-200106130-00051. [DOI] [PubMed] [Google Scholar]
- Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94:82–91. doi: 10.1016/j.drugalcdep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Fischer G, Podreka I, Schindler S, Brucke T, Jagsch R, Thurner M, Kastper S. Opioid addiction changes cerebral blood flow symmetry. Neuropsychobiology. 2002;45:67–73. doi: 10.1159/000048679. [DOI] [PubMed] [Google Scholar]
- Pocock SJ. Clinical Trials: A Practical Approach. John Wiley & Sons; New York: 1983. [Google Scholar]
- Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug Alcohol Depend. 2006;84:240–247. doi: 10.1016/j.drugalcdep.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapeli P, Fabritius C, Alho H, Salaspuro M, Wahlbeck K, Kalska H. Methadone vs. buprenorphine/naloxone during early opioid substitution treatment: a naturalistic comparison of cognitive performance relative to healthy controls. BMC Pharmacol Toxicol. 2007;7:5. doi: 10.1186/1472-6904-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. J Abnorm Child Psychol. 2008;36:825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Rass O, Schacht R, Marvel C, Mintzer M. Opioids. In: Allen D, Woods S, editors. Neuropsychological Aspects of Substance Use Disorders: Evidence-Based Perspectives. Oxford University Press; Oxford: 2014. pp. 231–253. [Google Scholar]
- Raven J. Progressive Matrices: A Perceptual Test of Intelligence. H.K. Lewis; London: 1939. [Google Scholar]
- Reitan RM. Investigation of the validity of Halstead’s measure of biological intelligence. AMA Arch Neurol Psychiatry. 1955;73:28–35. doi: 10.1001/archneurpsyc.1955.02330070030005. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139:5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Sahdra BK, MacLean KA, Ferrer E, Shaver PR, Rosenberg EL, Jacobs TL, et al. Enhanced response inhibition during intensive meditation training predicts improvements in self-reported adaptive socioemotional functioning. Emotion. 2011;11:299–312. doi: 10.1037/a0022764. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Green CE, Lane SD, Steinberg JL, Swann AC, et al. Baseline neurocognitive profiles differentiate abstainers and non-abstainers in a cocaine clinical trial. J Addict Dis. 2009;28:250–257. doi: 10.1080/10550880903028502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, DeYoung CG, Green AE, Reis DL, Johnson MR, Conway AR, Engle RW, Braver TS, Gray JR. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19:904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shipstead Z, Hicks KL, Engle RW. Cogmed working memory training: does the evidence support the claims? J Appl Res Mem Cogn. 2012;1:185–193. [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback User’s Guide: A Calendar Method for Assessing Alcohol and Drug Use. Addiction Research Foundation; Toronto: 1996. [Google Scholar]
- Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl) 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- Stuster J, Burns M. Validation of the Standardized Field Sobriety Test Battery at BACs Below 0.10 Percent. 1998. DOT-HS-808-839. [Google Scholar]
- Süß HM, Oberauer K, Wittmann WW, Wilhelm O, Schulze R. Working-memory capacity explains reasoning ability—and a little bit more. Intelligence. 2002;30:261–288. [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. Int J Neurosci. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Bergman Nutley S, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Dev Sci. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW. Is working memory capacity task dependent? J Mem Lang. 1989;28:127–154. [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, Malcolm R. Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. J Subst Abuse Treat. 2009;37:328–334. doi: 10.1016/j.jsat.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Verdejo A, Toribio I, Orozco C, Puente KL, Pérez-García M. Neuropsychological functioning in methadone maintenance patients versus abstinent heroin abusers. Drug Alcohol Depend. 2005;78:283–288. doi: 10.1016/j.drugalcdep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Vocci FJ. Cognitive remediation in the treatment of stimulant abuse disorders: a research agenda. Exp Clin Psychopharmacol. 2008;16:484–497. doi: 10.1037/a0014101. [DOI] [PubMed] [Google Scholar]
- von Bastian CC, Oberauer K. Distinct transfer effects of training different facets of working memory capacity. J Mem Lang. 2013;69:36–58. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; San Antonio: 1981. [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, Bartfai A. Computerized working memory training after stroke – a pilot study. Brain Inj. 2007;21:21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Klingberg T. Changes in cortical activity after training of working memory – a single-subject analysis. Physiol Behav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Douglas H, Hsu SH. Mindfulness-based relapse prevention for substance craving. Addict Behav. 2013;38:1563–1571. doi: 10.1016/j.addbeh.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Lustyk MK, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2012;27:351–365. doi: 10.1037/a0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CF, Silva K, Kecojevic A, Schrager SM, Bloom JJ, Iverson E, Lankenau SE. Coping and emotion regulation profiles as predictors of nonmedical prescription drug and illicit drug use among high-risk young adults. Drug Alcohol Depend. 2013;132:165–171. doi: 10.1016/j.drugalcdep.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA, Paulson MJ, Gorsuch RL. Estimating WAIS IQ from the Shipley Institute of Living scale using continuously adjusted age norms. J Clin Psychol. 1985;41:820–831. doi: 10.1002/1097-4679(198511)41:6<820::aid-jclp2270410616>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.