Introduction
Gastric acid hypersecretory states increase the risk for peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD) and gastrointestinal bleeding, and increase the morbidity and mortality related to these conditions. Gastrin is a key hormone that regulates gastric acid secretion; hypergastrinemia is an important diagnostic indicator of the level of gastric acid secretion. This review will provide an understanding of the clinical approaches to managing patients suspected of having gastric acid hypersecretory states and an approach to the differential diagnosis to better recognize the different disease states (Figure 1). Treatment options for treating gastric acid hypersecretion are discussed.
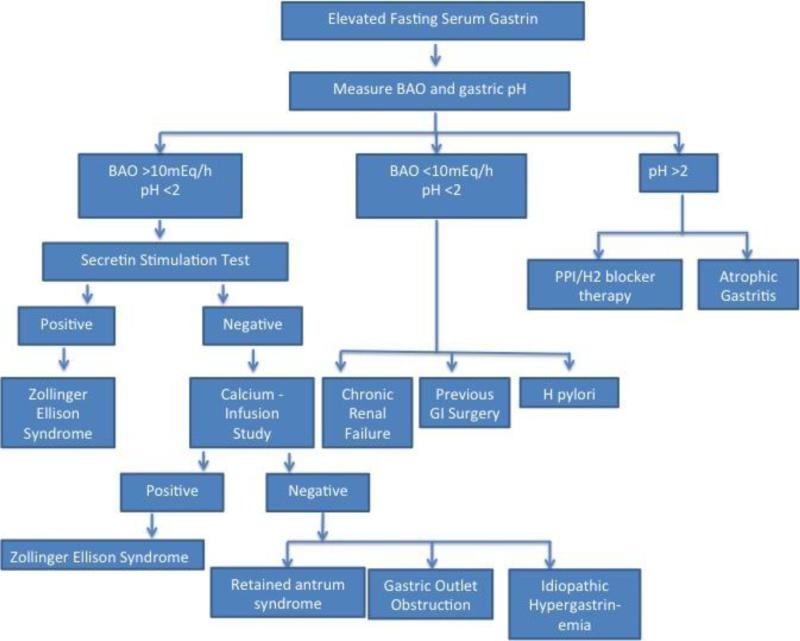
Figure 1.
Diagnostic Algorithm if Gastric Acid Hypersecretion Suspected
Physiology of Gastric Acid Secretion
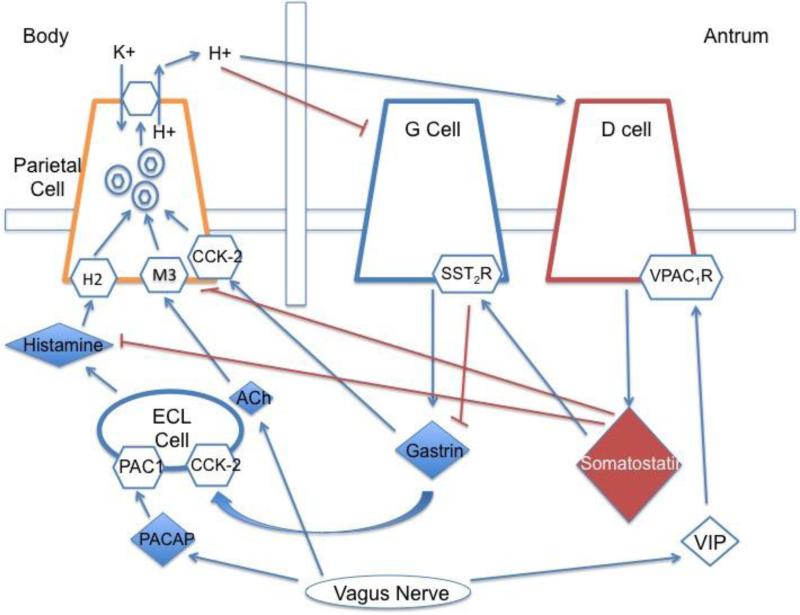
Gastric acid secretion by parietal cells occurs in the fundus of the stomach, and is intricately regulated by various neuronal (vagal), paracrine (histamine, somatostatin) and hormonal factors (Fig 2). Gastric acid secretion can be divided into a cephalic and gastric phase. The former occurs before the entry of food into the digestive system and is mediated by the central and peripheral nervous systems.1 During the cephalic phase, vagal efferents release acetylcholine, which acts on M3 muscarinic receptors that are expressed on parietal cells, resulting in increased acid secretion. Neural activation of pituitary adenylate cyclase activating polypeptide (PACAP) results in histamine release from the enterochromaffin-like (ECL) cells by activity at its specific receptor, PAC1.1 The release of histamine from the ECL cell activates the histamine H2 receptor expressed on the parietal cells. The gastric phase of gastric acid secretion occurs with the entry of nutrients into the gastrointestinal tract. Gastrin, released by antral G cells, is triggered by a protein meal and stimulates acid secretion via two mechanisms. Its major action indirectly causes acid secretion through binding to cholecystokinin-2 (CCK-2) receptors expressed on ECL cells, which in turn secrete histamine.1 Circulating gastrin may also bind to CCK-2 receptors on the basolateral membrane of parietal cells to promote acid secretion.
Figure 2.
Physiology of Gastric Acid Secretion
Gastric acid secretion is also under control of negative feedback mechanisms to maintain gastric acid homeostasis. When the intraluminal gastric pH drops, inhibitory feedback occurs through the release of somatostatin from the gastric D cells. Somatostatin acts in a paracrine manner to inhibit parietal cell secretion directly and indirectly through inhibiting both histamine and gastrin release. Vasoactive intestinal polypeptide (VIP) has a negative feedback inhibition of somatostatin release from D cells and may account for the hypergastrinemia associated with gastrointestinal dysmotility.2
Following activation by various secretagogues, the parietal cell undergoes multiple transformations to initiate secretion of HCl into the lumen. Tubulovesicles containing the H+/K+-ATPase (the proton pumps) are translocated to the apical membrane and fuse with the canalicular surface of the parietal cell.1 This allows for a 1:1 ATP-driven exchange of intracellular hydrogen ions for luminal potassium ions. At resting state, these acid-secreting pumps lie inactive within the cytoplasmic tubulovesicles. Recruitment of the proton pumps for activation on the secretory canaliculi is thought to be gastrin-driven and histamine-dependent.1 Secretagogues such as histamine and PACAP that generate intracellular cAMP are also important in the recycling of pumps and in the synthesis of new proton pumps in the Golgi apparatus.3
Hypergastrinemia
The fasting serum gastrin concentration in a healthy individual should be under 100 pg/ml. Following ingestion of a protein-containing meal, the serum gastrin increases from the basal level to a maximal level within approximately 20 minutes. In conditions such as atrophic gastritis, hypergastrinemia is expected in the setting of hypochlorhydria or achlorhydria due to lack of negative feedback on the release of gastrin. Hypergastrinemia is also associated with gastric outlet obstruction or gastric dysmotility states.2 Numerous medical conditions result in elevation of the serum gastrin level. Hypergastrinemia in the presence of an acidic intragastric pH suggests an inappropriate response that requires further investigation. There may exist other pathways that regulate G cell gastrin release that have yet to be described.
Zollinger-Ellison syndrome (ZES)
ZES was described in the 1950s by two surgeons: Drs. Zollinger and Ellison. In their surgical series, they discovered a subgroup of patients with recurrent peptic ulcer disease that was caused by overproduction of gastric acid. With the discovery of the gastrin radioimmunoassay test it was later shown that this syndrome was caused by overproduction of gastrin by a gastrin-producing neuroendocrine tumor (gastrinoma). ZES is a rare condition in which the tumor causing gastric acid hypersecretion is usually localized to the pancreas or duodenum. Significantly elevated serum gastrin levels (>1000 pg/ml) in the absence of achlorhydria is specific for the diagnosis of ZES. Although most gastrinomas occur sporadically, 25-30% of ZES cases are associated with multiple endocrine neoplasia type 1 (MEN-1) – an autosomal dominant familial syndrome.4 A subgroup of patients with MEN-1 also have hypercalcemia due to a parathyroid tumor that secretes parathyroid hormone (PTH). Elevated serum calcium concentrations may also stimulate gastric acid hypersecretion; thus the management of patients with ZES and MEN-1 may require higher doses of proton pump inhibitors (PPIs).5 Complications of unopposed gastrin release include severe and/or refractory peptic ulcer disease that is mainly localized to the post-bulbar regions of the duodenum, development of esophagitis or duodeno-jejunitis, and diarrhea.6 Although the exact reason for post-bulbar ulcers is unknown, one explanation may be the occurrence of hypertrophic, heterotopic gastric mucosa in the proximal duodenal bulb. Gastrin, acting as a growth factor for the oxyntic mucosa, is in line with the theory that gastrin has trophic effects on ECL and parietal cells.7 The expansion of the parietal cell mass results in increased basal and maximal acid output (BAO and MAO, respectively), which can lead to significant morbidity and mortality from ulcer-associated bleeding and perforation. Although surgical removal of a gastrinoma in patients with sporadic ZES may be the best option for a curative result, the initial management should be focused on the control of gastric acid secretion with PPIs, as is discussed later.
Retained Gastric Antrum Syndrome
Recurrent peptic ulcers following gastrectomy are rare unless there are common identifiable causes including incomplete vagotomy or NSAID use. Retention of the gastric antrum within the duodenal stump following gastric surgery, such as a Billroth II gastrectomy, can cause virulent ulcer recurrence. This results because the retained gastric antrum is not in communication with inhibitory pathways (e.g. VIP) that would be normally present when the antrum is contiguous with the gastric body.8 In addition, the isolated antrum is exposed to the neutral or alkaline environment of the duodenum thus promoting acid formation. The BAO and MAO are elevated as in ZES, although the increase in baseline serum gastrin is moderate in comparison (usually < 1000 pg/ml).8 99mTc scanning is the imaging modality of choice when suspecting the diagnosis of retained antrum; management includes antral resection and vagotomy.
Gastric Outlet Obstruction
Gastric outlet obstruction secondary to mechanical or functional etiologies causes antral distension, which induces neural activation of parietal cells through vagal release of acetylcholine. Affected patients will typically have hypergastrinemia. Therefore, the differential diagnosis of hypergastrinemia includes gastric outlet obstruction and gastric emptying disorders. VIP may play an important role in this process as recently described.2 In patients with chronic obstruction, ECL and parietal cell hyperplasia occurs through gastrin-mediated trophism. Coupled with dysmotility and gastric content stasis, breakdown of the mucosal protective barriers occurs leading to ulceration.9 The mechanism underlying the hypergastrinemia in these conditions deserves further exploration.
Heliobacter pylori Infection
Patients infected with H. pylori may have hypergastrinemia, which may return to normal baseline levels following eradication. Multiple mechanisms have been proposed to explain the relationship between this gastric bacterium and its manipulation of the gastric milieu. It is important to understand that H. pylori can colonize different areas of the stomach. Most infections involve the antrum; therefore, not surprisingly, both G and D cells are probably involved in the pathogenesis of hypergastrinemia. Mechanisms include H. pylori urease production causing urea hydrolysis and alkalization around G cells, diminished D cell density and somatostatin release secondary to surrounding inflammation or cytokine activation of G cells.10 Atrophic gastritis through direct damage of parietal cells by H. pylori occurs when the gastric corpus is heavily colonized.11 In addition, H. pylori may induce autoantibodies preventing activation of proton pumps within the parietal cells thus promoting negative feedback from hypochlorhydria. The reflux of bile and/or pancreatic juice may predispose individuals with antral-predominant H. pylori infection to develop gastric mucosal metaplasia. Thus, eradication of this organism is warranted in susceptible individuals.
Chronic Renal Failure
Patients with chronic renal failure or those undergoing dialysis will typically have an elevated serum gastrin.12 In experimental models, Ohning and co-workers demonstrated that gastrin mediates the increase in gastric cell growth in a rat model of hypertension and uremia.13 In this same rat model, uremia results in H+ back-diffusion in the stomach resulting in a reduction in the gastric mucus layer protection and a lower transmural potential difference, which may account for the greater mucosal injury in chronic renal failure.14 Gastrin is cleared by the kidney. In the absence of normal renal clearance, hypergastrinemia is commonly identified. Although the pathophysiological mechanisms are unknown, patients normally have asymptomatic peptic ulceration with a higher likelihood of post-bulbar bleeding as well as multiple ulcerations.15 Perhaps the mechanism is similar to that seen with ZES.
Idiopathic Gastric Acid Hypersecretion
Subgroups of patients with PUD have gastric acid hypersecretion despite having normal serum gastrin levels and the absence of a gastrinoma; they are described as having idiopathic gastric acid hypersecretion. By definition, these patients likely have a BAO >10-15 mEq/hr and a normal fasting serum gastrin level. In one study, Collen and Jensen compared 124 patients with idiopathic gastric acid hypersecretion to 137 patient with ZES and showed that the mean serum gastrin was 60 pg/ml compared to 3679 pg/ml, respectively, despite similar gastric acid output.16 Some of these patients may present with diarrhea or be misdiagnosed with inflammatory bowel disease.17 In one study, the authors reported an association between idiopathic gastric acid hypersecretion with MUTYH-associated colonic polyposis.18
Pharmacophysiology of Proton Pump Inhibitors
The ultimate step in gastric acid secretion is movement of the tubulovesciular proton pumps from the cytoplasm to the canalicular space. Recognition of this final step underlies the development of PPIs to target H+/K+-ATPase.1,3 PPIs are benzimidazole compounds delivered with some form of enteric coating. Inactive PPI molecules are released into the pH-optimal environment of the duodenum. The plasma half-life of PPIs is usually around one hour although the duration of acid suppression may be up to 48 hours due to covalent binding to proton pumps. The pKa of PPIs ranges from 3.8 to 4.9, enabling accumulation of the prodrug within the acidic space of the parietal cell canaliculi following diffusion across the basal membrane.2 The first step of metabolism is acid-dependent conversion of the accumulated prodrug to its activated species, which is dependent on parietal cell activation by a peptone meal and the secretagogues described above. The sulphenic acid active form of the PPI then covalently binds extracellularly to the proton pump resulting in irreversible inhibition of gastric acid secretion across the parietal cell membrane (Fig. 2).1,3 PPIs only inhibit around 70% of proton pumps with oral dosing since not all pumps are active at any one time, and around 25% of pumps are synthesized de novo every 24 hours.
The timing of PPI ingestion to around the time of maximal activation of the parietal cell is critically important to optimize its pharmacodynamic effect of inhibiting gastric acid secretion. Assuming normal gastric emptying, this should be approximately 30-45 minutes before ingestion of a protein-containing meal. This enables the inactive PPI molecules to be absorbed from the duodenum while the protein meal stimulates gastrin release from the G cells.
In order to resume acid secretion, the parietal cell must translocate de novo cytoplasmic pumps into the secretory canaliculus or reduce the disulfide bond between pump and PPI. Studies by Metz et al show that pump reactivation is dependent on an individual's parietal cell mass, with mean pump recovery time in healthy individuals of 37.1 (range 6.7 to 75) hours.1 Therefore, patients with increased acid secretory capability or higher MAO may not require rapid re-activation to maintain gastric homeostasis. The presumed irreversible binding of PPIs has also undergone reconsideration. Rodent studies performed in vitro have demonstrated discordance between half-life of pump gene transcription time (54 hours) and recovery of ATPase activity (15 hours), entertaining the possibility of disulfide bond reduction and pump recirculation.
Clinical Presentation of Hypersecretion
PUD is diagnosed in approximately 500,000 patients per year in the United States. Most common presentations for symptomatic ulcers are dyspepsia with occasional epigastric pain (74%), nausea (23%) and emesis (20%).1,3 On endoscopic evaluation for GERD, an average 46% of patients had peptic ulcerations. 25% of patients with PUD will experience significant ulcer-related complications, with increased likelihood in older patients and with NSAID use.5 The most common cause of surgical intervention and death is upper gastrointestinal bleeding which may be the first manifestation in patients with silent ulcers. Pyloric channel ulcers and recurrent duodenal ulcers can cause gastric outlet obstruction.
Chronic acid exposure of the esophagus can predispose to erosive esophagitis, strictures, and Barrett's esophagus – with possible progression to esophageal adenocarcinoma. Esophageal disease is present in up to 40% of ZES patients, although studies have shown equivalent lower esophageal sphincter pressures and esophageal motility as healthy patients.6,7 Therefore the exact link between hypergastrinemia and determinants of neoplastic potential require further investigation.
Further theoretical risks associated with hypergastrinemia include gastric carcinoids and an increased risk of colon adenomas, although these associations have not been substantiated. Gastrin, through its trophic actions on epithelia, is thought to pose some risk for the development of gastric adenocarcinoma.20 Gastrin is also trophic for the colonic mucosa, predisposing to colorectal cancer. Pancreatic and hematological malignancies have also been linked to hypergastrinemia.
Diagnosis of Gastrin Hypersecretion
Serum Gastrin Concentration
Measurement of fasting gastrin has an important role in delineating the various causes of high gastric acid output states. Serum gastrin levels >150 pg/ml are abnormal, and levels > 1000 pg/ml indicate a high probability of ZES. Fasting gastrin levels alone were non-diagnostic in two-thirds of ZES patients due to significant overlap with alternative upper gastrointestinal hypergastrinemic states such as atrophic gastritis and PUD.22
Secretin Provocation Test
In patients with non-diagnostic fasting serum gastrin levels in whom ZES is suspected, the secretin provocation test is used to differentiate patients with gastrinomas from those with alternative causes of hypergastrinemia. Secretin receptors are located on all gastrinomas; when secretin is infused, it provokes excessive gastrin release. The test is initiated by measuring baseline fasting gastrin levels, followed by rapid intravenous infusion of secretin (0.4 μg/ kg) and measurement of serum gastrin after 2, 5, and 10 minutes. An increase in serum gastrin by 200 pg/ml from baseline signifies a positive test and carries 83% overall accuracy for the diagnosis of ZES.22 Unfortunately, the availability of secretin is unreliable and alternatives to its use are required. Glucagon may be a possible alternative to secretin since gastrinomas also possess receptors for glucagon. It is administered intravenously at a dose of 20 μg/kg plus 20 μg/kg/hr for 1 hour with serial gastrin measurements.23
Since the validation of the secretin test was performed in the absence of antisecretory agents, guidelines suggest withdrawal of PPIs one week prior to secretin provocation. Abrupt interruption of PPIs places ZES patients at high risk of rebound acid hypersecretion from both tumoral hypergastrinemia and the hyperplastic parietal cell mass.22 PPI dependency eliminates gut defense mechanisms such as pancreatic and duodenal bicarbonate secretion; therefore ZES patients on PPIs cannot tolerate the high gastric acid load when PPIs are discontinued.22 Therefore the decision to perform the secretin test should be made judiciously - and especially in patients with flagrant feature of ZES who are on PPI treatment. Alternative diagnostic options include nasogastric tube acid aspiration tests or endoscopic gastric analysis (see below).
Calcium Infusion Study
Stimulation with calcium infusion causes gastrin release from gastrinomas, presumably mediated through calcium sensing receptors (CaSRs) on the tumor surface. In addition, CaSRs are expressed on antral G cells, and act to sense extracellular calcium and regulate gastrin release. One study compared the multiple provocative tests including rapid (2 mg Ca++/kg/min) versus long (5 mg Ca++/kg/3h) calcium infusion, secretin stimulation tests, and combination of calcium infusion immediately followed by secretin.22 Rapid calcium infusion followed by secretin provided the largest provocation of gastrin release in ZES. Although uncommon, the NIH literature has identified a 1.4% lack of gastrin response in ZES patients given bolus secretin, thereby identifying a population in which calcium infusion is useful given the degree of clinical suspicion. Change of >395 pg/ml from fasting gastrin or >50% from baseline is considered a positive calcium infusion study with sensitivity of 54-78% in diagnosing ZES.
Nasogastric tube-based Acid Aspiration Test
The conventional method to measure gastric acid output is through aspiration of gastric juice via a nasogastric tube during basal and stimulated acid secretion. The aspiration port of the nasogastric tube should lie in the gastric fundus, with positioning confirmed with fluoroscopy or the water recovery test in which 100 cc of water is instilled into the stomach and recovery of >90% by aspiration confirms the location within the gastric fundus. Gastric juice is continuously aspirated through suction of the aspiration port and is measured for volume and acidity through titration to pH of 7 with alkaline agents and chemical indicators. The BAO is averaged between four 15-minute collections in a fasting state and represents background vagal tone. MAO and peak acid output (PAO) are measured following administration of a gastric stimulant such as pentagastrin or tetragastrin, and reflect parietal cell mass. MAO totals the acid secretion within one hour whereas PAO reflects the two highest 15-minute collections multiplied by 2.25 In ZES, the BAO is normally >10 mmol/h and the MAO >30 mmol/h.
The aspiration method has been criticized for underestimating gastric acid output. The test involves removal of stomach acid, which in and of itself can alter measurements. In addition, acid is lost to gastric emptying or undergoes neutralization from alkaline secretions, which can falsely decrease output.25 Due to its invasive nature and patient discomfort; the aspiration test has decreased in favor of endoscopic options.
Endoscopic Gastric Analysis
Quantitative endoscopic gastric analysis was first validated in 2006 by Oh et al to diagnose high acid output states in patients with ZES. Multiple methods have been developed with attempts to shorten collection times with the initial 2 hour aspiration time with Yamaguchi et al, then the progressively shorter techniques with Eisenband and Wenger. Under direct endoscopic visualization developed by Oh et al, a single 15-minute sample of gastric juice is collected under fasting and stimulated states to assess for acidity and volume.26 When compared to conventional gastric aspiration tests, there was excellent agreement when measuring acid concentration with the acceptable difference of ±2.5 mEq/h. However the endoscopic method was found to overestimate acid volume. The authors argue the innate critique of under-measuring volume with blind aspiration and increased accuracy with direct visible aspirate.26 The endoscopic method has the advantage over nasogastric aspiration in that it permits the collection of gastric juice by directly observing the gastric juice and allows the endoscopist to exclude the presence of peptic ulcerations, especially useful in patients with ZES. This method is routinely used at our institution since the test is better tolerated and likely to provide more accurate results.
Qualitative endoscopic gastric evaluation involves inspection of gastric lumen acid production via a pH-dependent color change. Congo red dye is introduced onto the gastric mucosa following intravenous pentagastrin (6 μ/kg) stimulation, and the dye changes color from red to black or blue at pH <3. This approach helps diagnose patient with gastric atrophy and early gastric cancer.25
Intragastric pH Tests
Conventional intragastric pH testing involves insertion of a pH-measuring electrode by the nasogastric route with fluoroscopic or manometric guidance. Placement is essential given the variations of pH depending on stomach region, although optimum location is still under debate. The probe is usually placed 10 cm below the upper margin of the lower esophageal sphincter. However it is susceptible to movement based on the patient's activity or oral bolus composition. Patients may also alter their daily activities and diet because of probe-related discomfort.
Patient inconvenience was counteracted by the wireless Bravo ® pH monitoring system. The capsule, which continuously measures pH and wirelessly transmits the data via a radiofrequency signal, is inserted endoscopically into the gastric wall. The capsule monitors pH for 48 hours and is self-eliminated within 1-2 weeks. Early studies were complicated by premature dislodgement, which was rectified through the clipping technique developed by Chang and colleagues which reduced dislodgement rates by 70%.27 The Bravo® system is well tolerated and allows longer ambulatory pH monitoring, although is unable to quantitate gastric secretory volume. A new method has been recently described to measure gastric acid output more accurately using the SmartPill® wireless pH sensor. Accurate placement of sensor via nasogastric tube is confirmed through air auscultation or aspiration of 50 ml of instilled water. Gastric acid output is calculated based on the rate of acidification of an Ensure Plus® meal from pH of 5 to 2, therefore enabling the measurement of gastric emptying time and maximal acid output. The values from the SmartPill® sensor were validated against conventional evaluations.28
Serum VIP levels
Recent studies demonstrated a >5 fold increase in gastrin gene expression and serum gastrin levels in a murine VIP−/− model suggesting that VIP plays an important role in downregulating gastrin secretion. Future studies are required to validate measured VIP levels as a diagnostic tool for hypergastrinemia.2
Treatment of Gastrin Hypersecretion
Intravenous Proton Pump Inhibitors
Initial antisecretory therapy for patients with hypersecretory states consisted of H2-receptor antagonists. However management has been largely replaced by PPIs due to their greater antisecretory potency, blocking the final step of acid secretion. Although most hypersecretory patients are controlled on oral PPIs, a cohort of patients requires intravenous (IV) PPI treatment to reduce breakthrough acid hypersecretion on an oral PPI, or for perioperative transition. An initial IV bolus inactivates activated proton pumps, and subsequent continuous IV infusion provides a steady delivery of drug to inactive newly synthesized pumps. In a multi-center study, Metz and colleagues evaluated the appropriate IV regimen to transition ZES patients who were well-controlled on oral omeprazole or lansoprazole without breakthrough acid hypersecretion.29 Pantoprazole 80 mg IV every 12 hours as a 15 minute infusion was adequate in 93% of patients to reduce acid output to a goal of <10 mEq/h and within the first hour of infusion. Lau et al found that high dose IV omeprazole (80 mg bolus followed by infusion of 8 mg/hour for 72 hours) significantly reduced rebleeding following endoscopic treatment of bleeding peptic ulcers.30 IV PPIs can be used in clinical scenarios when oral formulations are unpredictable or delivery through nasogastric tube is not available.
Oral Proton Pump Inhibitors
Over 70% of ZES patients (even after curative surgical resection), and those with idiopathic gastric acid hypersecretory conditions, will require life-long acid suppressive therapy (Tables 1, 2). Numerous studies have demonstrated the efficacy of PPIs in hypersecretory conditions; consequently, these are the agents of choice to control hyperchlorhydria.
Table 1.
FDA recommendations and Pharmacokinetics of PPIs
| PPI | Brand Name | FDA-Approved Doses in Hypergastrinemic Hypersecretory States | IV Doses in Hypergastrinemic States | Bioavailability (%) | Time to peak Conc (hours) | Half-life (hours) |
|---|---|---|---|---|---|---|
| Omeprazole | Prilosec | 60 mg PO daily (starting dose) * Up to 120 mg PO TID has been administered |
30-40 | 0.5–3.5 | 0.5–3.0 | |
| Lansoprazole | Prevacid | 60 mg PO daily * Up to 180 mg PO daily has been administered |
80 | 1.5–3.0 | 0.5–2.5 | |
| Esomeprazole | Nexium | 40 mg PO BID * Up to 240 mg per day has been administered |
80 mg IV BID | 64-90 | 1.5–2.0 | 1.0–2.5 |
| Pantoprazole | Protonix | 40 mg PO BID or 80 mg IV BID for 7-10d * Up to 240 mg per day has been administered |
40 mg IV BID | 77 | 2.0–2.5 | 1.0 |
| Rabeprazole | Aciphex | 60 mg PO daily * Up to 100 mg daily or 60 mg PO BID have been administered |
52 | 2.0–5.0 | 1.0–2.0 |
Conc = concentration.
Table 2.
Recommended Dosing Schedules of PPIs - Pharmacokinetic Properties
| PPI | Dosing With Meals | Food Effect |
|---|---|---|
| Omeprazole (20mg) | Before eating | Not available |
| Lansoprazole (30,60mg) | With or without food | 12-55% increase in Cmax and 9-37% increase in AUC |
| Esomeprazole (40mg) | At least 1 hour before eating | 33-50% decrease in AUC after food intake compared to fasting conditions |
| Pantoprazole (40mg) | With or without food | Absorption may be delayed up to 2 hours or longer with food Cmax and AUC not altered |
| Rabeprazole (20mg) | With or without food | Absorption may be delayed up to 4 hours or longer when administered with a high fat meal. Tmax is variable, Cmax and AUC not altered |
Cmax = Maximum plasma concentration; Tmax = Time to reach maximum concentration
Omeprazole delayed-release capsules were studied in 136 patients with ZES with and without concomitant familial endocrinopathies. Doses ranging from 20 mg every other day to 360 mg daily suppressed BAO to <10 mEq/h in patients without prior gastric surgery and <5 mEq/h in those post-resection. In a prospective nine year trial by Metz et al, maintenance therapy with omeprazole 60 mg once- or twice-daily effectively controlled gastric acid hypersecretion while being well tolerated and without the apparent development of tachyphylaxis.31 The same author in a separate study argued for dose reduction down to 20 mg once- or twice-daily as sufficient for maintaining the goal acid output.30 Therefore, long term dose reduction should be attempted with close monitoring, especially in patients with non-resectable gastrinomas, for breakthrough symptoms.
A prospective study by Hirschowitz and colleagues followed 49 patients with and without ZES to study the long-term effects of oral lansoprazole. Starting at 60 mg/day, doses were titrated individually to the target BAO <5 mmol/h in healthy individuals and < 1 mmol/h (as opposed to the more customary 10 mmol/h), in post-antrectomy patients.33 On lansoprazole, 70% of patients were stable without relapse of symptoms through a nine year period; among those who became symptomatic, 90% were not associated with endoscopic evidence of hypersecretion. Post-antrectomy ZES patients had a 3.6-fold higher rate of relapse than non-operated patients suggesting that acid suppression in these patients is innately difficult to control.33 Lansoprazole was well tolerated for prolonged periods, although some studies show rebound hypergastrinemia at higher levels than prior to PPI initiation.31
The first study to examine the efficacy of esomeprazole in hypersecretory states was performed by Metz et al in both ZES and idiopathic hypergastrinemic patients. Adequate control of acid secretion was achieved on esomeprazole 40 mg twice daily to 80 mg three times daily.34 Depressed acid output was maintained in >90% of patients, with lack of erosive mucosa seen on yearly endoscopy. Esomeprazole was well tolerated in all 21 patients in the study, although there was evidence of significant hypomagnesemia in one patient.
Initial studies with oral pantoprazole showed that a dose of 40 mg per day was adequate in maintaining acid secretion <10 mmol/hour in normal individuals stimulated by pentagastrin. Extrapolation to hypergastrinemia states including ZES and idiopathic hypersecretion found that oral pantoprazole 40-80 mg every 12 hours suppressed acid output and was well tolerated over a study period of three years (Tables 1, 2). MEN-1 patients and those who had had gastric acid reduction surgery were included in the study, and required higher doses of PPI therapy to control gastric acid hypersecretion. In addition, dosage requirements remained stable following one year of therapy.35 This differs from reports on omeprazole and lansoprazole in which periodic measurement of gastric acid output was performed to adjust dosing.
Rabeprazole was studied in twelve individuals with idiopathic hypersecretion or ZES. At a dose of 20-120 mg per day, rabeprazole adequately inhibited gastric acid secretion with resolution of symptoms in patients with acid-peptic disease.36
Surgical Approaches
The ideal treatment of ZES is resection of the gastrinoma, although more than one half of patients present with non-curable disease. Roughly 20-30% of patients present with metastatic disease to lymph nodes or the liver, while another 20-25% have associated MEN-1 for which aggressive surgery remains controversial The current thinking is that all ZES patients without MEN-1 or metastatic disease should undergo surgical exploration for possible cure.37
The current standard for surgical approach for sporadic ZES is the Billroth II gastrectomy. Billroth II reconstruction involves anastomosis of the gastric remnant to the jejunum leaving loss of duodenal continuity. Careful palpation of the pancreas and duodenum is performed to survey for nodules. Typical adverse reactions include alkaline reflux gastritis, dumping syndrome, and the retained antrum syndrome.
Vagotomy transects direct cholinergic stimulation of gastric acid secretion, therefore decreasing the stimulus for antral gastrin. In one study, parietal cell vagotomy resulted in 75% decrease of BAO; however, only 9% of patients were able to remain off antisecretory agents.38 There remains controversy regarding truncal versus highly selective vagotomy. The anterior and posterior vagal nerves are isolated in the truncal procedure, and ligated at the proximal and distal end with removal of the interval nerves. Complications of this technique include marked alteration of gastric motility causing delayed emptying. Highly selective vagotomy blocks the vagal stimulation to the corpus alone, sparing the antrum and pylorus therefore preserving antral peristalsis.39
There is relative lack of information regarding management of pregnant patients with ZES. In a small study by Steward and colleagues, five pregnant women underwent either surgical or medical management of acid secretion. If patients were diagnosed with non-metastatic ZES prior to contraception, curative resection with parietal cell vagotomy obviated the need for acid suppressive therapy. However if resection was not a viable option or if metastatic disease was present then ranitidine or, for uncontrolled acid secretion, omeprazole was adequate.39
Opinion Statement.
Hypersecretory conditions affecting the stomach account for significant morbidity and mortality and manifest in some cases with peptic ulcer, gastrointestinal hemorrhage and/or gastroesophageal reflux disease (GERD). The diagnosis of gastric acid hypersecretory states can be challenging and relies on the use of quantitative assays to measure gastric acid secretion and serum gastrin. The most common etiology for hypergastrinemia is the use of potent gastric acid inhibitors such as the proton pump inhibitors. The differential diagnosis of this condition is of critical importance, which will dictate management decisions. Conditions such as atrophic gastritis are relatively benign and can lead to hypergastrinemia without the presence of gastric acid hypersecretion. Zollinger Ellison syndrome, on the other hand, causes hypergastrinemia with profound gastric acid hypersecretion. More common causes of hypergastrinemia include gastric outlet obstruction, ileus and chronic renal failure. In most cases, proton pump inhibitors will be used to manage the condition. In some instances, surgical therapy may be required. This chapter will review the important clinical causes of gastric acid hypersecretion and provide insights to the best medical management options to better care for patients with these disorders.
Acknowledgements
This work received grant support from: Department of Veterans Affairs RR&D Merit Review (JRP); Human Studies CORE through CURE: Digestive Diseases Research Center supported by NIH grant P30DK41301; NIH NIDDK T32 NIH# is 5T32DK07180 (JNB).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Jennifer Phan declares that she has no conflict of interest.
Jihane N. Benhammou has a patent, Measurement of VIP, pending.
Joseph R. Pisegna has received speaker fees from Takeda and has a patent, VIP Measurement for Hypergastrinemia, pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Metz DC, Ferron GM, Paul J, Turner MB, Soffer E, Pisegna JR, Bochenek WJ. Proton Pump Activation in Stimulated Parietal Cells Is Regulated by Gastric Acid Secretory Capacity: A Human Study. Journal of Clinical Pharma. 2002;42:512–519. doi: 10.1177/00912700222011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benhammou J, Jacob N, Vu J, Ohning GV, Germano PM, Waschek J, Pisegna JR. Gastroenterology. 2015;148(4)(Suppl 1):S–734. [Google Scholar]; Schubert M. Gastric Secretion. Current Opinion in Gastroenterology. 2010;26(6):598–603. doi: 10.1097/MOG.0b013e32833f2010. [DOI] [PubMed] [Google Scholar]
- 3.Metz DC, Comer GM, Soffer E, Forsmark CE, Cryer B, Chey W, Pisegna JR. Three-year Oral Pantoprazole Administration is Effective for Patients with Zollinger-Ellison Syndrome and Other Hypersecretory Conditions. Alimentary Pharmacology and Therapeutics. 2006;23(3):437–444. doi: 10.1111/j.1365-2036.2006.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Jensen RT. Control of gastric acid hypersecretion in the management of patients with Zollinger-Ellison syndrome. World J Surg. 1993 Jul-Aug;17(4):468–80. doi: 10.1007/BF01655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strader DB, Benjamin SB, Orbuch M, Lubensky TA, Gibril F, Weber C, Fishbeyn VA, Jensen RT, Metz DC. Esophageal Function and Occurrence of Barrett's Esophagus in Zollinger-Ellison Syndrome. Digestion. 1995;56(5):347–56. doi: 10.1159/000201258. [DOI] [PubMed] [Google Scholar]
- 6.Pisegna J, Holtmann G, Howden CW, Katelaris PH, Sharma P, Spechler S, Triadafilopoulos G, Ytygat G. Esophageal complications and consequences of persistent gastro-esophageal reflux disease. Aliment Pharmacology and Therapy. 2004;20(9):47–56. doi: 10.1111/j.1365-2036.2004.02240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisegna JR. The effect of Zollinger-Ellison syndrome and neuropeptide-secreting tumors on the stomach. Curr Gastroenterol Rep. 1999 Dec;1(6):511–7. doi: 10.1007/s11894-999-0012-8. [DOI] [PubMed] [Google Scholar]
- 8.Kohan E, Oh D, Wang H, Hazany S, Ohning G, Pisegna JR. Duodenal Bulb Mucosa with Hypertrophic Gastric Oxyntic Heterotopia in Patients with Zollinger Ellison Syndrome. Diagnostic and Therapeutic Endoscopy. 2009 doi: 10.1155/2009/298381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster MW, Barnes EL, Stremple JF. Serum Gastrin Levels in the Differential Diagnosis of Recurrent Peptic Ulceration due to Retained Gastric Antrum. The American Journal of Surgery. 1978;135(2):248–252. doi: 10.1016/0002-9610(78)90110-1. [DOI] [PubMed] [Google Scholar]
- 10.Fisher JC, Masiakos PT, Oviedo J, Burch M, Kondi ES, Wolfe MM, Becker JM. Gastric Outlet Obstruction as a Consequence of a Duodenal Web Masquerading as Gastrinoma in an Adult. Current Surgery. 2003;60(6):602–606. doi: 10.1016/S0149-7944(03)00080-1. [DOI] [PubMed] [Google Scholar]
- 11.Chuang CH, Sheu BS, Yang HB, Kao AW, Cheng HC, Yao WJ. Hypergastrinemia after Helicobacter pylori infection is Associated with Bacterial Load and Related Inflammation of the Oxyntic Corpus Mucosa. Journal of Gastroenterology and Hepatology. 2004;19(9):988–993. doi: 10.1111/j.1440-1746.2004.03416.x. [DOI] [PubMed] [Google Scholar]
- 12.McGowan CC, Cover TL, Blaser MJ. Helicobacter pylori and Gastric Acid: Biological and Therapeutic Implications. Gastroenterology. 1996;110:926–938. doi: 10.1053/gast.1996.v110.pm8608904. [DOI] [PubMed] [Google Scholar]; Kes P. Serum gastrin concentration in chronic renal failure. Acta Med Croatica. 1992;46(1):47–58. [PubMed] [Google Scholar]
- 13.Quintero E, Ohning GV, Del Rivero M, Wong HC, Walsh JH, Guth PH. Gastrin mediates the increase in gastric cell growth in uremic rats. Am J Physiol. 1995;268(4):586–91. doi: 10.1152/ajpgi.1995.268.4.G586. [DOI] [PubMed] [Google Scholar]
- 14.Quintero E, 1, Kaunitz J, Nishizaki Y, De Giorgio R, Sternini C, Guth PH. Uremia increases gastric mucosal permeability and acid back-diffusion injury in the rat. Gastroenterology. 1992 Dec;103(6):1762–8. doi: 10.1016/0016-5085(92)91432-4. [DOI] [PubMed] [Google Scholar]
- 15.Fallone CA, Mayrand S. Gastroesophageal reflux and hyperacidity in chronic renal failure. Perit Dial Int. 2001;21(Suppl 3):S295–9. [PubMed] [Google Scholar]
- 16.Collen MJ, 1, Jensen RT. Idiopathic gastric acid hypersecretion. Comparison with Zollinger-Ellison syndrome. Dig Dis Sci. 1994 Jul;39(7):1434–40. doi: 10.1007/BF02088045. [DOI] [PubMed] [Google Scholar]
- 17.Blonski WC, 1, Katzka DA, Lichtenstein GR, Metz DC. Idiopathic gastric acid hypersecretion presenting as a diarrheal disorder and mimicking both Zollinger-Ellison syndrome and Crohn's disease. Eur J Gastroenterol Hepatol. 2005 Apr;17(4):441–4. doi: 10.1097/00042737-200504000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Jin G, Westphalen CB, Hayakawa Y, Worthley DL, Asfaha S, Yang X, Chen X, Si Y, Wang H, Tailor Y, Friedman RA, Wang TC. Progastrin stimulates colonic cell proliferation via CCK2R-and β-arrestin-dependent suppression of BMP2. Gastroenterology. 2013 Oct;145(4):820–30. doi: 10.1053/j.gastro.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reggoug S, Ropert A, Blayau M, Zeddini A, Dugast C, Péquin P, Meunier B, Bretagne JF. Idiopathic gastric acid hypersecretion in a patient with MUTYH-associated polyposis. Am J Gastroenterol. 2009 Oct;104(10):2648–9. doi: 10.1038/ajg.2009.344. [DOI] [PubMed] [Google Scholar]
- 20.Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum Gastrin in Zollinger-Ellison Syndrome: I. Prospective Study of Fasting Serum Gastrin in 309 Patients from the National Institutes of Health and Comparison with 2229 Cases from the Literature. Medicine (Baltimore) 2006;85(6):295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shibata C, Funayama Y, Fukushima K, Ueno T, Kohyama A, Satoh K, Shimosegawa T, Yamagiwa T, Sasaki I. The glucagon provocative test for the diagnosis and treatment of Zollinger-Ellison syndrome. J Gastrointest Surg. 2008 Feb;12(2):344–9. doi: 10.1007/s11605-007-0372-z. [DOI] [PubMed] [Google Scholar]
- 22.Poitras P, Gingras MH, Rehfeld JF. The Zollinger-Ellison Syndrome: Dangers and Consequences of Interrupting Antisecretory Treatment. Clinical Gastroenteroogy and Hepatology. 2012;10(2):199–202. doi: 10.1016/j.cgh.2011.08.012. [This article provides recommendations for the management of gastric acid hypersecretion in patients with ZES.] [DOI] [PubMed] [Google Scholar]
- 23.Ghosh T, Lewis DI, Axon ATR, Everett SM. Review Article: Methods of Measuring Gastric Acid Secretion. Alimentary Pharmacology and Therapeutics. 2011;33(7):768–781. doi: 10.1111/j.1365-2036.2010.04573.x. [DOI] [PubMed] [Google Scholar]
- 24.Oh DS, Wang HS, Ohning GV, Pisegna JR. Validation of a New Endoscopic Technique to Assess Acid Output in Zollinger-Ellison Syndrome. Clinical Gastroenterology and Hepatology. 2006;4(1):1467–73. doi: 10.1016/j.cgh.2006.08.015. [This article provides a useful approach to the measurement of gastric acid secretion using standard endoscopic approaches.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JH, Choi MG, Yim DS, Cho YK, Park JM, Lee IS, Kim SW, Chung IS. A Novel Placement Method of the Bravo Wireless pH Monitoring Capsule for Measuring Intragastric pH. Digestive Diseases and Sciences. 2009;54(3):578–85. doi: 10.1007/s10620-008-0399-3. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein DH, deRijke S, Chow CC, Foruraghi L, Zhao X, Wright EC, Whatley M, Maass-Moreno R, Chen CC, Wank SA. A New Method for Determining Gastric Acid Output using a Wireless pH-sensing Capsule. Alimentary Pharmacology and Therapeutics. 2013;37(12):1198–1209. doi: 10.1111/apt.12325. [This article provides an innovative approach to the measurement of gastric acid secretion in patients using the wireless pH sensing capsule.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metz DC, Forsmark C, Lew EA, Starr JA, Soffer EF, Bochenek W, Pisegna JR. Replacement of Oral Proton Pump Inhibitors With Intravenous Pantoprazole to Effectively Control Gastric Acid Hypersecretion in Patients with Zollinger-Ellison Syndrome. The American Journal of Gastroenterology. 2001;96(12):3274–3280. doi: 10.1111/j.1572-0241.2001.05325.x. [DOI] [PubMed] [Google Scholar]
- 28.Lau JYW, Sung JJ, Lee KK. Effect of Intravenous Omeprazole on Recurrent Bleeding after Endoscopic Treatment of Bleeding Peptic Ulcers. New England Journal of Medicine. 2000;343:310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 29.Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of Omeprazole in Zollinger-Ellison Syndrome: a Prospective Nine-Year Study of Efficacy and Safety. Alimentary Pharmacology and Therapeutics. 1993;7(6):597–610. doi: 10.1111/j.1365-2036.1993.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 30.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, Feigenbaum KM, Koviack PD, Jensen RT. Currently Used Doses of Omeprazole in Zollinger-Ellison Syndrome Are Too High. Gastroenterology. 1992;103(5):1498–508. doi: 10.1016/0016-5085(92)91170-9. [DOI] [PubMed] [Google Scholar]
- 31.Hirschowitz BI, Simmons J, Mohnen J. Clinical Outcome Using Lansoprazole in Acid Hypersecretors With and Without Zollinger-Ellison Syndrome: a 13-year Prospective Study. Clinical Gastroenterology and Hepatology. 2005;3(1):39–48. doi: 10.1016/s1542-3565(04)00606-8. [DOI] [PubMed] [Google Scholar]
- 32.Metz DC, Sostek MB, Ruszniewski P, Forsmark CE, Monyak J, Pisegna JR. Effects of Esomeprazole on Acid Ouptut in Patients with Zollinger-Ellison Syndrome or Idiopathic Gastric Acid Hypersecretion. American Journal of Gastroenterology. 2007:2648–2654. doi: 10.1111/j.1572-0241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 33.Metz DC, Soffer E, Forsmark CE, Cryer B, Chey W, Bochenek W, Pisegna JR. Maintenance Oral Pantoprazole Therapy Is Effective for Patients with Zollinger-Ellison Syndrome and Idiopathic Hypersecretion. The American Journal of Gastroenterology. 2003;98(2) doi: 10.1111/j.1572-0241.2003.07262.x. [DOI] [PubMed] [Google Scholar]
- 34.Carswell CI, Goa KL. Rabeprazole: An Update of Its Use in Acid-related Disorders. Drugs. 2001;61(15):2327–56. doi: 10.2165/00003495-200161150-00016. [DOI] [PubMed] [Google Scholar]
- 35.Norton JA. Intra-operative Procedures to Localize Endocrine Tumours of the Pancreas and Duodenum. Italian Journal of Gastroenterology and Hepatology. 1999;31(2):195–97. [PubMed] [Google Scholar]
- 36.McArthur KE, Richardson CT, Barnett CC, Eshaghi N, Smerud MJ, McClelland RN, Feldman M. Laparotomy and Proximal Gastric Vagotomy in Zollinger-Ellison Syndrome: Results of a 16-year Prospective Study. American Journal of Gastroenterology. 1996;91(6):1104–11. [PubMed] [Google Scholar]
- 37.Stewart CA, Termanini B, Sutliff VE, Corleto VD, Weber HC, Gibril F, Jensen RT. Management of the Zollinger-Ellison Syndrome in Pregnancy. American Journal of Obstetrics and Gynecology. 1997;176(1):224–33. doi: 10.1016/s0002-9378(97)80041-5. [DOI] [PubMed] [Google Scholar]