Abstract
Background
Preventing or postponing tobacco use initiation could greatly reduce the number of tobacco-related deaths. While evidence suggests that exercise is a promising treatment for tobacco addiction, it is not clear whether exercise could prevent initial vulnerability to tobacco use. Thus, using an animal model, we examined whether exercise attenuates vulnerability to the use and reinforcing effects of nicotine, the primary addictive chemical in tobacco.
Methods
Initial vulnerability was assessed using an acquisition procedure wherein exercising (unlocked running wheel, n = 10) and sedentary (locked or no wheel, n = 12) male adolescent rats had access to nicotine infusions (0.01-mg/kg) during daily 21.5-hr sessions beginning on postnatal day 30. Exercise/sedentary sessions (2-hr/day) were conducted prior to each of the acquisition sessions. The effects of exercise on nicotine’s reinforcing effects were further assessed in separate groups of exercising (unlocked wheel, n = 7) and sedentary (no wheel, n = 5) rats responding for nicotine under a progressive-ratio schedule with exercise/sedentary sessions (2-hr/day) conducted before the daily progressive-ratio sessions.
Results
While high rates of acquisition of nicotine self-administration were observed among both groups of sedentary controls, acquisition was robustly attenuated in the exercise group with only 20% of exercising rats meeting the acquisition criterion within the 16-day testing period as compared to 67% of the sedentary controls. Exercise also decreased progressive-ratio responding for nicotine as compared to baseline and to sedentary controls.
Conclusions
Exercise may effectively prevent the initiation of nicotine use in adolescents by reducing the reinforcing effects of nicotine.
Keywords: Adolescent, Nicotine, Self-administration, Exercise, Acquisition, Motivation
1. INTRODUCTION
Early smoking initiation is associated with heavy smoking (Taioli and Wynder, 1991), nicotine dependence (Breslau et al., 1993; Van De Ven et al., 2010), and difficulty quitting later in life (Breslau and Peterson, 1996; Khuder et al., 1999), suggesting that the earlier one begins to smoke, the more likely they will die from smoking-related diseases (Hegmann et al., 1993; McCarron et al., 2001). Although rates of smoking initiation among adolescents have decreased over the years, rates of use remain high among adolescents. The Substance Abuse and Mental Health Services Administration (2014) estimated that in the past year, 3 million adolescents (ages 12–17) smoked cigarettes and each day 3,701 adolescents smoked for the first time. In addition, the popularity of electronic cigarettes and hookah has rapidly increased among adolescents resulting in no net change in tobacco and nicotine use among adolescents since 2011 (Arrazola et al., 2015). These statistics indicate that further investigation into potential treatments that prevent the initiation of tobacco and nicotine use in adolescents is needed. In this regard, animal models are useful to identify treatments that prevent the initiation of nicotine self-administration, a model with good predictive validity for tobacco use (O’Dell and Khroyan, 2009).
Clinical and preclinical data suggest that physical activity may protect against the initiation of drug use. Epidemiological studies have consistently demonstrated that adolescents involved in some sort of physical activity are less likely to be current smokers as compared to their less active peers (for a review see Lynch et al., 2013). A major confound in this literature is that most physically active teens participate in organized sports. Since some reports have found that participation in specific sports may increase risk for use of smokeless tobacco, alcohol, or other drugs (Aaron et al., 1995; Castrucci et al., 2004; Kirkcaldy et al., 2002; Mattila et al., 2012; Moore and Werch, 2005; Pate et al., 2000; Rainey et al., 1996; Terry-McElrath and O’Malley, 2011; for review see Lisha and Sussman, 2010), it is likely that psychosocial influences associated with being an athlete also affect drug use. However, in prospective studies in adult smokers, acute bouts of exercise have been shown to decrease desire to smoke and cigarette craving (for review see Haasova et al., 2013; Roberts et al., 2012; Taylor et al., 2007) indicating that exercise itself may decrease nicotine use. Further work is necessary to determine if exercise, without confounding social factors, can prevent nicotine use initiation.
Factors that may delay or prevent the initiation of regular drug use in humans can be difficult to determine in prospective studies since it is unethical to expose drug-naïve individuals, particularly adolescents, to drugs. The use of animal models circumvents this issue and, like in humans, many variables have been shown to influence the risk of drug acquisition (Campbell and Carroll, 2000). Acquisition is usually modeled in drug and experimentally naïve animals allowed to respond for drug in operant sessions. In rats, wheel running, a model of aerobic exercise, has been shown to be effective in reducing the acquisition of cocaine and methamphetamine self-administration (Engelmann et al., 2013; Smith and Pitts, 2011), suggesting that there is a biological basis for the effect of exercise in preventing drug use. Furthermore, wheel running has been found to reduce motivation to self-administer cocaine under a progressive ratio (PR) schedule indicating that exercise may prevent drug use initiation by reducing the reinforcing effects of these drug (Smith et al., 2008). However, the efficacy of wheel running exercise in decreasing the acquisition of nicotine self-administration and its reinforcing effects are not yet known. Thus, one goal of this study was to test the hypothesis that voluntary wheel running exercise would prevent the initiation of nicotine self-administration in adolescent rats. A second goal of this study was to determine if exercise decreases the reinforcing efficacy of nicotine, as has been observed with other drugs of abuse. To address these goals, drug naïve adolescent rats were permitted daily bouts of wheel running exercise followed by nicotine self-administration sessions under acquisition testing conditions or PR testing conditions in rats that had previously acquired nicotine self-administration. 2.
2. METHODS
2.1 Animals
Male Sprague-Dawley (Charles River Laboratories, Portage, ME, USA) rats (N = 34; experiment 1, n = 22; experiment 2, n = 12) were shipped on day of weaning (postnatal day 21) and arrived at the laboratory on postnatal day 22. Upon arrival, animals were individually housed in self-administration chambers, and were maintained on a 12-hour light/dark (house lights on at 0700) cycle with ad libitum access to water and food except during exercise sessions during which only water was available. Animals were habituated in chambers for 3–4 days prior to any training and 6 days prior to surgery. All procedures were approved by The Animal Care and Use Committee at the University of Virginia and were in accordance with the guidelines set by the National Institutes of Health.
2.2 Apparatus
Individual 31 cm × 24 cm × 21 cm self-administration chambers (ENV-008CT, Med Associates, St. Albans, VT, USA) were equipped with a house light (4.76 W), water bottle holder, food-hopper, retractable active (drug-associated) lever, a cue light (4.74 W) above the active lever, and a stationary inactive lever. Each chamber was centered within a ventilated sound-attenuating box (ENV-018M, Med Associates, St. Albans, VT, USA) along with a pump (PHM-100, Med Associates, St. Albans, VT, USA). A 10-ml drug syringe was mounted on the pump and connected to Tygon tubing that attached to swivel (Instech Laboratories Inc., Plymouth Meeting, PA, USA) embedded in a counterbalanced metal arm above the chamber. A polyethylene tube encased in a metal spring (C313CS; PlasticsOne, Roanoke, VA, USA) was attached to the swivel and to a 22-gauge guide (C313G; PlasticsOne, Roanoke, VA, USA) embedded within an infusion harness (CIH95AB; Instech Laboratories Inc., Plymouth Meeting, PA, USA) the animal wore following surgery and thereafter until study completion. The house light was illuminated from 0700 to 1900 daily to maintain a 12-hour light dark cycle. Running wheels (ENV-046; Med Associates, St. Albans, VT, USA) with polycarbonate cage attachment were equipped with a revolutions counter.
2.3 Surgery
On postnatal day 28, rats underwent surgery to implant a chronic indwelling silastic catheter (0.51 and 0.94 mm o.d.; Dow Corning Corporation) into the right jugular vein as described previously (Lynch, 2008). Rats were given ketoprofen (2–5 mg/kg, subcutaneous) and gentamicin (5.5 mg/kg, i.v.) on the day of surgery and for 2 subsequent days. Rats were given 2 days to recover from surgery prior to nicotine self-administration testing. Catheter patency was assessed by flushing a small amount of heparinized saline into the catheter and pulling back to check for the presence of blood. This check was conducted for 2 days following surgery and thereafter every Monday, Wednesday, and Friday prior to self-administration sessions.
2.4 Drug
Nicotine bitartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% sterile saline (pH 7.4) and passed through a microfilter with dose expressed as the free base weight. A single moderate dose (10 μg/kg/infusion) of nicotine was selected based on previous work demonstrating this dose leads to rapid and high rates of acquisition of nicotine self-administration in adolescent males (Lynch, 2009). Infusions were delivered at a rate of 0.1 ml/sec and infusion volume was adjusted for animal’s weight. Nicotine solution was stored in the dark at 4°C but was available at room temperature during self-administration.
2.5 Experiment 1: Acquisition study
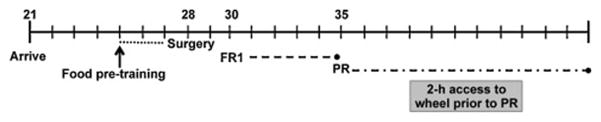
Rats were randomly assigned to either an exercise (n = 10) or control condition (n = 12). Controls included a group that did not have access to a wheel (no wheel; n = 6) and as a control for environmental enrichment, and a group that had access to a running wheel that was stationary (locked wheel; n = 6). Prior to surgery, on postnatal days 23–24, rats were acclimated to their assigned wheel condition for 2 hours each day (0930–1130). Beginning on postnatal day 30, rats were moved from their self-administration chambers to their assigned wheel condition. During the 2-hour access session, both wheel groups could move freely between the wheel and the polycarbonate cage. Rats were then moved back to their self-administration chambers at 1130. Daily nicotine self-administration sessions began at noon with the presentation of the active lever into the chamber. Responses on the active lever during each session were reinforced under a fixed ratio (FR) 1 schedule and each infusion was paired with the illumination of a cue light above the active lever. Sessions were terminated when the rat obtained all 20 infusions that were available or after 21.5 hours. Acquisition was defined as 2 consecutive days of 20 infusions with a 2:1 preference of the active lever over the inactive lever. Figure 1 outlines the experimental timeline.
Figure 1.
Experimental timeline used to examine the effect of exercise on the acquisition of nicotine self-administration in adolescent rats. Rats were given 2-hour access to their exercise or sedentary condition each day prior to nicotine self-administration beginning on postnatal day 30.
2.6 Experiment 2: Progressive ratio study
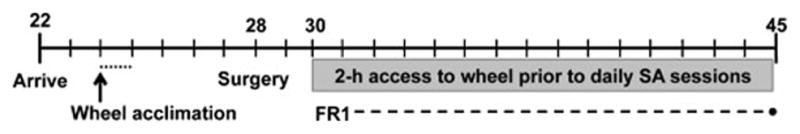
In this experiment, rapid acquisition of nicotine self-administration was desired and therefore a separate cohort of male adolescent rats (n = 12) were food pre-trained to lever press for sucrose pellets under an FR1 schedule until 2 consecutive days of 50 pellet deliveries as described previously (Sanchez et al., 2013). Rats then underwent surgery on postnatal day 28 as described above. Beginning on postnatal day 30, rats were trained to self-administer nicotine under an FR1 schedule with a maximum of 20 deliveries each day for 5 days. Acquisition of nicotine self-administration was defined as 2 consecutive days of receiving all 20 infusions with at least a 2:1 preference for the active lever over the inactive lever. All rats reached acquisition criteria within 5 days, at which point they were randomly assigned to exercise (n = 7) or sedentary (n = 5) conditions. In this experiment, the sedentary controls were not exposed to a running wheel since our previous experiment did not reveal a significant effect of exposure to a locked running wheel (see Fig. 3). Following acquisition, rats began a PR schedule of reinforcement for nicotine infusions. Under this schedule the lever response requirement increases (i.e., 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, etc.) for each subsequent infusion. The final ratio completed, or the breakpoint, is thought to be a sensitive measure of reinforcing efficacy or motivation for the drug (Arnold and Roberts, 1997). Breakpoints were recorded after each session, and were typically reached approximately 2 hours after the start of the session. A stable baseline of PR responding was defined as no increasing or decreasing trends in breakpoints for 3 consecutive sessions. All rats reached stable responding on PR schedule quickly, within 3–4 days, and rates did not differ between groups. Once a stable baseline was reached, rats were given 2-hour access to their exercise condition before PR sessions for 5 consecutive sessions. An additional 3 PR sessions without access to exercise were performed to determine the persistence of the effects of exercise. During this experiment, 1 rat from the exercise condition lost catheter patency and was removed from all statistical analyses of data (final n = 6). Figure 2 outlines the experiment timeline.
Figure 3.
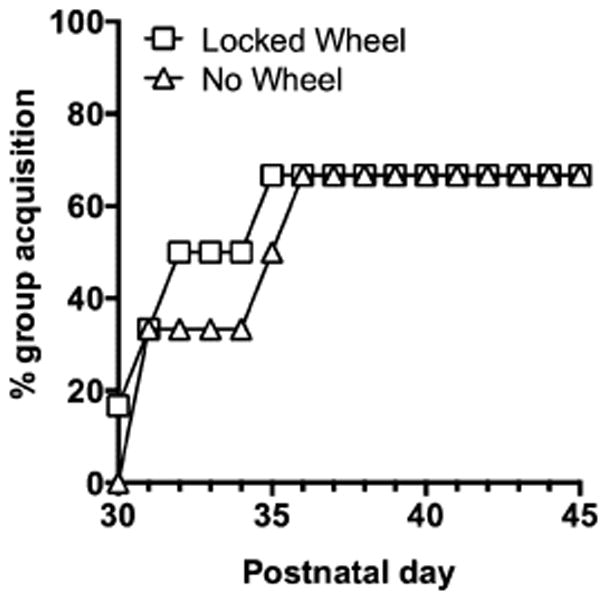
Acquisition of nicotine self-administration in male adolescent rats with exposure to a locked wheel (squares) or no wheel (triangles) conditions for 2 hours prior to each self-administration session.
Figure 2.
Timeline for Experiment 2 in which the effect of exercise on nicotine self-administration under a progressive ratio schedule in adolescent rats was examined. Following self-administration acquisition and stable baseline PR responding, adolescent rats were given 2-hour access to their exercise or sedentary condition each day prior to the PR session for 5 days. The persistent effect of exercise was determined in 3 post-exercise sessions, in which the rats did not have access to sedentary or exercise conditions prior to PR sessions.
2.7 Data analysis
Rate of acquisition and percentage of rats acquiring were compared between wheel condition groups using a Kaplan-Meier survival analysis and the Log-rank (Mantel-Cox) statistic as previously described by (Lynch, 2009; see also, Aarde et al., 2015; Burke and Miczek, 2015; Cambell et al., 2002). In experiment 1, the two sedentary control groups (i.e. the no wheel and locked wheel groups) were combined into one sedentary group since no differences were observed between these groups in any measures examined in this study (i.e., body weights and rates of acquisition; also see Fig 3). Weights at the beginning (postnatal day 30) and at the end (postnatal day 44) were compared between groups using an ANOVA and are reported as the mean ± SEM. In experiment 2, the number of infusions during the 3 stable baseline, 5 treatment, and the 3 post-treatment PR sessions was compared between the exercise and control group using a repeated measures ANOVA with day as a within subject factor and treatment as a between subject factor. We also performed within group comparison of the number of infusions obtained at baseline (averaged across 3 baseline sessions) versus those obtained on each of the exercise/control and post exercise/control sessions using repeated measures ANOVA followed by paired-sample t-tests. A Pearson product-moment correlation was computed to determine the association between the average daily distance run in the wheel and the percent change from baseline PR breakpoint. Statistics were run using SPSS 20 with the alpha defined as 0.05.
3. RESULTS
3.1 Experiment 1: Exercise prevents acquisition of nicotine self-administration
Acquisition rates were similar between the locked and no wheel groups with 67% of rats in each group reaching acquisition criteria within 7 days (χ2 = 0.0825, p > 0.05; Fig 3). Of the rats that acquired, it took on average 3 ± 1 days for those in the locked wheel condition and 4 ± 1 days for those in the no wheel condition to reach the acquisition criteria. Weights were also similar between locked and no wheel groups at the beginning (96.0 ± 3.2 versus 93.6 ± 5.0 g, respectively) and at the end of this experiment (203 ± 6 versus 194 ± 9 g, respectively; Table 1). These control groups were subsequently combined and termed the sedentary control group for comparison with the wheel running exercise group in this experiment.
Table 1.
The average ± SEM weight of animals within exercise and sedentary groups are listed for postnatal day (PND) 30, 35, 39 and 44. The 2 wheel conditions for the sedentary group are also listed individually.
| PND 30 | PND 35 | PND 39 | PND 44 | |
|---|---|---|---|---|
| Exercise Group | 93.3 ± 3.9 | 128.0 ± 6.9 | 157.0 ± 8.3 | 193.3 ± 10.1 |
| Sedentary Group | 94.9 ± 3.4 | 130.0 ± 4.0 | 160.0 ± 4.5 | 199.0 ± 5.2 |
| Locked Wheel | 96.0 ± 3.2 | 132.7 ± 5.6 | 161.7 ± 6.1 | 203.0 ± 6.3 |
| No Wheel | 93.6 ± 5.0 | 126.8 ± 6.0 | 157.5 ± 7.4 | 194.2 ± 9.0 |
Fewer rats in the exercise group acquired nicotine self-administration as compared to the sedentary control group (Fig 4). Acquisition of nicotine self-administration was achieved by 8 of 12 (67%) rats in the sedentary group, but only 2 of 10 (20%) rats in the exercise group. A survival analysis revealed a significant effect of exercise group (χ2 = 5.195, p < 0.05) indicating a significant difference in percent group acquisition. Of the rats that acquired self-administration, rates of acquisition were similar between the sedentary and exercise groups. Specifically, in the exercise group, the 2 rats that acquired did so on day 2 and 3 and in the sedentary group, rats acquired in an average of 3.6 ± 0.8 days. Thus, exercise significantly attenuates percent group acquisition of nicotine self-administration in this adolescent-onset model. Importantly, no effect of exercise group was observed on inactive lever responding during acquisition (sedentary, 2.6 ± 1.1; exercise 1.1 ± 0.4). Rats within the exercise group ran on average 0.3 ± 0.1 km/2-hour exercise session. All rats gained weight over the course of the experiment with no differences observed between the exercise and sedentary groups on initial (93.3 ± 3.9 versus 94.9 ± 3.4 g, respectively) or final (193.3 ± 10.1 versus 199.0 ± 5.2 g, respectively) weights (Table 1).
Figure 4.
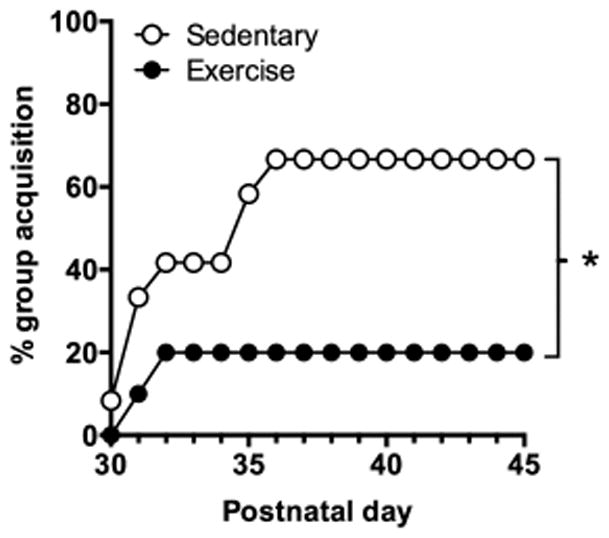
Percent group reaching nicotine self-administration acquisition criteria in male adolescent rats with exposure to sedentary (locked or no wheel condition; open circles) or exercise (unlocked wheel; black circles) groups for 2-hours prior to each 21.5-hour self-administration session. Asterisk indicates a significant difference between the exercise and control groups.
3.2 Experiment 2: Exercise attenuates the reinforcing effects of nicotine
In the rats trained to self-administer nicotine under a PR schedule, wheel-running exercise prior to nicotine self-administration sessions significantly attenuated breakpoints from baseline levels while exposure to a sedentary (no wheel) environment did not (Fig 5). Specifically, a repeated measures ANOVA revealed a significant exercise group effect (F (1,9) = 8.423, p < 0.05), a significant day effect (F (10,90) = 5.759, p < 0.001), and a significant day by exercise group interaction (F (10, 90) = 4.048, p <0.001). While no differences were observed between the two groups on any of the three baseline sessions, significant differences were observed on each of the 5 exercise days (exercise day 1, t9 = 4.005, p < 0.01; exercise day 2, t9 = 5.007, p < 0.01; exercise day 3, t9 = 6.332, p < 0.001; exercise day 4, t9 = 2.857, p < 0.05; exercise day 5, t9 = 3.434, p < 0.01), and on post-exercise day 1 (t9 = 2.363, p < 0.05). No differences were observed between the 2 groups on post-exercise days 2 and 3. However, further analysis within the exercise group comparing the average number of infusions obtained at baseline to those obtained on each of the exercise and post exercise sessions revealed significant differences for each of the exercise and post-exercise sessions (overall effect of day, F (8,40) = 9.05, p < 0.001; exercise day 1, t10 = 8.27, p < 0.001; exercise day 2, t10 = 5.30, p < 0.01; exercise day 3, t10 = 4.90, p < 0.01; exercise day 4, t10 = 5.39, p < 0.01; exercise day 5, t10 = 5.06, p < 0.01; post day 1, t10 = 4.43, p < 0.01, post day 2, t10 = 2.57, p = 0.05, post day 3, t10 = 3.56, p < 0.05) indicating a robust and persistent effect of exercise. In contrast, no significant differences were observed within the sedentary group with similar breakpoints observed at baseline and throughout the control and post-control components of the experiment. Inactive lever responding was modest and variable and did not differ by exercise group, day, or by phase of study. Rats within the exercise group ran on average 0.2 ± 0.05 km/2-hour exercise session. A Pearson product-moment correlation revealed a strong negative association between the average daily distance run in the wheel and the percent change from baseline PR breakpoint (Fig 6; r2 = 0.96; p < 0.01) indicating that the more an individual ran, the less motivated they were to self-administer nicotine. Taken together, these results indicate that exercise dose-dependently decreases the reinforcing effects of nicotine and that these effects persist beyond period of exposure.
Figure 5.
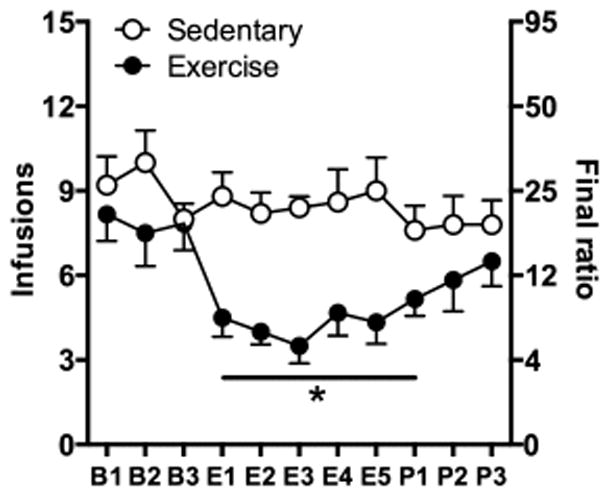
Number of infusions during daily PR nicotine self-administration sessions in male adolescent rats are shown at baseline (B1-B3), following 2-hours of wheel running exercise (E1-E5), and after removal of the exercise session (P1-P3) for sedentary (no wheel; open circles) and exercise (unlocked wheel; black circles) groups. Asterisk indicates a significant difference between the exercise and control groups (p < 0.05).
Figure 6.
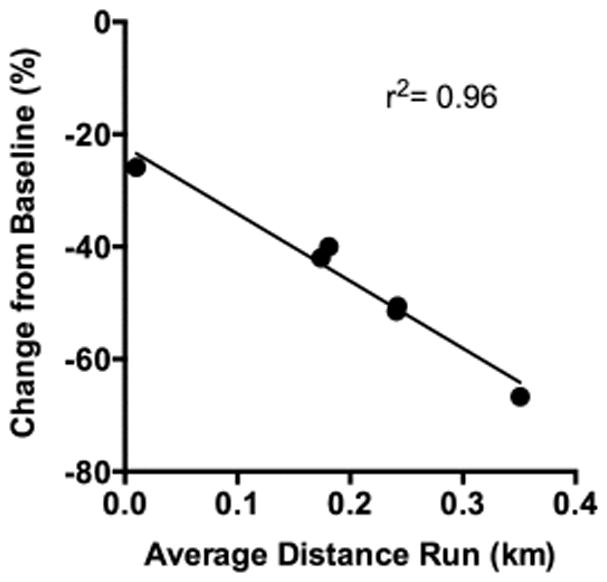
Scatter plot and Pearson product moment correlation of average daily distance run in wheel and percent change from baseline PR breakpoint during exercise sessions. A strong negative correlation between these two variables was observed (p < 0.01).
4. DISCUSSION
The primary goal of this study was to determine if voluntary wheel running exercise would attenuate vulnerability to nicotine self-administration in adolescent rats by assessing its effects on initiation of nicotine self-administration and motivation for nicotine following acquisition. In support of our hypothesis, significantly fewer animals in exercise group acquired nicotine self-administration as compared to those in the sedentary groups. These results appear to be attributable to exercise and not environmental enrichment since the locked wheel group, which was housed in a similar environment to the exercise group, did not differ from the controls that had no access to wheels. We also found that exercise attenuates the primary reinforcing effects of nicotine in adolescent rats with results showing that exercising significantly reduced breakpoints to obtain nicotine infusions under a PR schedule. Interestingly, more distance run was associated with a greater reduction in the reinforcing effects of nicotine under a PR schedule with distance run accounting for 96% of the variability in the efficacy of exercise at reducing nicotine self-administration. Taken together, these findings suggest that exercise in the running wheel attenuates the initiation of nicotine self-administration in adolescent rats by reducing the rewarding effects of nicotine.
This study is the first to demonstrate a preventative effect of wheel running exercise on nicotine self-administration in adolescents. Our findings are consistent with a previous report demonstrating that wheel-running exercise prevents acquisition of cocaine self-administration (Smith and Pitts, 2011). In this previous study, rats were permitted to run 24-hours/day during a prolonged exercise training phase that spanned adolescence and into adulthood and they were able to run in their home cages following self-administration sessions. Other reports have demonstrated that exercise can decrease cocaine self-administration when the exercise is concurrently available as an alternative reinforcer during self-administration sessions (Cosgrove et al., 2002; Zlebnik et al., 2012). The current report suggests that extensive training is not necessary for the beneficial effects of wheel-running exercise on acquisition of drug self-administration to emerge and that it need not be concurrently available. The present findings also suggest that even limited exercise (2-h sessions) during adolescence effectively deters acquisition of nicotine self-administration. This finding is consistent with previous work using limited exercise, in which 2-h sessions robustly decreased nicotine-seeking behavior in rats (Sanchez et al. 2013a, 2013b).
The acquisition data in the present study indicate that wheel running exercise may decrease the positive reinforcing effects of nicotine, a hypothesis that is supported by the second experiment in which 2-hours of daily exercise also decreased responding for nicotine under a more demanding PR schedule of reinforcement. Interestingly, this effect persisted after wheel running exposure, suggesting that exercise may have lasting beneficial effects. Previous work by others has also demonstrated that exercise reduces the positive reinforcing effects of cocaine (Smith et al., 2008, 2011). Taken together, it appears that exercise decreases the reinforcing effects of psychostimulant drugs leading to decreased drug use. The neurobiological mechanism underlying the effectiveness of exercise in preventing the initiation of nicotine self-administration is largely unknown, but may be due to the ability to act as an alternative reward and modulate dopamine signaling that is critical for the initial rewarding effects of nicotine (for review see Lynch et al., 2013).
In this study two controls for the effects of exercise were used, one that had access to a locked running wheel and one that did not have access to a running wheel. In the literature examining the effects of wheel running exercise on drug self-administration there have been inconsistencies on control conditions. Some studies utilized a locked wheel condition (Lynch et al., 2010; Sanchez et al. 2013a, 2013b; Zlebnik et al. 2010, 2012) and others used a polycarbonate cage condition without a wheel (Smith et al., 2012, 2011, 2008; Smith and Pitts, 2012, 2011; Smith and Witte, 2012). The locked wheel condition controls for the presence of the wheel and the environmental enrichment this might provide; however, under these conditions, it is possible that animals may perform “alternative” forms of exercise within the wheel (e.g., climbing and hanging). While the polycarbonate cage condition without a wheel eliminates the “alternative exercise” issue it does not account for potential environmental enrichment effects. Our work is novel in that we used both control groups and found no difference between them in adolescent males. Therefore, the effect observed in the unlocked wheel group was not due to environmental enrichment provided by the wheel. This work also suggests either control (no wheel or locked wheel) is appropriate for examining the effects of wheel running exercise in adolescent male Sprague-Dawley rats.
In human adolescents it is difficult to determine whether the suppressive effect of physical activity on smoking initiation is due to social influences or biological effects. The robust preventative effect of wheel running exercise on nicotine self-administration that we observed in adolescent male rats supports the notion that the biological effects of exercise may at least in part explain the negative association of physical activity and smoking in human adolescents. Further research is needed to determine if there are sex differences in this preventative effect of exercise.
Highlights.
Exercise prevents acquisition of nicotine self-administration in adolescent rats.
Acquisition rates are not affected by access to locked running wheel.
Exercise decreases motivation to self-administer nicotine in adolescent rats.
Exercise may prevent acquisition by reducing the reinforcing effects of nicotine.
Acknowledgments
Role of Funding Source
Financial support for this study was provided by grants from Virginia Foundation for Healthy Youth 8520667 and 8520893 (D.H.B.), NIDA RO1 DA039093 (W.J.L.), and NIH training grants T32 HD007323 (V.S.) and NIDA F31 DA033087 (V.S.). Funding sources had no role in study design; in the collection, analysis, or interpretation of data; in writing of the report; or in the decision to submit the publication.
Footnotes
Contributors
Wendy Lynch and Darlene Brunzell served as principal investigators. Victoria Sanchez wrote the manuscript. Victoria Sanchez, Wendy Lynch, and Matthew Lycas designed the project, and collected and analyzed the data. All author approved the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Dickerson TJ, Taffe MA. Binge-like acquisition of 3,4-methylenedioxypyrovalerone (MDPV) self-administration and wheel activity in rats. Psychopharmacology (Berl) 2015;232:1867–1877. doi: 10.1007/s00213-014-3819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaron DJ, Dearwater SR, Anderson R, Olsen T, Kriska AM, Laporte RE. Physical activity and the initiation of high-risk health behaviors in adolescents. Med Sci Sports Exerc. 1995;27:1639–1645. [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS. Tobacco use among middle and high school students - United States, 2011–2014. MMWR. 2015;64:381–385. [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Fenn N, Peterson EL. Early smoking initiation and nicotine dependence in a cohort of young adults. Drug Alcohol Depend. 1993;33:129–137. doi: 10.1016/0376-8716(93)90054-t. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–220. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology (Berl) 2015;232:3067–3079. doi: 10.1007/s00213-015-3947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8:312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002;66:61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Castrucci BC, Gerlach KK, Kaufman NJ, Orleans CT. Tobacco use and cessation behavior among adolescents participating in organized sports. Am J Health Behav. 2004;28:63–71. doi: 10.5993/ajhb.28.1.7. [DOI] [PubMed] [Google Scholar]
- Cosgrove K, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats Sex differences. Pharmacol Biochem Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2013;219:657–672. doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Byron-Daniel J, Everson-Hock ES, Oh H, Taylor AH. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction. 2013;108:26–37. doi: 10.1111/j.1360-0443.2012.04034.x. [DOI] [PubMed] [Google Scholar]
- Hegmann KT, Fraser AM, Keaney RP, Moser SE, Nilasena DS, Sedlars M, Higham-Gren L, Lyon JL. The effect of age at smoking initiation on lung cancer risk. Epidemiology. 1993;4:444–448. doi: 10.1097/00001648-199309000-00010. [DOI] [PubMed] [Google Scholar]
- Khuder SA, Dayal HH, Mutgi AB. Age at smoking onset and its effect on smoking cessation. Addict Behav. 1999;24:673–677. doi: 10.1016/s0306-4603(98)00113-0. [DOI] [PubMed] [Google Scholar]
- Kirkcaldy BD, Shephard RJ, Siefen RG. The relationship between physical activity and self-image and problem behaviour among adolescents. Soc Psychiatry Psychiatr Epidemiol. 2002;37:544–550. doi: 10.1007/s00127-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Lisha NE, Sussman S. Relationship of high school and college sports participation with alcohol, tobacco, and illicit drug use: a review. Addict Behav. 2010;35:399–407. doi: 10.1016/j.addbeh.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci Biobehav Rev. 2013;37:1622–1644. doi: 10.1016/j.neubiorev.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila VM, Raisamo S, Pihlajamäki H, Mäntysaari M, Rimpelä A. Sports activity and the use of cigarettes and snus among young males in Finland in 1999–2010. BMC Public Health. 2012;12:230. doi: 10.1186/1471-2458-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron P, Smith GD, Okasha M, McEwen J. Smoking in adolescence and young adulthood and mortality in later life: prospective observational study. J Epidemiol Community Health. 2001;55:334–335. doi: 10.1136/jech.55.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Werch CEC. Sport and physical activity participation and substance use among adolescents. J Adolesc Health. 2005;36:486–493. doi: 10.1016/j.jadohealth.2004.02.031. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Trost SG, Levin S, Dowda M. Sports participation and health-related behaviors among US youth. Arch Pediatr Adolesc Med. 2000;154:904–911. doi: 10.1001/archpedi.154.9.904. [DOI] [PubMed] [Google Scholar]
- Rainey CJ, McKeown RE, Sargent RG, Valois RF. Patterns of tobacco and alcohol use among sedentary, exercising, nonathletic, and athletic youth. J Sch Health. 1996;66:27–32. doi: 10.1111/j.1746-1561.1996.tb06254.x. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C, Prapavessis H. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology (Berl) 2012;222:1–15. doi: 10.1007/s00213-012-2731-z. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology (Berl) 2013a;227:403–411. doi: 10.1007/s00213-012-2964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl) 2013b;231:1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pennock MM, Walker KL, Lang KC. Access to a running wheel decreases cocaine-primed and cue-induced reinstatement in male and female rats. Drug Alcohol Depend. 2012;121:54–61. doi: 10.1016/j.drugalcdep.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Access to a running wheel inhibits the acquisition of cocaine self-administration. Pharmacol Biochem Behav. 2011;100:237–243. doi: 10.1016/j.pbb.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Wheel running decreases the positive reinforcing effects of heroin. Pharmacol Rep. 2012;64:960–964. doi: 10.1016/s1734-1140(12)70891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC. The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl) 2011;218:357–369. doi: 10.1007/s00213-011-2321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Witte MA. The effects of exercise on cocaine self-administration, food-maintained responding, and locomotor activity in female rats: importance of the temporal relationship between physical activity and initial drug exposure. Exp Clin Psychopharmacol. 2012;20:437–446. doi: 10.1037/a0029724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. 2014. pp. 14–4863. NSDUH Series H-48, HHS Publication No. (SMA) [Google Scholar]
- Taioli E, Wynder EL. Effect of the age at which smoking begins on frequency of smoking in adulthood. N Engl J Med. 1991;325:968–969. doi: 10.1056/NEJM199109263251318. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O’Malley PM. Substance use and exercise participation among young adults: parallel trajectories in a national cohort-sequential study. Addiction. 2011;106:1855–65. doi: 10.1111/j.1360-0443.2011.03489.x. discussion 1866–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Ven MOM, Greenwood PA, Engels RCME, Olsson CA, Patton GC. Patterns of adolescent smoking and later nicotine dependence in young adults: a 10-year prospective study. Public Health. 2010;124:65–70. doi: 10.1016/j.puhe.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Zlebnik NE, Anker JJ, Carroll ME. Exercise to reduce the escalation of cocaine self-administration in adolescent and adult rats. Psychopharmacology (Berl) 2012;224:387–400. doi: 10.1007/s00213-012-2760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]