Abstract
Physical activity and non-exercise activity thermogenesis (NEAT) are crucial factors accounting for individual differences in body weight, interacting with genetic predisposition. In the brain, a number of neuroendocrine intermediates regulate food intake and energy expenditure (EE); this includes the brain melanocortin (MC) system, consisting of melanocortin peptides as well as their receptors (MCR). MC3R and MC4R have emerged as critical modulators of EE and food intake. To determine how variance in MC signaling may underlie individual differences in physical activity levels, we examined behavioral response to MC receptor agonists and antagonists in rats that show high and low levels of physical activity and NEAT, that is, high- and low-capacity runners (HCR, LCR), developed by artificial selection for differential intrinsic aerobic running capacity. Focusing on the hypothalamus, we identified brain region-specific elevations in expression of MCR 3, 4, and also MC5R, in the highly active, lean HCR relative to the less active and obesity-prone LCR. Further, the differences in activity and associated EE as a result of MCR activation or suppression using specific agonists and antagonists were similarly region-specific and directly corresponded to the differential MCR expression patterns. The agonists and antagonists investigated here did not significantly impact food intake at the doses used, suggesting that the differential pattern of receptor expression may by more meaningful to physical activity than to other aspects of energy balance regulation. Thus, MCR-mediated physical activity may be a key neural mechanism in distinguishing the lean phenotype and a target for enhancing physical activity and NEAT.
Keywords: Non-exercise activity thermogenesis, (NEAT), obesity, spontaneous physical activity, HCR/LCR
1
The problem of obesity continues to plague society, decreasing the quality of life and increasing mortality rates (Allison et al., 1999, Ogden et al., 2014). An obesogenic environment interacts with genetic predisposition to make an individual more or less susceptible to becoming obese. However, only a small proportion of human obesity can be accounted for by monogenic mutations; instead, genetic polymorphisms and their associated intermediates contribute to weight gain (Cauchi et al., 2009, Zegers et al., 2012). The brain leptin-melanocortin (MC) system is one of the most common systems to be a target of polymorphisms affecting energy homeostasis (Fan et al., 1997, Zemel and Shi, 2000, Butler, 2006, Lee et al., 2006). Of its five known receptors, MC receptors (MCRs) 3, 4, and 5 are present in the adult mammalian brain. MC4R is a common target for alterations related to both monogenic and polygenic obesity (Santini et al., 2009, Tao, 2010). Their roles in energy balance, particularly for MC4R and MC3R, have been demonstrated through studies using gene deletions as well as reports of single nucleotide polymorphisms (SNP) in human obesity (Bell et al., 2005). MC4R mutations are among the most common monogenic causes of human obesity, with over 150 reported mutations (Tao, 2010), and genome-wide complex trait analysis indicating significant SNP signals surrounding MC4R as part of the “missing heritability” for BMI (Locke et al., 2015).
Daily physical activity influences both weight management and cardiovascular disease risk. The energy expenditure (EE) of daily living, called non-exercise activity thermogenesis (NEAT), contributes to inter-individual differences in body weight and resistance to fat gain during overfeeding (Levine et al., 1999). The tendency to be physically active is a biologically regulated, heritable trait [reviewed in (Novak and Levine, 2007, Garland et al., 2011)], but also interacts with known genetic determinants of obesity risk. Evidence links the brain MC system to physical activity as well, for example, in obese animal models, deletion of MCRs results in lower physical activity along with obesity (Chen et al., 2000a, Coll et al., 2004, Loos et al., 2005, Yako et al., 2012). Altogether, ample evidence implicates the brain MC system as a major contributing factor for individual differences in energy balance and physical activity.
In both rats and humans, high intrinsic aerobic capacity strongly associates with high levels of physical activity (Levine et al., 1999, Novak et al., 2009); both of these traits could serve to identify the lean phenotype, and could share common underlying mechanisms. In a rat model of leanness (Koch and Britton, 2001, Koch et al., 2012) that demonstrates consistently elevated levels of physical activity and NEAT (Novak et al., 2009, Novak et al., 2010, Shukla et al., 2012, Gavini et al., 2014, Smyers et al., 2015), we previously reported elevated levels of MCR expression in the hypothalamus (Shukla et al., 2012). The relevance of the divergent receptor profiles to behavior, including physical activity levels, is unexplained. Here, we determined the site specificity of receptor expression by using a highly specific methodology, laser capture microdissection (LCM). We then used MC receptor subtype-specific agonists and antagonists to examine the relevance of regional differences in MCR expression, including MC5R, to intrinsic differences in daily physical activity levels.
2. Experimental Procedures
2.1 Rats
High-capacity runner (HCR) and low-capacity runner (LCR) female rats (generation 27–31) with overlapping ranges of body weight were provided by the International Aerobic Rat Resource maintained at the University of Michigan (Koch and Britton, 2001, Wisloff et al., 2005, Ren et al., 2013). Compared to males, HCR and LCR females show less change in body weight with age and less group difference in body weight, thus minimizing potential group bias stemming from differences in body weight and composition (Goran, 2005, White et al., 2009, Butler and Kozak, 2010, Tschop et al., 2012). Female rats were used throughout these studies unless noted otherwise. Although sex differences in metabolism and subtle variations in daily physical activity that are estrous-cycle related may exist (Smyers et al., 2015), the measured differences in activity between LCR and HCR rats are robust and not dependent on sex (Novak et al., 2010, Smyers et al., 2015), and variance related to estrous cycle has minimal effect on EE (Giles et al., 2010). Rats were housed individually and placed on a 12:12 h light:dark cycle with lights on at 0700 EST, and received ad libitum water and rodent chow (Lab Diet 5001; Lab Diet, Richmond, Indiana, USA). A total of 148 rats (HCR + LCR) were used in these studies; all procedures and handling were in accordance with and approved by Kent State University’s Institutional Animal Care and Use Committee.
2.2 Laser capture microdissection (LCM) and gene expression
Brains from 12 HCR and 12 LCR rats were sectioned at 12 μm sections on a cryostat and mounted onto SuperfrostPlus slides. Sections were stained using a quick protocol to allow visual identification of the arcuate nucleus, perifornical lateral hypothalamus (PeFLH), paraventricular nucleus (PVN), ventromedial nucleus (VMN), and dorsomedial nucleus (DMN). We chose these regions because of their documented presence of MC receptors in these areas and the actions of MC on metabolism. Briefly, sections were fixed in a 75% EtOH (30 sec), rinsed in water, and immersed in Hemotoxylin (90 sec), followed by serial dehydration (75%, 95%, and 100% EtOH for 30 sec each) and immersion in xylenes (5 min). The LCM machine (Arcturus XT™) was used to identify and capture brain regions onto CapSure® HS LCM Caps (Molecular Devices), pooling 6–12 captures from one nucleus onto one cap for every sample. Pre- and post-capture images of the tissue confirmed accuracy of nuclei captured; we estimate that extra cells comprised less than 1% of the total captured material.
RNA from the LCM samples was isolated and measured using quantitative real-time PCR (Q-PCR). The samples were purified and total RNA was extracted using an RNA purification kit (Ribopure; Ambion Life Technologies, Grand Island, New York, USA). RNA concentration and purity were measured using NANODROP (ND-1000; Nanodrop Technologies, Wilmington, Delaware, USA) with A260/280 ratio ranging from 1.8 – 2.1; only samples with optimum RNA integrity numbers were used for further processing. Purified total RNA was reverse transcribed using the Applied Biosystems reverse transcription reagents kit (Carlsbad, California, USA), using random hexamers with thermal cycling at 25°C for 10 minutes, 48°C for 30 minutes, 95°C for 5 minutes. Next, 20–100 ng of cDNA was used for quantifying the expression of the genes of interest using Taqman probes (Applied Biosystems); starting concentration of cDNA was kept the same within the nuclei examined. All samples were run in triplicate on the StratageneMx3005P Real-Time PCR System (Agilent, Carlsbad, California, USA), with annealing temperature of 60°C, for 40 cycles. The housekeeping gene, glyceraldehyde phosphate dehydrogenase (GAPDH) was used as control for all assays and the relative expression was calculated using the comparative Ct method (ΔCt) method (Schmittgen and Livak, 2008).
2.3 Brain micropunches and Western blot
10 HCR and 10 LCR male rats were euthanized and brains were rapidly removed, frozen in cooled isopentane, and stored at −80°C. Brains were sectioned at 100 μm on a cryostat; sections were placed onto slides and frozen on dry ice. Tissue sites containing the PVN and PeFLH were then micropunched using a 2 mm (midline, PVN) or a 1 mm (PeFLH, bilateral) micropunch tool (Fine Science Tools, Foster City, CA), similar to a method described previously (Novak et al., 2010). We focused on these two hypothalamic nuclei to validate our findings that show significant RNA differences in these regions. All punches were flash frozen with liquid nitrogen and stored at −80°C.
Punches were sonicated in 35 μl of ice-cold radioimmunoprecipitation buffer (Thermo Scientific) supplemented with protease inhibitor cocktail (Roche Diagnostics) followed by 30-min incubation on ice. Total homogenates were then centrifuged, and the supernatant (total lysate) was transferred to new clear tubes for analysis. Equal quantities of total lysate were resolved by SDS-PAGE and used for Western blot analysis. MC3R, MC4R, and MC5R protein levels were examined using actin as a loading control. Equal quantities of supernatant and sample buffer (150 mM tris-HCl pH 6.8, Trizma-base for pH, 6% SDS, 30% glycerol, 0.03% pyronin-Y, DTT) were mixed and tubes were heated at 90°C for 3 minutes. Samples containing equal quantities of protein were loaded onto a gradient gel (4–15%; Bio Rad) and electrophoresed using SDS running buffer (0.384 M glycine, 0.05 M Trizma base, 0.1% SDS) at constant voltage (150V) for 30 minutes. The gel was blotted onto a PVDF membrane using a semi-dry blotting apparatus and transfer buffer (49.6 mM Trizma base, 384 mM glycine, 17.5% methanol, 0.01% SDS) at constant current (400 mAmp). The blot was incubated overnight in a blocking solution of 5% Blotto in 1XPBST (Phosphate buffered saline; 84 mM sodium hydrogen phosphate, 16 mM sodium dihydrogen phosphate, 100 mM sodium chloride, Tween20), then rinsed using 1X PBST. Primary antibodies used for MC3R, MC4R, MC5R, and actin (Santa Cruz Biotechnology sc6878, 6879, and sc7644, sc1616 respectively) were diluted in blocking solution at a ratio of 1:2000 and incubated overnight at 4°C. After three washes in PBST, the membrane was exposed to a 1:5000 dilution of a donkey anti-goat IgG-HRP secondary antibody (Santa Cruz Biotechnology sc2020) and incubated for 1 hour at room temperature. After washing, the blots were developed using a chemiluminiscence detector using an Amersham kit (GE Healthcare, UK; 1 ml solution A, 1 ml solution B) and imaged with the image reader LAS-3000. The expression levels relative to β-actin were plotted as a percent of the reference value with HCR as 100%. To further validate on-target specificity of our MCR antibodies used for immunoblots, we used hindbrain samples from MC4R-deficient rats and wild-type controls (Mul et al., 2012) (Transpogen Biopharmaceuticals, Inc. Lexington, KY) to measure MC4R and MC5R protein expressions using the same antibodies.
2.4 Hypothalamic cannulation, microinjection, and calorimetry
Guide cannulae aimed at the PVN or PeFLH (Novak et al., 2006, Novak et al., 2010) were implanted into 10–12 each of HCR and LCR using stereotaxic surgery, as described previously (Shukla et al., 2012). Briefly, rats were anesthetized using inhaled isoflurane and placed in the stereotaxic apparatus. The following coordinates were used for PVN: anterior-posterior, −1.92 mm; medial-lateral, +0.5; dorsal-ventral, 7 to −7.3; injection needle, 1mm projection. For the PeFLH, the stereotaxic coordinates were anterior-posterior, −2.6 to −2.7 mm; medial-lateral, +1.7; dorsal-ventral, −7.3; injection needle, 1mm projection. The guide cannula was affixed to the skull using a sterile Michel wound clip and dental cement. Following surgery, rats were allowed to recover for a minimum of 7 days, after which fat and lean mass were measured using an EchoMRI-700 (Echo Medical Systems, Houston, Texas) (Nixon et al., 2010).
2.4.1 Measurement of physical activity and energy expenditure
Before the start of any experiment, rats were acclimated to their testing cage (7.5 × 12 × 9 inches) housed in a calorimetry room (isolation chamber) for a minimum of 48 hours, with the room temperature set at thermoneutral (25.9 °C) (Brown, 2008, Overton, 2010) and food and water available ad libitum. Although in mice, activity-associated EE is not a significant contributor to total daily EE (Virtue et al., 2012), this was assessed in mice housed in temperatures below thermoneutral. However in our studies, temperatures were maintained at thermoneutral (Overton, 2010); further, rats are larger than mice, and the relative contribution of physical activity to energy balance increases with body size. Rats were randomly divided into groups of four, with concurrent measurements of 2 HCR and 2 LCR randomly assigned to each of four calorimetry chambers. Small-animal indirect calorimetry was performed using a 4-chamber Oxymax FAST system (Columbus Instruments, Columbus, OH) after treating the rats with site-specific microinjections. Rats were microinjected with either a vehicle (artificial cerebrospinal fluid, aCSF) or a drug (MCR agonist or antagonist) over 15 seconds; the needle was left in place for an additional 10–15 seconds to minimize potential flow up the cannula track. The final volume and concentration of each compound administered was 10pm/200nl.
MC compounds used were melanotan II (MTII; Phoenix Pharmaceuticals), MC3R agonist (D-Trp8-γ-MSH; Tocris Biosciences), MC4R agonist (Cyclo(βAla-His-D·Phe-Arg-Trp·Glu)NH2; Phoenix Pharmaceuticals), MC4R antagonist (HS014; Tocris Biosciences), MC5R agonist (Ac-Nle-cyclo(Asp-Oic-D-4,4′-Bip-Pip-Trp-Lys)-amide; (Bednarek et al., 2007), and MC5R antagonist (Tyr-Val-Nle-Gly-His-DNal(2′)-Arg-Dtrp-Asp-Arg-Phe-Gly-NH2; Dr. Victor J. Hruby) (Grieco et al., 2008). Table 3 summarizes a comparison of these drugs describing the specificity against each receptor subtype. Different sets of rats were used to test each region to avoid the possibility of damage from repeated microinjections, thus minimizing the total number of injections per rat to a maximum of 8–10. There was a minimum 48–72 hour gap between each injection providing a sufficient washout period to avoid any residual effects. The day before their first injection, each rat was lightly restrained and given a sham injection to accustom rats to handling and injection. The few commercially available non-selective MC3R antagonists cannot effectively differentiate against MC4R and MC5R, therefore we were not able to completely test the functional role of MC3R in the PVN.
Table 3.
Melanocortin receptor agonists and antagonists used along with a comparison of their IC50 values and selectivity for MC3R, MC4R, and MC5R.
| Drug | IC50 (nM) | Selectivity ratio | Reference | |||
|---|---|---|---|---|---|---|
| MC3R | MC4R | MC5R | ||||
| MTII | 3.1 | 0.18 | 1.2 | 3/5–2.8 | 4/5–0.8 | (Bednarek, MacNeil et al. 2007) |
| MC3R agonist | 6.7 | 600 | 340 | 3/4–300 | 3/5–250 | (Grieco, Balse et al. 2000) |
| MC4R agonist | 490 | 4.3 | 4600 | 3/4–114 | 5/4–1070 | (Bednarek, MacNeil et al. 2001) |
| MC4R antagonist | 10.1 | 0.075 | unavailable | 3/4–17 | 5/4–11 | (Schioth, Muceniece et al. 1999) |
| MC5R agonist | 4000 | 2500 | 0.74 | 5/3–5400 | 5/4–3380 | (Bednarek, MacNeil et al. 2007) |
| MC5R antagonist | >1000 | >5000 | 130 | unavailable | unavailable | (Grieco, Cai et al. 2008) |
MC3R, MC4R, MC5R: melanocortin receptors 3, 4, and 5, IC50: half maximal inhibitory concentration.
This data was compiled from published studies that calculated average specificities using binding assays.
On the day of injection, rats were weighed, given the microinjection, and placed back in the testing cages, after which the chamber was sealed. The rats had ad libitum food and water present during all studies with the antagonist treatment; the drug was microinjected just before lights-off time, and the data were collected overnight for 12-hour period until right after lights-on the following morning. For the agonist treatment, 3–4 h measurements were taken post-injection (during the daytime) and any food was removed from the cages while they still had access to water. This was done to factor out any variations in thermic effect of food or in movements due to feeding episodes secondary to potential agonist-induced changes in appetite.
The calorimeter was calibrated using primary gas standards. Air was pumped into the chamber between 1.5–2.5 liters/min (depending on the weight of the rat), and chamber air was sampled at 0.4 liters/min. Gas exchange (percent O2 and CO2) was measured every 30 seconds, and reference values were measured over 3.5 min after each 60-sample interval. Physical activity data were collected, using infrared beam-break counts, every 10 seconds in the x- and z-axes; the initial 20 minutes of data were excluded from the analysis to account for handling-induced activity and to allow the air exchange to settle. EE (kcal/h), respiratory exchange ratio (RER; VCO2/VO2), and physical activity (counts/min) were measured for a total of 3 hours.
To validate that the MC5R agonist and antagonist were not exerting effects via off-target actions on MC4R, we measured 3-hour daytime activity after MC5R and MC5R agonist and antagonist microinjections into the PeFLH of 8 female HCR. Rats were given several combinations of 2 microinjections separated by 15 min: vehicle/vehicle, vehicle/MC5R agonist, MC5R antagonist/MC5R agonist, MC4R antagonist/MC5R agonist; veh/MC4R agonist, MC5R antagonist/MC4R agonist. We used targeted t-tests for specific comparisons to probe for off-target effects using a less conservative p-value as a cut-off (p<0.10) to maximize the identification of potential actions of the MC5R agents on MC4R.
2.5 Measurement of food intake
The impact of MCR agonists and antagonists on food intake was also assessed. For treatment with the agonists, each rat was deprived of food overnight (18 h) before being microinjected with the drug or a vehicle (aCSF), in order to detect a decrease in food intake. For antagonist treatment, each rat was tested in the absence of food deprivation. After the microinjections, rats were placed back into their home cages along with weighed amounts of food. Food intake was determined at 2 h, 4 h, 6 h, 12 h, 24 h, and 48 hours post injection; food spillage was weighed and accounted for when calculating intake. Body weight measurements were also taken. The experiment was repeated after a 7-day period such that each rat was injected with vehicle and drug (order counterbalanced), in addition to allowing sufficient washout time to prevent any residual drug effects. Possible variability in feeding efficiency due to minor differences in body weights of rats within a group is accounted for by the repeated-measures design and by measuring both vehicle and drug compounds within the same rat on different days.
2.6 Injection site verification
Rats were euthanized with FatalPlus, and 200 nl of dye (India Ink) was injected into the guide cannula to allow easy identification of the microinjection site. The brains were removed and fixed in formalin for ~2 days, then placed in 10% phosphate buffered formalin with 30% sucrose. Brains were sectioned at 50 μm using a cryostat, and mounted onto slides; sections were stained with cresyl violet, and the injection sites were determined using a microscope. Only rats in which the dye injection site corresponded to the stereotaxic coordinates (within 250 μm) were used for data analysis.
2.7 Statistical analyses
For the Q-PCR and western blot analyses, the HCR mean ΔCt (change in threshold detection compared to GAPDH) values were used to define 100%, and each animal’s data were calculated as a percentage of this mean. Unpaired two-tailed t-tests were used to compare gene expression between HCR and LCR, with differences of p<0.05 considered significant.
For physical activity and EE experiments, for each rat measurements of EE (kcal/h), RER, and physical activity (mean beam breaks/min) were obtained for the 2 hypothalamic nuclei examined and analyzed separately using 2×4 analysis of variance (ANOVA), with dose as the within-subjects independent variable, and rat line (HCR-LCR) as the between-subjects independent variable. In order to statistically factor out the influence of body weight and lean mass on EE, we used analysis of covariance (ANCOVA), with body weight or lean mass as the covariate.
The impact of MCR agonists and antagonists on food intake of treated rats was compared between HCR and LCR, and agonist or antagonist vs. vehicle (aCSF) treatment, using a 2X2 mixed (split-plot) ANOVA: with rat line as the between-subjects independent variable, drug-vehicle as the within-subjects independent variable, and food intake or body weight (both in grams) as dependent variables in separate analyses.
3. Results
3.1 Lean HCR rats have greater site-specific melanocortin receptor expression
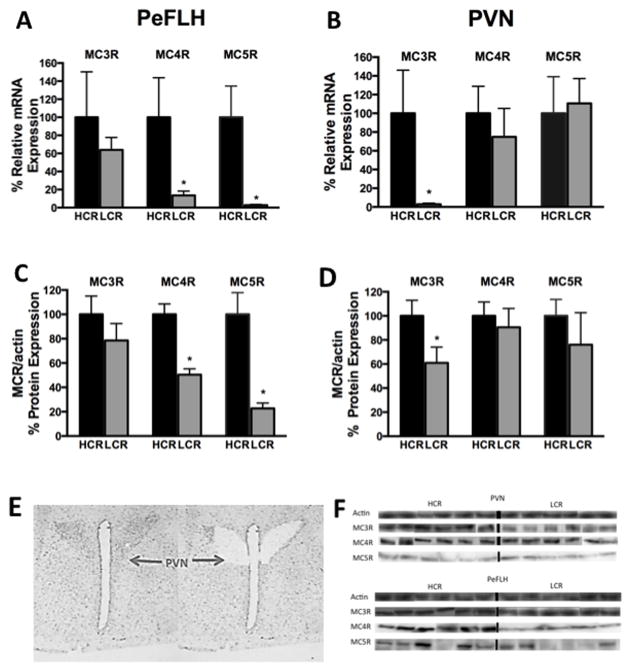
We examined the baseline expression of MCRs 3, 4, and 5 in two hypothalamic nuclei (PeFLH and PVN) of HCR and LCR rats. Q-PCR data is shown in Figure 1A–D and Table 1. In the PVN (Figure 1 B), HCR rats had significantly higher MC3R mRNA expression compared to LCR (p= 0.02) while no significant differences were detected in MC4R or MC5R expression. In the PeFLH (Figure 1A), both MC4R (p= 0.04) and MC5R (p= 0.01) were significantly higher in HCR compared to LCR rats. We also examined the DMN and VMN regions and expression of MCRs was not found to be significantly different between lean HCR and the obese LCR rats (Table 1). To verify if the differential gene expression corresponds to differences in protein expression pattern in the hypothalamic regions identified, we used western blot analysis to compare the brain MCR protein expression in PVN and PeFLH. On comparing HCR vs. LCR in the PeFLH, protein expression of MC4R and MC5R were significantly higher in HCR, while the MC3R was higher in the PVN region of HCR (Figure 1C). No differences were seen in the MC3R protein expression in the PeFLH (Figure 1C), or MC4R and MC5R in the PVN (Figure 1D), consistent with the predictions based on the mRNA profile (Figure 1A and B). Though the levels of MC5R (mRNA and protein) were lower compared to MC4R and MC3R (Figure 1A–D) in all brain regions, MC5R was found to be present in significant quantities in brain and in muscle and was detectable using our methods in all the tissues. Range of Ct values detected were: MC5R, 29–38 cycles; MC4R, 28–35 cycles; MC3R, 28–36 cycles; and GAPDH, 24–33 cycles.
Figure 1. Melanocortin receptor expression in hypothalamic nuclei.
Region-specific elevations in melanocortin receptors subtypes in the perifornical lateral hypothalamus (PeFLH) and the paraventricular nucleus (PVN) in lean, active high-capacity rats (HCR) compared to low-capacity rats (LCR) measured by quantitative PCR (Q-PCR) and Western blots. (A) Heightened levels of melanocortin receptors 4 and 5 (MC4R, MC5R) were seen in HCR in PeFLH. (B) In the PVN, melanocortin 3 receptor (MC3R) was higher in HCR. This pattern was also seen in protein expression (C) and (D). (E) Photomicrographs of the hypothalamus before and after laser capture microdissection of the PVN. (F) Representative blots of MC3R, MC4R, and MC5R from micropunched samples of HCR and LCR from PVN and PeFLH with actin as the loading control. *p<0.05, different than HCR for the same receptor and brain region. N= 8–12 per group, data represent mean plus SEM. Immunoblots for each protein subtype represent bands from the same experiment and may have been spliced to reorder and show parallel comparisons between MC3, 4, and 5 receptors within the same animal.
Table 1.
Quantitative mRNA measurement in hypothalamic nuclei obtained from laser capture microdissection (LCM).
| Brain Region LCM | Q-PCR Probe | HCR:LCR | p-value |
|---|---|---|---|
| Arcuate nucleus | PC1 | 0.76 | 0.20 |
| PC2 | 1.23 | 0.45 | |
| POMC | 1.08 | 0.13 | |
| AgRP | 1.61 | 0.08 | |
| Perifornical lateral hypothalamus (PeFLH) | MC3R | 1.56 | 0.20 |
| MC4R | 7.14 | 0.04* | |
| MC5R | 50.00 | 0.02* | |
| BDNF | 7.14 | 0.05* | |
| Paraventricular nucleus (PVN) | MC3R | 33.33 | 0.03* |
| MC4R | 1.33 | 0.67 | |
| MC5R | 0.91 | 0.99 | |
| Ventromedial nucleus (VMN) | MC3R | 0.75 | 0.40 |
| MC4R | 1.00 | 0.99 | |
| MC5R | 0.60 | 0.26 | |
| Sirt1 | 0.89 | 0.39 | |
| SF1 | 0.87 | 0.37 | |
| ADCYAP1 | 0.64 | 0.10 | |
| BDNF | 0.71 | 0.07 | |
| Dorsomedial nucleus (DMN) | MC3R | 0.91 | 0.10 |
| MC4R | 1.42 | 0.56 | |
| MC5R | Low levels/undetectable | Low levels/undetectable |
PC1, PC2: prohormone convertase 1 and 2; POMC: proopiomelanocortin; AgRP: agouti-related peptide; MC3R, MC4R, MC5R: melanocortin receptors 3, 4, and 5; Sirt1: sirtuin 1; SF-1: steroidogenic factor 1; ADCYAP: adenylate cyclate activating polypeptide 1 or prepro-PACAP; BDNF: brain-derived neurotrophic factor.
p<0.05 (HCR>LCR).
To validate the specificity of our antibodies used, we focused on MC4R and MC5R; the possible non-specific actions with MC3R or its antibodies are unlikely given its distinct expression pattern, compared to the overlap seen between MC4R and MC5R (Figure 1A–D and Table 1). On examining protein expression in MC4R-deficient and wild-type rats, MC4R antibody showed a signal in the wild-type rats significantly more than the MC4R-deficient rats (~650 times, p= 0.017); any labeling in the MC4R deficient tissues was probably due to the remaining truncated protein from a premature stop codon; in this MC4R-deficient rat model, the protein generally fails to localize in the membrane (Mul et al., 2012). No significant difference was observed for expression of MC5R (p= 0.817) in these rats, indicating that the antibodies used in our studies can specifically differentiate between these receptor subtypes. The MC5R antibody used in our study has also been shown by others to specifically differentiate against other receptor subtypes (Hatta et al., 2001, Zhang et al., 2006).
3.2 MCR agonists and antagonists in specific brain nuclei enhance or suppress short-term physical activity and energy expenditure
Previously, using a non-specific MCR agonist MTII, we showed that HCR are more responsive to an MTII-induced enhancement of activity (Shukla et al., 2012). Here, using MCR subtype-specific agonists and antagonists, we further identify the role of melanocortins in the hypothalamic regulation of energy homeostasis.
3.2.1 PeFLH
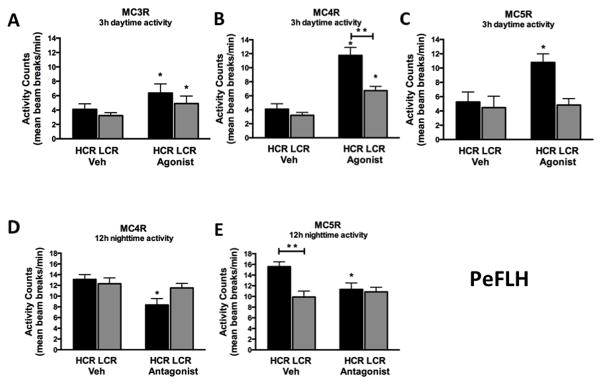
Compared to vehicle, a 10pmol/200 nl dose of MC5R agonist in the PeFLH significantly increased 3-h physical activity and EE in HCR but not in LCR rats (Figure 2C). Further, physical activity and associated EE in the HCR rats was significantly suppressed with an MC5R antagonist treatment (Table 2). For nighttime physical activity, the MC5R antagonist suppressed HCR physical activity to the level of LCR (Figure 2 E), but no significant change was seen in the activity of LCR. These results implicate brain MC5R in the elevated physical activity associated with high endurance capacity.
Figure 2. Perifornical region of hypothalamus PeFLH.
(A, B) High-capacity runners (HCR) were more responsive to the activity-inducing effects agonists to melanocortin receptor 4 and 5 (MC4R, MC5R), (C) but not 3 (MC3R), microinjected into the perifornical lateral hypothalamus (PeFLH). (D, E) Antagonists for MC4R and MC5R significantly suppressed nighttime physical activity in HCR; antagonists did not significantly impact nighttime activity levels in LCR. N= 7–10 per group, data represent mean + SEM, *significant change from vehicle, **HCR>LCR within treatment (p<0.05).
Table 2.
Energy expenditure: Differential activation/suppression in lean high-capacity rats (HCR) vs. low-capacity rats (LCR) after melanocortin receptor agonists and antagonists compared to vehicle treatment.
| Region | MCR drug used | EE (kcal/h) | Interaction (p value) | Main effect (p value) | ||||
|---|---|---|---|---|---|---|---|---|
| HCR | LCR | Drug | Group HCR/LCR | |||||
| veh | drug | veh | drug | |||||
| PeFLH | MC3R Agonist | 1.92±0.08 | 2.27±0.06 | 1.64±0.09 | 2.09±0.07 | 0.3 | <0.01 | 0.6 |
| MC4R Agonist | 1.53±0.05 | 2.22±0.05 | 1.57±0.092 | 1.95±0.15 | 0.5 | <0.001 | <0.05 | |
| MC4R Antagonist | 2.21±0.11 | 1.85±0.15 | 1.99±0.07 | 1.89±0.04 | <0.05 | <0.01 | 0.1 | |
| MC5R Agonist | 1.80±0.06 | 2.53±0.09 | 1.72±0.11 | 1.96±0.05 | <0.05 | <0.01 | <0.01 | |
| MC5R Antagonist | 2.2±0.07 | 1.85±0.02 | 1.93±0.11 | 1.91±0.01 | <0.05 | <0.01 | <0.05 | |
| PVN | MC4R Agonist | 1.57±0.01 | 1.97±0.06 | 1.53±0.08 | 1.83±0.06 | 0.5 | <0.05 | 0.2 |
| MC4R Antagonist | 2.19±0.01 | 2.11±0.02 | 1.94±0.01 | 1.92±0.04 | 0.1 | 0.1 | <0.05 | |
| MC5R Agonist | 1.7±0.02 | 2.15±0.01 | 1.64±0.03 | 2.09±0.08 | 0.4 | <0.01 | 0.1 | |
| MC5R Antagonist | 2.2±0.06 | 2.1±0.1 | 1.93±0.06 | 1.91±0.01 | 0.7 | <0.05 | <0.05 | |
PeFLH: perifornical lateral hypothalamus; PVN: hypothalamic paraventricular nucleus; MC3R, MC4R, MC5R: melanocortin receptors 3, 4, and 5; HCR: high capacity runners; LCR: low capacity runners; EE: energy expenditure; veh: vehicle;
p<0.05 (HCR>LCR). N=8–12, data represent mean ± SEM.
Using a MC4R-specific agonist in the PeFLH, both groups showed a significant increase in short-term activity, however HCR rats once again showed an enhanced response compared to LCR; HCR activity increased about 3-fold with the MC4R agonist compared to vehicle treatment (Figure 2B). Similarly, EE showed a significantly greater increase in the HCR vs. LCR rats (Table 2). This clearly indicates that the HCR showed a stronger response, potentially driven by their receptor expression in the PeFLH (Figure 1, Figure 2, Table 2). Using a highly specific MC4R antagonist, HS014, we also show a significant decrease in 12-h nighttime physical activity only in HCR rats, compared to vehicle (aCSF) treatment (Figure 2D). No significant change in nighttime activity was detected in the LCR. The HS014-induced suppression of activity seen in the HCR was also significant at the 3-h time point (data not shown). Enhanced nighttime activity seen in HCR under vehicle conditions was nullified by PeFLH treatment with either MC4R or MC5R antagonists. Lastly, the MC4R antagonist treatment significantly reduced the nighttime EE in HCR but not in LCR rats (Table 2).
With the MC3R agonist in PeFLH, both physical activity and EE increased in both HCR and LCR in a similar fashion (Figure 2A and Table 2). This supports the hypothesis that the MCR-driven activity and EE is regulated by the brain in a region- and receptor subtype-specific manner.
The MC4R antagonist in the PeFLH induced a significant increase in RER compared to vehicle; in HCR, RER increased from 0.87 ±0.01 to 0.89 ±0.01 and in LCR, from 0.89 ±0.01 to 0.93 ±0.01 (Table 4). There were no other significant effects of drug or group (HCR/LCR) on RER. Means for RER across the groups and conditions ranged from 0.82 to 0.93, and SEMs ranged from 0.01 to 0.02 (Table 4).
Table 4.
Comparison of respiratory exchange ratios (RER) in lean high-capacity rats (HCR) vs. low-capacity rats (LCR) after melanocortin receptor agonists and antagonists compared to vehicle treatment
| Region | MCR drug used | RER | |||
|---|---|---|---|---|---|
| HCR | LCR | ||||
| veh | drug | veh | drug | ||
| PeFLH | MC3R Agonist | 0.85±0.01 | 0.86±0.02 | 0.84±0.01 | 0.85±0.02 |
| MC4R Agonist | 0.85±0.01 | 0.84±0.01 | 0.84±0.01 | 0.83±0.02 | |
| MC4R Antagonist | 0.87±0.01 | 0.89±0.01 | 0.89±0.01 | 0.93±0.01 | |
| MC5R Agonist | 0.84±0.01 | 0.82±0.02 | 0.83±0.01 | 0.82±0.02 | |
| MC5R Antagonist | 0.90±0.01 | 0.89±0.01 | 0.91±0.01 | 0.91±0.01 | |
| PVN | MC4R Agonist | 0.85±0.01 | 0.84±0.01 | 0.85±0.01 | 0.85±0.02 |
| MC4R Antagonist | 0.85±0.01 | 0.83±0.02 | 0.84±0.01 | 0.84±0.02 | |
| MC5R Agonist | 0.86±0.02 | 0.84±0.01 | 0.84±0.02 | 0.83±0.01 | |
| MC5R Antagonist | 0.88±0.02 | 0.88±0.02 | 0.90±0.02 | 0.91±0.01 | |
PeFLH: perifornical lateral hypothalamus; PVN: hypothalamic paraventricular nucleus; MC3R, MC4R, MC5R: melanocortin receptors 3, 4, and 5; RER: respiratory exchange ratio; veh: vehicle; HCR: high capacity runners; LCR: low capacity runners. N=8–12, data represent mean ± SEM.
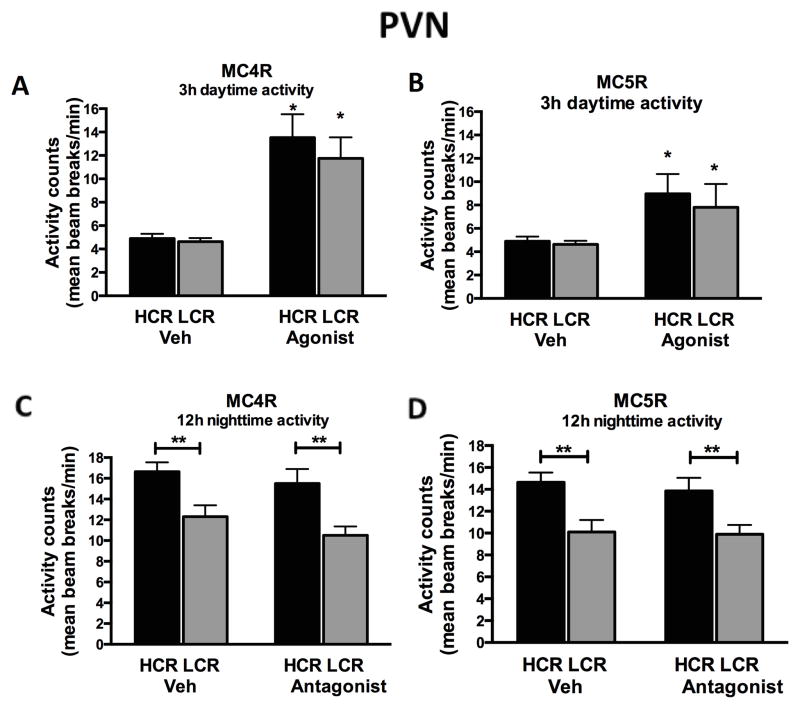
3.2.2 PVN
There was a significant main effect of the MC4R and MC5R agonists on physical activity but this response did not differ significantly between HCR and LCR (Figure 3A and 3B). The overall spontaneous physical activity was higher in HCR after the vehicle and antagonist treatments during the nighttime, which reflects the intrinsic high-activity phenotype of HCR rats (Figure 3C and 3D). Since experiments with the antagonists were performed over the 12-h dark cycle, this allowed sufficient time for such activity differences to emerge, compared to shorter 3-h periods (such as in agonist experiments) where average overall HCR activity counts are rarely significantly elevated.
Figure 3. Paraventricular nucleus of the hypothalamus.
(A, B) Agonists to melanocortin receptors 4 and 5 (MC4R, MC5R) microinjected into the paraventricular nucleus of the hypothalamus (PVN) induced significant increases in short-term (3h) daytime physical activity levels in rats. No differences were found between high-capacity runners (HCR) and low-capacity runners (LCR). (C, D) Antagonists to MC4R and MC5R in the PVN did not suppress nighttime (12h) physical activity levels in either HCR or LCR. N= 6–9 per group, data represent mean plus SEM, *significant change from vehicle, **HCR>LCR within treatment (p<0.05).
3.2.3 Drug interactions
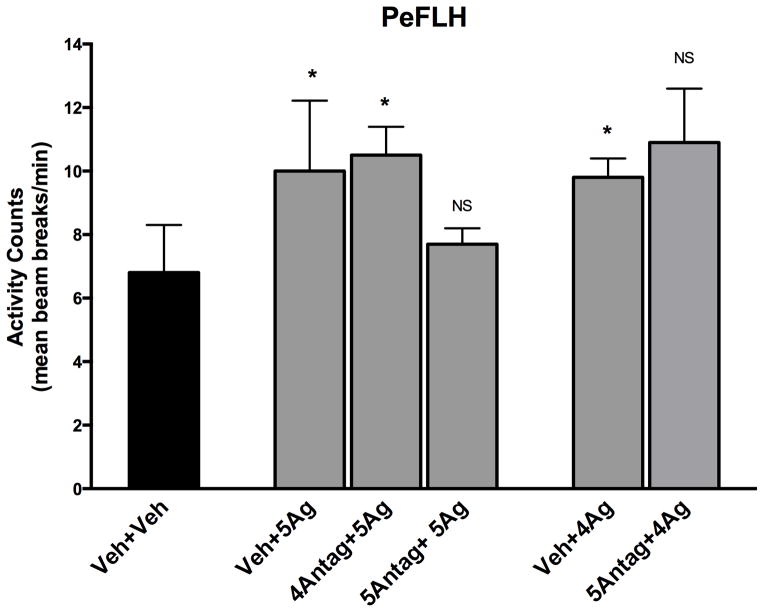
We used a combination of MCR compounds to validate the agonist/antagonist-mediated effects, and found no interaction between microinjections of melanocortin 4 and 5 receptor (MC4R, MC5R) agonists and antagonists in the PeFLH. Seven out of eight rats had successful cannula placements; one rat’s activity data after vehicle+vehicle injections were not included in the analysis because the data points were statistical outliers (>2 SDs above the mean). As shown in Figure 4, pretreatment with the MC4R antagonist did not significantly decrease MC5R-induced activity (p=0.425); rats receiving the MC4R antagonist with the MC5R agonist showed activity levels significantly elevated over vehicle+vehicle treatment (p<0.01). Similarly, microinjection of the MC4R agonist significantly increased activity over vehicle+vehicle levels (p<0.05), and pre-treatment with the MC5R antagonist did not significantly decrease activity (p=0.252).
Figure 4. Lack of interaction between melanocortin 4 and 5 receptor (MC4R, MC5R) pharmacologic agents.
Increases in physical activity induced by melanocortin receptor agonists were not suppressed by pre-treatment with antagonists to the alternate receptor in perifornical lateral hypothalamus (PeFLH) of high-capacity runner (HCR) rats. Veh: vehicle; 4Ag: MC4R agonist; 4Antag: MC4R antagonist; 5Ag: MC5R agonist; 5Antag: MC5R antagonist. N=7, data represent mean plus SEM *Different from vehicle (p<0.05), NS not significant.
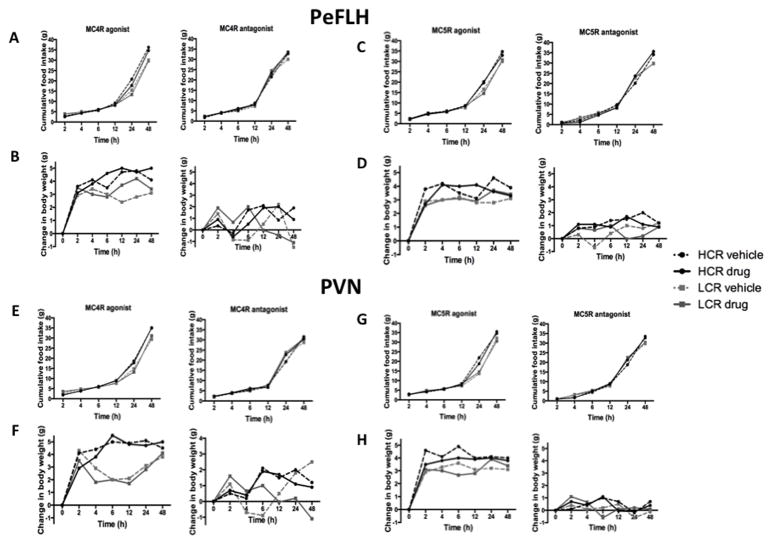
3.3 No significant increase or suppression of food intake with the MCR specific agonists and antagonists
In HCR-LCR rats, the MC4R and MC5R agonists in the PeFLH did not significantly alter food intake (Figure 5A and 5C) or body weight (Figure 5B and 5D) up to 48 hours after treatment, compared to the vehicle (aCSF) after overnight food restriction. The LCR rats weighed slightly but significantly more than the HCR to start with, but no significant difference was seen for change in body weight over time in either HCR or LCR rats. Similarly, we did not detect any change in food intake or body weight as a function of MC5R antagonist in the PeFLH (Figure 5C and 5D). In a separate experiment, the MC4R and MC5R agonist (after overnight food deprivation) and antagonist were injected into the PVN and similar results were found, that is, no significant induction or suppression of food intake was observed (Figure 5E and 5G). There was also no significant difference in the feed efficiency (food intake/weight gain) between the two groups as an effect of agonist or antagonist treatment. Means for feed efficiency across the groups and conditions were 0.8 – 1.15, and SEMs ranged from 0.07–0.1.
Figure 5. Food intake and body weight was not impacted by melanocortin receptor agents.
Cumulative food intake with MC4R agonist and antagonist treatments in (A) perifornical lateral hypothalamus (PeFLH) and (E) paraventricular nucleus (PVN); MC5R and antagonist treatments in (C) PeFLH and (G) paraventricular nucleus (PVN) of high-capacity runner (HCR) and low-capacity runner (LCR) rats. Change in body weight with MC4R agonist and antagonist in PeFLH (B) and PVN (F), and MC5R agonist and antagonist in PeFLH (D) and PVN (H). No significant differences in body weight and cumulative food intake over 48 hours after microinjecting with MC4R/MC5R agonists (after an overnight fast) or antagonists compared to vehicle treatment between HCR vs. LCR rats. Dose: 20 pmol/200nl. N=7–10 per group, SEMs range from 0.05 to 0.2 for change in body weight, and from 0.2 to 2.6 for food intake.
4. Discussion
The significance of the brain leptin-melanocortin system in energy balance, and the consequences to energy balance of its malfunction, are apparent from experimental perturbations of this system in animals (Chen et al., 2000a, Chen et al., 2000b, Butler et al., 2001) and genetically in human obesity (Farooqi et al., 2000, Bell et al., 2005, Tao, 2010). While the importance of MC4R gene mutations and polymorphisms to human obesity is extensively documented, the specific mechanisms underlying are not known. Perturbing and activating the brain melanocortin system alter physical activity (Huszar et al., 1997, Chen et al., 2000a, Butler et al., 2001, Challis et al., 2004, Shukla et al., 2012). Moreover, physical activity and NEAT are heritable traits that confer resistance to obesity (Levine et al., 1999, Levine and Kotz, 2005, Church et al., 2007, Hamilton et al., 2007, Woolf et al., 2008). We have previously shown that lean rats with intrinsically high levels of physical activity (HCR) (Novak et al., 2010, Smyers et al., 2015) show region-specific enhancement of MCR expression (Shukla et al., 2012). Here, we demonstrate that HCR rats not only exhibit higher brain MCR expression (Figure 1), but also show corresponding responsiveness to the physical activity-activating effects of MCR agonists, and the activity-suppressing effects of MCR antagonists (Figure 2). This suggests the possibility that a lean genetic profile impacts the brain melanocortin system, including regional receptor expression, which results in differential levels of physical activity and NEAT. For example, pre-ganglionic neurons in the sympathetic nervous system are also important in regulating energy expenditure; melanocortin receptors, particularly MC4R, have been shown to be important in regulating not just spontaneous activity but also energy expenditure through the sympathetic tone (Sohn et al., 2013, Garfield et al., 2015).
Because of its relevance to the genetics of human obesity (Zegers et al., 2012) there has been tremendous focus on the brain melanocortin system, including MCR, and most of this has centered on appetite regulation. We find that there are regional differences in melanocortin receptor expression in the hypothalamus of lean rats that have elevated physical activity levels, but do not demonstrate decreased appetite (Shukla et al., 2012, Smyers et al., 2015). Specifically, in the lean HCR, the PVN displays elevated expression of MC3R but not MC4R or MC5R; the PeFLH shows the opposite pattern, that is, high expression levels of MC4R and MC5R but not MC3R. Brain regions were isolated using laser-capture microdissection ruling out potential contamination from nearby hypothalamic regions. This study supports and expands upon previous evidence of differential expression of MCRs in high-activity HCR rats (Shukla et al., 2012). The gene expression pattern is mirrored by protein expression (Figure 1), supporting the assertion that the differential expression contributes to altered function. PVN is known to be important in energy balance, promoting satiety, EE, sympathetic nervous system outflow, and physical activity. Recent evidence emphasizes the role of PVN and its interaction with arcuate AgRP neurons in modulating hunger (Krashes et al., 2014). Though peptides including corticotropin releasing hormone (CRH) and thyrotropin releasing hormone (TRH) have similar actions as melanocortin peptides (reviewed in (Novak and Levine, 2007), evidence is inconsistent regarding the extent to which these peptides mediate melanocortin peptide function, at least with respect to appetite (Fekete et al., 2000, Sarkar et al., 2002, Lu et al., 2003, Siljee et al., 2013). Melanocortins alter TRH with respect to thyroid hormone release, but we do not see any phenotypic difference in this peptide in HCR-LCR rats (Shukla et al., 2012); further, the melanocortin function on TRH may be more relevant to fasting or refeeding whereas appetite is related to glutamatergic neuronal activation in PVN (Shah et al., 2014). In the PeFLH, MC may also interact with the hypothalamic neuropeptide orexin, and the pathways for orexin’s function on physical activity (Teske et al., 2013). Melanocortins may also interact with other peptides in the PeFLH important in energy balance, including melanin-concentrating hormone (Pissios et al., 2006) and neurotensin (Cui et al., 2012), both important in integrating energy balance consistent with the role of melanocortins in activity, EE, autonomic function, and satiety, integrating physiological and behavioral aspects of energy balance (Brown et al., 2015). Overall, our findings implicate MC3R in the PVN, and MC4R and MC5R in the PeFLH, as potential targets underlying the elevated levels of daily physical activity seen in lean HCR rats (Novak et al., 2010, Shukla et al., 2012, Gavini et al., 2014, Smyers et al., 2015).
In order to determine the functional significance of the elevated regional MCR expression in the HCR relative to LCR, we used specific agonists and antagonists for individual MCR subtypes and targeted regions showing robust phenotype-associated differences in expression, namely the PVN and PeFLH. The ability of MCR agonists to alter physical activity mirrored the expression patterns. In the PeFLH, where HCR showed enhanced expression of MC4R and MC5R, agonists of these receptors significantly elevated activity levels in HCR but not in LCR; the MC3R agonist did not have this effect (Figure 2). In contrast, in the PVN, where MC3R (but not MC4R or MC5R) expression was elevated in HCR, the MC4R and MC5R agonists both increased physical activity to the same extent in both HCR and LCR (Figure 3). The potential influence of these receptor expression patterns is further supported by the ability of antagonists to suppress nighttime physical activity. Inhibition of either MC4R or MC5R activation in the PeFLH significantly suppressed nighttime activity, but only in the HCR, bringing their activity down to the level of LCR (Figure 2). Lastly, the ability of the agonist- and antagonist-induced changes in physical activity to significantly influence EE strengthens the assertion that differential expression of MCRs in the hypothalamus may underlie phenotypic differences in NEAT. The match between brain MCR expression profiles and the physical-activity response to receptor activation is particularly intriguing in light of emerging recognition of the contributions of spontaneous physical activity and NEAT to metabolic and cardiovascular health, in contrast to sitting for long durations (Dunstan et al., 2012, Moore et al., 2012, van der Ploeg et al., 2012, George et al., 2013).
The “lean” melanocortin receptor expression profile encompasses not only MC3R and MC4R, but also MC5R. Though others have demonstrated the relevance of MC3R and MC4R in the regulation of energy balance, satiety, and human obesity, the potential importance of brain MC5R is yet to be considered (Mountjoy and Wild, 1998, Butler et al., 2000, Bromberg-Martin and Hikosaka, 2009). In this study we identified pronounced differences specifically in MC5R expression in the PeFLH, where levels in HCR were almost 1000 times higher than LCR (Figure 1). There is limited information on MC5R or its role in energy balance since the structure of this receptor was characterized in rat only in 1994 (Griffon et al., 1994). However, since MC5R is present in the brain, as well as in other tissues, and has a strikingly similar sequence structure to receptors MC3R and MC4R, its potential role in metabolism cannot be ignored (Fathi et al., 1995). We show that, while the MC5R expression is relatively low in the brain, it shows a distinct expression pattern that differs according to a physical-activity-related phenotype. We previously identified a substantial elevation in MC5R expression in the PeFLH region in genetically lean and active HCR relative to LCR (Shukla et al., 2012). Here, we further establish that the high-activity phenotype of lean HCR rats may be partly attributed to a MC5R-mediated response—suppression of MC5R activity in the PeFLH decreased nighttime activity levels to that of LCR, which have very low levels of MC5R expressed in this region. This implicates MC5R in the high-activity phenotype seen in the intrinsically lean HCR. It is likely that we were able to detect these differences in MCR expression because we focused on smaller regions specifically implicated in modulation of physical activity, while these differences may remain obscured if tested as an overall expression in the brain. The contribution of the HCR-LCR model is further highlighted by the fact that complex differences among neuropeptides and their receptors associated with the lean phenotype might not be identifiable using standard rodent models housed in a sedentary environment with unrestricted food supply and limited physical challenges (Martin et al., 2010). With respect to activity EE, use of MC5R has the potential to overcome the barrier associated with MC4R compounds that are currently used to target obesity. Over the years, multiple drug discovery attempts for anti-obesity MC4R agonists have been curbed by emerging evidence linking MC4R activation with therapeutically adverse effects such as hypertension, erectile dysfunction, and inflammation (King et al., 2007, Corander et al., 2009, Greenfield et al., 2009, Maier and Hoyer, 2010, Sayk et al., 2010).
Because the in vivo effects of MC5R agonist and antagonist have not been characterized previously and have potential off-target effects on MC4R, we verified the specificity of the MC5R agents used. Though the in vitro affinities of the MC5R agonist and antagonist are very low for MC4R compared to MC5R (Bednarek et al., 2007, Grieco et al., 2008), the similar effects of MC4R and MC5R agonists, and the MC4R and MC5R antagonists on activity suppression, suggested an in vivo study to discount actions of the MC5R agents on MC4R. The MC5R antagonist did not decrease the MC4R-agonist-induced increase in activity. Similarly, the MC4R antagonist did not decrease the ability of the MC5R agonist to stimulate activity (Figure 4). This supports the assertion that the ability of the MC5R agonist microinjection into the PeFLH to increase physical activity is due to MC5R activation, and not to actions on the MC4R. Similarly it is unlikely that the MC5R antagonist exerted its effects through off-target interactions with MC4R. In summary, these data support the behavioral relevance of brain MC5R, especially in the PeFLH where expression levels drastically differ between HCR and LCR, as a potential mechanism underlying intrinsically elevated physical activity levels. The findings strongly suggest that region-specific differences in MC5R, specifically within the PeFLH, may contribute to the inter-individual differences in physical activity. While we see acute effects on activity and energy expenditure with MC4R and MC5R compounds in the PeFLH, daily injections of MC5R and MC4R agonists in the PeFLH would be needed to demonstrate the ability of induced physical activity to alter long-term energy balance.
One potential source of the differential receptor expression patterns seen in HCR and LCR could be up- or down-regulation secondary to altered MC “tone.” For example, increased POMC results in up-regulation of α-MSH and decreased MC receptor expression (Zemel and Shi, 2000, Pritchard et al., 2002). However, we found no significant difference in expression levels of POMC mRNA expression or of its processing enzymes (Table 1). Further, contrary to our findings, globally elevated MC release would be expected to alter MC receptors similarly regardless of subtype or brain region. Altogether, these data suggest that a specific MC receptor expression profile may be intrinsic to the high-activity, high-endurance phenotype. One possible mechanism is epigenetic regulation of expression. There are instances where one neurotransmitter or peptide can drive changes in the promoter region (histone modifications such as acetylation) in a tissue-specific manner (Gozen et al., 2013). A number of theoretical models exist that explain how the nervous system utilizes the kinetics of epigenetic changes to direct neurogenesis or changes in neuronal cell populations (Tan et al., 2013). This may also affect epigenetic modifications leading to differential activation of brain regions, ultimately affecting behaviors including physical activity (Kumsta et al., 2013). These epigenetic effects may act in concert with the underlying genetic differences between HCR and LCR rats (Ren et al., 2013).
Unlike studies utilizing mixed MCR agonists and antagonists like MTII and SHU9119 (Murphy et al., 1998), the present findings did not detect effects of specific MCR agonists or antagonists on food intake (Table 2). It is possible that the ability of MTII to suppress food intake is dependent on MC3R or a combination of receptor activation. The lack of a significant effect of the MC4R agonist and antagonist on food intake is at odds with others (Benoit et al., 2000, Balthasar et al., 2005, Noble et al., 2011). This may be due to the site-specificity of our injections (vs. intracerebroventricular injections), the animal model used, or the dose employed, though our doses were sufficient to induce significant changes in physical activity (Benoit et al., 2000, Fehm et al., 2001, Benoit et al., 2003). Our food-intake studies were done with regular chow which could also be one of the differences from some of the earlier works that have demonstrated a function of MC4R in PVN with high-fat diet (Garza et al., 2008). Previous work suggests the importance of MC3R in the PVN in regulating food intake, however we did not test this in our studies (Rowland et al., 2010). Overall, our data support the importance of the differential MCR receptor expression in the HCR and LCR to the modulation physical activity rather than appetite, and suggest that MC5R expression in the hypothalamus may be particularly relevant to physical activity instead of food intake.
The importance of the brain MC system is underscored by the abundant point mutations and polymorphisms identified in genetic studies of human obesity (Mountjoy et al., 1994, Chen et al., 2000a, MacKenzie, 2006, Cauchi et al., 2009). Using an animal model of polygenic obesity, we have identified promising targets in the brain MC system that may contribute to the variability in energy balance via physical activity and EE. This has implications for how we consider metabolism and intrinsic physical activity when attempting to prevent or treat obesity; targeting pathways that enhance daily activity levels in an individual may take advantage of already-existing mechanisms, endogenously employed to a greater extent in naturally lean people (as mimicked in HCR rats). If sites for different MCR subtypes actions on energy homeostasis are anatomically distinct from those affecting cardiovascular functions, differential modulation of their activation could also be attempted. Our findings, which clearly show a pattern of differential expression of MC3R and MC5R along with MC4R in the brain, may help overcome some setbacks in MC drug discovery so far.
5. Conclusions
Region- and MCR subtype-specific differences were detected between the lean and obesity-prone rats. Physical activity differences seen as a result of treatment with MCR agents were region-specific and directly correspond to the differential expression of MCRs in the hypothalamic nuclei. Thus, hypothalamic MCR expression is integral to the high-activity phenotype and MCR-mediated physical activity may be a key neural mechanism in distinguishing the lean phenotype and a target for enhancing physical activity and NEAT.
Highlights.
We investigated the role of brain melanocortin receptors (MCR) in the lean phenotype which shows high physical activity.
Lean rats showed elevated MCR expression specific to receptor subtype and brain region.
Lean rats’ physical activity was more responsive to MCR agonists and antagonist treatment.
There was correspondence between MCR expression pattern and regional response to brain MCR agonist and antagonist.
Hypothalamic MCR expression is integral to the high-activity phenotype.
Acknowledgments
7. Acknowledgements and funding
C.S. was funded by AHA 11PRE7320029, C.M.N. by NIH R01NS055859 and R15DK097644-01A1, and V.J.H and M.C. by NIH R01 GM10840. We thank Lydia Heemstra for her help with the western blots on MC4R rats. The LCR-HCR rat model system was supported by the Office of Research Infrastructure Programs/OD grant R24OD010950 (LGK, SB) from the National Institutes of Health (NIH). We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact LGK (lgkoch@med.umich.edu) or SLB (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, Michigan.
Abbreviations
- ADCYAP
Adenylate cyclate activating polypeptide 1
- AgRP
Agouti-related peptide
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- aCSF
Artificial cerebrospinal fluid
- BDNF
Brain-derived neurotrophic factor
- CRH
Corticotropin releasing hormone
- DMN
Dorsomedial nucleus
- EE
Energy expenditure
- GAPDH
Glyceraldehyde phosphate dehydrogenase
- HCR
High-capacity runner
- IACUC
Institutional Animal Care and Use committee
- LCM
Laser capture microdissection
- LCR
Low-capacity runner
- MC
Melanocortin
- MCR
Melanocortin receptor
- MTII
Melanotan II
- NEAT
Non-exercise activity thermogenesis
- PVN
Paraventricular nucleus
- PeFLH
Perifornical lateral hypothalamus
- PC
Prohormone convertase
- POMC
Proopiomelanocortin
- Q-PCR
Quantitative PCR
- RER
Respiratory exchange ratio, VCO2/VO2
- SNP
Single nucleotide polymorphisms
- Sirt1
Sirtuin
- SF-1
Steroidogenic factor 1
- TRH
Thyrotropin releasing hormone
- VMN
Ventromedial nucleus
Footnotes
6. Author contributions: C.S. and C.M.N. conceived and designed the experiments. C.S performed all experiments. L.G.K. and S.L.B. developed the HCR-LCR rat model and provided input on study interpretation, M.B., M.C., and V.J.H, developed the non-commercial MCR agonists and antagonists used in these studies. C.S. and C.M.N. wrote and edited the manuscript for content. All authors have read and approved the final article.
8. Conflicts of interest
There are no conflicts of interest.
References
- Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van der Ploeg LH, Weinberg DH. Potent and selective peptide agonists of alpha-biological evaluation in vitro. Biochem Biophys Res Commun. 2001;286(3):641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Fong TM, Angeles Cabello M, Maroto M, Teran A. Potent and selective peptide agonists of alpha-melanocyte stimulating hormone (alphaMSH) action at human melanocortin receptor 5; their synthesis and biological evaluation in vitro. Chem Biol Drug Des. 2007;69:350–355. doi: 10.1111/j.1747-0285.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221–234. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Barrera JG, Seeley RJ, Woods SC. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52:2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Schwartz MW, Lachey JL, Hagan MM, Rushing PA, Blake KA, Yagaloff KA, Kurylko G, Franco L, Danhoo W, Seeley RJ. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:3442–3448. doi: 10.1523/JNEUROSCI.20-09-03442.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009;63:119–126. doi: 10.1016/j.neuron.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Woodworth HL, Leinninger GM. To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Frontiers in systems neuroscience. 2015;9:9. doi: 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N-MPLaJW. Characterization of the Thermoneutral Zone of the Laboratory Rat. The FASEB Journal. 2008:22. [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–290. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nature neuroscience. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- Cauchi S, Stutzmann F, Cavalcanti-Proenca C, Durand E, Pouta A, Hartikainen AL, Marre M, Vol S, Tammelin T, Laitinen J, Gonzalez-Izquierdo A, Blakemore AI, Elliott P, Meyre D, Balkau B, Jarvelin MR, Froguel P. Combined effects of MC4R and FTO common genetic variants on obesity in European general populations. J Mol Med (Berl) 2009;87:537–546. doi: 10.1007/s00109-009-0451-6. [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O’Rahilly S. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36) Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4695–4700. doi: 10.1073/pnas.0306931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000a;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000b;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. The Journal of clinical endocrinology and metabolism. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- Corander MP, Fenech M, Coll AP. Science of self-preservation: how melanocortin action in the brain modulates body weight, blood pressure, and ischemic damage. Circulation. 2009;120:2260–2268. doi: 10.1161/CIRCULATIONAHA.109.854612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. The Journal of comparative neurology. 2012;520:4168–4183. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O’Rahilly S. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi Z, Iben LG, Parker EM. Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype. Neurochem Res. 1995;20:107–113. doi: 10.1007/BF00995160. [DOI] [PubMed] [Google Scholar]
- Fehm HL, Smolnik R, Kern W, McGregor GP, Bickel U, Born J. The melanocortin melanocyte-stimulating hormone/adrenocorticotropin(4-10) decreases body fat in humans. The Journal of clinical endocrinology and metabolism. 2001;86:1144–1148. doi: 10.1210/jcem.86.3.7298. [DOI] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Tatro JB, Rand WM, Lechan RM. alpha-Melanocyte stimulating hormone prevents fasting-induced suppression of corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Neuroscience letters. 2000;289:152–156. doi: 10.1016/s0304-3940(00)01256-8. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, Elmquist JK, Tannous BA, Krashes MJ, Lowell BB. A neural basis for melanocortin-4 receptor-regulated appetite. Nature neuroscience. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W, Lu XY. Adeno-associated virus-mediated knockdown of melanocortin-4 receptor in the paraventricular nucleus of the hypothalamus promotes high-fat diet-induced hyperphagia and obesity. The Journal of endocrinology. 2008;197:471–482. doi: 10.1677/JOE-08-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and Heightened Non-Resting Energy Expenditure: Role of Skeletal Muscle Activity Thermogenesis. Am J Physiol Endocrinol Metab. 2014 doi: 10.1152/ajpendo.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George ES, Rosenkranz RR, Kolt GS. Chronic disease and sitting time in middle-aged Australian males: findings from the 45 and Up Study. Int J Behav Nutr Phys Act. 2013;10:20. doi: 10.1186/1479-5868-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles ED, Jackman MR, Johnson GC, Schedin PJ, Houser JL, MacLean PS. Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1634–1642. doi: 10.1152/ajpregu.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI. Estimating energy requirements: regression based prediction equations or multiples of resting metabolic rate. Public Health Nutr. 2005;8:1184–1186. doi: 10.1079/phn2005803. [DOI] [PubMed] [Google Scholar]
- Gozen O, Balkan B, Yildirim E, Koylu EO, Pogun S. The epigenetic effect of nicotine on dopamine D1 receptor expression in rat prefrontal cortex. Synapse. 2013;67:545–552. doi: 10.1002/syn.21659. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Grieco P, Cai M, Liu L, Mayorov A, Chandler K, Trivedi D, Lin G, Campiglia P, Novellino E, Hruby VJ. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. Journal of medicinal chemistry. 2008;51:2701–2707. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochemical and biophysical research communications. 1994;200:1007–1014. doi: 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- Hatta N, Dixon C, Ray AJ, Phillips SR, Cunliffe WJ, Dale M, Todd C, Meggit S, Birch-MacHin MA, Rees JL. Expression, candidate gene, and population studies of the melanocortin 5 receptor. The Journal of investigative dermatology. 2001;116:564–570. doi: 10.1046/j.0022-202x.2001.01286.x. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. 2007;7:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med. 2012;22:29–34. doi: 10.1016/j.tcm.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, Hummel E, Chen FS, Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell metabolism. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283:212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- Levine JA, Kotz CM. NEAT--non-exercise activity thermogenesis--egocentric & geocentric environmental factors vs. biological regulation. Acta Physiol Scand. 2005;184:309–318. doi: 10.1111/j.1365-201X.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Magi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Hua Zhao J, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Mateo Leach I, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stancakova A, Strawbridge RJ, Ju Sung Y, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Arnlov J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Bluher M, Bohringer S, Bonnycastle LL, Bottcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Ida Chen YD, Clarke R, Daw EW, de Craen AJ, Delgado G, Dimitriou M, Doney AS, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Grassler J, Gronberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson A, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindstrom J, Sin Lo K, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PK, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Muller G, Muller-Nurasyid M, Musk AW, Nagaraja R, Nothen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Vernon Smith A, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundstrom J, Swertz MA, Swift AJ, Syvanen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, LifeLines Cohort S, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gadin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee JY, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JR, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, Van’t Hooft FM, Vinkhuyzen AA, Westra HJ, Zheng W, Zondervan KT, Mu TC, Heath AC, Arveiler D, Bakker SJ, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrieres J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hypponen E, Illig T, Jacobs KB, Jarvelin MR, Jockel KH, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJ, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimaki T, Lyssenko V, Mannisto S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PA, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PE, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tonjes A, Tregouet DA, Tremblay A, Tremoli E, Virtamo J, Vohl MC, Volker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PI, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, Marz W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njolstad I, Oostra BA, Palmer CN, Pedersen NL, Perola M, Perusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann HE, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJ, Speliotes EK Consortium AD, Group A-BW, Consortium CAD, Consortium CK, Glgc, Icbp, Investigators M, Consortium MI, Consortium P, ReproGen C, Consortium G, International Endogene C. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos RJ, Rankinen T, Tremblay A, Perusse L, Chagnon Y, Bouchard C. Melanocortin-4 receptor gene and physical activity in the Quebec Family Study. Int J Obes (Lond) 2005;29:420–428. doi: 10.1038/sj.ijo.0802869. [DOI] [PubMed] [Google Scholar]
- Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:7863–7872. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie RG. Obesity-associated mutations in the human melanocortin-4 receptor gene. Peptides. 2006;27:395–403. doi: 10.1016/j.peptides.2005.03.064. [DOI] [PubMed] [Google Scholar]
- Maier T, Hoyer J. Modulation of blood pressure by central melanocortinergic pathways. Nephrol Dial Transplant. 2010;25:674–677. doi: 10.1093/ndt/gfp644. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9:e1001335. doi: 10.1371/journal.pmed.1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Wild JM. Melanocortin-4 receptor mRNA expression in the developing autonomic and central nervous systems. Brain Res Dev Brain Res. 1998;107:309–314. doi: 10.1016/s0165-3806(98)00015-7. [DOI] [PubMed] [Google Scholar]
- Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW, Garner KM, Adan RA, Cuppen E. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity. 2012;20:612–621. doi: 10.1038/oby.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B, Nunes CN, Ronan JJ, Harper CM, Beall MJ, Hanaway M, Fairhurst AM, Van der Ploeg LH, MacIntyre DE, Mellin TN. Melanocortin mediated inhibition of feeding behavior in rats. Neuropeptides. 1998;32:491–497. doi: 10.1016/s0143-4179(98)90077-4. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity. 2010;18:1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1053–1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, Koch LG, Levine JA. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PloS one. 2009;4:e5869. doi: 10.1371/journal.pone.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. Journal of neuroendocrinology. 2007;19:923–940. doi: 10.1111/j.1365-2826.2007.01606.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Zhang M, Levine JA. Neuromedin U in the paraventricular and arcuate hypothalamic nuclei increases non-exercise activity thermogenesis. Journal of neuroendocrinology. 2006;18:594–601. doi: 10.1111/j.1365-2826.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton JM. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int J Obes (Lond) 2010;34(Suppl 2):S53–58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocrine reviews. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. The Journal of endocrinology. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- Ren YY, Overmyer KA, Qi NR, Treutelaar MK, Heckenkamp L, Kalahar M, Koch LG, Britton SL, Burant CF, Li JZ. Genetic analysis of a rat model of aerobic capacity and metabolic fitness. PloS one. 2013;8:e77588. doi: 10.1371/journal.pone.0077588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Fakhar KJ, Robertson KL, Haskell-Luevano C. Effect of serotonergic anorectics on food intake and induction of Fos in brain of mice with disruption of melanocortin 3 and/or 4 receptors. Pharmacology, biochemistry, and behavior. 2010;97:107–111. doi: 10.1016/j.pbb.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F, Maffei M, Pelosini C, Salvetti G, Scartabelli G, Pinchera A. Melanocortin-4 receptor mutations in obesity. Adv Clin Chem. 2009;48:95–109. [PubMed] [Google Scholar]
- Sarkar S, Legradi G, Lechan RM. Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain research. 2002;945:50–59. doi: 10.1016/s0006-8993(02)02619-7. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Muceniece R, Mutulis F, Bouifrouri AA, Mutule I, Wikberg JE. Further pharmacological characterization of the selective melanocortin 4 receptor antagonist HS014: comparison with SHU9119. Neuropeptides. 1999;33(3):191–196. doi: 10.1054/npep.1999.0760. [DOI] [PubMed] [Google Scholar]
- Sayk F, Heutling D, Dodt C, Iwen KA, Wellhoner JP, Scherag S, Hinney A, Hebebrand J, Lehnert H. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. The Journal of clinical endocrinology and metabolism. 2010;95:1998–2002. doi: 10.1210/jc.2009-2297. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, Lowell BB. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla C, Britton SL, Koch LG, Novak CM. Region-specific differences in brain melanocortin receptors in rats of the lean phenotype. Neuroreport. 2012;23:596–600. doi: 10.1097/WNR.0b013e328354f5c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A. Melanocortin 4 receptor distribution in the human hypothalamus. European journal of endocrinology/European Federation of Endocrine Societies. 2013;168:361–369. doi: 10.1530/EJE-12-0750. [DOI] [PubMed] [Google Scholar]
- Smyers ME, Bachir KZ, Britton SL, Koch LG, Novak CM. Physically active rats lose more weight during calorie restriction. Physiol Behav. 2015;139:303–313. doi: 10.1016/j.physbeh.2014.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Zong C, Xie XS. Rare event of histone demethylation can initiate singular gene expression of olfactory receptors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:21148–21152. doi: 10.1073/pnas.1321511111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocrine reviews. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske JA, Perez-Leighton CE, Billington CJ, Kotz CM. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1337–1345. doi: 10.1152/ajpregu.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172:494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell metabolism. 2012;16:665–671. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Blackburn TM, Seymour RS. Phylogenetically informed analysis of the allometry of Mammalian Basal metabolic rate supports neither geometric nor quarter-power scaling. Evolution. 2009;63:2658–2667. doi: 10.1111/j.1558-5646.2009.00747.x. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Woolf K, Reese CE, Mason MP, Beaird LC, Tudor-Locke C, Vaughan LA. Physical activity is associated with risk factors for chronic disease across adult women’s life cycle. J Am Diet Assoc. 2008;108:948–959. doi: 10.1016/j.jada.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Yako YY, Hassan MS, Erasmus RT, van der Merwe L, Janse van Rensburg S, Matsha TE. Associations of MC3R Polymorphisms with Physical Activity in South African Adolescents. J Phys Act Health. 2012 doi: 10.1123/jpah.10.6.813. [DOI] [PubMed] [Google Scholar]
- Zegers D, Van Hul W, Van Gaal LF, Beckers S. Monogenic and complex forms of obesity: insights from genetics reveal the leptin-melanocortin signaling pathway as a common player. Crit Rev Eukaryot Gene Expr. 2012;22:325–343. doi: 10.1615/critreveukargeneexpr.v22.i4.60. [DOI] [PubMed] [Google Scholar]
- Zemel MB, Shi H. Pro-opiomelanocortin (POMC) deficiency and peripheral melanocortins in obesity. Nutr Rev. 2000;58:177–180. doi: 10.1111/j.1753-4887.2000.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li WH, Anthonavage M, Eisinger M. Melanocortin-5 receptor: a marker of human sebocyte differentiation. Peptides. 2006;27:413–420. doi: 10.1016/j.peptides.2005.05.030. [DOI] [PubMed] [Google Scholar]