Abstract
Background
Because the role of dopamine (DA) D3 receptors has been investigated primarily in relation to cocaine-related behaviors little is known of the role of these receptors in heroin seeking.
Purposes
To investigate the effect of the selective DA D3 receptor antagonist, SR 21502, on cue-induced reinstatement of heroin seeking and heroin conditioned place preference (CPP).
Methods
In experiment 1, rats were trained to self-administer intravenous heroin for 15 days followed by extinction. Following extinction animals were treated with one of several SR 21502 doses (0, 7.5, 10 or 15 mg/kg) and a cue-induced reinstatement test was conducted. In Experiment 2, animals were conditioned to experience heroin in one compartment of a CPP apparatus and saline in the other. On the test day animals were treated with 0, 3.75, 7.5, 10 or 15 mg/kg of SR 21502 and tested for their CPP.
Results
The results from Experiment 1 showed a significant dose-related reduction in cue-induced reinstatement of active lever pressing in the 7.5 and 10 mg groups and an absence of the reinstatement effect in the 15 mg group. In experiment 2, animals treated with vehicle or 3.75 mg of SR 21502 showed significant heroin place preferences but those treated with the higher doses showed no CPP.
Conclusions
Our findings suggest that DA D3 receptors play a significant role in heroin approach behaviors driven by conditioned stimuli. As such, we propose that SR 21502 holds potential as an effective pharmacotherapeutic agent for relapse prevention and should be studied further.
Keywords: D3 receptor antagonist, Heroin, Addiction, Reward, Reinstatement
1. Introduction
Heroin, like other drugs of abuse, can activate the brain’s reward circuits and produce reinforcing and incentive motivational effects (Hubner and Kornetsky, 1992; Koob, 1992; Koob et al., 1975; Wise, 1996; Wise and Bozarth, 1982; Wise and Rompré, 1989; Zito et al., 1985). As such, heroin use can escalate into addiction, a state characterized by compulsive use, withdrawal and relapse (Himmelsbach, 1943; Koob and Le Moal, 1997; Nichols et al., 1956; Solomon, 1977).
A major problem in heroin addiction is the high rate of relapse, which is thought to be driven by incentive motivational (positive reinforcement; Bozarth and Wise, 1984) as well as aversive state (negative reinforcement) factors (Himmelsbach, 1943; Jaffe and Sharpless, 1968; Koob et al., 1989; Shaham et al., 1996; for review see Stewart et al., 1984; Wise and Koob, 2014). In heroin addicts, drug-related cues can support compulsive drug taking, elicit drug-associated physiological responses, prompt craving and trigger relapse (Childress et al., 1988; O’Brien et al., 1992, 1984; Sherman et al., 1989; Sideroff and Jarvik, 1980; Wikler, 1973). In animal models of addiction, opiate-associated cues can reinforce intravenous drug self-administration (Davis and Smith, 1976; Di Ciano and Everitt, 2004; Dymshitz and Lieblich, 1987), enhance locomotor activity (Mucha et al., 1981), facilitate the acquisition of opiate tolerance (Siegel, 1975), elicit conditioned place preference (Bardo et al., 1984; Bardo and Neisewander, 1986; Schenk et al., 1983) and reinstate drug seeking (McFarland and Ettenberg, 1997; Peck and Ranaldi, 2014; Schuster and Woods, 1968).
Several lines of evidence suggest that drug-related behaviors that are driven by cues (i.e., conditioned stimuli) require the stimulation of DA D3 receptors. First, blockade of DA D3 receptors reduces cue-induced reinstatement of nicotine (Aujla and Beninger, 2005; Khaled et al., 2010; Micheli et al., 2007), cocaine (Cervo et al., 2007; Galaj et al., 2014; Gilbert et al., 2005; Xi and Gardner, 2007), methamphetamine (Chen et al., 2014; Higley et al., 2011) or alcohol seeking (Vengeliene et al., 2006). Second, DA D3 receptor antagonists such as SB-277011A, NGB 2904, YQA14 or SR 21502 can reduce conditioned placed preference (CPP) established with nicotine (Micheli et al., 2007), cocaine (Cervo et al., 2005; Hachimine et al., 2014; Song et al., 2013; Vorel et al., 2002) or amphetamine (Aujla and Beninger, 2005). Blockade of DA D3 receptors also reduced the expression of morphine- (Frances et al., 2004; Hu et al., 2013) or heroin-induced CPP (Ashby et al., 2003; but see Duarte et al., 2003). The DA D3 antagonist, YQA14, also reduces the reactivation of morphine CPP (Hu et al., 2013). Third, exposure to drug cues can up-regulate DA D3 receptors, but only if these receptors are not blocked by antagonists during cue presentation (Le Foll et al., 2003, 2002). Context-specific behavioral sensitization arising from chronic morphine is associated specifically with up-regulation of nucleus accumbens DA D3 receptor mRNA and this behavioral sensitization can be reduced with intra-accumbens injections of SB-277011A (Liang et al., 2011). Cocaine cue-induced hyperlocomotion and accompanying cortical and limbic c-fos expression is reduced with DA D3 receptor antagonist treatment (Le Foll et al., 2002). Lastly, we have recently found that blockade of DA D3 receptors in the presence of the cocaine-associated environment of a CPP apparatus facilitates the extinction of an established cocaine CPP (Galaj et al., under review) demonstrating the importance of the role of D3 receptor stimulation in the maintenance of cue-driven behavior.
Thus, compelling evidence suggests that stimulation of DA D3 receptors is necessary for drug-related behaviors driven by conditioned cues. However, to our knowledge, no one has investigated the role of DA D3 receptors in cue-induced reinstatement of heroin seeking. Similar to psychostimulants, opiate cues have been shown to cause release of DA in the mesocorticolimbic system (Bassareo et al. 2007, 2011) and also similar to psychostimulants, DA antagonists can block the behavioral effects of opiate cues (Bossert et al. 2007, See 2009). This suggests an overlap in neural substrates, perhaps even in DA D3 receptor-related mechanisms, in the rewarding effects of psychostimulant and opiate cues. This leads us to hypothesize that DA D3 receptor stimulation is necessary for cue prompted heroin seeking and for approach behavior elicited by heroin cues. In the present set of experiments we tested these ideas specifically in regards to heroin cue-induced reinstatement of heroin seeking and expression of heroin CPP. We predicted that antagonism of DA D3 receptors, with SR 21502, would reduce cue-induced reinstatement of heroin seeking as well as the expression of heroin CPP.
2. METHODS
2.1 Subjects
The housing conditions and care of the animals were consistent with those specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). All experiments were approved by the Queens College Institutional Animal Care and Use Committee.
Subjects consisted of Long Evans rats obtained from our in-house colony bred from males and females obtained from Charles River Laboratories (Wilmington, MA, US). All animals were housed individually and maintained on a reversed 12:12 hour light/dark cycle (lights turned off 10 am). The rats weighed between 350–400 g and had free access to food and water. All experiments were conducted during the animal’s active period (dark cycle). Animals were handled for three days prior to experimentation.
2.2 Surgery
Prior to jugular vein catheterization each animal, weighing between 350 and 450 g, was injected intraperitoneally (IP) with 0.54 μg of atropine sulfate concentrated in 0.1 ml of distilled water and anesthetized with sodium pentobarbital (65 mg/kg, IP). A small incision was made on the neck to the right of the midline. The jugular vein was isolated, cleaned and opened and a silastic catheter (Dow Corning, Midland, MI) was inserted into it such that its tip penetrated to a position just short of the right atrium. The catheter was secured to the vein by tying sutures around it and its free end was passed subcutaneously to the back of the neck and exited through an incision made on the scalp. Next, the catheter was connected to a bent 22-gauge stainless steel tube that served as a connector between it and the fluid line; the connector was mounted to the rat’s skull using four stainless steel screws and dental acrylic. To maintain its patency, the catheter was filled with a heparin saline solution (200 U/ml) immediately after surgery and daily thereafter. The rats were allowed to recover for two to three days before self-administration training began.
2.3 Apparatus
2.3.1 Self-administration chambers
Heroin self-administration sessions were conducted in eight operant conditioning chambers, each measuring 26 x 26 x 30 cm (l x w x h) and housed in a sound-attenuating ventilated box. Each chamber was equipped with two retractable levers, a white light above each lever and a drug line consisting of a metal tether covering a polyethylene tubing which, through a fluid swivel, was connected to a syringe pump (Razel, 3.33 rpm) loaded with a 20 ml syringe.
2.3.2 Conditioned place preference chambers
Conditioning took place in six conditioned place preference (CPP) chambers, each placed in a sound-attenuating ventilated box equipped with a fan that provided both ventilation and a constant source of masking noise. CPP chambers, measuring 43 x 43 x 30 cm, consisted of two compartments with distinct walls and floors (each chamber had a different combination of white or striped walls; rod or grid floor). The two compartments were separated by a removable partition that was removed during the pre-exposure and test sessions. The CPP chambers were equipped with photo-beam detectors tracking the position of the rats in one compartment or the other.
2.4 Drugs
Heroin (a generous gift from NIDA) was dissolved in 0.9% physiological saline to achieve a dose of 0.05 mg/kg for the self-administration experiment and a dose of 1.0 mg/kg for the CPP experiment. The selective DA D3 antagonist, SR 21502 (a gift from Southern Research Institute), was dissolved in distilled water to achieve the doses of 3.75, 7.5, 10 and 15 mg/kg and was injected by the intraperitoneal (IP) route. These doses were chosen because they produced significant behavioral effects in our previous studies (Galaj et al., 2014; Hachimine et al., 2014).
2.5 Procedures
2.5.1 Experiment 1: Cue-induced reinstatement of heroin seeking
Experiment 1 consisted of three phases: self-administration, extinction and the reinstatement test. Animals were trained to self-administer heroin (0.05 mg/kg/injection) under a fixed ratio 1 (FR1) schedule of reinforcement during daily 3-h sessions. Responding on the active lever activated the injection pump for 4.5 s and turned on the light cue above the active lever for 20 s. The 20-s period constituted a time-out period during which active lever presses did not activate the pump or cue light. Responding on the inactive lever was counted but had no programmed consequences. The active and inactive levers were counterbalanced across animals and remained constant for the duration of the experiment. After the animals showed stable responding (defined as three consecutive sessions where the total number of infusions taken per session did not vary by more than ±10% of the mean of the three sessions and with no ascending or descending trends) they were allowed to self-administer heroin for an additional 15 sessions. This was followed by the extinction phase consisting of 15 sessions. Each extinction session was 3 h long and responding on either lever produced no consequences; heroin was not delivered and heroin-related cues (light/ pump activation) were not presented. The cue-induced reinstatement test occurred one day following the last extinction session. Twenty minutes before the test, animals were injected with one of several SR 21502 doses [vehicle (n=10), 7.5 (n=10), 10 (n=9) or 15 (n=9) mg/kg]. At the beginning of the session the heroin-related cue (20-s presentation of the light above the active lever and 4.5-s activation of the syringe pump) were presented twice, each 2 min apart. Each response on the active lever was reinforced with the drug cues (light/pump activation) but heroin was not delivered. Responding on the inactive lever produced no consequences. The reinstatement test session lasted 60 min.
To test the possibility that any observed effects of SR 21502 on cue-induced reinstatement of lever pressing may be due to motoric effects, specifically on lever pressing, we evaluated the effects of SR 21502 lever pressing reinforced by food, a procedure that produces many times more lever pressing than the reinstatement procedure. A group of 10 rats were trained to lever pressed reinforced by food pellets under a progressive ratio schedule of reinforcement using our standard procedure (see Galaj et al., 2014). After rats demonstrated stable responding they were treated with the 15 mg/kg dose of SR 21502 and the number of lever presses during 60 min, the same period of time as in the reinstatement test, was recorded for each rat.
2.5.2 Experiment 2: Heroin conditioned place preference
Baseline preferences were assessed by placing animals in the CPP apparatus and allowing them free access to both compartments for 15 min and recording the time spent in each. Based on the initial preference half of the animals were conditioned with heroin to their preferred compartment and the remaining half to the non-preferred compartment. During conditioning animals were injected with heroin intraperitoneally (IP) 5 minutes prior to the session and then placed in one of the two compartments. On four alternate days animals received a saline injection (IP) and were placed in the other compartment. There were a total of 8 conditioning sessions, each 30 min long and held one per day. One day following the last conditioning session the rats were tested for the conditioned place preference. Twenty minutes prior to the test session the rats were injected with one of the doses of SR 21502 (vehicle, 3.75, 7.5, 10 and 15 mg/kg, N=10 for each group) and then placed in the CPP apparatus. The dividing partition was removed and the animals had access to both compartments for 15 min.
2.6 Data Analysis
For Experiment 1 the data consisted of the average number of active and inactive lever presses during the first 60 min of each of the last three extinction sessions and the number of active and inactive lever presses during the 60-min reinstatement test. Groups were compared on lever pressing across extinction without cues and reinstatement with cues by analyzing active and inactive lever presses using a three-way (cue x lever x dose) analysis of variance (ANOVA). A significant three-way interaction was followed by cue by lever interaction comparisons at each level of dose and by lever by dose interaction comparisons at each level of cue (extinction and reinstatement). Significant two-way interactions were followed by tests of simple effect of dose at each level of lever. Significant simple effects were followed by post hoc Dunnett’s tests. To correct for multiple comparisons we used a conservative alpha level of .01. In experiment 2, we measured time spent in the heroin compartment during the pre-exposure and test sessions. Planned comparisons, consisting of repeated measures t-tests, were used to analyze the time spent in the heroin compartment during the pre-exposure and test sessions for each group. To correct for these multiple planned comparisons we used a conservative alpha level of .01 to determine significance.
3. RESULTS
3.1 Experiment 1: Heroin cue-induced reinstatement
The left panel of Fig. 1 shows responding on the active and inactive levers averaged across the first hour of each of the last three extinction sessions in all rats grouped by the dose of SR 21502 that they would eventually be tested with. Responding on the active lever was similar among all dose groups. Further, responding was higher on the active than on the inactive lever in all dose groups (see Fig. 1, left panel). The right panel of Fig. 1 shows responding on the active and inactive levers during the reinstatement test. In all groups responding increased in the reinstatement test compared to extinction sessions and the increases on the active lever were greater than on the inactive lever. However, animals treated with SR 21502 showed less responding on the active lever than animals treated with vehicle. This lower responding was related to the dose of SR 21502; the greater the dose, the less active lever responding that was observed (see Fig. 2, right panel). Responding on the inactive lever during the reinstatement test was similar in all dose groups and always less than responding on the active lever. Statistical analysis with a three-way ANOVA [cue (no cue: extinction, cue: reinstatement) and lever as repeated measures factors and SR 21502 dose as a between-groups factor] revealed a significant cue by lever by dose interaction [F (3, 34) = 4.28, p < .01]. Cue by lever interaction comparisons at each level of dose revealed significant interactions at all doses except the 15 mg dose [Fs (1,34) = 40.72, 10.35 and 6.05, all ps < .01, for the vehicle, 7.5 and 10 mg doses, respectively]. Tests of simple effect of cue at each level of lever revealed significant cue effects on the active lever in the vehicle [F(1,34) = 109.37], 7.5 mg [F(1,34) = 33.68] and 10 mg [F(1,34) = 29.13] dose groups (all ps < .01). These analyses suggest that the vehicle, 7.5 and 10 mg groups showed reinstatement effects (cue by lever interactions) and that the 15 mg group did not. However, they do not inform on whether or not the 7.5 and 10 mg groups show significantly reduced reinstatement effects compared to the vehicle group, something that the right panel of Fig. 1 suggests and something that is an important aspect of this study. Therefore, to further explore the cue by lever by group interaction we conducted lever by dose interaction comparisons at each level of cue (extinction and reinstatement). These analyses revealed a significant lever by dose interaction in the cue (reinstatement) condition [F (3, 34) = 8.65, p < .01, but not in the no cue (extinction) condition. Tests of simple effect of dose at each level of lever revealed a significant dose effect at the active lever [F (3,34)=25.72, p< .01] but not at the inactive lever. Dunnett’s tests revealed that responding on the active lever was significantly lower in 7.5, 10 and 15 mg groups than in the vehicle group, ps < .01.
Figure 1.
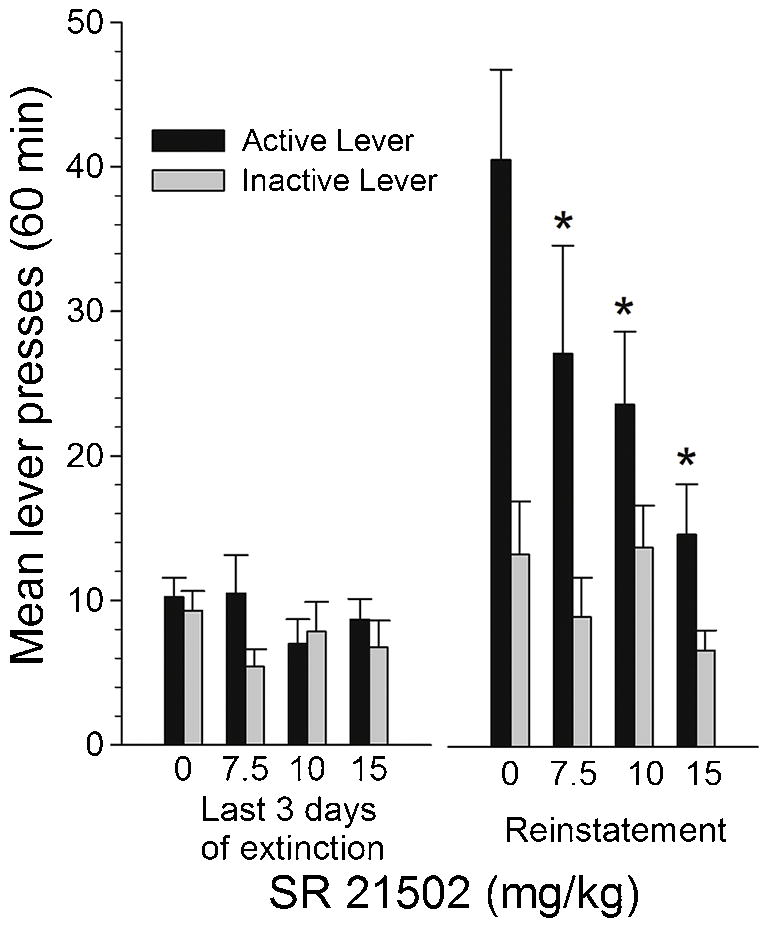
Left panel: Mean (± SEM) number of presses on the active and inactive levers averaged across the last three extinction sessions far all animals separated into eventual SR 21502 dose groups. Right panel: Mean (± SEM) number of presses on the active and inactive levers during the reinstatement test for all groups treated with vehicle or a dose of SR 21502. * represents active lever pressing significantly different from vehicle during reinstatement at p < .01.
Figure 2.
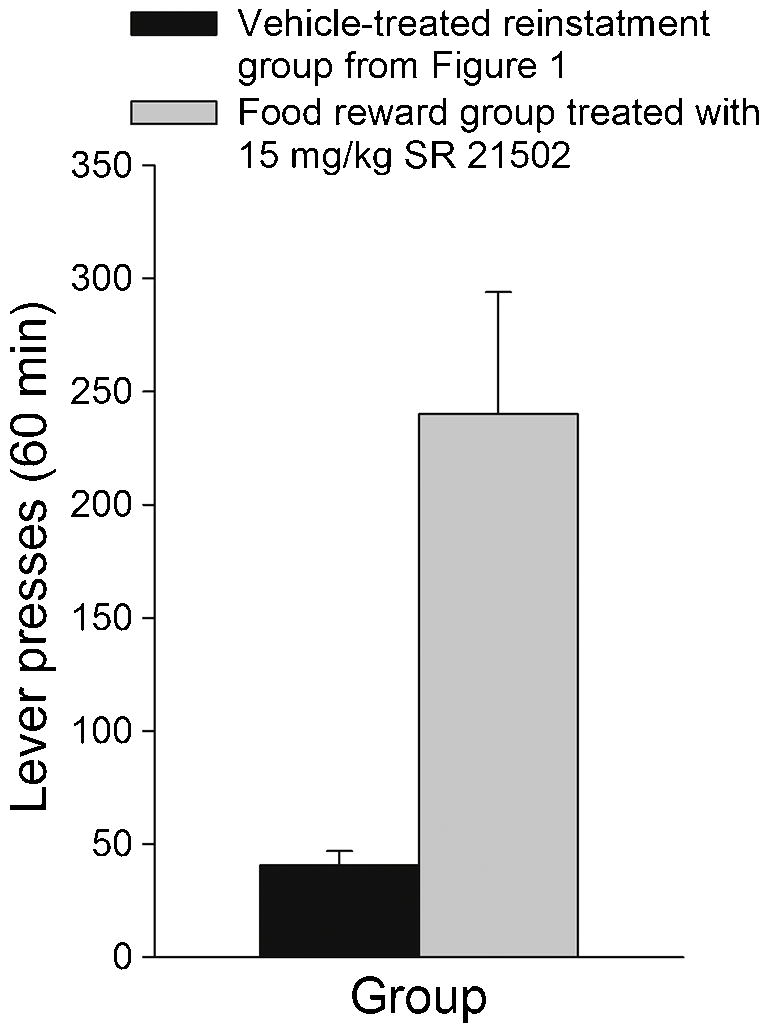
Mean (± SEM) number of lever presses for the same vehicle-treated reinstatement group shown in Figure 1 and a group lever pressing under a progressive ratio schedule of food reinforcement and treated with the 15 mg/kg dose of SR 21502 .
Fig. 2 shows that animals treated with the 15 mg/kg dose of SR 21502 could make at least as many, and in fact many times more, lever presses than the vehicle-treated group in the reinstatement experiment during a similar 60-min period. The numbers of responses for individual rats in the SR 21502-treated food reward group ranged from 96 to 679, all of which were higher than for any of the animals in the vehicle-treated reinstatement group.
3.2 Experiment 2: Heroin conditioned place preference
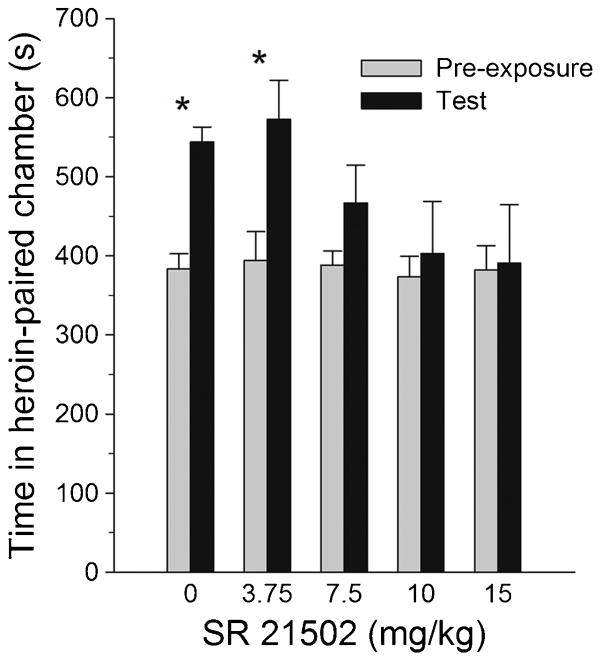
During the pre-exposure session all groups spent the same amount of time in the CPP compartment that would later be paired with heroin. The groups treated with vehicle or the 3.75 mg/kg dose of SR 21502 prior to the test session spent more time in the heroin-paired compartment during the test session than they did during the pre-exposure session. In contrast, the 7.5 mg group spent less time in the heroin side than did the 0 and 3.75 mg groups and the 10 and 15 mg groups spent as much time in the heroin side during the test as they did during pre-exposure (see Fig. 3). These observations were confirmed by the planned comparisons. Separate repeated measures t-tests revealed significant phase effects (preference) for the vehicle and 3.75 mg groups, t(9) = −6.7 and t(9) = −3.27, ps <.01, respectively, but not for the 7.5, 10 and 15 mg groups.
Figure 3.
Mean (± SEM) time spent in the heroin-paired compartment of the CPP apparatus during pre-exposure and preference test. * represents significantly more time spent in the heroinpaired environment after the conditioning compared to pre-exposure (ps < .01).
4. DISCUSSION
During cue-induced reinstatement, rats treated with SR 21502 showed a dose-related reduction in active lever pressing compared to vehicle-treated rats, suggesting a reduction in the cue-induced reinstatement effect. The group treated with the highest SR 21502 dose showed responding during the reinstatement test that was similar to during extinction, suggesting an absence of the reinstatement effect. A different group of animals treated with the 15 mg/kg dose of SR 21502 but pressing for food reward demonstrated that rats under the influence of the highest SR 21502 dose used here can press the lever at least as often, and on average about 5 times more, than the vehicle-treated reinstatement group, suggesting that reductions in lever pressing in SR 21502 reinstatement groups were not due to motoric effects on lever pressing. Instead, the effects of SR 21502 were more likely incentive motivational. Therefore, we conclude that blockade of DA D3 receptors reduces, and at higher doses can eliminate, cue-induced reinstatement of heroin seeking. Furthermore, SR 21502 attenuated and eliminated the expression of heroin CPP such that rats treated with higher doses of SR 21502 spent equal amounts of time in the heroin-paired compartment during the pre-exposure and test sessions. Again, we interpret these as incentive motivational reductions in the effects of heroin-associated stimuli.
Our results suggest that heroin-related behaviors driven by conditioned cues require stimulation of DA D3 receptors. This is a unique finding given that the focus in DA D3 receptor research has been in relation to stimulant- – primarily cocaine – related behavior. DA D3 receptor antagonists and partial agonists have the capacity to reduce stimulant self-administration maintained on a higher fixed ratio schedule (Ross et al., 2007; Xi et al., 2005), a second-order schedule (Di Ciano et al., 2008; Xi et al., 2006) or progressive ratio schedule (Chen et al., 2014; Galaj et al., 2014; Higley et al., 2011; Song et al., 2011). DA D3 receptor agents also can reduce cue-induced reinstatement of stimulant seeking (Aujla and Beninger, 2005; Cervo et al., 2007; Chen et al., 2014; Galaj et al., 2014; Gilbert et al., 2005; Higley et al., 2011; Khaled et al., 2010; Micheli et al., 2007; Xi and Gardner, 2007). In a number of studies, DA D3 receptor agents reduce the expression of stimulant- (Aujla and Beninger, 2005; Cervo et al., 2005; Hachimine et al., 2014; Song et al., 2013; Vorel et al., 2002) and opiate-induced CPP (Ashby et al., 2003; Frances et al., 2004; Hu et al., 2013), suggesting that DA D3 receptors are necessary for stimulant and opiate-related behavior controlled by conditioned cues. Here, we provide evidence that DA D3 receptor stimulation also is necessary for reinstatement of heroin seeking and expression of heroin CPP, behaviors elicited by heroin-conditioned cues and contexts.
There is some evidence suggesting that opiate conditioned cues, just like opiates, acquire the ability to activate the reward mesolimbic DA system (Bozarth and Wise, 1986; Matthews and German, 1984; Wise et al., 1995). DA neurons fire in response to heroin-related cues (Kiyatkin and Rebec, 2001), a result of which DA is released in the nucleus accumbens and prefrontal cortex (Bassareo et al., 2007, 2011). Rats can discriminate morphine from saline and self-administer the drug into the VTA or nucleus accumbens upon presentation of drug cues, suggesting that morphine-related cue effects are mediated by mesolimbic sites (Shoaib and Spanagel, 1994). Blockade of DA D1-family receptors reduces context- and cue-induced reinstatement of heroin seeking (Bossert et al. 2007, 2009;) as well as morphine CPP (Acquas et al., 1989), implicating the DA mesolimbic system in opiate-related behavior driven by cues. Furthermore, opiates and opiate conditioned cues can induce the expression of c-fos and other immediate-early genes in the mesolimbic system (Bontempi and Sharp, 1997; Koya et al., 2006; Liu et al., 1994). The activation of these cortico-limbic regions in response to heroin cues has been also shown by functional imaging studies (Li et al., 2012; Sell et al. 1999, 2000). Altogether, this suggests that the present effects of SR 21502 on heroin cue-mediated behavior might occur through inhibition of heroin-cue enhancement of mesolimbic DA activity. This adds support for the idea that heroin cues, just like psychostimulant and natural reward (e.g., food, sex) cues (Ettenberg and Camp, 1986; Gerber et al., 1981; Hernandez and Hoebel, 1988; Pfaus et al., 1995), mediate at least some of their behavioral effects through enhancement of DA neurotransmission in the mesocorticolimbic DA system (Bassareo et al., 2007, 2011).
It appears that DA D3 receptor stimulation may not only play a role in opiate cue-induced behaviors but also in opiate-induced behaviors. In support of this claim are the findings that mice chronically treated with morphine show up-regulation of DA D3 receptor mRNA in the mesolimbic system (Spangler et al., 2003). DA D3 receptor knockout mice do not show a deficit in heroin-induced sensitization (Li et al., 2010) while wild-type mice treated with the DA D2/D3 antagonist, nafadotride, or the partial DA D3 receptor agonist, BP897, show a reduction in morphine-induced sensitization (Cook and Beardsley, 2003; Li et al., 2010). In contrast, others report that genetically modified mice with DA D3 receptor deletions show enhancement of morphine-induced locomotor activity (Narita et al., 2003). The discrepancy in these findings might be explained by the developmental changes that occur in genetically modified animals to compensate for the absence of DA D3 receptors. In addition, the DA D3 receptor antagonist, NGB 2904, inhibits heroin-enhanced brain stimulation reward, although the effect is small (Xi and Gardner, 2007). And lastly, the DA D3 antagonist, SB-277011A, and the partial DA D3 receptor agonist, BP897, reduce the expression of opiate-induced CPP (Ashby et al., 2003; Frances et al., 2004; Hu et al., 2013).
In conclusion, we have demonstrated the importance of DA D3 receptor stimulation in cue-induced reinstatement of heroin seeking and expression of heroin CPP. Antagonism of DA D3 receptors with SR 21502 significantly reduced, and at the highest doses eliminated, heroin seeking and the preference for a heroin-associated environment. Therefore, we provide additional evidence that SR 21502, a selective DA D3 receptor antagonist, is effective in reducing drug-seeking behavior (heroin and cocaine; Galaj et al., 2014) and conditioned responses driven by drug cues (Hachimine et al., 2014). As such, we propose that SR 21502 holds potential as an effective pharmacotherapeutic agent for relapse prevention and should be studied further.
Highlights.
The selective dopamine D3 receptor antagonist, SR 21502, blocks cue-induced reinstatement of heroin seeking
The selective dopamine D3 receptor antagonist, SR 21502, blocks expression heroin conditioned place preference
SR 21502 may have potential as an anti-relapse treatment
Acknowledgments
Role of Funding
This research was supported in part by NIH Grant R01DA024675 (to S.A.) from the National Institute on Drug Abuse (NIDA).
Footnotes
Conflict of Interest
No conflict declared.
Contributors
Ewa Galaj participated in the design and analysis of the study, was the principle collector of the data and wrote the complete first draft of the manuscript and all revisions. Monica Manuszak and Sandra Babic participated in data collection and assisted in editing the manuscript. Subramaniam Ananthan designed and synthesized the test compound (SR 21502) and assisted in writing the manuscript. Robert Ranaldi served as principle investigator, participated in the design and analysis of the study and assisted in writing all drafts of the manuscript. All authors have approved the final version of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acquas E, Carboni E, Leone P, Di Chiara G. SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology (Berl) 1989;99:151–155. doi: 10.1007/BF00442800. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jr, Paul M, Gardner EL, Heidbreder CA, Hagan JJ. Acute administration of the selective D3 receptor antagonist SB-277011A blocks the acquisition and expression of the conditioned place preference response to heroin in male rats. Synapse. 2003;48:154–156. doi: 10.1002/syn.10188. [DOI] [PubMed] [Google Scholar]
- Aujla H, Beninger RJ. The dopamine D(3) receptor-preferring partial agonist BP 897 dose-dependently attenuates the expression of amphetamine-conditioned place preference in rats. Behav Pharmacol. 2005;16:181–186. doi: 10.1097/00008877-200505000-00007. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Miller JS, Neisewander JL. Conditioned place preference with morphine: the effect of extinction training on the reinforcing CR. Pharmacol Biochem Behav. 1984;21:545–549. doi: 10.1016/s0091-3057(84)80037-4. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Neisewander JL. Single-trial conditioned place preference using intravenous morphine. Pharmacol Biochem Behav. 1986;25:1101–1105. doi: 10.1016/0091-3057(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Musio P, Di Chiara G. Reciprocal responsiveness of nucleus accumbens shell and core dopamine to food- and drug-conditioned stimuli. Psychopharmacology (Berl) 2011;214:687–697. doi: 10.1007/s00213-010-2072-8. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Sharp FR. Systemic morphine-induced Fos protein in the rat striatum and nucleus accumbens is regulated by mu opioid receptors in the substantia nigra and ventral tegmental area. J Neurosci. 1997;17:8596–85612. doi: 10.1523/JNEUROSCI.17-21-08596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. NIDA Res Monogr. 1986;67:190–196. [PubMed] [Google Scholar]
- Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Cervo L, Cocco A, Petrella C, Heidbreder CA. Selective antagonism at dopamine D3 receptors attenuates cocaine-seeking behaviour in the rat. Int J Neuropsychol. 2007;10:167–181. doi: 10.1017/S1461145705006449. [DOI] [PubMed] [Google Scholar]
- Chen Y, Song R, Yang RF, Wu N, Li J. A novel dopamine D3 receptor antagonist YQA14 inhibits methamphetamine self-administration and relapse to drug-seeking behaviour in rats. Eur J Pharmacol. 2014;743:126–132. doi: 10.1016/j.ejphar.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Cook CD, Beardsley PM. The modulatory actions of dopamine D2/3 agonists and antagonists on the locomotor-activating effects of morphine and caffeine in mice. Pharmacol Biochem Behav. 2003;75:363–371. doi: 10.1016/s0091-3057(03)00090-x. [DOI] [PubMed] [Google Scholar]
- Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Bio Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose, implications for the persistence of addictive behaviour. Neuropharmacology. 2004;47:202–213. doi: 10.1016/j.neuropharm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiebot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- Dymshitz J, Lieblich I. Opiate reinforcement and naloxone aversion, as revealed by place preference paradigm, in two strains of rats. Psychopharmacology (Berl) 1987;92:473–477. doi: 10.1007/BF00176481. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. A partial reinforcement extinction effect in water-reinforced rats intermittently treated with haloperidol. Pharmacol Biochem Behav. 1986;25:1231–1235. doi: 10.1016/0091-3057(86)90117-6. [DOI] [PubMed] [Google Scholar]
- Frances H, Le Foll B, Diaz J, Smirnova M, Sokoloff P. Role of DRD3 in morphine-induced conditioned place preference using drd3-knockout mice. Neuroreport. 2004;15:2245–2249. doi: 10.1097/00001756-200410050-00021. [DOI] [PubMed] [Google Scholar]
- Galaj E, Ananthan S, Saliba M, Ranaldi R. The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology. 2014;231:501–510. doi: 10.1007/s00213-013-3254-y. [DOI] [PubMed] [Google Scholar]
- Galaj E, Haynes J, Nisanov N, Ananthan S, Ranaldi R. The dopamine D3 receptor antagonist, SR 21502, facilitates extinction of cocaine conditioned place preference. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Sing J, Wise RA. Pimozide attenuates lever pressing for water reinforcement in rats. Pharmacol Biochem Behav. 1981;14:201–205. doi: 10.1016/0091-3057(81)90243-4. [DOI] [PubMed] [Google Scholar]
- Gilbert JG, Newman AH, Gardner EL, Ashby CR, Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–28. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachimine P, Seepersad N, Ananthan S, Ranaldi R. The novel dopamine D3 receptor antagonist, SR 21502, reduces cocaine conditioned place preference in rats. Neurosci Lett. 2014;569:137–41. doi: 10.1016/j.neulet.2014.03.055. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Higley AE, Spiller K, Grundt P, Newman AH, Kiefer SW, Xi ZX, Gardner EL. PG01037, a novel dopamine D3 receptor antagonist, inhibits the effects of methamphetamine in rats. J Psychopharmacol. 2011;25:263–273. doi: 10.1177/0269881109358201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelsbach C. Morphine, with reference to physical dependence. Fed Proc. 1943;2:201–203. [Google Scholar]
- Hu R, Song R, Yang R, Su R, Li J. The dopamine D3 receptor antagonist YQA14 that inhibits the expression and drug-primed reactivation of morphine-induced conditioned place preference in rats. Eur J Pharmacol. 2013;720:212–217. doi: 10.1016/j.ejphar.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Kornetsky C. Heroin, 6-acetylmorphine and morphine effects on threshold for rewarding and aversive brain stimulation. J Pharmacol Exp Ther. 1992;260:562–567. [PubMed] [Google Scholar]
- Jaffe JH, Sharpless SK. XVII. Pharmacological denervation supersensitivity in the central nervous system, a theory of physical dependence. Res Publ Assoc Res Nerv Ment Dis. 1968;46:226–246. [PubMed] [Google Scholar]
- Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacol. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Rebec GV. Impulse activity of ventral tegmental area neurons during heroin self-administration in rats. Neuroscience. 2001;102:565–580. doi: 10.1016/s0306-4522(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Spector NH, Meyerhoff JL. Effects of heroin on lever pressing for intracranial self-stimulation, food and water in the rat. Psychopharmacologia. 1975;42:231–234. doi: 10.1007/BF00421261. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Increased dopamine D3 receptor expression accompanying behavioral sensitization to nicotine in rats. Synapse. 2003;47:176–183. doi: 10.1002/syn.10170. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, Wu N, Chang H, Zheng Y, Qin W, Zhao L, Yuan K, Liu J, Wang W, Tian J. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012;1469:63–72. doi: 10.1016/j.brainres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Li T, Hou Y, Yan CX, Chen T, Zhao Y, Li SB. Dopamine D3 receptor knock-out mice display deficits in locomotor sensitization after chronic morphine administration. Neurosci Lett. 2010;485:256–260. doi: 10.1016/j.neulet.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Liang J, Zheng X, Chen J, Li Y, Xing X, Bai Y, Li Y. Roles of BDNF, dopamine D(3) receptors, and their interactions in the expression of morphine-induced context-specific locomotor sensitization. Eur Neuropsychopharmacol. 2011;21:825–834. doi: 10.1016/j.euroneuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Liu J, Nickolenko J, Sharp FR. Morphine induces c-fos and junB in striatum and nucleus accumbens via D1 and N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A. 1994;91:8537–8541. doi: 10.1073/pnas.91.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl) 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Fabio RD, Donati D, Gentile G, Gribble A, et al. 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem. 2007;50:5076–5089. doi: 10.1021/jm0705612. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Volkovskis C, Kalant H. Conditioned increases in locomotor activity produced with morphine as an unconditioned stimulus, and the relation of conditioning to acute morphine effect and tolerance. J Comp Physiol Psychol. 1981;95:351–362. doi: 10.1037/h0077778. [DOI] [PubMed] [Google Scholar]
- Narita M, Mizuo K, Mizoguchi H, Sakata M, Tseng LF, Suzuki T. Molecular evidence for the functional role of dopamine D3 receptor in the morphine-induced rewarding effect and hyperlocomotion. J Neurosci. 2003;23:1006–1012. doi: 10.1523/JNEUROSCI.23-03-01006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JR, Headlee CP, Coppock HW. Drug addiction. I Addiction by escape training. J Am Pharm Assoc. 1956;45:788–791. [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Ehrman RN, Ternes JW. Classical conditioning in opiate dependence. NIDA Res Monogr. 1984;49:35–46. [PubMed] [Google Scholar]
- Peck JA, Ranaldi R. Drug abstinence: exploring animal models and behavioral treatment strategies. Psychopharmacology (Berl) 2014;231:2045–2058. doi: 10.1007/s00213-014-3517-2. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Ross JT, Corrigall WA, Heidbreder CA, LeSage MG. Effects of the selective dopamine D3 receptor antagonist SB-277011A on the reinforcing effects of nicotine as measured by a progressive-ratio schedule in rats. Eur J Pharmacol. 2007;559:173–179. doi: 10.1016/j.ejphar.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Colle L, Amit Z. Isolation versus grouped housing in rats: differential effects of low doses of heroin in the place preference paradigm. Life Sci. 1983;32:1129–1134. doi: 10.1016/0024-3205(83)90118-2. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Woods JH. The conditioned reinforcing effects of stimuli associated with morphine reinforcement. Int J Addict. 1968;3:223–230. [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JE, Zinser MC, Sideroff SI, Baker TB. Subjective dimensions of heroin urges: influence of heroin-related and affectively negative stimuli. Addict Behav. 1989;14:611–623. doi: 10.1016/0306-4603(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R. Mesolimbic sites mediate the discriminative stimulus effects of morphine. Eur J Pharmacol. 1994;252:69–75. doi: 10.1016/0014-2999(94)90576-2. [DOI] [PubMed] [Google Scholar]
- Sideroff SI, Jarvik ME. Conditioned responses to a videotape showing heroin-related stimuli. Int J Addict. 1980;15:529–536. doi: 10.3109/10826088009040035. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Solomon RL. An opponent-process theory of acquired motivation: the effective dynamics of addiction. In: Masser JD, Seligman MEP, editors. Psychopathology: Experimental Models. W.H. Freeman and Company; San Francisco: 1977. pp. 124–145. [Google Scholar]
- Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL. YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol. 2011;17:14. doi: 10.1111/j.1369-1600.2011.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Zhang HY, Peng XQ, Su RB, Yang RF, Li J, Xi ZX, Gardner EL. Dopamine D3 receptor deletion or blockade attenuates cocaine-induced conditioned place preference in mice. Neuropharmacology. 2013;72:82–87. doi: 10.1016/j.neuropharm.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Elevated D3 dopamine receptor mRNA in dopaminergic and dopaminoceptive regions of the rat brain in response to morphine. Molec Brain Res. 2003;111:74–83. doi: 10.1016/s0169-328x(02)00671-x. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;19:251–268. [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, Gebicke-Haerter P, Drescher K, Gross G, Spanagel R. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB J. 2006;20:2223–2233. doi: 10.1096/fj.06-6110com. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Action of drugs of abuse on brain reward systems: an update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17:239–243. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbredr CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, Gitajn L, Gardner EL. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Zito KA, Vickers GJ, Roberts DCS. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]