Abstract
Objective
The SELF Trial examined the effect of adding individual, self-efficacy (SE) enhancement sessions to standard behavioral weight loss treatment (SBT).
Methods
Participants were randomly assigned to SBT or SBT plus SE sessions (SBT+SE). Outcome measures were weight loss maintenance, quality of life, intervention adherence and self-efficacy at 12 and 18 months.
Results
The sample (N=130) was female (83.08%) with a mean (SD) body mass index of 33.15 (4.11) kg/m2. There was a significant time effect for percent weight change (p=.002), yet no significant group or group-by-time effects. The weight loss for the SBT+SE group was 8.38% (7.48) at 12 months and 8.00% (7.87) at 18 months, with no significant difference between the two time points (p=.06). However, weight loss for the SBT group was 6.95% (6.67) at 12 months and 5.96% (7.35) at 18 months, which was significantly different between the two time points (p=.005) indicating that the SBT group had significant weight regain.
Conclusions
Both groups achieved clinically significant weight loss. The group receiving an intervention targeting enhanced self-efficacy had greater weight loss maintenance whereas the SBT group demonstrated significant weight regain possibly related to the greater attention provided to the SBT+SE group.
Keywords: behavioral weight loss, self-efficacy, obesity, weight loss maintenance
Introduction
Obesity is a chronic health problem associated with an extremely high rate of relapse.1,2 The greatest challenge in obesity treatment is to identify strategies to improve long-term weight loss maintenance. Approximately 80% of adults who intentionally lose weight regain 50% of the weight within a year.3,4 Standard behavioral treatment (SBT) protocols have been used in clinical trials of weight loss for nearly two decades with little improvement in the rates of weight loss maintenance.5-9 However, increasing evidence that self-efficacy is a factor that influences maintenance makes it a research focus.10-12 The premise of self-efficacy is that one’s confidence will determine their ability to initiate and continue in a specific behavior. The strength of perceived self-efficacy is particularly important; individuals are more likely to continue their efforts until success is achieved if their perceived self-efficacy is higher.13
Earlier work by our group demonstrated that self-efficacy improved significantly during an 18-month standard behavioral intervention trial for weight loss and that self-efficacy was significantly associated with weight loss.12 However, in this same trial, we observed self-efficacy decreasing over time. Others have reported that improved self-efficacy led to increased fruit and vegetable intake14, that changes in self-efficacy scores mediated the effects of weight loss combined with physical activity, and that cross-sectionally, eating and exercise self-efficacy beliefs were strongly associated with concurrent weight loss behaviors; moreover, these beliefs predicted weight control and weight change during active treatment but not subsequently.15,16 These findings suggest that it is possible to increase self-efficacy; however, it has not been demonstrated whether increased self-efficacy can be sustained and improve weight loss maintenance. The current study intervention was designed to enhance participants’ perception of their capability to make and sustain lifestyle changes by intentionally reinforcing mastery performance–the strongest source of self-efficacy enhancement.17,18 Our hypothesis is that a supplemental intervention to increase one’s self-efficacy for making lifestyle changes would enhance weight loss maintenance, health-related quality of life, and adherence to treatment.
Methods
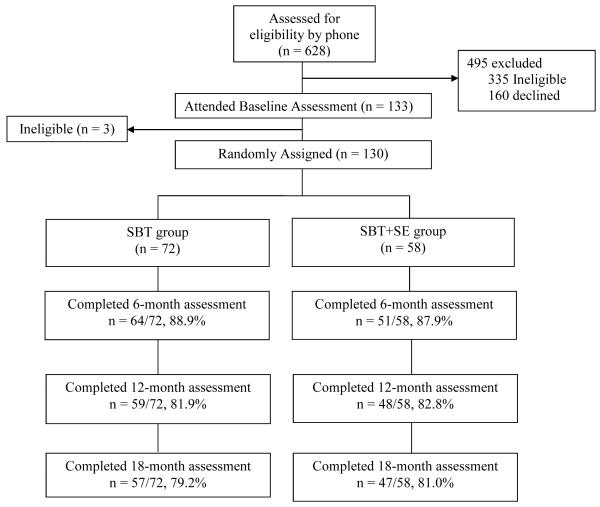
Design of the Self-Efficacy Lifestyle Focus (SELF) Trial was a 2-group, single center, 18-month clinical trial targeting weight-loss maintenance (see Figure 1). The University of Pittsburgh Institutional Review Board approved this study; all participants provided informed consent. Participant Recruitment and Screening
Figure 1.
CONSORT Figure
Table 1 lists the inclusion and exclusion criteria. The screening protocol was used in previous trials. We recruited from the Greater Pittsburgh area to increase the diversity of the sample, e.g., purchased mailing lists with zip codes that included underrepresented groups. We narrowed the pool of potential participants through a 5-step screening process: 1. Telephone screening (e.g., age); 2. Mailed questionnaire packet (including health history); 3. In-person body mass index (BMI) verification and orientation; 4. Test run of 5-day self-monitoring; 5. Physician clearance.
Table 1.
Inclusion and Exclusion Criteria for the SELF Trial
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Randomization
Randomization used the minimization method
Treatment assignments were determined considering gender and ethnicity (White vs. non-White) to ensure balance across the treatment groups.
Interventions
Participants in the SBT group received the same standard calorie, fat gram, physical activity and self-monitoring goals used in previous trials19,20 and that are part of established SBT today.21 The calorie goal was based on weight and gender (women: 1200 kcal for <200 lbs. or 1500 kcal for ≥200 lbs.; men: 1500 kcal for <200 lbs. or 1800 kcal for ≥200 lbs.). Participants were asked to limit their fat intake to 25% of their total calories and to self-monitor their calorie and fat intake. The home-based physical activity goal advanced from 150 minutes/week at 12 weeks to 180 minutes/week at 6 months, then 210 minutes/week at 12 months. Participants also were asked to record minutes of physical activity and number of daily steps using the pedometer provided.
Participants in the SBT+SE received an SBT weight loss intervention supplemented with 30 self-efficacy enhancing, one-on-one sessions. Participants in the SBT+SE group met with their interventionist prior to the first group session to collaboratively develop their calorie and activity goals with a target date for goal achievement. Lack of progress towards goals triggered an identification of possible barriers and engaged the participant in problem-solving.22 Each individual session began with participants completing a one-page assessment of perceived self-efficacy and the level of importance for the behavior change on a scale of 0-10. Interventionists were trained to use motivational interviewing strategies if the participant expressed ambivalence about the behavior change.23
During the first 12 months, one-on-one meetings were held every two weeks to review progress and establish new diet and activity goals; thereafter, sessions were held at least monthly. Sources of self-efficacy were incorporated through collaborative goal-setting of specific, proximal and attainable goals; cognitive behavioral and problem solving strategies were offered to increase the participant’s confidence in attaining the newly established goal; vicarious experience occurred by using credible models to demonstrate behavior change (e.g., Leslie Sansone™ exercise tapes) and physiological cues (e.g., less fatigue with physical exertion) were highlighted as evidence that supported behavior changes were occurring. Telephone sessions were available as an alternative to in-person at participant request; 11% of the sessions were conducted by phone. To ensure treatment fidelity, all one-on-one sessions were audio-recorded. A random sampling of 10% of these sessions was reviewed first by one investigator and then, a second investigator reviewed a 10% subset. Issues revealed during reviews were discussed at weekly intervention team meetings and additional training was provided if indicated. Individual sessions lasted, on average, 23 minutes.
Group intervention sessions
All participants attended group sessions weekly the first month, bi-weekly the second month, monthly for next ten months, and every 6 weeks for months 13-18. Prior to the group session, participants were weighed in a private room and submitted self-monitoring diaries and pedometers for data uploading. Interventionists reviewed, recorded feedback and returned the diaries to participants at the next session. Group sessions covered nutrition and reinforced principles of behavior change (goal setting, self-monitoring, feedback).4 Individual goal setting was not conducted during the group sessions; goal adjustments for SBT group participants were provided by written feedback on their paper diaries. Group sessions lasted one hour. To ensure treatment fidelity, the study project director attended and evaluated a random sample of 10% of these sessions.
All interventionists were Master’s trained health professionals with previous experience in SBT. Each interventionist led a specific group in each wave and rotated to the other treatment condition in the next wave. For the SBT+SE, the same interventionist led the group and one-to-one sessions.
Measurements
Data were collected at the research center by trained staff using standardized procedures and questionnaires. Equipment was standardized and routinely calibrated. Participants completed assessments at baseline, 6, 12, and 18 months.
The outcome variables were weight loss maintenance, health-related quality of life, adherence to the intervention protocol and self-efficacy. Weight loss maintenance at 18 months was determined by comparing the percent weight changes from baseline to 12 months and to 18 months (Tanita Scale, Tanita Corporation of America, Inc., IL, USA). Health-related quality of life was measured with the Medical Outcomes Study Short Form-36, version 2 (MOS SF-36).
Intervention adherence was measured by 5 variables: attendance, calorie and fat goals, activity goal, and dietary self-monitoring. Attendance adherence was determined by percent of the 20 group sessions attended for both groups, and the percent of the 30 individual sessions attended for the SBT+SE group. Dietary adherence was assessed via two unannounced 24-hour dietary recalls (1 weekend, 1 weekday) within a 6-week window around each assessment guided by the Nutrition Data System for Research program and conducted by blinded and trained research staff.19 Dietary adherence was defined as achieving 85%-100% of the dietary goal. Non-adherence was represented as exceeding 100% or attaining <85% of the recommended goal. Activity adherence was defined as ≥7500 steps/day and ≥85% of the weekly activity goal. Dietary self-monitoring adherence was calculated as the proportion of weeks that the diaries were completed.
Perceived self-efficacy was assessed using the 20-item Weight Efficacy Lifestyle (WEL) Scale24, where participants rate their confidence in their ability to avoid eating in various contexts (e.g., home, work, social settings) and in a range of mood states (e.g., depressed, stressed, celebratory). Responses are made using a 10-point Likert scale, 0 (not confident) to 9 (very confident). For each item a total score is calculated as the sum of all responses. Thus, possible scores range from 0 to 180 with higher scores indicating greater levels of self-efficacy. Cronbach’s alpha coefficients ranged from 0.70 to 0.90.24
Statistical Analysis
Sample size justification
A sample size of 60 per group was determined based on published effect sizes for the primary endpoint of weight change observed for interventions similar to the intervention proposed.25 With a sample size of 48 per group, we would have at least .80 power to detect an effect size as small as d=0.637 when comparing the SBT+SE and SBT groups on mean weight change from baseline to 12 and 18 months using linear contrasts via linear mixed modeling at a test-wise significance level of .025. To ensure that at least 96 subjects would complete the study, 120 participants were enrolled to account for up to 20% attrition.
Statistical analysis
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). The significance level was set at .05 for two-sided hypothesis testing. Analyses followed the intention-to-treat (ITT) model. Summary statistics were reported as mean (SD) and frequency count (%). Independent samples t-tests were used to compare baseline characteristics and weight loss maintenance at 18 months between treatment groups. Chi-square tests of independence were used to compare categorical subject characteristics between groups.
Assuming missing at random, linear mixed modeling with linear contrasts was applied to assess treatment group, time and interactions effects on weight loss maintenance, quality of life, intervention adherence and self-efficacy. Sensitivity analysis of potentially influential cases supported the robustness of our findings based on the full sample. Generalized linear mixed modeling was employed to examine treatment group, time and interaction effects on adherence to intervention protocols.
Results
The sample (N=130) was predominantly female (83.08%), White (71.54%), married or cohabiting (63.85%), and had a mean BMI of 33.15 (4.11) kg/m2. Participants had completed, on average, 15.88 (3.07) years of education and were 53.02 (9.57) years of age; 64.84% had a household income >$50,000. No significant differences were found between SBT and SBT+SE groups at baseline.
Percent weight change
Table 2 provides results from the linear mixed modeling of percent change in weight over time. We observed no significant group-by-time interaction or group effect on percent weight change over time. However, there was a significant time effect for percent weight change (p=.002) and a significant percent weight change in each group at each time point (p’s <.001). For the SBT+SE group, there was no significant difference in percent weight loss between 12 months (8.38% ± 7.48) and 18 months (8.00% ± 7.87), indicating that the SBT+SE group maintained their weight loss at 18 months (p=.06); while the SBT group had a significantly lower weight loss at 18 months (5.96% ± 7.35) compared with that at 12 months (6.95% ± 6.67), indicating that the SBT group had a significant weight regain at 18 months (p=.005).
Table 2.
Percent Weight Change Over Time by Treatment Group
| SBT (n=72) | SBT+SE (n=58) | p-values | |||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Group × Time |
Group | Time | |
| 6 months | −6.32% (5.27) | −7.30% (5.40) | |||
| 12 months | −6.95% (6.67) | −8.38% (7.48) | .58 | .19 | .002 |
| 18 months | −5.96% (7.35) | −8.00% (7.87) | |||
SD = Standard Deviation; SBT=standard behavioral treatment; SE=self-efficacy
When we examined weight loss from baseline to 12 months, there were 95 subjects (73.08% of sample) who lost weight between baseline and 12 months, 53 (89.8%) were in the SBT group and 42 (87.5%) were in the SBT+SE group. A linear contrast revealed that among participants who lost weight from baseline to 12 months, the mean weight-loss maintenance at 18 months was not significantly different between the SBT+SE group (8.04kg ± 6.93) and the SBT group (6.00 kg ± 6.73) (p=0.16).
Health-related quality of life
The MOS SF-36 Physical and Mental Component scores are described in Table 3. We did not observe a significant group-by-time interaction or group main effect in higher component scores. The Physical Component Summary (PCS) scores revealed an improvement over time (p=.01), although we observed no significant time-effect for the Mental Component Summary (MCS) scores (p=.10).
Table 3.
Summary Statistics for Outcomes over 18 months by Treatment Group
| SBT (n=72) | SBT+SE (n=58) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Physical component scores | ||
| Baseline | 50.34 (7.44) | 52.34 (6.76) |
| 6 months | 51.82 (7.05) | 53.93 (5.33) |
| 12 months | 52.57 (6.90) | 54.37 (6.47) |
| 18 months | 52.75 (6.66) | 55.09 (4.36) |
| Mental component scores | ||
| Baseline | 49.53 (10.15) | 48.84 (10.75) |
| 6 months | 52.44 (7.89) | 49.83 (8.10) |
| 12 months | 52.01(8.23) | 50.98 (9.65) |
| 18 months | 52.49 (7.37) | 50.83 (10.02) |
| Weight (kg) | ||
| Baseline | 91.32 (13.65) | 90.82 (13.27) |
| 6 months | 84.07 (12.92) | 84.33 (14.48) |
| 12 months | 82.79 (13.26) | 83.63 (15.89) |
| 18 months | 84.05 (13.90) | 83.71 (16.24) |
| Fat (gram) | ||
| Baseline | 83.04 (42.41) | 85.88 (33.68) |
| 6 months | 59.12 (35.51) | 59.17 (25.01) |
| 12 months | 58.25 (26.60) | 60.61 (20.36) |
| 18 months | 59.62 (25.10) | 61.92 (24.36) |
| Calorie (kcal) | ||
| Baseline | 2097.35(808.42) | 2117.26(597.11) |
| 6 months | 1645.72(668.88) | 1695.25(504.73) |
| 12 months | 1636.87(481.64) | 1676.16(410.68) |
| 18 months | 1718.39(525.72) | 1698.34(498.46) |
| MET hr/wk | ||
| Baseline | 13.96 (12.37) | 12.22 (11.57) |
| 6 months | 21.45 (17.23) | 19.56 (16.28) |
| 12 months | 20.79 (21.71) | 21.24 (18.62) |
| 18 months | 18.70 (15.39) | 22.04 (18.30) |
| WEL total score | ||
| Baseline | 99.11 (32.58) | 100.03 (34.90) |
| 6 months | 117.17 (32.37) | 112.61 (32.52) |
| 12 months | 115.72 (32.03) | 111.64 (36.28) |
| 18 months | 117.02 (33.19) | 116.15 (35.00) |
Note: There were no group × time and group effects for any of the variables, but there was a time effect for each variable (ps <.01) except the Mental Component Summary score of the MOS-SF36 (p =.10). MET = metabolic equivalent unit, equal to 3.5 milliliters of oxygen per kilogram of body weight per minute; WEL= Weight Efficacy Lifestyle; SD = Standard Deviation; SBT = standard behavioral treatment; SE = self-efficacy
Adherence to intervention protocol
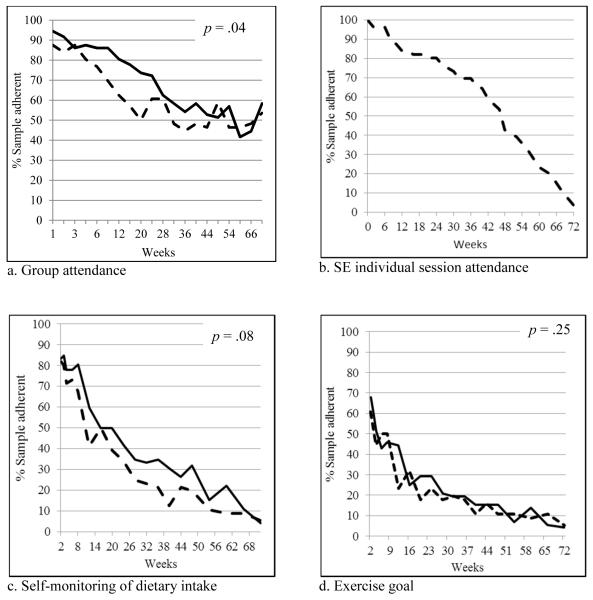
Overall, the SBT group attended 68.75% of the 20 group sessions while the SBT+SE group attended 60.00% of the group sessions and 65.11% of the 30 one-on-one sessions. Figure 2 illustrates adherence to the intervention protocol. The p values noted represent group effects. We found that group session attendance over time differed between SBT+SE and SBT groups, (p<.001, interaction). The percentage of the sample attending group sessions declined from 87.5% to 53.6% in SBT+SE group, compared to 94.4% to 58.3% in SBT group over 18 months. The SBT+SE group attended on average 65% of the individual sessions. Figure 2a illustrates the rapid decline in the percentage of the SBT+SE group attending the group sessions in the first 20 weeks of the study followed by an upward trend that remained above 40% until the end of the study. Concurrent with this change, the percentage of the SBT+SE group attending the individual sessions steadily declined over the course of the study (Figure 2b). There was a trend towards better adherence to self-monitoring by the SBT+SE group compared to the SBT group (p=.08) (Figure 2c). There was a significant group effect for adherence to the calorie goal (p=.02) with the SBT+SE group having significantly better adherence on average than the SBT group (see Table 4). There was no significant group, time, or group-by-time effect for adherence to the fat gram goal (p’s >.05). We observed a significant time effect in adherence to physical activity minutes and pedometer steps (p <.01); however, we did not observe group-by-time or group differences.
Figure 2.
Adherence to attendance and intervention goals (y-axis=% of sample adherent; x-axis=weeks in protocol). Solid line = SBT group; Dashed line = SBT+SE group.
Table 4.
Proportion of the Sample Adherent to Calorie and Fat Gram Goals by Treatment Group over Time (N = 130)
| SBT (n=72) | SBT+SE (n=58) | p-values | |||
|---|---|---|---|---|---|
| n (%) | n (%) | Group × Time | Group | Time | |
| Adherence to calorie goal | |||||
| 6 months | 8 (13.11) | 13 (26.00) | |||
| 12 months | 9 (16.07) | 16 (33.33) | 0.71 | 0.02 | 0.26 |
| 18 months | 7 (12.28) | 8 (17.02) | |||
| Adherence to fat goal | |||||
| 6 months | 12 (19.67) | 5 (10.00) | |||
| 12 months | 5 (8.93) | 7 (14.58) | 0.25 | 0.99 | 0.15 |
| 18 months | 3 (5.26) | 3 (6.38) | |||
Note: SD=Standard Deviation; SBT=standard behavioral treatment; SE=self-efficacy Adherence to the dietary goals was defined as 85% to 100% of each dietary goal.
Self-efficacy
WEL scores for weight-related behaviors were the same at baseline for both groups (SBT 99.11±32.58 vs. SBT+SE 100.03±34.90) with increases over time (Table 3). At 12 months they were 115.72±32.03 vs. 111.64±64 and at 18 months 117.02±33.10 vs. 116.15±35.00 for SBT vs. SBT+SE, respectively. There were no significant differences between groups but there was a significant change over time (p <.001).
Discussion
The SELF Trial demonstrated excellent weight loss maintenance at 18 months in the SBT+SE (8.00%) and SBT (5.96%) groups; however, the between-group difference was not significant. Our findings are consistent with others26,27 who used face-to-face contact and did well with maintaining weight loss.
Perri et al.26 conducted a trial with post-menopausal women testing two approaches to improve weight loss maintenance, face-to-face vs. telephone vs. newsletter (control). At 18 months, the intervention groups had the same regain (1.2 kg), which was significantly less than the control group (3.75 kg). While the regain was the same for both conditions, interestingly, only 53% of the face-to-face sessions were attended, while 81% of the telephone sessions were completed. In our study, during months 13 to 18, attendance at the group sessions by the SBT+SE group was 45-55% and the group had a regain of .35 kg while the SBT only group attended 50-60% of the sessions and had a regain of .90 kg. Similar to the Perri et al., our study with increased face-to-face contact did not result in greater weight loss maintenance. Kiernan designed a study that compared a 6-month intervention focusing on maintenance skills prior to a weight loss intervention compared to a group that focused on weight loss first followed by a 12-month no-contact follow-up period.28 This innovative approach included a focus on self-efficacy enhancement, skill development and mastery and demonstrated that at 6 months there was no difference in the weight loss; however, the Maintenance First group regained significantly less weight at 18 months than the Weight Loss First group. Also similar to our study, there were multiple components to the intervention in the Kiernan study that cannot be identified as the sole factor contributing to the improved maintenance.
We found no significant group or group-by-time interaction effects in either MOS SF-36 PCS or MCS. These findings are similar to the results by Blissmer and colleagues29, where 144 individuals were assessed at 12 and 24 months after a 6-month weight-loss program and neither group effect or group-by-time effect was significant. Blissmer and colleagues29 reported a significant time effect in both the physical and mental scores, while in our study, the time effect was only significant in the PCS in the SBT group. Similarly, Sarwer et al. reported30 a significant improvement only in the PCS over time. One hypothesis is that the psychological burdens for individuals with obesity are often multidimensional and the MOS SF-36 may lack obesity-related specificity and sensitivity.
The SBT+SE group had excellent attendance at the one-on-one sessions during the first 20 weeks of the study but their attendance at group sessions declined to 50% during the same period of time. This pattern changed after 20 weeks with an upward trend observed in group session attendance that was sustained to the end of the study while attendance at the one-on-one sessions declined continuously. We observed the SBT+SE participants choosing to attend only one of the sessions (one-on-one vs. group) and encouraged them to attend the group sessions for their content and peer support. We posit that participants derived what was needed from the one-on-one sessions and then began to see that the group sessions provided additional benefits beyond those gained in the one-on-one sessions.
Compared to the SBT group, adherence to dietary self-monitoring was not significantly better in the SBT+SE group. A clinical trial that conducted individual intervention sessions based on behavioral choice theory also reported that self-monitoring of dietary intake adherence did not differ between groups.31 Both groups in SELF used paper diaries and demonstrated declining adherence to self-monitoring over time at a typical rate.32-34 In a previous trial, we observed greater adherence to dietary self-monitoring with a handheld computer or mobile device versus a paper diary.34,35
The SBT+SE group demonstrated better adherence to the calorie goal than the SBT group but no difference in fat goal adherence. The adherence to the fat goal was low and declined over time. We have demonstrated in previous studies,32,36 that of the five protocol components in SBT (self-monitoring, group attendance, and calorie, fat and exercise goals) adherence to the fat goal is lower than to the other targets. Other behavioral intervention trials have not reported adherence to the dietary goals.
In the PREMIER Trial, 30% of participants self-monitored their physical activity, on average, less than once a week, and approximately 38% self-monitored an average of 1.0 to 2.9 days per week.37 Initially 70% of our sample was adherent to the exercise goal. This declined to approximately 5% by the end of the study suggesting a need for greater emphasis and improved strategies to increase adherence to this crucial part of maintenance.
We hypothesized that delivering an intervention based on self-efficacy enhancement would improve weight loss maintenance in the SBT+SE group. However, we observed no significant change in self-efficacy, as measured by the WEL, over time or between the two groups. We believe this is not a measurement issue as we have used the WEL in previous studies and have demonstrated significant change in WEL scores over time among those who have participated in SBT for weight loss.12 The absence of the anticipated effect may be the result of several small differences beyond the increased attention the SBT+SE group received. Attendance at the group sessions by the SBT+SE group declined rapidly until the 20th week when it increased and leveled off; missing some of the initial group support might have contributed to this reduced effect. Also, only 66% of the individual sessions were completed, which resulted in a reduced dose effect. The participant perception of the ratio of ‘burden to benefit’ of the two types of sessions may have changed at 20 weeks, at which time the group sessions were moving to a less burdensome monthly schedule and the benefit of the bi-weekly individual sessions also may have been maximized for individuals. SBT+SE participants were more adherent to the energy intake restriction, which may have contributed to their improved maintenance; however, there was no difference in other adherence measures.
Limitations and strengths
A limitation to this study is that our sample was comprised of predominantly White, middle-age, well-educated women. Thus our findings may not be generalizable to certain populations. Strengths include the randomized 2-group design and the excellent retention of 80% at 18 months. Additionally, we had a 28% representation of non-Whites in the sample.
Conclusion
We compared SBT to a modified SBT (SBT+SE) in which participants were provided 30 one-on-one sessions focusing on collaborative goal setting for achieving dietary and exercise goals and self-efficacy enhancement. Participants in both groups achieved significant weight loss and maintained over 5% of the weight loss at the end of the study. However, the SBT+SE group had a larger, but not statistically significant different weight loss maintenance. This difference may be due to several factors; however, the design of the study and the multi-component intervention precludes us from identifying the self-efficacy enhancement intervention as the main contributor to the nonsignificant difference. Future studies need to design interventions incorporating maximal optimization approaches such as multi-factorial designs so that one can tease apart the contribution of each intervention component.
What is already known about this subject
Long-term maintenance following intentional weight loss remains a significant challenge in addressing the high prevalence of overweight and obesity. Over 75% of adults who intentionally lose weight regain most of the weight. Standard behavioral treatment (SBT) protocols have been used for several years with numerous variations but have shown little improvement in weight loss maintenance rates.
Increased self-efficacy is associated with behaviors related to weight loss (e.g., improved adherence to dietary and physical activity goals). Individuals who have higher perceived self-efficacy are more likely to continue their weight loss efforts until the desired goal is reached. Self-efficacy can be increased within behavioral weight loss interventions. There is evidence that applying the sources of self-efficacy (e.g., mastery performance, modeling) can lead to an increase in one’s perception of his/her capability to perform a behavior.
What this study adds
Explores the effect of supplementing group-delivered behavioral weight loss interventions with one-to-one sessions designed to target self-efficacy enhancement to support weight loss maintenance
Describes patterns of adherence to group sessions concurrent to one-on-one sessions for the experimental group
Reinforces the importance of face-to-face contact in behavioral weight loss interventions as a tool for weight-loss maintenance
Acknowledgements
This study was supported by NIH through P01NR010949 and K24NR010742.
Funding: P01NR010949 and L. E. Burke, MidCareer Mentorship Award NIH K24NR010742
Footnotes
Disclosure: No conflicts of interest to report.
Authors contributions: Lora E Burke-study design and implementation, data interpretation, manuscript development; Linda J. Ewing-intervention consultation, data interpretation; Lei Ye-data analysis; Mindi Styn-project director; Yaguang Zheng-manuscript contribution; Edvin Music-data manager; India Loar-study coordinator, data collector; Juliet Mancino-manuscript contribution, data interpretation; Christopher C. Imes-manuscript contribution; Lu Hu-manuscript contribution; Rachel Goode-manuscript contribution; and Susan Sereika-senior biostatistician.
Registered with ClinicalTrials.gov, ID NCT00896194.
References
- 1.National Heart Lung and Blood Institute . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. National Institutes of Health; Bethesda, MD: 1998. pp. 98–4083. [PubMed] [Google Scholar]
- 2.Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol. 2002 Jun;70(3):510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- 3.Perri MG. The maintenance of treatment effects in the long-term management of obesity. Clinical Psychology: Science and Practice. 1998;5:526–543. [Google Scholar]
- 4.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of Obesity: Clinical Applications. 2nd ed Marcel Dekker; New York: 2004. pp. 147–167. [Google Scholar]
- 5.Jeffery RW, Drewnowski A, Epstein LH, et al. Long term maintenance of weight loss: Current status. Health Psychol. 2000;19(Suppl 1):5–16. doi: 10.1037/0278-6133.19.suppl1.5. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J, Konz E, Frederick R, Wood C. Longterm weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 7.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(suppl):S151–S162. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 8.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005 Jul;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 9.Franz MJ, Vanwormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association. 2007 Oct;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Pinto BM, Clark MM, Cruess DG, Syzmanski L, Pera V. Changes in self-efficacy and decisional balance for exercise among obese women in a weight management program. Obes Res. 1999;7(3):288–292. doi: 10.1002/j.1550-8528.1999.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 11.Dennis KE, Tomoyasu N, McCrone SH, Goldberg AP, Bunyard L, Qi BB. Self-efficacy targeted treatments for weight loss in postmenopausal women. Scholarly Inquiry for Nursing Practice. 2001;15(3):259–276. [PubMed] [Google Scholar]
- 12.Warziski MT, Sereika SM, Styn MA, Music E, Burke LE. Changes in self-efficacy and dietary adherence: the impact on weight loss in the PREFER study. J Behav Med. 2008 Feb;31(1):81–92. doi: 10.1007/s10865-007-9135-2. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A. Self-Efficacy Mechanism in Human Agency. Am Psychol. 1982;37(2):122–147. [Google Scholar]
- 14.Anderson-Bill ES, Winett RA, Wojcik JR, Winett SG. Web-based guide to health: relationship of theoretical variables to change in physical activity, nutrition and weight at 16 months. J Med Internet Res. 2011;13(1):e27. doi: 10.2196/jmir.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW. Weight loss and self-regulatory eating efficacy in older adults: the cooperative lifestyle intervention program. The journals of gerontology. Series B, Psychological sciences and social sciences. 2011 May;66(3):279–286. doi: 10.1093/geronb/gbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol. 2006;25(3):282–291. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- 17.Bandura A, Adams N. Analysis of self-efficacy theory of behavioral change. Cognitive Therapy and Research. 1977;1:287–310. [Google Scholar]
- 18.Bandura A. Self-Efficacy: The Exercise of Control. W.H. Freeman and Company; New York: 1997. [Google Scholar]
- 19.Burke LE, Styn MA, Glanz K, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp Clin Trials. 2009 Nov;30(6):540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke LE, Warziski M, Acharya S, et al. PREFER Trial: A randomized clinical trial testing treatment preference and two dietary options combined with behavioral weight management. Obesity. 2006;14((Suppl.):A32. doi: 10.1038/oby.2006.235. [DOI] [PubMed] [Google Scholar]
- 21.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006 May;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Zurilla TJ, Goldfried MR. Problem solving and behavior modification. J Abnorm Psychol. 1971;78(1):107–126. doi: 10.1037/h0031360. [DOI] [PubMed] [Google Scholar]
- 23.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20(1):68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 24.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS. Self-efficacy in weight management. J Consult Clin Psychol. 1991;59:739–744. doi: 10.1037//0022-006x.59.5.739. [DOI] [PubMed] [Google Scholar]
- 25.Carels RA, Darby LA, Rydin S, Douglass OM, Cacciapaglia HM, O’Brien WH. The relationship between self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Ann Behav Med. 2005;30(3):182–190. doi: 10.1207/s15324796abm3003_2. [DOI] [PubMed] [Google Scholar]
- 26.Perri MG, Limacher MC, Durning PE, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008 Nov 24;168(21):2347–2354. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355(15):1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 28.Kiernan M, Brown SD, Schoffman DE, et al. Promoting healthy weight with “stability skills first”: a randomized trial. J Consult Clin Psychol. 2013 Apr;81(2):336–346. doi: 10.1037/a0030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blissmer B, Riebe D, Dye G, et al. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow up effects. Health & Quality of Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarwer DB, Moore RH, Diewald LK, et al. The impact of a primary care-based weight loss intervention on the quality of life. Int J Obes (Lond) 2013 Aug;37(Suppl 1):S25–30. doi: 10.1038/ijo.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sbrocco T, Nedegaard RC, Stone JM, Lewis EL. Behavioral choice treatment promotes continuing weight loss: preliminary results of a cognitive-behavioral decision-based treatment for obesity. J Consult Clin Psychol. 1999 Apr;67(2):260–266. doi: 10.1037//0022-006x.67.2.260. [DOI] [PubMed] [Google Scholar]
- 32.Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya SD, Elci OU, Sereika SM, et al. Self-monitoring influences diet quality among obese adults in a weight loss trial. Circulation. 2009 Nov;120(18):S460. [Google Scholar]
- 34.Burke LE, Ambeba E, Ye L, et al. Changes in adipokines with weight loss and regain in a 24-month behavioral weight loss study: the SMART trial. Circulation. 2012;125(Supp):AP373. [Google Scholar]
- 35.Wang J, Sereika SM, Chasens ER, Ewing LJ, Matthews JT, Burke LE. Effect of adherence to self-monitoring of diet and physical activity on weight loss in a technology-supported behavioral intervention. Patient Prefer Adherence. 2012;6:221–226. doi: 10.2147/PPA.S28889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke LE, Choo J, Music E, et al. PREFER study: a randomized clinical trial testing treatment preference and two dietary options in behavioral weight management--rationale, design and baseline characteristics. Contemp Clin Trials. 2006 Feb;27(1):34–48. doi: 10.1016/j.cct.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Bartfield JK, Stevens VJ, Jerome GJ, et al. Behavioral transitions and weight change patterns within the PREMIER trial. Obesity (Silver Spring) 2011 Aug;19(8):1609–1615. doi: 10.1038/oby.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]