Abstract
Estrogen receptor beta (ERβ) is highly expressed in normal breast epithelium and a putative tumor suppressor. Atypical hyperplasia substantially increases breast cancer risk, but identification of biomarkers to further improve risk stratification is needed. We evaluated ERβ expression in breast tissues from women with atypical hyperplasia and association with subsequent breast cancer risk. ERβ expression was examined by immunohistochemistry in a well-characterized 171 women cohort with atypical hyperplasia diagnosed 1967–1991. Nuclear ERβ percent and intensity was scored in the atypia and adjacent normal lobules. An ERβ sum score (percent + intensity) was calculated and grouped as low, moderate or high. Competing risks regression was used to assess associations of ERβ expression with breast cancer risk. After 15 years median follow-up, 36 women developed breast cancer. ERβ expression was lower in atypia lobules than normal lobules, by percent staining and intensity (both p<0.001). Higher ERβ expression in the atypia or normal lobules, evaluated by percent staining, intensity or sum score, decreased the risk of subsequent breast cancer by 2 (p=0.04) and 2.5-fold (p=0.006). High normal lobule ERβ expression conferred the strongest protective effect in pre-menopausal women: the 20-year cumulative incidence of breast cancer was 0% for women <age 45 with high versus 31% for low-moderate ERβ expression (p=0.0008). High ERβ expression was associated with a significantly decreased risk of breast cancer in women with atypical hyperplasia. These data suggest ERβ may be a useful biomarker for risk stratification and a novel therapeutic target for breast cancer risk reduction.
Keywords: breast cancer risk prediction, estrogen receptor beta, atypical hyperplasia
Introduction
Estrogen receptor beta (ERβ) is a member of the nuclear receptor superfamily of transcription factors that was identified in 1996 as the product of the ESR2 gene on chromosome 14q22-24.[1,2] ERβ is distinct from ERα (ESR1 gene on chromosome 6), the form of the estrogen receptor that is assayed in routine clinical practice for all newly diagnosed breast cancers and used to determine treatment and prognosis.[3,4] ERα and ERβ share 96% homology in their DNA-binding domains, but differ considerably at the hinge region, AF1 domain and ligand binding domain.[2,5] Unlike ERα, ERβ is highly expressed in normal breast epithelium but declines in expression in pre-cancerous and cancerous breast lesions.[6–13]
Further, ERβ (specifically the full-length form, termed ERβ1) is postulated to function as a tumor suppressor of breast cancer as well as other cancers.[14–17] Its expression is diminished or absent in invasive breast cancers compared to preinvasive or benign lesions.[8,9,12,18–20] In breast cancer models, ERβ expression alone, or in combination with ERα, inhibits breast cancer cell proliferation and enhances sensitivity of ERα expressing breast cancer cells to the anti-proliferative effects of selective estrogen receptor modulators.[14,21–24] Tumoral expression of ERβ is also associated with increased effectiveness of tamoxifen and aromatase inhibitor therapy.[14,25–30]
There is little data on the association of ERβ expression in benign breast disease with subsequent breast cancer risk. Atypias of the breast increase the lifetime risk of breast cancer four-fold.[31] In particular, given its possible tumor suppressor role, ERβ expression in breast tissues with atypia and a possible role for ERβ in mitigating breast cancer risk in high-risk individuals are of interest. The identification of novel biomarkers for risk prediction within this high-risk group of patients is desirable to ascertain those individuals at the highest risk, to better guide women in their choice of pharmacologic or surgical risk reduction strategies, and to identify pharmacologic targets to lower risk.
To better understand the role of ERβ in benign breast disease and its possible impact on future breast cancer risk, we undertook this study in women with atypical hyperplasia and known long-term outcome with regard to subsequent development of breast cancer. Our aim was to examine the relationship of ERβ expression in the atypical epithelium and in the adjacent histologically normal lobules with the risk of future breast cancer.
Materials and Methods
Patient Cohort
With IRB approval, we studied breast tissues from women with atypical ductal hyperplasia (ADH) or atypical lobular hyperplasia (ALH) from the Mayo Clinic Benign Breast Disease Cohort. The cohort and verification of atypical hyperplasia has been described previously.[32] Among 334 women in the cohort diagnosed with atypia between 1967 and 1991, adequate tissue for ERβ staining was available for the 171 women who form the basis of this study. Median follow-up of this atypia subcohort was 15 (range 1 to 36) years.
Histology and Immunohistochemistry
The atypia tissue samples were characterized for the presence of unifocal versus multifocal atypia defined as more than one terminal ductal lobular unit clearly containing atypical hyperplasia.[33] Additionally, the degree of involution of the breast tissue was categorized as none, partial or complete.[34] Immunostaining and assessment for ERα and Ki-67 was performed as previously described.[32,35] For this analysis of ERβ expression the tissues studied included the epithelia of the atypical hyperplasia lesion(s), referred to as “the atypia”, and epithelia of histologically normal lobules from the same tissue section as the atypia, hereafter called normal or adjacent normal lobules.
The ERβ subcohort was defined after review of all the tissue sections by two pathologists (JMC and DWV). Subsequently, ERβ immunohistochemistry was performed on archival formalin-fixed paraffin-embedded tissue sections which were stained with an ERβ specific monoclonal antibody (PPG5/10, Thermo Scientific) which is known to detect only the full length form of ERβ (termed ERβ1) without cross reacting with any of its splice variant forms.[11,36] This antibody also has been shown to be the most sensitive and specific commercially available ERβ antibody for use in immunohistochemical studies.[11,36,37] In brief, sections were first deparaffinized in xylene, washed in decreasing concentrations of ethanol and rehydrated in distilled water. Antigen retrieval was performed by treatment with a pre-heated, citrate-based solution (low-pH Target Retrieval Solution (Dako) in a steamer at 98° C for 40 minutes. Staining was performed in a Dako Autostainer Plus as previously described.[11]
Nuclear ERβ staining in epithelia was scored semi-quantitatively by a single pathologist (JMC) blinded to patient identity as well as clinical characteristics and outcome. Both the atypical and adjacent histologically normal breast epithelia were scored for percentage of nuclei stained (scores 0–4 for <1%, 1–25%, 26–50%, 51–75%, and >75%, respectively) and staining intensity (scores 0–3 for negative, weak, intermediate, and strong, respectively). All epithelial atypical foci were scored for categorization, on average this was one focus per case. Similarly, all background adjacent normal lobules were reviewed and scored for categorization, an average of 3 adjacent normal lobules per case. An ERβ expression sum score (percent plus intensity, range 0–7) was created and grouped as negative/low (score 0–2), moderate (score 3–5), and high (score 6–7) as previously described.[14]
Statistical Analysis
Descriptive statistics were reported with frequency and percentage for categorical variables and median (range) for continuous variables. Paired comparisons of ERβ expression between the atypia and adjacent normal lobules were performed using Wilcoxon signed-rank tests. Independent sample comparisons between the two types of atypia (ADH versus ALH) used Wilcoxon rank-sum tests. Follow-up time was calculated from the date of the index benign biopsy until the diagnosis of breast cancer or until the earliest of the following: prophylactic mastectomy, last follow-up, or death. The cumulative incidence estimator was used to estimate the incidence of breast cancer while taking the competing risk of death into account.[38] The association of ERβ expression with breast cancer risk was assessed using Fine and Gray competing risks regression with the Firth penalized bias-reduction method and was summarized with hazard ratio (HR) and 95% profile-likelihood confidence intervals.[39] P-values < 0.05 were considered statistically significant. Correlation coefficients (ρ) were calculated using the Spearman’s rank method. Analysis was performed using SAS (Version 9.3) with the %pshreg macro and R (http://www.r-project.org) including the cmrsk package.[40,41]
Results
The median patient age was 56 years (range 28–84 years). All women underwent excisional biopsy of the atypia lesion. Seventy-nine women had ADH and 92 had ALH. No women in this cohort received chemoprevention with endocrine therapy. One patient underwent bilateral prophylactic mastectomy 3 years after her atypia biopsy. During follow-up, 36 of the 171 women developed breast cancer at a median of 13 (range 1–29) years.
ERβ expression, assessed by nuclear percent staining, intensity and sum score, was lower in the atypia than in adjacent normal lobules (all p < 0.0001) as shown in Table 1 and illustrated Figure 1 which shows low and high ERβ expression, respectively, in the atypias (Panels A and B) and in the adjacent normal lobules (Panels C and D). ERβ expression was slightly lower in ADH than ALH (supplemental Table 1S), particularly for nuclear percent staining (p=0.0002), sum score (p=0.0004) and sum score category (p=0.001). However, ERβ expression in the adjacent normal lobules did not differ between ADH and ALH cases (data not shown).
Table 1.
ERβ staining in the atypia and adjacent normal lobules.
| Atypia N=171 |
Normal Lobule N=171 |
p-value | ||
|---|---|---|---|---|
| Nuclear % Staining | p<0.0001 | |||
| <1% | 8 (4.7%) | 0 | ||
| 1–25% | 41 (24.0%) | 1 (0.6%) | ||
| 26–50% | 59 (34.5%) | 12 (7.0%) | ||
| 51–75% | 56 (32.7%) | 81 (47.4%) | ||
| >75% | 7 (4.1%) | 77 (45.0%) | ||
| Nuclear Intensity | p<0.0001 | |||
| Negative | 2 (1.2%) | 0 | ||
| Weak | 109 (63.7%) | 21 (12.3%) | ||
| Intermediate | 48 (28.1%) | 73 (42.7%) | ||
| Strong | 12 (7.0%) | 77 (45.0%) | ||
| Sum Score, median (range) | 3 (0–7) | 6 (2–7) | p<0.0001 | |
| Sum Score Group | p<0.0001 | |||
| Negative/Low | 44 (25.7%) | 1 (0.6%) | ||
| Moderate | 117 (68.4%) | 74 (43.3%) | ||
| High | 10 (5.8%) | 96 (56.1%) | ||
Figure 1.
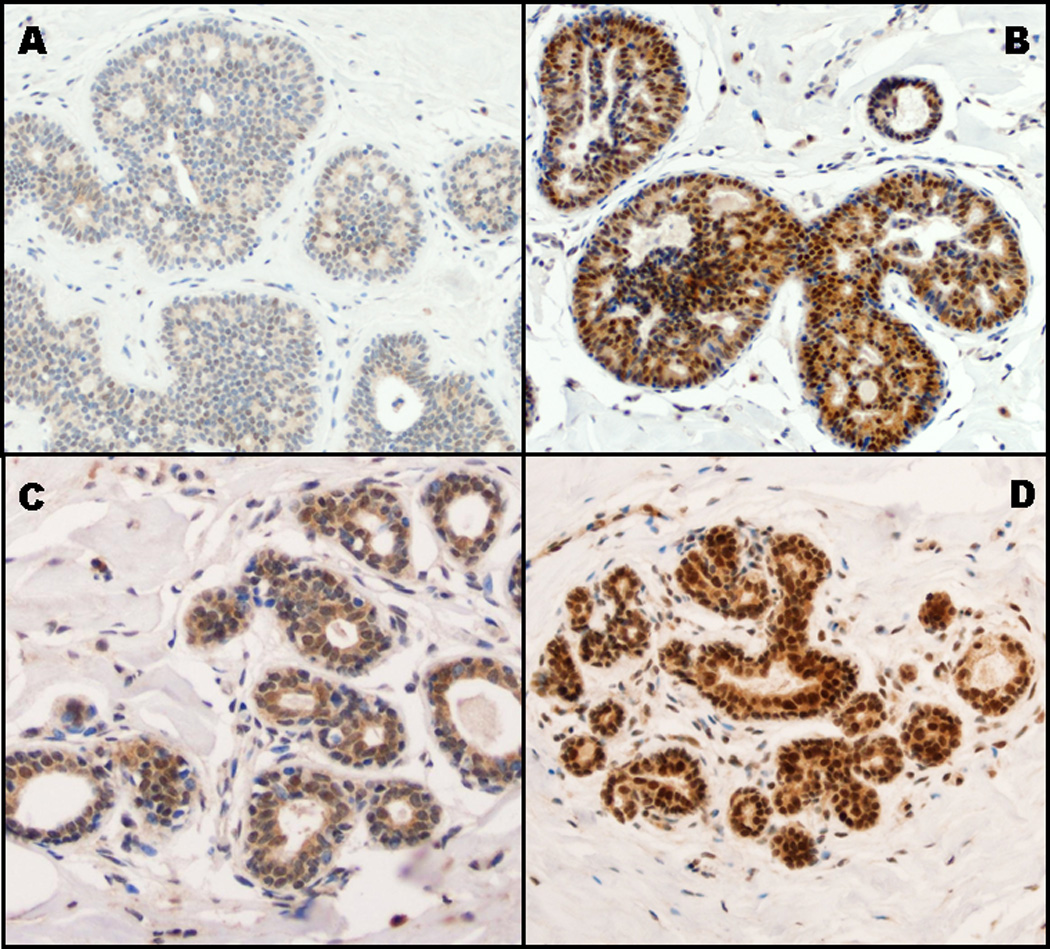
Illustration of ERβ nuclear immunostaining stratified by sum score incorporating extent and intensity of staining in both atypical and normal lobules. (A) Low ERβ expression in atypia: nuclear percentage score of 1 and intensity score of 1, representing a sum score of 2. (B) High ERβ expression in atypia: nuclear percentage score of 3 and intensity score of 3, representing a sum score of 6. (C) Low ERβ expression in normal lobule: nuclear percentage score of 1 and intensity score of 1, representing a sum score of 2. (D) High ERβ expression in normal lobule: nuclear percentage score of 4 and intensity score of 3, representing a sum score of 7.
Association of ERβ Expression with Other Risk Factors
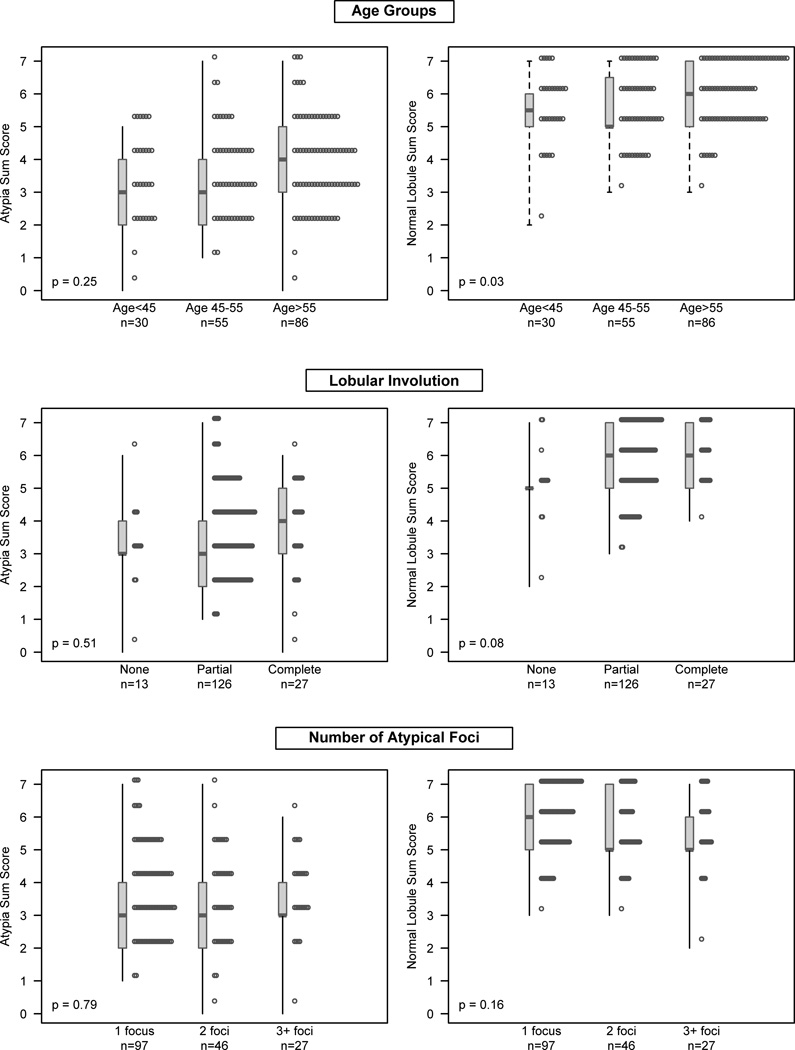
The association of ERβ expression with age, lobular involution and the number of atypical foci is summarized in Figure 2. Generally, ERβ expression in either the atypia or normal lobules was independent of these factors. The only significant association was for age category with normal lobule ERβ expression, with significantly lower ERβ expression in women ≤ 55 years as compared to women > age 55 (p = 0.03). ERβ expression in the atypia was not significantly associated with the three variables.
Figure 2.
The relationship between ERβ expression in the atypia (left panels) and in the normal lobules (right panels) with age, lobular involution and the number of atypical foci.
Correlation of ERβ expression with other markers
Data were available on ER-α and Ki-67 for the atypia lesion only. ERα in the atypia was not correlated with ERβ expression in the atypia for either percent staining (ρ = 0.02) or intensity (ρ = 0.08). ERβ expression in the atypia lesion did show a trend (p=0.09) toward an inverse association with Ki-67 expression with 39% (12/31), 24% (20/86), and 14% (1/7) showing elevated Ki-67 expression (≥2% cells positive in the atypia) across the levels of low, moderate, and high ER-β sum score, respectively.
Association of ERβ with Breast Cancer Risk
ERβ in the atypia
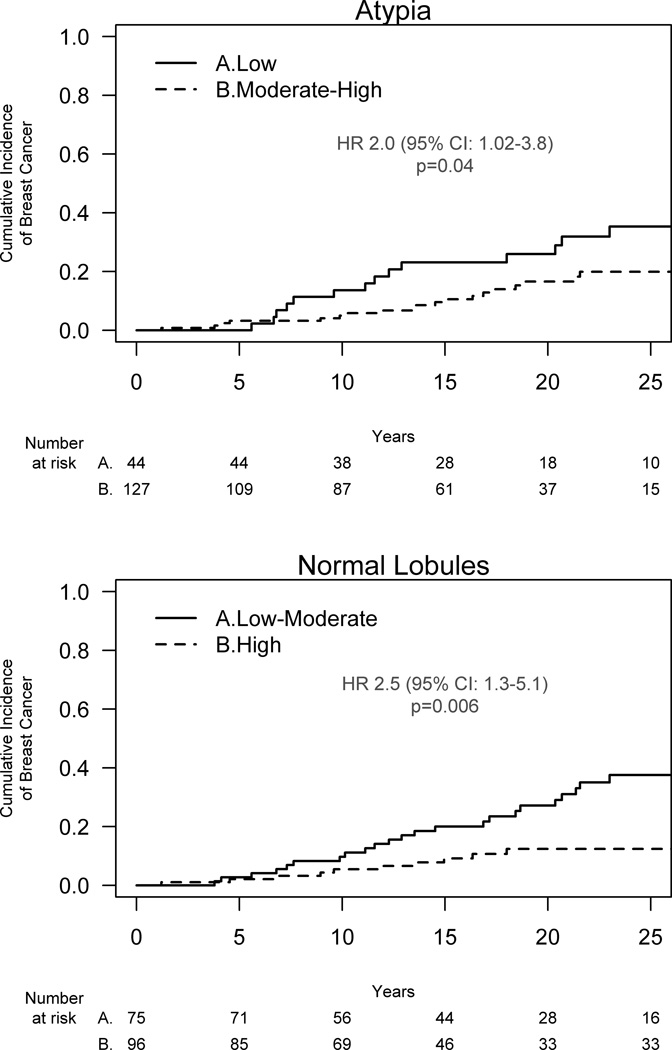
By sum score, 44 (26%) of atypias had low ERβ expression, 117 (68%) moderate expression, and 10 (6%) high expression. Higher nuclear ERβ expression in the atypia was associated with decreased breast cancer risk for all methods of evaluation including nuclear percent staining (p=0.03), intensity of staining (p=0.03) and sum score (p=0.01). Low nuclear ERβ expression (sum score 0–2) in the atypia was associated with a 2-fold increased risk of subsequent breast cancer (HR 2.0, 95% CI: 1.02–3.8, p = 0.04) compared to atypias with moderate-high expression (sum score 3–7) (Figure 3A).
Figure 3.
ERβ expression in the atypia (top panel) and adjacent normal lobule (lower panel) and subsequent breast cancer risk.
ERβ in the normal lobules
In the adjacent normal lobules ERβ expression assessed by sum score was high in 96 (56%), moderate in 74 (43%) and low in 1 (0.6%). As the distribution was substantially different in normal lobules as compared to the atypia, low-moderate expression was compared to high expression in further analysis of the normal lobule scores. The normal lobule sum score showed a moderate correlation of ρ = 0.39 with atypia sum score within subject. Of those with low expression in the atypia, 28/44 (64%) showed low-moderate expression in the adjacent normal lobules, which was significantly more frequent (p=0.002) than for the moderate-high atypia expression group at 47/127 (37%). However, there were also a substantial number of subjects discordant between the atypia and the normal lobules in terms of favorable ERβ expression (kappa = 0.22), with a favorable score for the normal lobules but not the atypia in 16/171 (9%) and a favorable score for the atypia but not the normal lobules in 47/171 (27%).
High ERβ expression in the normal lobules was also protective against future development of breast cancer, and as with the atypia lesion, nuclear percent staining (p=0.002), intensity of staining (p=0.01), and sum score (p=0.001) were each significantly associated with risk. A low-moderate sum score (score 0–5) conferred a HR of 2.5 (95% CI 1.3–5.1) for future breast cancer risk versus a high sum score (score 6–7), p=0.006 (Figure 3B).
Multivariate analysis
The potential for multivariate analysis was somewhat limited due to the small number of breast cancer events (n = 36). Yet, after adjustment for the key risk factors of age, degree of lobular involution, and number of foci of atypia, lower ERβ expression remained significantly associated with increased breast cancer risk, in both the atypia (adjusted HR 2.3, 95% CI: 1.1–4.5, p=0.007) and in the normal lobules (adjusted HR 2.3, 95% CI: 1.1–4.8, p = 0.006). When both atypia and normal lobule ERβ expression were included in the same model with the above mentioned covariates, their respective hazard ratios showed some attenuation (adjusted HRs 1.91 and 1.96, p=0.07 and p=0.08, respectively), yet each measure continued to show a trend.
We noted a slight shift in ERβ scores over time with higher scores observed in more recent years (normal lobule median sum score 6 versus 5 and atypia median sum score 4 versus 3 for 1982–1991 versus 1967–1981, respectively, p <0.01 for both). However, although these differences were statistically significant, the absolute differences were modest and hazard ratio estimates adjusting for year of BBD biopsy remained significant with only small attenuations relative to the unadjusted estimates [HR 1.9 (95% CI: 0.9–3.6) for low versus high-moderate scores in the atypia, and HR 2.3 (95% CI: 1.2–4.9) for low-moderate versus high scores in normal lobules].
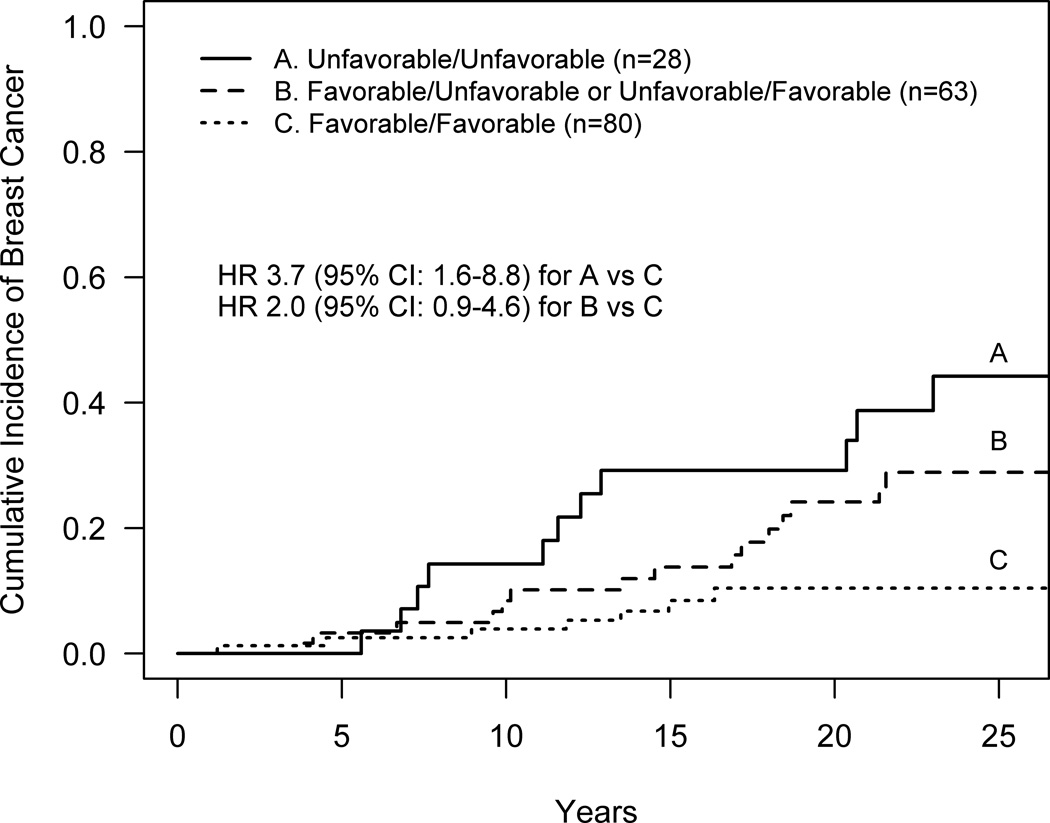
To further explore whether considering ERβ expression from both the atypia and normal added value to either alone, three categories of ERβ expression were compared, derived from the combination of the binary variables for the atypia lesion (low or moderate-high) and the normal lobules (low-moderate or high), (Figure 4). Those with low expression in the atypia and low-moderate expression in the normal lobules (poor/poor) had the highest risk of future breast cancer with an estimated cumulative incidence of 29.2% at 20 years and an unadjusted hazard ratio of 3.7 (95% CI: 1.6–8.8) or an adjusted HR of 3.8 (95% CI: 1.6–9.5, p=0.001) as compared to subjects with moderate-high expression in the atypia and high expression in the normal lobules (favorable/favorable) who had an estimated cumulative incidence of 10.4% at 20 years. Those with favorable expression levels for either the atypia or normal lobules but not both showed an intermediate degree of risk that was not significantly different from the most and least favorable categories (data not shown), although these analyses are limited by sample size.
Figure 4.
Breast cancer risk for combinations of ERβ expression in the atypia and adjacent normal lobules. Unfavorable/Unfavorable refers to those women with low expression in the atypia and low-moderate expression in normal lobules; Favorable/Favorable refers to those with moderate-high expression in the atypia and high expression in the normal lobules; the intermediate category captures those with favorable expression in either the atypia or normal lobules but not both.
Effect of age on ERβ expression and breast cancer risk
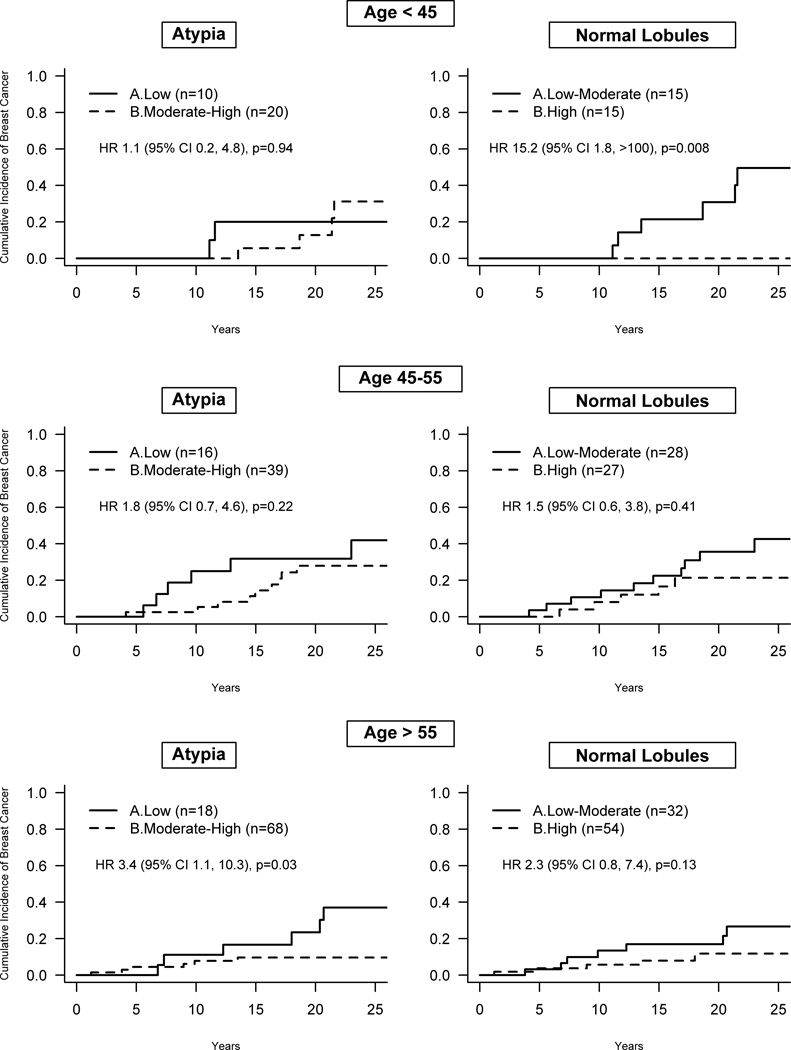
To explore the effect of hormonal status on the protective effect of higher ERβ expression, associations of ERβ expression and breast cancer development were determined within age strata roughly corresponding to pre-, peri-, and post-menopausal states based on age at benign biopsy. The effect of low ERβ expression in the atypia lesion was greatest for older patients. For women over age 55 the HR was 3.4 (95% CI 1.1–10.3), for those age 45 to 55 the HR was 1.8 (95% CI 0.7–4.6) and for those less than age 45 the HR was 1.1 (95% CI 0.2–4.8) as shown in Figure 5. In contrast, lower ERβ expression in normal lobules showed a stronger effect in younger women. In women under age 45 at biopsy, low-moderate versus high ERβ expression was strongly associated with future breast cancer risk with a HR of 15.2 (95% CI 1.8 to >100). Smaller effects of ERβ expression in normal lobules were observed for individuals 45 to 55 (HR 1.5, 95% CI 0.6–3.8) and over age 55 (HR 2.3, 95% CI 0.8–7.4) at the time of benign biopsy. Strikingly, no patient (0 of 15) less than age 45 with high normal lobule ERβ expression developed breast cancer during follow-up versus 40% (6 of 15) with low-moderate normal lobule ERβ expression, p=0.008, as shown in Figure 5.
Figure 5.
Association of ERβ expression in the atypia and adjacent normal lobules with subsequent breast cancer risk stratified by age groups.
Discussion
Atypical hyperplasia is identified in 4 to 10% of benign breast biopsies.[42–44] Recent data suggest that the absolute risk of future breast cancer imparted after a biopsy demonstrating atypia is about 30% at 25 years, comparable to the risk associated with mantle radiation prior to age 31, a well-recognized indication for breast cancer risk reduction interventions.[33,45–47] While a prior benign breast biopsy showing atypia confers a substantial risk for the future development of breast cancer, currently utilized models to predict breast cancer risk are poorly applicable to these women, although a risk model incorporating benign breast biopsy histology recently has been published.[48] In current practice, the use of cumulative incidence data is recommended when counseling women with atypical hyperplasia on their future risk of breast cancer and the number of atypical foci is reported as the best further discriminant of risk for these high-risk women.[47] Despite these advances, further individualization of risk prediction is needed, as the majority of these very high-risk women will not be diagnosed with breast cancer. To this end, we evaluated ERβ expression using a sum score incorporating both the extent and intensity of staining and found that preserved nuclear ERβ expression in the atypia and in the normal lobules of women with atypical hyperplasia was significantly associated with a decreased risk of subsequent breast cancer.
In our longitudinal cohort study we found that when ERβ expression was diminished within the atypical epithelium, the risk of future breast cancer was doubled. Low ERβ expression in the adjacent normal lobules also was associated with a 2.5 -fold increase in future breast cancer risk. Further, high ERβ expression in the normal lobules was most protective against future breast cancer for women who were under 45 years of age (and likely premenopausal) at the time of their biopsy. To the best of our knowledge, this is the first study to evaluate ERβ expression and breast cancer risk in a well-defined cohort of women with long-term follow-up.
Shaaban et al reported a case-control study in which they evaluated patients who were diagnosed with breast cancer at least 6 months after a prior benign breast biopsy and compared them to an age and year of biopsy-matched group of controls who had a benign breast biopsy between 1979 and 1999 and who did not develop cancer.[20] In this study, ERβ expression was assessed in 54 cases and 71 controls in foci of usual type hyperplasia without atypia using the same antibody we utilized, specific for the full-length ERβ1 receptor. In the hyperplastic lesions of cases versus controls they found a non-statistically significant difference in mean nuclear percent staining for ERβ: 68% in the cases versus 81% in the controls. In 116 normal lobules evaluated for ERβ expression, 56 from patients who later developed cancer and 60 who did not, mean percentage nuclear staining was 89% in the cases versus 97% in the controls. These findings lend support to our hypothesis that high levels of ERβ expression in both the atypical lesion and adjacent normal breast tissue are protective against future breast cancer. To the best of our knowledge, no other case-control studies evaluating ERβ expression and subsequent breast cancer risk have been reported.
We confirmed that ERβ expression was lower in the atypia than in adjacent normal lobules whether assessed by nuclear percent staining, staining intensity or sum score. Roger and colleagues evaluated 13 cases of atypia (12 ADH and one ALH) of which two were from patients with coincident cancer. They evaluated total ERβ expression with a polyclonal ERβ503 antibody and described a statistically significant decrease in the ERβ to ERα ratio in the lesional epithelia versus both adjacent normal epithelia and normal lobules from reduction mammoplasties.[8] Ellis et al reported similar findings on ERβ expression in a study of ADH grouped with DCIS, and ALH grouped with LCIS from both patients with and without cancer combined.[10] This study used the 14C8 ERβ antibody which also detects total ERβ, including splice variants, and described similar levels of ERβ expression in normal lobules from breast reduction specimens and cancer patients and a steady decline in expression from benign breast disease with usual hyperplasia to atypia combined with carcinoma in situ. Using a different ERβ1 specific antibody and categorization of staining similar to our methodology, Chantzi et al found that ERβ1 expression was slightly, but not significantly, higher in 14 samples of normal breast tissue versus 16 cases of atypical hyperplasia.[12] Unlike our study and another [10] in which there was no association between age and ERβ expression in the atypia or adjacent normal lobule, they further found that ERβ1 expression declined after menopause in normal breast lobules and benign breast disease including atypical hyperplasia as well as in DCIS.
Our finding of the dramatic protective effect on future breast cancer risk of high ERβ expression in the normal adjacent breast lobules in premenopausal patients is intriguing and raises several interesting hypotheses for further investigation. Might early intervention to preserve or create a high ERβ breast environment prior to menopause confer a risk-reducing benefit that extends for decades? Why is it that ERβ is so effective when patients are younger (pre-menopausal) and why is the effect so long lasting even after menopause? Is this analogous to the tamoxifen effect of five years of treatment affecting 20-year event rate? Would therapeutic targeting of ERβ represent a novel treatment for breast cancer prevention? These findings lend further support to the notion that ERβ functions as a potent tumor suppressor in the breast and lay the foundation for future studies aimed at addressing these questions.
Strengths of our study include use of an established and well-characterized patient cohort with long-term follow-up information on breast cancer events, as well as careful central pathology review and verification of cases. Limitations include the relatively modest absolute number of events and use of a manual, semi-quantitative scoring system for assessing ERβ expression. Along with development of digital quantitation algorithms to permit semi-automated reads of immunohistochemical ERβ expression, confirmation of our findings in a validation cohort is needed prior to clinical application of these data.
Conclusions
Here we show for the first time that preserved ERβ expression in atypical hyperplasia and within normal adjacent breast epithelium is protective against the future development of breast cancer. These data suggest that ERβ may be both a biomarker of elevated breast cancer risk and a potential target for therapeutic intervention. Analysis in a validation cohort to confirm our findings, and further investigation in a larger group of women at lower risk for breast cancer, is indicated to confirm the role of ERβ as a novel biomarker of breast cancer risk. Studies are warranted to further elucidate the mechanisms by which ERβ functions in normal breast epithelial cells and/or alters the microenvironment surrounding atypical breast lesions, in order to elicit a breast cancer preventative effect. Finally, strategies aimed at enhancing ERβ expression levels and activating the tumor suppressive effects of this receptor appear promising for the prevention of breast cancer.
Supplementary Material
Acknowledgments
The authors thank T. Allers, J. Johnson, M. Campion, M. Kasner, and A. Harris and the Mayo Survey Research Center for data collection.
Financial Support: This research was supported by the Mayo Clinic Breast Cancer Specialized Program of Research Excellence (SPORE) grant CA116201 (LC Hartmann and DC Radisky) and R01 CA132879 from the National Cancer Institutes (LC Hartmann) and by the Fred C. and Katherine B. Andersen Foundation (LC Hartmann). The content is the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health.
Footnotes
Disclosures: None.
References
- 1.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 3.Osborne CK, Schiff R. Estrogen-receptor biology: continuing progress and therapeutic implications. J Clin Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907–922. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce ST, Jordan VC. The biological role of estrogen receptors alpha and beta in cancer. Crit Rev Oncol Hematol. 2004;50:3–22. doi: 10.1016/j.critrevonc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Speirs V, Skliris GP, Burdall SE, Carder PJ. Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol. 2002;55:371–374. doi: 10.1136/jcp.55.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvinen TA, Pelto-Huikko M, Holli K, Isola J. Estrogen receptor beta is coexpressed with ER alpha and PR and associated with nodal status, grade, and proliferation rate in breast cancer. Am J Pathol. 2000;156:29–35. doi: 10.1016/s0002-9440(10)64702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roger P, Sahla ME, Makela S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–2541. [PubMed] [Google Scholar]
- 9.Shaaban AM, O'Neill PA, Davies MP, Sibson R, West CR, Smith PH, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32:513–523. doi: 10.1097/PAS.0b013e318161d1a5. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, et al. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem. 2012;113:711–723. doi: 10.1002/jcb.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chantzi NI, Palaiologou M, Stylianidou A, Goutas N, Vassilaros S, Lourea HP, et al. Estrogen receptor beta2 is inversely correlated with Ki-67 in hyperplastic and noninvasive neoplastic breast lesions. J Cancer Res Clin Oncol. 2014;140:1057–1066. doi: 10.1007/s00432-014-1652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B, Omoto Y, Iwase H, Yamashita H, Toyama T, Coombes RC, et al. Differential expression of estrogen receptor alpha, beta1, and beta2 in lobular and ductal breast cancer. Proc Natl Acad Sci U S A. 2014;111:1933–1938. doi: 10.1073/pnas.1323719111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, et al. ERbeta1: characterization, prognosis, and evaluation of treatment strategies in ERalpha-positive and -negative breast cancer. BMC Cancer. 2014;14:749. doi: 10.1186/1471-2407-14-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengupta D, Bhargava DK, Dixit A, Sahoo BS, Biswas S, Biswas G, et al. ERRbeta signalling through FST and BCAS2 inhibits cellular proliferation in breast cancer cells. Br J Cancer. 2014;110:2144–2158. doi: 10.1038/bjc.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edvardsson K, Strom A, Jonsson P, Gustafsson JA, Williams C. Estrogen receptor beta induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol Endocrinol. 2011;25:969–979. doi: 10.1210/me.2010-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Giorgi V, Gori A, Gandini S, Papi F, Grazzini M, Rossari S, et al. Oestrogen receptor beta and melanoma: a comparative study. Br J Dermatol. 2013;168:513–519. doi: 10.1111/bjd.12056. [DOI] [PubMed] [Google Scholar]
- 18.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- 19.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of estrogen receptor beta1, beta2, and beta5 messenger RNAs in human breast tissue. Cancer Res. 1999;59:1175–1179. [PubMed] [Google Scholar]
- 20.Shaaban AM, Jarvis C, Moore F, West C, Dodson A, Foster CS. Prognostic significance of estrogen receptor Beta in epithelial hyperplasia of usual type with known outcome. Am J Surg Pathol. 2005;29:1593–1599. doi: 10.1097/01.pas.0000184807.38037.75. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13:R27. doi: 10.1186/bcr2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64:423–428. doi: 10.1158/0008-5472.can-03-2446. [DOI] [PubMed] [Google Scholar]
- 23.Sotoca AM, van den Berg H, Vervoort J, van der Saag P, Strom A, Gustafsson JA, et al. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105:303–311. doi: 10.1093/toxsci/kfn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci U S A. 2004;101:1566–1571. doi: 10.1073/pnas.0308319100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honma N, Horii R, Iwase T, Saji S, Younes M, Takubo K, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26:3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 26.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Huges TA, Harkins L, et al. Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin Cancer Res. 2008;14:5228–5235. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 27.Novelli F, Milella M, Melucci E, Benedetto A, Sperduit I, Perrone-Donnorso R, et al. A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 2008;10:111. doi: 10.1186/bcr2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madeira M, Mattar A, Logullo AF, Soares FA, Gebrim LH. Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer. 2013;13:425. doi: 10.1186/1471-2407-13-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Li X, Blanchard A, Bramwell VH, Pritchard KI, Tu D, et al. Expression of both estrogen receptor-beta 1 (ER-β1) and its co-regulator steroid receptor RNA activator protein (SRAP) are predictive for benefit from tamoxifen therapy in patients with estrogen receptor-alpha (ER-α)-negative early breast cancer (EBC) Ann Oncol. 2013;24:1986–1993. doi: 10.1093/annonc/mdt132. [DOI] [PubMed] [Google Scholar]
- 30.Motomura K, Ishitobi M, Komoike Y, Koyama H, Nagase H, Inaji H, et al. Expression of estrogen receptor beta and phosphorylation of estrogen receptor alpha serine 167 correlate with progression-free survival in patients with metastatic breast cancer treated with aromatase inhibitors. Oncology. 2010;79:55–61. doi: 10.1159/000319540. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 32.Barr FE, Degnim AC, Hartmann LC, Radisky DC, Boughey JC, Anderson SS, et al. Estrogen receptor expression in atypical hyperplasia: lack of association with breast cancer. Cancer Prev Res (Phila) 2011;4:435–444. doi: 10.1158/1940-6207.CAPR-10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25:2671–2677. doi: 10.1200/JCO.2006.09.0217. [DOI] [PubMed] [Google Scholar]
- 34.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 35.Santisteban M, Reynolds C, Barr Fritcher EG, Frost MH, Vierkant RA, Anderson SS, et al. Ki67: a time-varying biomarker of risk of breast cancer in atypical hyperplasia. Breast Cancer Res Treat. 2010;121:431–437. doi: 10.1007/s10549-009-0534-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, et al. Assessment of multiple different estrogen receptor-beta antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat. 2006;100:23–31. doi: 10.1007/s10549-006-9229-5. [DOI] [PubMed] [Google Scholar]
- 37.Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang X-RAY, Andrulis IL, et al. ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat. 2014;146:657–667. doi: 10.1007/s10549-014-3050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 40.Kohl M, Heinze G. PSHREG: A SAS macro for proportional and nonproportional substribution hazards regression with competing risk data. Technical report 08/2012, Center for Medical Statistics, Informatics and Intelligent Systems. 2013 [Google Scholar]
- 41.Gray B. cmprsk. Subdistribution Analysis of Competing Risks. R package version 2.2-6. 2013 Available at: http://CRANR-projectorg/package=cmprsk. [Google Scholar]
- 42.Ghosh K, Melton LJ, 3rd, Suman VJ, Grant CS, Sterioff S, Brandt KR, et al. Breast biopsy utilization: a population-based study. Arch Intern Med. 2005;165:1593–1598. doi: 10.1001/archinte.165.14.1593. [DOI] [PubMed] [Google Scholar]
- 43.Allison KH, Abraham LA, Weaver DL, Tosteson AN, Nelson HD, Onega T, et al. Trends in breast biopsy pathology diagnoses among women undergoing mammography in the United States: a report from the Breast Cancer Surveillance Consortium. Cancer. 2015;121:1369–1378. doi: 10.1002/cncr.29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson JF. Update on atypical epithelial hyperplasia and ductal carcinoma in situ. Pathology. 2009;41:36–39. doi: 10.1080/00313020802568097. [DOI] [PubMed] [Google Scholar]
- 45.Hartmann LC, Radisky DC, Frost MH, Santen RJ, Vierkant RA, Benetti LL, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila) 2014;7:211–217. doi: 10.1158/1940-6207.CAPR-13-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Bruin ML, Sparidans J, van't Veer MB, Noordijk EM, Louwman MW, Zijlstra JM, et al. Breast cancer risk in female survivors of Hodgkin's lymphoma: lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast--risk assessment and management options. N Engl J Med. 2015;372:78–89. doi: 10.1056/NEJMsr1407164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pankratz VS, Degnim AC, Frank RD, Frost MH, Visscher DW, Vierkant RA, et al. Model for individualized prediction of breast cancer risk after a benign breast biopsy. J Clin Oncol. 2015;33:923–929. doi: 10.1200/JCO.2014.55.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.