Abstract
Abnormal accumulation of amyloid β (Aβ), α-synuclein (α-syn), and microtubule associated protein tau (tau) have been implicated in neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Pick’s disease (PiD). The mechanisms through which aggregated versions of α-syn, Aβ, and tau may lead to neurodegeneration are not entirely clear, however, there is emerging evidence that neuronal calcium dysregulation is at play. Two-photon microscopy is a powerful tool that can be used to measure in vivo alterations of calcium transients using animal models of neurodegeneration, and when coupled with statistical methods to characterize functional signals, can reveal features that identify and discern between distinct mouse types. We studied four mouse models of neurodegenerative diseases, wild-type (WT) α-syn, E57K α-syn, amyloid precursor protein (APP), and triple-repeat (3R)-Tau and Non-tg littermates using two-photon microscopy. We found that for calcium transients, simple measures such as area under the curve (AUC) and peak width in the 1 Hz whisker pad stimulation paradigm, were significantly increased for WT α-syn, E57K α-syn and APP mice across all cortical depths compared to Non-tg mice. A similar result was found in the 3 Hz paradigm in E57K α-syn mice. Spontaneous calcium transient AUC was significantly higher in WT α-syn mice and lower for APP and 3R Tau mice at 150 µm depth. Going beyond simple measure differences such as group means for AUC, signal peak width, and spontaneous calcium activity counts, we built statistical classifiers to characterize neuronal calcium signals to identify and discern, with quantified measures of confidence, all mouse types. We tested our classifier with FK506, which regulates mitochondrial calcium and found that this drug modulated the WT α-syn calcium transients to such an extent that the classifier easily identified the calcium transients as belonging to Non-tg mice. The coupling of two photon microscopy data and statistical classifiers serves to effectively create a bioassay where the number of animals and scientific resources can be reduced without compromising the results of the experiment.
Keywords: bio-assay, classifier, FK506, neurodegeneration, statistical pattern recognition, two-photon
1. Introduction
Abnormal accumulation of amyloid β (Aβ), α-synuclein (α-syn), and microtubule associated protein tau (tau) have been implicated in neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Pick’s disease (PiD). Although the specific mechanisms through which these proteins lead to neuronal vulnerability are not completely clear, previous studies have suggested that intracellular calcium homeostasis is dysregulated in each of these disease states (Richard et al., 1978, Thibault et al., 2007, Zundorf and Reiser, 2011).
In order to investigate the proteins associated with neurodegenerative diseases, including Aβ, α-syn, and tau, mouse models of neurodegenerative diseases have been used to gain invaluable insight in to the mechanisms and progression of the diseases, in addition to providing a way to evaluate potential treatment strategies. For instance, the wild-type (WT) α-syn transgenic (tg) mouse model (Line 61) is used to model PD and accumulates various forms of α-syn (Rockenstein et al., 2002), and more recently, an E57K mutant α-syn oligomer-prone model of PD has become available (Rockenstein et al., 2014). There are a number of AD animal models (Gotz and Ittner, 2008) including an amyloid precursor protein (APP) mouse model, which develops mature plaques at an early age and has high levels of Aβ42 compared to Aβ40 (Rockenstein et al., 2001). In contrast, few models of the familial forms of PiD with three-repeat Tau are available. Most recently a tg mouse model expressing three-repeat Tau was developed bearing the mutations associated with familial forms of PiD (L266V and G272V mutations), which has notable mitochondrial alterations (Rockenstein et al., 2015).
The mechanisms through which aggregated versions of α-syn, Aβ, and tau may lead to neurodegeneration are not entirely clear, however, calcium dysregulation may be implicated (Bezprozvanny, 2009, Zundorf and Reiser, 2011). Two-photon microscopy is a powerful tool used to analyze calcium dynamics in vivo, and has been employed to analyze changes in calcium signaling in some models of neurodegenerative disease. Previous reports have shown using in vivo two-photon imaging in awake behaving mice that Aβ plaques were associated with altered calcium homeostasis in APP tg mice (Busche et al., 2008, Busche et al., 2012), but not in anaesthetized PS1 tg animal models (Kuchibhotla et al., 2008), nor were neurofibrillary tangle (NFT)-bearing neurons affected in a tau (P301L) mutant model (Kuchibhotla et al., 2014). Recently, abnormal calcium transients were analyzed for visibly apparent changes such as the size of the transient (peak width, peak height, and area under the curve) and frequency of spontaneous calcium transients in an α-syn tg mouse model of synucleinopathy (Reznichenko et al., 2012). The transgenic mice exhibited augmented, long-lasting calcium transients characterized by considerable deviation from the exponential decay when stimulated using a 1 Hz paradigm (Reznichenko et al., 2012). At the same time, these results raised the possibility that abnormal calcium transients may be present in other transgenic mouse models of neurodegenerative diseases, and that there may be other features present in the calcium trace data that are not visibly apparent.
In neuroscience, statistical methods to characterize functional signals, such as calcium transients, have been evolving as advances in statistical data processing proliferate and flow into other fields. MRI and EEG signal processing have paved the way as the earlier and prominent examples of neuroscience studies to benefit from emerging statistical methods (Bell and Sejnowski, 1995, Makeig S et al., 1996). More recently, calcium transients and other temporal signals commonly used in neuroscience are being analyzed using statistical pattern recognition (SPR) methods (Mukamel et al., 2009). While SPR is well established, the techniques are still novel in their application to neuroscience and as such can provide new approaches to analyzing data beyond widely utilized standard measures like the group mean and standard deviation. Neuroscience has relied primarily on visible features in the data leaving the non-visual feature space largely unexplored. We used established SPR methods in order to access and use the information embedded in the data space to look beyond anomalies we can see visually in transient shape and size when we plot the calcium transients. Often there are patterns embedded in the data that can help discriminate one signal class from another, but they are not necessarily discernible in the raw form of the data . SPR can transform the data space to unmask subtle characteristics and allow for discrimination of two groups that otherwise might “look” similar. Dimensionality reduction methods such as principal component analysis (Bishop, 2007) or cluster analysis (Duda et al., 2001) are common methods used to transform high-dimensional data. Cluster analysis on calcium data, in particular, can group together calcium traces that are similar to arrive at a small number of sub-groups (i.e. calcium traces from non-transgenic mice group) to capture a new representation of the data for a mouse type. With this more compact representation, a classifier function (Bishop, 2007) can be constructed based on the cluster centers to recognize a particular mouse type from its calcium traces. The classifier function outputs a binary ‘1’ or ‘0’ value determining if the calcium traces of a novel mouse, specifically a mouse that is not part of the training the classifier but does belong to one of the mouse types that the classifier has been trained to recognize, is similar to a known model and if there are multiple models in question, which is the most likely fit (Bishop, 2007).
We evaluated four different mouse models of neurodegenerative diseases (PD, AD, and PiD) and Non-tg littermates using two-photon microscopy in order to identify features in calcium transients that can serve as features across different neurodegenerative disease models, and tested a calcium modulatory drug (FK506) in one of the mouse models that showed calcium transient aberrations. We found that the AUC and peak widths were significantly different for WT α-syn, E57K α-syn and APP tg mouse types, but not for 3R Tau compared to Non-tg mice. Transforming the data into different signal spaces using cluster analysis effectively reduced the dimensionality of the data. Then by building classifiers for each mouse type we were able to assess whether a novel mouse was statistically similar to the training data, essentially answering the question, “Does this mouse belong the mouse type X?”. We found that the classifier distinguished between different mouse types, and that when FK506 was added as a treatment for the WT α-syn mouse, the classifier categorized the treated mouse as Non-tg.
2. Experimental procedures
2.1 Generation of mThy-1 wild-type α-syn, E57K α-syn, APP, and 3R Tau tg mice
The University of California at San Diego’s animal subjects committee approved all experiments. WT α-syn Line 61 tg mice expressing human (h)α-syn under the neuronal murine Thy-1 promoter (mThy-1) (provided by Dr. H. van der Putten, Ciba-Geigy, Basel, Switzerland) were generated as previously described (Rockenstein et al., 2002). E57K α-syn Line 16 mice expressing (h)α-syn-bearing the oligomer-prone E57K mutation under the neuronal mThy-1 promoter cassette were generated, as previously described (Rockenstein et al., 2014). APP line 41 mice expressing hAPP751 cDNA containing the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the murine Thy-1 promoter (mThy1) were generated as previously described (Rockenstein et al., 2001). 3R Tau Line 13 mice expressing (h)3R Tau mutations associated with familial PiD (L266V and G272V) under the neuronal mThy-1 promoter cassette were generated, as previously described (Rockenstein et al., 2015). A total of 5–9 female mice per group were utilized (9–12 months of age).
2.2 Animal procedures
Mice were anesthetized with isoflurane [3% initially, followed by 1–2% during surgical procedures (tracheotomy, cannulation of femoral artery, and cortical exposure)]. Surgical procedures were as previously described (Devor et al., 2007, Reznichenko et al., 2012). During data acquisition, anesthesia was maintained with α-chloralose (50 mg*kg−1) and pancuronium (0.4 mg/kg). Mice were ventilated with 30% O2 in air (~100 bpm). Expired CO2 (CI240, Columbus instruments), heart rate, blood pressure (BP1, World Precision Instruments), and body temperature (Homeothermic blanket, Harvard Apparatus) were monitored continuously. Blood gas (Rapidlab 248, Siemens) was measured at the start of data acquisition to adjust the respiration parameters to achieve pCO2 35–45 mmHg, pO2 100–180 mmHg, and pH 7.35–7.45.
In order to test the ability of our classifier to determine if a transgenic mouse treated with a drug was statistically more likely to be categorized as belonging to the transgenic (suggesting the drug had no effect on calcium transients) or Non-tg mouse types (suggesting the drug normalized the calcium transients), WT α-syn mice (N=3) were treated with FK506 (5 mg/kg dissolved in 10% ethanol 90% saline, intra-arterial) 1 hour before imaging.
2.3 Two-photon imaging
Calcium indicator Oregon Green 488 BAPTA-1 AM (OGB, green) was loaded by microinjection (Stosiek et al., 2003) and glial marker sulforhodamine 101 (SR101, red) was applied topically to the brain. Images were obtained using an Ultima two-photon laser scanning microscopy system from Prairie Technologies, equipped with an Ultra II femtosecond laser (Coherent) tuned to 800 nm. Two stimulus types, a single electrical stimulus (100 µs, 1 mA) and a train of 3 stimuli at 3 Hz, were delivered to the contralateral whisker pad. Within each ~50 × 100 µm field of view (FOV), neuronal cell bodies were imaged in a frame scan mode with a target acquisition rate of 10 Hz. Fifteen stimulus trials with interstimulus intervals (ISI) of 15 s were presented for each FOV and for each of the 2 stimulus conditions.
For extraction of time courses, masks corresponding to individual neuronal bodies were segmented from the images. For an individual neuron, calcium signal per frame was calculated as an average of all pixels within its cell body mask. This calculation was repeated for each frame in the time series to generate a single-neuron time course. The fluorescent signal (F) was expressed as the percent change relative to the prestimulus baseline (ΔF/F). For calculation of averages across neurons, we grouped the single-neuron data according to 3 cortical depths: 100 µm (the upper boundary of layer II), 150 µm (layer II), and 250 µm (layer III). For the WT-α-syn mice treated with FK506, we followed the same data collection process, but optimized our data collection to use the 1 Hz stimulus paradigm and a depth of 150 µm.
2.4 Data analysis
Two-photon data were analyzed in MATLAB if they responded to stimulus in at least 13 of 15 stimulus trials using the classic measures of AUC, peak width (30% peak amplitude), and the highest peak (first or third), and spontaneous calcium transients as previously described (Reznichenko et al., 2012). For classical shape analysis, calcium transients were aligned based on maximum peak slope and normalized to the maximum peak height. Two-photon imaging results were expressed as the mean ± SEM. For evaluation of statistical significance, independent datasets were compared using One-way ANOVA followed by Dunnett’s post hoc analysis. P-values < 0.05 were considered significant. Additional statistical analyses were performed in MATLAB using the MATLAB statistical and bioinformatics toolboxes. For each mouse-type, a binary classifier was developed using K-means cluster analysis combined with one of five different measures to determine if a novel dataset belonged to the Non-tg mouse type or to the rest of the mouse types: Method 1, distance to the training set for the mean of all traces for a given mouse; Method 2, Likelihood ratio test (Bishop, 2007) which statistically combined the probability of the distances (to the training set) for the novel traces having come from the training set; Method 3, the AUC for all individual traces spanning 1 second of time from the max peak height in the novel data set; Method 4, was similar to Method 2, but used the average trace to perform the likelihood ratio test; Method 5, was similar to Method 3, but used the mean trace to perform AUC. We used a cluster size of 4, which was arrived at empirically as a tuning parameter with the best results in classification tasks. Since the datasets for the mouse types were small due to the nature of working with tg mice, novel datasets were generated using the leave-one-out cross validation method (Bishop, 2007). While we trained the classifiers based on individual calcium traces, the classification task was defined as generating a single score resulting from a set of traces collected from an individual mouse. We used a simple approach of one-versus-rest binary classifiers (Bishop, 2007), which effectively asked, “Is the novel dataset Non-tg?”, “Is it WT α-syn?”, and so on, iteratively testing one group at a time against the rest of the mouse types until the most-likely mouse type was identified. Receiver Operator Characteristic (ROC) Curves quantitatively assessed how well the classifiers correctly identified to which mouse-type the novel dataset belonged (Bishop, 2007).
3. Results
The barrel cortices of female mice (9–12 months of age) were imaged for WT α-syn (n = 5), E57K α-syn (n = 6), APP (n = 5) and 3R Tau (n = 7) tg, and age-matched Non-tg controls (n = 9) (Fig 1A). The number of neurons imaged varied by depth with fewer neurons imaged at 100 µm compared to 150 and 250 µm depths. For illustrative purposes, using the 1 Hz stimulus paradigm and a depth of 150 µm, we analyzed the following numbers of neurons: Non-tg (344), E57K α-syn (257), APP (247), and 3R Tau (217). For WT α-syn mice we collected additional data at 1 Hz and 150 µm depth and therefore imaged 1047 neurons. The numbers of neurons were in a similar range for the other Hz/depth combinations with on average 266 neurons per mouse/depth/Hz combination. Neurons, labeled with calcium indicator OGB1, appeared in green. Astrocytes, labeled with both OGB and SR101, appeared in yellow (Fig 1B). The masks for the ROIs for neurons and astrocytes (Fig 1C) were used for the initial processing of individual calcium traces (Fig 1D).
Figure 1.
Representative examples of calcium activity from barrel cortex neurons in Non-tg, WT α-syn, E57K α-syn, APP, and 3RTau Tg mice. A. Composite two photon image of the calcium indicator dye, OGB (green), and astrocyte marker, SR101 (yellow-orange). B. Respective two photon images indicating the SR101-positve structures. C. Regions of interest for each image. Neurons are numbered and indicated in green; astrocytes are in red. D. The time course for the calcium signal was extracted based on regions of interest identified in the previous panel for 1 and 3 Hz, respectively. The representative neuron number is indicated to the left of each trace. The arrows indicate the onset of the stimulus. Scale bar for photomicrographs is 10 µm.
3.1 One-dimensional evaluation of calcium transient indicated differences between transgenic and Non-tg mice
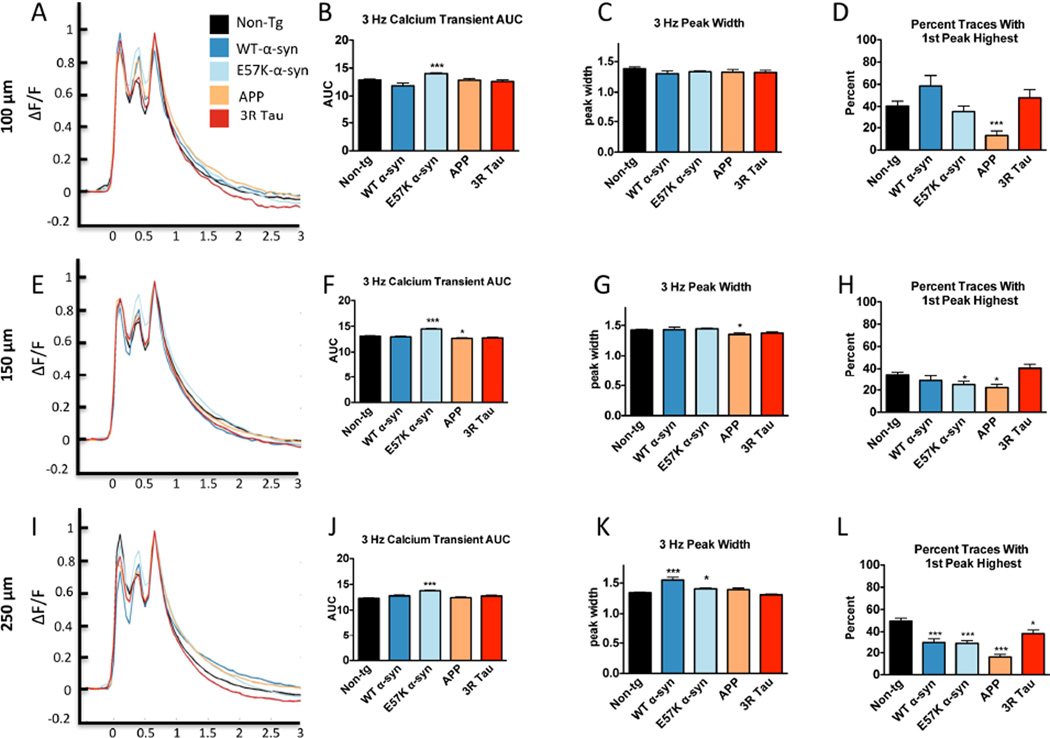
Calcium transients were normalized for peak shape in order to evaluate the peak shapes using the classical measures of AUC, peak width, and in the 3 Hz stimulus paradigm, peak height. Under the 1Hz stimulus paradigm and at a depth of 100 µm, the WT α-syn, E57K α-syn, and APP 1 Hz calcium transients had “shoulders” in their geometric shapes, while the 1 Hz calcium transients from the 3R Tau tg mice had geometric shapes indistinguishable to the Non-tg mice (Fig 2A). Similarly, the AUC and peak widths were significantly different from Non-tg mice for WT α-syn, E57K α-syn and APP tg mouse types, but not for 3R Tau (Fig 2B, C). These findings were similar at depths of 150 (Fig 2 D–F) and 250 µm (Fig 2 G–I) for WT α-syn, E57K α-syn and APP mice for peak shape, AUC, and peak width. In addition, the AUC for 3R Tau was also significantly different from Non-tg mouse type at 150 µm (Fig 2E) and 250 µm (Fig 2H). These findings are similar to previous reports of a shoulder in the calcium transients, as well as the AUCs and peak widths of WT α-syn mice compared to Non-tg mice across depths (Reznichenko et al., 2012). Using the 3 Hz stimulus paradigm at a depth of 100 µm, the most notable visual difference was observed for the middle peak (Fig 3A); however only the E57K α-syn mouse type had a significant difference for the AUC parameter (Fig 3B, C). While there were subtle visual differences in heights between the first and third peaks, WT α-syn and 3R Tau mice had a non-significant trend toward the 1st peaks being higher then the 3rd peaks, and the APP tg mouse more often had 3rd peaks which were higher then the 1st peaks (Fig 3D). At 150 µm depth (Fig 3E–G), E57K α-syn and APP mouse types had significantly different AUCs compared to the Non-tg mouse type, and the peak width was significantly different for the E57K α-syn and APP mouse types when compared to the Non-tg mouse types (Fig 3H). At the 250 µm depth (Fig 3I–K), the E57K α-syn tg mouse type was significantly different from Non-tg mouse type for both AUCs (Fig 3J) and peak width (Fig 3K). At this depth the WT α-syn, E57K α-syn and APP mouse types appeared to have the highest frequency of the 3rd peak being higher then the 1st peak (Fig 3L).
Figure 2.
Visual representation and simple quantification of average calcium peak shapes for Non-tg, WT α-syn, E57K α-syn, APP, and 3R Tau mice using 1Hz stimulus frequency across different depths. Normalized calcium traces from barrel cortex neurons were quantified using area under the curve (AUC) and peak width. A. Calcium transient peak shape, B AUC, and C peak width from 100 µm depth. D. Calcium transient peak shape, E. AUC, and F. peak width from 150 µm depth. G. Calcium transient peak shape, H. AUC, and I. peak width from 250 µm depth. Statistical analysis performed using Oneway ANOVA with a Dunnett’s post hoc analysis compared to Non-tg mice. * = p-value < 0.05; ** = p-value < 0.01; *** = p-value < 0.001.
Figure 3.
Visual representation and simple quantification of average calcium peak shapes for Non-tg, WT α-syn, E57K α-syn, APP, and 3RTAU mice using 3Hz stimulus frequency across different depths. Normalized calcium traces from barrel cortex neurons were quantified using area under the curve (AUC) and peak width. A. Calcium transient peak shape, B AUC, C peak width, and D percent of traces where the first peak is higher then the third peak from 100 µm depth. E. Calcium transient peak shape, F. AUC, G. peak width, and H percent of traces where the first peak is higher then the third peak from 150 µm depth. I. Calcium transient peak shape, J. AUC, K. peak width, and L percent of traces where the first peak is higher then the third peak from 250 µm depth. Statistical analysis performed using One-way ANOVA with a Dunnett’s post hoc analysis compared to Non-tg mice. * = p-value < 0.05; ** = p-value < 0.01; *** = p-value < 0.001.
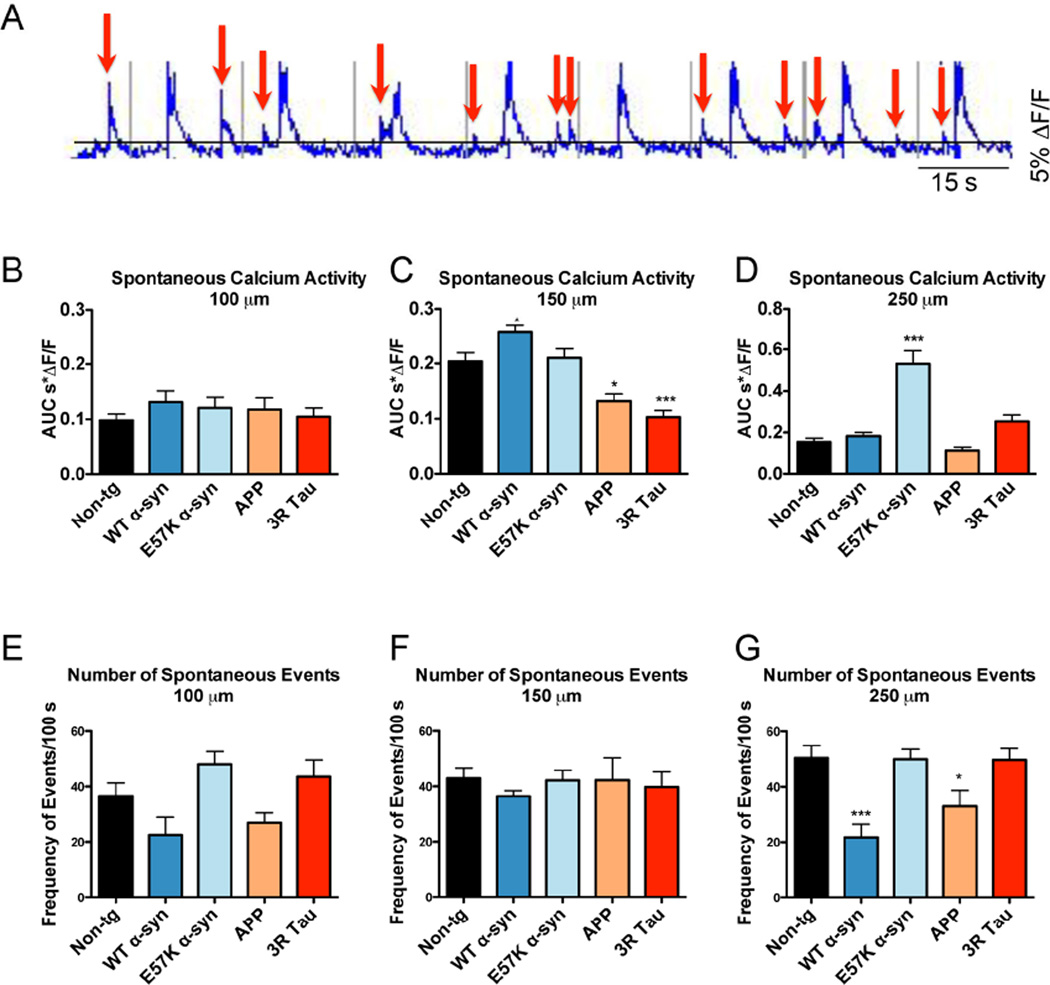
In addition to the evoked calcium transient analysis, spontaneous activity, defined as a transient having a percent calcium increase greater than 5% above baseline, was evaluated across all depths (Fig 4A). While there was no statistically significant change in spontaneous activity for any of the transgenic mouse types at 100 µm depth (Fig 4B), at 150 µm WT α-syn had a statistically significant increase in spontaneous activity, while APP and 3R Tau had statistically significant decreases in spontaneous activity compared to Non-tg mice (Fig 4C). At 250 µm depth only E57K α-syn had a statically significant increase in spontaneous activity compared to Non-tg mice (Fig 4D). Along with the AUC, the frequency of spontaneous events was evaluated per 100 s. While there were no significant differences in the number of spontaneous events at depths of 100 (Fig 4E) and 150 µm (Fig 4F), at 250 µm both WT α-syn and APP mouse types had significant reductions in the numbers of spontaneous events compared to Non-tg mice (Fig 4G). In addition to the classical outcome measures, which offered insight into three mouse models that had previously not been imaged under these paradigms, we asked if the different mouse types could be classified using the high-dimensional data captured in the calcium transients.
Figure 4.
Spontaneous calcium transient activity. A Spontaneous events were defined as a greater than 5% ΔF/F increase from the baseline and are indicated by red boxes. The AUC was calculated as an integral over time periods above the threshold. Spontaneous events were evaluated at B 100 µm, C 150 µm, and D 250 µm depths. The frequency of the spontaneous events per 100 s were evaluated at E 100 µm F 150 µm, and G 250 µm. Statistical analysis performed using One-way ANOVA with a Dunnett’s post hoc analysis compared to Non-tg mice. * = p-value < 0.05; *** = p-value < 0.001.
3.2 Developing the Non-tg statistical model
Modeling the Non-tg mouse data was the first step towards looking at the ensemble of data since the first set of comparisons were made between each tg mouse type and the Non-tg mouse type. Using our previously reported findings on Non-tg calcium transients (Reznichenko et al., 2012), the Non-tg data set from the present study was carefully evaluated to ensure the capture of the highest fidelity signal to represent this data class. This is particularly important since cluster analysis and dimensionality reduction relies on consistent structure within each mouse type. Lowering the threshold toward allowing more noisy/erratic traces into the training set would result in higher numbers of clusters and higher complexity of the classifier models. This would result in effectively asking the classifier to “learn” the form of erratic traces for each mouse type. The primary objective of classifiers is to capture low complexity but highly descriptive and consistent features for each mouse type. Lowering the threshold would decrease the accuracy of our classifier for the given amount of training data. To that end, we would want our representative traces be describable via a lower number of representative cluster vectors. Therefore, all datasets were reviewed for non-responding and poor responding neurons as defined by responding in less than 13 of 15 stimulus trials.
3.3 Discriminating Different Mouse Models of Neurodegenerative Diseases by Building Multi-Class Classifiers
Since calcium transients are inherently high dimensional, in these experiments a single trace had 250 samples/dimensions per trial-averaged trace, an alternative analysis was undertaken to determine if features existed that were not necessarily visibly apparent, but could be used to classify different mouse types. An overview of the steps used to create the classifiers is presented in Figure 3. It is important to keep in mind that this schematic representation presents the process in two dimensions; however, these analyses were actually performed in high dimensional space, for which there is no way to visually represent the data. The Non-tg training set (Fig 5A) was created using the leave-one-out method, and several iterations of the cluster analysis (Fig 5B) were performed using the Non-tg training data in order to empirically determine that 4 clusters were the most reasonable. Each point in the cluster was a trace with 250 dimensions. The cluster analysis (via implementation of k-means clustering in MATLAB) grouped the traces into fixed number of cluster sets such that the distance between the traces within each of the clusters was minimized. At the center of each cluster was the average trace for that cluster that represents one of the four basis vectors for the particular mouse type (Fig 5C). The basis set was used to create the subspace for the training set (Fig 5D). This subspace reduces the complexity of the data while still being able to describe the entire data set (Bishop, 2007). Novel traces (Fig 5E) from the leave-one-out method were plotted in the Non-tg training subspace and the orthogonal distance from the novel trace to the subspace was calculated. The orthogonal distances for the Non-tg basis set and the transgenic mice were plotted as Gaussian distributions (Fig 5G), along with the orthogonal distances for the novel traces. The likelihood ratio test was used to determine the probability that a trace belonged to the Non-tg subspace or not. Other measures were also tested, but in general did not perform as well, including mean distance for all traces to the training space; likelihood ratio test on the average trace; AUC during the 1 second interval following stimulus per trace, and the AUC during the 1 second interval on the average trace. These steps combined together to form the Non-tg classifier (Fig 5H), which was then used to determined if a set of traces from a mouse belonging to one of the 5 types of mice was Non-tg or transgenic. The ROC curve (Fig 5I) was generated for the Non-tg classifier and used to set the threshold for the classifier. ROC curves are a measure of how well the classifier identifies a novel/independent dataset correctly (true positive) versus falsely identifying a known non-training class sample as belonging to the training-class (false positive), with the goal of maximizing the true positive rate and minimizing the false positive rate. These steps were reiterated to build a classifier for each mouse type.
Figure 5.
Schematic overview of the data processing for the classifier. In this schematic, Non-tg and Tg are used for clarity; however, these two groups are reiteratively replaced as the algorithm proceeds through different mouse type classifiers until the mouse type is determined. In the event of multiple completing positive mouse type identifications, we invoke the policy of choosing the model with the highest ROC fidelity score (ROC AUC). A. Following the leave-one-out method, training data sets were created and along with novel (data not in training set) test sets to be used in for assessing classification performance. B. Cluster analysis was used to group the calcium traces into 4 representative groups, creating C. a basis set consisting of cluster centers where every element in the basis set represents the cluster of traces that it belongs to. D. A two-dimensional graphical representation of how a one-dimensional subspace (a single line vector) can represent data points in two dimensions. In our case we use the 4 cluster center vectors as the basis for data set of calcium time traces, each 250 dimensional vectors. E. The novel set is incorporated. F. The orthogonal distance from the novel trace to the Non-tg training subspace was calculated and used to create G Gaussian distributions for the two groups (Non-tg and Tg). The likelihood ratio test was then employed to determine if each novel trace was more likely part of the Non-tg or Tg group. H. The abovementioned steps formed the binary classifier against which the novel data sets of both Tg and Non-tg types were quantitatively characterized. Based on the outcome, the dataset was either classified as belonging to the same mouse type as the training set, or belonging to another group. I. The quantitative performance of the classifier is described with ROC curves, demonstrating the ratio of our classifier’s successful Non-tg detections (Non-tg correctly identified as Non-tg) vs. false positives (transgenic identified as Non-tg).
3.4 Cluster Analysis From Non-tg Mice Discriminates Non-obvious Features Between Transgenic Models of Neurodegeneration
The first classifier was built to detect Non-tg mice. The training set of normalized Non-tg traces (Fig 6A) underwent k-means cluster analysis and 4 clusters were induced. The traces for each cluster and the basis set that resulted (Fig 6B–E) show how traces that visually look similar can have non-visually apparent features that can be used to sort traces into different groups.
Figure 6.
Example of cluster analysis using Non-tg training data. A. Following leave-one-out method, the training data was combined into a dataset. B-E. Basis set (heavy line), and individual traces that belong to each cluster.
3.5 Iterative Application of the Classifiers Identified Novel Calcium Transient Datasets in the Transgenic and Non-tg Mice
Each novel dataset was iteratively tested using the 5 differ mouse-type classifiers. For each iteration, the algorithm was effectively asked, “Is this novel dataset detected by this classifier?”. The output was either one (yes the classifier identified the novel dataset as belonging to the same subspace as the training dataset) or zero (no, the classifier did not identify the novel dataset as belonging to the training dataset). Each novel dataset was tested against all classifiers, and in most cases there was no ambiguity about which mouse type the novel dataset belonged.
ROC curves quantify the accuracy of the classifier, where an AUC of 1 (100%) indicates a perfect test, which correctly identifies if a novel dataset belongs to the correct group 100% of the time. Using traditional academic grading criteria, an AUC between 90 and 100% is considered excellent; 80–90% is good; and 70–80% is fair. Several methods were tested for each classifier using the one-vs.-rest approach, each classifier method had a corresponding ROC curve (Fig 6). For all but one classifier there was at least one method that produced an ROC curve with an AUC greater than 0.8, which is considered good (Fig 7A). The WT α-syn vs. rest classifier had a maximum AUC of 75% (Fig 7B). For each iteration of a classifier, the best method for that classifier was used. For the Non-tg (Fig 7A,B), WT α-syn (Fig 7B), and 3R Tau (Fig 7B) classifiers the per-trace likelihood ratio test was used (method 2), while for E57K α-syn classifier the AUC on the average trace was used (method 3, Fig 7B). The APP classifier used the AUC on individual traces as the best method (method 5, Fig 7B).
Figure 7.
Receiver Operator Characteristic (ROC) Curves using the one-vs.-rest approach. For each mouse-type detector 5 different methods were evaluated. A. ROC curve for Non-tg vs. the rest of the mouse types. B. The AUC for each method as an indicator of how well the detector correctly identified a novel data set as belonging to the training data set (true positive), compared to misidentifying a novel data set as belonging to the training data when it does not (false positive).
3.6 FK506 Normalized Wt α-syn Calcium Transients as a Proof of Concept
In order to test our classifier approach to discriminate calcium transient in tg vs. Non-tg mice we tested FK506 normalized calcium transients in WT α-syn mice. We chose for these experiments the WT α-syn mice because among the different models of neurodegenerative disease showed robust calcium transients alterations. FK506 was chosen because this immunomodulatory drug has been shown to bind calcineurin and regulate mitochondrial calcium homeostasis (Rusnak and Mertz, 2000). Following analysis of the data (Fig 8A, B) FK506 visibly altered the shape of the calcium transient (Fig 8C); however analysis of AUC created ambiguity since the AUC was significantly reduced from the WT α-syn AUC, but also significantly increased from the Non-tg AUC (FIG 8D). Therefore, we applied our statistical classifier to the transients from each mouse in this data set and asked if the data were more similar to untreated WT α-syn or Non-tg mice. We found that FK506 altered the calcium transient normalizing it to such an extent that our classifier consistently assigned the transients from these mice to the Non-tg mouse type (Fig 8E). A more detailed analysis of the number of traces required to for the classifier to assign a Non-tg mouse type 100% of the time appeared to converge by 30 traces indicating that by collecting and analyzing 30 traces the classifier was always assigning the mouse type as Non tg for each of the three drug-treated mice. In conclusion, these data provide a proof of concept that we can utilize 2 photon microscopy combined with statistical classifiers to create a bioassay. Using this bioassay we were able to evaluate whether or not a drug normalized calcium transients in a transgenic mouse such that the transients were indistinguishable from Non-tg mice calcium transients.
Figure 8.
FK506 significantly modulated WT α-syn tg calcium transients to be indistinguishable with Non-tg mice. A. Representative examples of calcium activity from barrel cortex neurons in Non-tg, WT α-syn, and FK506-treated WT α-syn mice. B. The time course for the calcium signal was extracted based on regions of interest for 1 Hz at a depth of 150 µm. The representative neuron number is indicated to the left of each trace. The arrows indicate the onset of the stimulus. Scale bar for photomicrographs is 10 µm. C Calcium transient peak shape and D AUC for Non-tg, WT α-syn, and FK506-treated WT α-syn mice. E. Scatter plots for each FK506-treated WT α-syn mouse showing how increasing the number of traces used by the classifier led to a 100 % convergence for the classifier identifying the FK506-treated WT α-syn mice as indistinguishable from Non-tg mice. Statistical analysis performed using One-way ANOVA test with a Dunn’s Multiple Comparison post hoc analysis compared to Non-tg *** = p-value < 0.001 and WT α-syn ### = p-value <0.001 mouse types. Scale bar = 10 µm
4. Discussion
The present study characterized four transgenic mouse models of neurodegeneration (WT α-syn, E57K α-syn, APP, and 3R Tau), of which only WT α-syn tg along with the Non-tg control mice, have been previously characterized using two-photon microscopy of calcium transients. Using classical measurements such as AUC and peak width, we found that WT α-syn, E57K α-syn, and APP tg mice had significantly increased AUC compared to Non-tg mice across all depth in the 1 Hz stimulus paradigm. Similarly, APP mouse line also had statistically significant differences for peak width when compared to Non-tg mice except at the 250 µm depth. In addition, 3R Tau tg mice also had significantly increased AUCs at the 150 and 250 µm depth. The present results are in-line with previously published results for WT α-syn tg and Non-tg mice (Reznichenko et al., 2012), which showed a pattern of increased AUC and peak width in the 1 Hz stimulus paradigm for WT α-syn tg mice compared to Non-tg mice. Likewise utilizing different experimental paradigms, two photon analyses have shown calcium dysregulation in APP tg models (Busche et al., 2008, Kuchibhotla et al., 2008).
While the cause(s) of the aberrations in the calcium transients for these tg mouse types are not certain, there are a number of possible mechanisms including increase in spiking, increase in the amount of calcium that enters the cell, and failure of organelles or cytosolic calcium buffer to sequester calcium (Berridge, 1998). Several of these mechanisms have been previously ruled out for the WT α-syn mouse type (Reznichenko et al., 2012), and at the same time, mitochondrial disruption has been suggested as a possible explanation for aberrant the calcium signals in the WT α-syn mouse (Reznichenko et al., 2012). Since the E57K α-syn is related to the WT α-syn mouse type, and the E57K α-syn oligomers have been shown to accumulate in the synapse (Rockenstein et al., 2014), it is also possible that the calcium aberrations may also be caused by mitochondrial defects in the synapse. The cause(s) for the abnormal calcium transients in the APP and 3R Tau mice are less clear, although APP (Anandatheerthavarada and Devi, 2007) and Tau (Reddy, 2011) have also been implicated in mitochondrial dysfunction which could also lead to abnormal calcium function.
As with all animal models of diseases, one of the major goals is to modulate features of the diseased state such that they return to non-diseased levels. While comparing the means of treated and untreated groups is one of the most common approaches in animal studies, it can be labor and cost intensive, particularly in the case of using two-photon microscopy to perform in vivo imaging. Therefore, we investigated a method for using statistical classifiers to determine on a per-animal level whether that animal looks like a diseased mouse-type or not. This approach is more similar to a standard bio-assay with a binary outcome, such as those used in the clinic, where even a single sample can be tested to determine if the sample is positive or not. To this end we used well-established statistical methods to build a classifier for each mouse type to ask the question, “Does this novel dataset belong to the X mouse-type?”, where X one of the 5 mouse-types in our transgenic mouse population.
For the size of the datasets we were able to obtain, our classifiers worked well, as evidenced by the scores from the ROC curves. Our current experiments also indicated that there are features, which are sensitive to the temporal resolution. We believe future experiments with higher temporal resolution can improve our classifiers performances far beyond current levels. Our current data set was collected at 10 frames/sec, effectively sampling the sample pixel 10 times in one second. Another potential way to maximize the quality and quantity of our data is to focus the experimental conditions to on one or two Hz-depth combinations. The current data were collected from 6 different Hz-depth combinations at 4 independent locations within the OGB-injected barrel cortex. Based on the classical outcome measures, the most notable differences in calcium transients were evident at the 150 µm depth. Therefore for the current mouse types, we may limit data collection to this depth, dramatically increasing the amount of data collected within a limited timeframe.
We chose the calcineurin inhibitor FK506 since it has been shown in other mouse models of neurodegenerative disease to reduce calcium overloading (Kuchibhotla et al., 2008). We hypothesized that FK506 might normalize the WT α-syn calcium transients. We found that the while the AUC for WT α-syn mice treated with FK506 was significantly reduced compared to untreated WT α-syn mice, the AUC remained significantly increased compared to Non-tg mice. Therefore, following this analysis we are only able to say that FK506 reduced AUC, but not all the way down to Non-tg levels. However, after applying our statistical classifier, we are able to unambiguously state that FK506 normalized calcium transients such that they were indistinguishable from Non-tg calcium transients. Moreover, we discovered that while over 100 calcium transients were available for analysis from each of the FK506-treated mice, we only needed 30 transients per mouse for the classifier to have 100 % convergence on assigning the mouse type as indistinguishable from Non-tg mice.
The potential benefits of using classifiers in this manner are provocative. Whether using a power analysis or empirical determination to decide the number of subjects per group, it is reasonable the most experiments will require at least n=6 to compare the means of each group. With a statistical classifier the number of animals can be reduced because the novel animal is evaluated compared to already existing training data. One example of this is the experimental design where the investigator wants to test a single drug, such as we did with FK506, at one concentration against a control group using in vivo imaging with two-photon microscopy. We showed that with three mice and no more than 30 traces per mouse that the statistical classifier proved that FK506 normalized calcium transients in WT α-syn mice so that they were indistinguishable from Non-tg mice. Since the characteristics, such as false positive and true positive rates, of the classifier are already determined, the investigator can save time and resources using a classifier to determine if the treated mice are statistically similar to the training set. Using a classifier is also beneficial in cases where a pilot study might be used to generate data for a grant or to determine if a particular treatment is worth further evaluation.
4.1 Conclusions
We created access to features within the data that can disambiguate two types of mice that were previously indistinguishable by going beyond differences in simple measures (such as group means for area under the curve (AUC), signal peak width, and spontaneous calcium activity counts) and building statistical classifiers to characterize functional signals. We effectively and with reasonable accuracy were able to correctly answer the question, “Does this novel animal belong the animal type X?”. Using these classifiers we created a bioassay, which provided evidence that FK506 normalized calcium transients from WT-α-syn tg mice.
Highlights.
In vivo two-photon microscopy data collected from WT α-syn, E57K α-syn, APP, 3R–Tau and Non-tg mice
Calcium transients AUCs were significantly increased for WT α-syn, E57K α-syn and APP mice with 1 Hz stimulus
Two-photon microscopy data coupled to statistical classifiers creates a bioassay to identify different mouse types
Our statistical classifier bioassay confirmed that FK506 normalized WT α-syn calcium transients
Acknowledgments
We gratefully acknowledge support from the NIH: AG18440, NS044233, AG010435 to EM, S10RR029050 to Anders M. Dale, and the 2 Photon Laser Scan Facility at UCSD directed by Anna Devor.
Abbreviations
- 3R
triple-repeat
- Aβ
amyloid β
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- α-syn
α-synuclein
- AUC
area under the curve
- FOV
field of view
- h
human
- ISI
interstimulus intervals
- F
fluorescent signal
- m
murine
- NFT
neurofibrillary tangle
- OGB
Oregon Green 488 BAPTA-1 AM
- PD
Parkinson’s disease
- PiD
Pick’s disease
- ROC
Receiver Operator Characteristic
- SPR
statistical pattern recognition
- SR101
sulforhodamine 101
- tau
microtubule associated protein tau
- tg
transgenic
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Anandatheerthavarada HK, Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer’s disease. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13:626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends in molecular medicine. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CM. Pattern Recognition and Machine Learning. New York City: Springer; 2007. [Google Scholar]
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27:4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda RO, Hart PE, Stork DG, editors. Pattern Classification. New York: John Wiley & Sons, Inc; 2001. [Google Scholar]
- Gotz J, Ittner LM. Animal models of Alzheimer’s disease and frontotemporal dementia. Nature reviews Neuroscience. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, Spires-Jones TL, Bacskai BJ, Hyman BT. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A. 2014;111:510–514. doi: 10.1073/pnas.1318807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Bell AJ, Jung TP, TJ S. Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst. 1996;8:145–151. [Google Scholar]
- Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Abnormal tau, mitochondrial dysfunction, impaired axonal transport of mitochondria, and synaptic deprivation in Alzheimer’s disease. Brain res. 2011;1415:136–148. doi: 10.1016/j.brainres.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznichenko L, Cheng Q, Nizar K, Gratiy SL, Saisan PA, Rockenstein EM, Gonzalez T, Patrick C, Spencer B, Desplats P, Dale AM, Devor A, Masliah E. In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J Neurosci. 2012;32:9992–9998. doi: 10.1523/JNEUROSCI.1270-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J, Constantinidis J, Tissot R. [Calcium EDTA treatment of Pick’s disease] Nouv Presse Med. 1978;7:1304. [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1–42) J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Trejo-Morales M, Gerez J, Picotti P, Jensen PH, Campioni S, Riek R, Winkler J, Gage FH, Winner B, Masliah E. Accumulation of oligomer-prone alpha-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain. 2014;137:1496–1513. doi: 10.1093/brain/awu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Bisquert A, Trejo-Morales M, Spencer B, Masliah E. A Novel Triple Repeat Mutant Tau Transgenic Model That Mimics Aspects of Pick’s Disease and Fronto-Temporal Tauopathies. PLoS One. 2015;10:e0121570. doi: 10.1371/journal.pone.0121570. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]