Abstract
Several studies have documented shifts in humoral immune parameters (e.g., immunoglobulins) across the menstrual cycle in healthy women. It is thought that these shifts may reflect dynamic balancing between reproduction and pathogen defense, as certain aspects of humoral immunity may disrupt conception and may be temporarily downregulated at ovulation. If so, one could expect maximal cycle-related shifts of humoral immunity in individuals invested in reproduction – that is, women who are currently sexually active – and less pronounced shifts in women who are not reproductively active (i.e., abstinent). We investigated the interaction of sexual activity, menstrual cycle phase, and humoral immunity in a sample of 32 healthy premenopausal women (15 sexually active, 17 abstinent). Participants provided saliva samples during their menses, follicular phase, ovulation (as indicated by urine test for LH surge), and luteal phase, from which IgA was assayed. Participants also provided blood samples at menses and ovulation, from which IgG was assayed. Sexually active participants provided records of their frequency of sexual activity as well as condom use. At ovulation, sexually active women had higher IgG than abstinent women (d = 0.77), with women reporting regular condom use showing larger effects (d = 0.63) than women reporting no condom use (d = 0.11). Frequency of sexual activity predicted changes in IgA (Cohen’s f2 = 0.25), with women reporting high frequency of sexual activity showing a decrease in IgA at ovulation, while women reporting low frequency or no sexual activity showing an increase in IgA at ovulation. Taken together, these findings support the hypothesis that shifts in humoral immunity across the menstrual cycle are associated with reproductive effort, and could contribute to the mechanisms by which women’s physiology navigates tradeoffs between reproduction and immunity.
Keywords: immunity, reproduction, menstrual cycle, menstrual cycle, sexual behavior, immunoglobulins
Introduction
Natural variations in adaptive immunity across the menstrual cycle are complex, yet critical for understanding determinants of women’s health. Research characterizing these variations is inconsistent, with some studies documenting significant decline in lymphocytes such as B-cells around ovulation [1–6], while others show increases (e.g., [7]) or no significant change across the menstrual cycle (e.g., [8–10]). Menstrual cycle-related variations could reflect a dynamic balance between prioritizing reproduction and defense: reducing certain aspects of immunity around ovulation may reduce immune disruption of conception. If so, we would expect such shifts only in women who are reproductively active – that is, regularly engaging in sexual activity. Few studies of women’s immune response report on sexual activity status; however, this variable may explain the inconsistencies observed across studies. In the present study, we examined the impact of sexual activity on variations in circulating immunoglobulins across the menstrual cycle.
Within the humoral immune system, there are a variety of antibodies whose activity corresponds to environment of the local sites in which they are predominantly expressed. Immunoglobulin A (IgA) is an antibody predominantly expressed in mucosa, and acts primarily by blocking pathogen entry into epithelium. Individuals with IgA deficiencies are at dramatically increased risk of infection, reflecting IgA’s role as the “first line defense” [11]. There is increased expression of IgA during the follicular phase relative to the luteal phase [3]; this pattern ultimately promotes fertility as infections early in the menstrual cycle can prevent ovulation [12]. However, at ovulation, high IgA appears to disrupt conception [13, 14] by altering mucosal composition in ways that impair sperm motility [15] or, in rare cases, directly attacking sperm [16]. Accordingly, in healthy women there is a midcycle decline in IgA, corresponding to ovulation [2].
In contrast to IgA, Immunoglobulin G (IgG), the most common antibody expressed in blood, acts directly on pathogens by either lysing target cells or immobilizing and marking them for disposal [17]. Low IgG at ovulation is not associated with better reproductive outcomes [18, 19], and in fact IgG may support conception by regulating systemic inflammation that is potentially damaging to a pre-placental embryo [20]. Accordingly, in healthy females, there are increases in IgG in the day prior to ovulation [2], lasting through the luteal phase [21]. Notably, although cycle variations in IgA and IgG have been primarily studied in the female reproductive tract, these effects translate into non-reproductive tract sites such as lymph nodes [22] and salivary mucosa [3].
Examining changes in IgA and IgG can reflect the relative balance of immune priorities: mucosal or/and systemic defense. Moreover, given the unique role each plays in reproduction, tracking cycle-related changes in IgA and IgG may reflect ongoing redistribution within the immune system to balance the conflicts between reproduction and immune defense [23–26]. Immunoredistribution – that is, temporary shifts that move immune cells to sites where they are most useful – can be triggered by reproductive behavior [23] such as courtship behaviors or mate competition. [27, 28]. Shifting immune resources from high IgA production during the follicular phase to high IgG production at mid-to-late cycle may reflect the need to avoid disrupting conception (i.e., lowering IgA) while maintaining systemic immunity (i.e., increasing IgG). In the present study, we predicted that a decline in IgA at ovulation would correspond to an increase in IgG.
If balancing reproductive priorities is the driving force for shifts in humoral immune factors across the menstrual cycle, we would expect such shifts to be especially critical, and potentially exaggerated, in women who are sexually active relative to those who are abstinent. To date, only a few studies have examined the association between sexual activity and humoral immunity in healthy women. In one study using a sample of commercial sex workers, there was no association between frequency of sexual activity (1–3 partners daily vs. 4+ partners daily) and total IgA or IgG among HIV-negative women. However, given the very high rates of sexual activity in this sample (up to 14×/daily), there could have been a ceiling effect on degree of variation observable in humoral immune factors [29]. Another study found lower IgA levels associated with increasing frequency of sexual activity in healthy women; however, these analyses did not account for cycle phase [30]. Similarly, one study showed significantly higher IgA levels in women who were sexually abstinent as compared to women who were sexually active with a male or female partner [31]. While this study found no association between sexual activity status and cycle phase on IgA levels, frequency of sexual activity within sexually active participants was not considered. Finally, in a sample of male and female college students, there was a curvilinear pattern between IgA and sexual frequency, with individuals reporting sexual activity 1–2×/week showing significantly greater IgA than any other group (abstinent, <1×/week, >2×/week) [32]. However, these analyses treated both men and women in the same group, despite evidence of sex/gender differences in humoral immunity [33] and self-reported sexual frequency [34]. Collectively, these studies point to a potential effect of sexual activity on humoral immunity, but the patterns are far from clear.
The association of sexual activity and humoral immunity appears to hold regardless of the sex/gender of the sexual partner [31], and does not appear to differ between women reporting consistent vs. inconsistent condom use [29], suggesting exposure to ejaculate is not a necessary factor. Nevertheless, exposure to an intimate partner’s microbiome may broadly alter immune response [35]. Thus, as an exploratory sub-analysis, we examined the potential role of barrier use in humoral immune parameters across the menstrual cycle in sexually active women. Given the presumably local effects of ejaculate on immune response, we predicted greater impact of barrier use in mucosal immunity (i.e., IgA) than systemic immunity (i.e., IgG).
In sum, we predicted that IgA would decrease at ovulation, particularly for sexually active women. Correspondingly we predicted that IgG would increase at ovulation, again, particularly for sexually active women. Finally, we predicted that, for sexually active women, exposure to ejaculate (that is, infrequent condom use) would moderate cycle-related changes in IgA, but not IgG.
Methods
Participants
Healthy, premenopausal women were recruited from the local community. Interested participants were screened via telephone, and again at the first lab session, to ensure they met study criteria. Exclusion criteria included: current illness or history of medical conditions known to impact immune function (e.g., cancer), use of psychoactive or immunoactive medications, use of hormonal medications (including oral contraceptives), pregnancy/lactation within the past year, and history of sexual assault (which may influence neuroendocrine response to adult sexual activity [36]. Inclusion criteria across groups included: self-reported good health, with regular menstrual cycles (26 – 34 day cycles with no more than 1 missed period in the last 6 months). Sexually abstinent participants were required to report no partnered genital sexual activity in the past four months; however, participants reporting masturbation and/or lifetime history of partnered sexual activity could be included. Sexually active participants were required to report vaginal intercourse at least once per week with a single partner. As women taking hormonal contraceptives were not enrolled, sexually active participants reported using either condoms or a non-hormonal intra-uterine device as contraception.
Three participants dropped out after the first lab session, leaving 32 participants (17 sexually abstinent, 15 sexually active) in the present sample (see Table 1 for demographics). Participants were on average 23.56 years old (SD = 5.54 years). Participants indicated their race, which was dummy-coded as White (N = 21), or non-White (N = 11, which included 5 women who indicated predominantly Asian racial background and 6 who indicated multiple racial backgrounds). There were no significant differences between groups in age, race/ethnicity, or body fat percentage. All participants provided informed consent and were compensated $30 for participating; study procedures were approved by the Institutional Review Board at Indiana University.
Table 1.
Demographics
| Sexually Active (N = 15) |
Sexually Abstinent (N = 17) |
Total (N = 32) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 24.96 | 7.05 | 22.16 | 2.85 | 23.56 | 5.54 |
| Years of education | 15.96 | 4.12 | 15.53 | 2.42 | 15.73 | 3.24 |
| Body fat percentage | 27.69 | 5.65 | 26.02 | 8.67 | 26.88 | 7.21 |
| N | % | N | % | N | % | |
| Race | ||||||
| White | 12 | 80% | 9 | 53% | 21 | 66% |
| Asian | 0 | 0% | 5 | 29% | 5 | 16% |
| Mixed Race/Other | 3 | 20% | 3 | 18% | 6 | 18% |
| Ethnicity | ||||||
| Hispanic/Latina | 1 | 7% | 0 | 0% | 1 | 3% |
| Not Hispanic/Latina | 14 | 93% | 17 | 100% | 31 | 97% |
Biomarker collection
Participants attended two laboratory sessions timed to their cycle: at menses (within two days of onset of menstrual bleeding) and at ovulation (within two days of ovulation). Ovulation was estimated via backwards counting using date of menses onset and self-reported typical cycle length [37]. To further confirm ovulation, participants also completed commercially available urine tests for luteinizing hormone (OneStep Urine Ovulation Test, BlueCross Biomedical, Beijing, China) for four days surrounding the expected date of ovulation. There is a surge of luteinizing hormone approximately 24–36 hours prior to ovulation; thus, if participants detected early ovulation (or had not detected ovulation by the morning of their second lab session) they were instructed to contact the lab to reschedule their “ovulation” laboratory session to occur within 24 hours of a positive ovulation test. Participants were also measured for body composition with a floor scale (FitScale 585F, Tanita Corporation, Illinois USA); average percent body fat was 27.69% (SD = 5.67%).
Blood was collected during laboratory visits via standard venipuncture procedures, taking blood from the anterior cubita fossa into uncoated tubes (Vacutainers, Becton Dickson & Company, Franklin Lakes, NJ). Whole blood was allowed to sit at room temperature for 30 minutes, then centrifuged for 15 minutes at 2200 RPM; serum was drawn from the resultant supernatant. Serum was frozen immediately following separation and aliquoting. There were two serum samples per participant (menses, ovulation).
Saliva samples were collected via passive drool into polypropylene tubes; participants were instructed not to eat, drink, or chew gum for the hour prior to providing a saliva sample. Participants completed saliva samples at laboratory visits, and samples were frozen immediately after collection. Participants provided two additional samples: in their follicular phase (7–10 days following onset of menses) and luteal phase (7–10 days following ovulation). Follicular and luteal phase samples were collected by the participant at home, and frozen in their home freezer immediately following collection. Samples were transported to the lab in Styrofoam boxes packed with deep-freeze packs [37]. Thus, there were four saliva samples per participant (menses, follicular, ovulation, and luteal).
All samples (serum and saliva) were kept at −80C until analysis, and no sample was subjected to more than 2 freeze-thaw cycles. Serum samples were assayed for IgG, and saliva samples for IgA, using commercially available enzyme-linked immunosorbent assay (ELISA) kits, using procedures recommended by kit manufacturers (IgG: eBioscience, Inc, San Diego, CA; IgA: Salimetrics LLC, Pennsylvania, USA). Intra- and inter-assay coefficients of variance were low (IgG: 16.02%, and 4.58%, respectively; IgA: 5.10%, and 2.14%, respectively). Salivary IgA served as our index of mucosal humoral immunity, while serum IgG served as our index of circulating humoral immunity.
Sexual event diaries
Sexually active participants completed a short online questionnaire following each partnered sexual event, in which they indicated the sexual behaviors in which they engaged and whether or not they used a condom. Intercourse events were coded as a positive response to the “vaginal penetration by your partner” item. From these diaries, we tallied number of intercourse events over the course of the study period (sexual frequency); mean sexual frequency was 6.67 events (SD = 4.93 events, range: 1 – 18 events). Menstrual cycle phase did not predict frequency of sexual events reported in this sample (F(1,3) = 0.08, p = 0.96) indicating women did not report a higher rate of intercourse around ovulation. Sexual frequency for abstinent participants was coded as 0. All participants were included in analyses of sexual frequency.
Of the sexual event diaries indicating intercourse, 54% indicated no condom use during that sexual event while 46% indicated a condom was used1. We calculated each participant’s condom use rate across intercourse events; mean condom use frequency was 44% (with 5 of participants reporting no condom use for any sexual event, 6 reporting condom use at every sexual event, and 4 reporting inconsistent condom use). Condom use data were used in analyses for sexually active participants only.
Statistical analyses
One participant could not provide saliva volume sufficient for IgA analysis, and seven participants did not complete all saliva samples (10% of total data missing). Similarly, blood could not be drawn from participants in two sessions for logistical reasons (3% of total data missing). These missing values were considered missing at random (MAR), and treated by using statistical methods robust to MAR values (see below). Values that were outside of assay limits (1 value above and 1 below limit of detection, 1% of total data), which are by definition not MAR, were replaced with the lower or upper limit of detection. We corrected IgA concentrations by the salivary flow rate [38]; correspondingly, values are expressed in terms of µg/min. The distribution of IgG values was roughly normal (Kolmogorov-Smirnov statistic = .099, p = .20), but the distribution of IgA values was right-skewed (Kolmogorov-Smirnov statistic = .130, p = .013). We used a Box-Cox transformation to normalize IgA only, and back-transformed values for presentation. As we used a repeated measures analysis, we used Cohen’s f2 as index of effect sizes, and set our threshold for interpreting these effect sizes as: very small, < 0.10; moderate, 0.10 – 0.20; large, 0.20 – 0.40; and very large, > 0.40 (equivalent to Cohen’s d < 0.20; 0.20 – 0.50; 0.50 – 0.80, and > 0.80, respectively). P-values are also reported for comparison; however, given the small N these values should be considered less reliable estimates of substantive significance than effect sizes [39].
To examine changes in IgA and IgG across the cycle, we used repeated measures mixed generalized linear models, specifying an unstructured correlations covariance structure and a random subject-level intercept (controlling for individual differences in baseline IgA or IgG). We entered the sexual variable (group, sexual frequency, or condom use frequency), cycle phase as a repeated measure, and their interaction as fixed effects, controlling for age, race, and body fat percentage. Analyses were performed with IBM SPSS Statistics v22.0.
Results
Systemic humoral immunity: IgG
The effect of sexual group predicting total IgG was large (F(1,59.92) = 4.03, Cohen’s f2 = 0.28, p = 0.05). On average, sexually active women had higher levels of IgG than sexually abstinent women (Figure 1). Follow-up specific contrasts revealed that at menses, the difference between sexual groups was moderate (Mdiff = −5.01, SE = 6.34, d = 0.28, p = 0.44); but at ovulation, this difference was large, with mean IgG lower in sexually abstinent than sexually active women (Mdiff = −13.05, SE = 6.08, d = 0.77, p = 0.04). However, the interaction between cycle phase and group had a very small effect size (F(1,59.87) = 0.88, Cohen’s f2 = 0.01, p = 0.35), as did the effect of frequency of sexual activity (F(1,30.03) = 1.30, Cohen’s f2 = 0.03, p = 0.26).
Figure 1.
Total serum immunoglobulin G (IgG) by group and menstrual cycle phase.
Among sexually active participants, frequency of condom use had a moderate effect size in predicting changes in IgG (F(1,27.83) = 5.92, Cohen’s f2 = 0.18, p = 0.02; Figure 2). Women who reported using condoms on every sexual event diary had a large increase in IgG from menses to ovulation (Mdiff = 22.88, SE = 11.02, d = 0.63, p = 0.05), while the change in IgG in women who reported no condom use on any sexual event diary was very small (Mdiff = −13.52, SE = 8.78, d = 0.11, p = 0.14).
Figure 2.
Total serum immunoglobulin (IgG) by frequency of condom use and menstrual cycle phase among sexually active participants only (N = 15). Frequency of condom use was coded from sexual event diaries. Grouping of frequency of condom use is presented for illustrative purposes only; analyses treated frequency of condom use as a continuous variable.
Mucosal humoral immunity: IgA
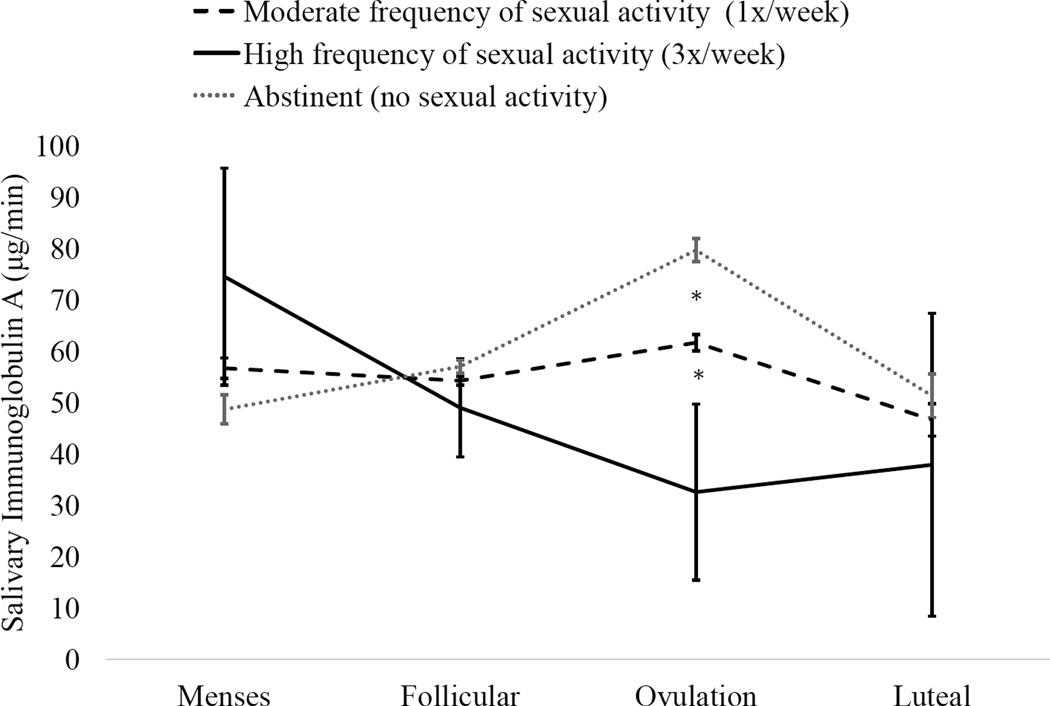
The interaction of group and cycle phase had a moderate effect size in predicting IgA (F(3,28.52) = 2.20, Cohen’s f2 = 0.16, p = 0.11). Follow-up specific contrasts indicated that, among sexually abstinent women, there was a moderate to large increase in IgA at ovulation relative to menses (Mdiff = 4.12, SE = 0.83, d = 0.63, p= 0.03), follicular phase (Mdiff = 1.88, SE = 0.34, d = 0.42, p= 0.03) and luteal phase (Mdiff = 4.12, SE = 0.86, d = 0.62, p= 0.04). Among sexually active women, differences between phases were very small to moderate (all ds < 0.25). The interaction of cycle phase and frequency had a large effect size, F(3, 27.82) = 4.05, Cohen’s f2 = 0.25, p = 0.02. Follow-up tests revealed that low sexual frequency was associated with an increase in IgA at ovulation, while high sexual frequency was associated with a decrease in IgA at ovulation (Figure 3). Finally, the effect size of condom use in predicting changes in IgA was very small (F(3,15.39) = 0.24, Cohen’s f2 = 0.09, p = 0.86).
Figure 3.
Interaction of sexual activity frequency and menstrual cycle phase on salivary immunoglobulin A (IgA). Frequency of sexual activity was tallied from sexual event diaries. Grouping of sexual frequency is presented for illustrative purposes only; analyses treated sexual frequency as a continuous variable.
Discussion
Insofar as menstrual cycle-related changes in humoral immunity are driven by tradeoffs between reproduction and immunity, they would be most critical for individuals investing in reproduction – that is, females who are sexually active. In the present study we examined cycle-related changes in two humoral immune parameters – mucosal (salivary) IgA and circulating (serum) IgG – and their interaction with sexual activity in healthy women. At menses, sexually active and abstinent women had similar levels of both IgA and IgG. However, at ovulation, sexually active women had higher IgG, but lower IgA, than did sexually abstinent women. Frequency of sexual activity moderated cycle-related changes in IgA, with increased frequency of sexual activity associated with increased ovulation-related decline in IgA. Finally, among sexually active women, condom use moderated changes in IgG but not IgA; specifically, women reporting always using condoms showed increases in IgG at ovulation while women reporting never using condoms had very little change in IgG across the cycle. This is the first study to document sociosexual behavior may moderate changes in humoral immune cells across the menstrual cycle in healthy females.
The immune system must support several critical processes during menses, including stimulation of uterine endometrium breakdown through recruitment of leukocytes leading to local inflammation [40] and heightened presence of antibodies in the reproductive tract [10], potentially to support maintenance of the vaginal microbiome during a period of altered pH associated with menstrual bleeding [41]. However, conception (and relatedly, ovulation) requires a different set of immune actions, including reducing inflammatory processes, particularly in mucosa [42, 43] while maintaining high vigilance against infections that could contribute to early pregnancy loss [18]. Our findings suggest that the balance between vigilance and conception may be achieved by shifting available immunoglobulins from IgA to IgG. In essence, this may shift immune activity further up the line, from mucosa (where antibody activity may interfere with fertilization or implantation) to general circulation (where antibody activity is less disruptive while maintaining high level of defense). That the greatest differences between sexually active and abstinent women were seen at ovulation further supports the hypothesis that these shifts are related to balancing reproductive/immune tradeoffs.
It is notable that there were group-wise differences in IgG, with sexually active women showing higher levels of total serum IgG than did sexually abstinent women, particularly at ovulation. On the other hand, there were different patterns in IgA in women reporting low vs. high frequency of sexual activity. These findings suggest that sexual activity may act differentially on circulating and mucosal humoral immunity. This may be due to different timelines for the maturation and degradation of IgG (~20 days [44]) and IgA (3 – 6 days [45]): IgA may require more frequent input from environmental cues – including from the social environment – to adjust to current conditions.
Sexual activity likely has multiple routes of influence on the immune system. Most notably, several studies have suggested that sexually active women have significantly higher estrogen and luteal-phase progesterone than do abstinent women [46–49]; given the powerful immune effects of ovarian hormones, it is reasonable to suspect the effects reported here are at least partially mediated by endocrine mechanisms. However, given the complex patterns of immune effects (e.g., different patterns in condom users vs. non-users), the effects of sexual activity are likely not driven solely by hormonal differences. Some fine-tuning of immunomodulation may come from transient physiological effects of sexual intercourse, such as increased prolactin following orgasm with a partner [50, 51] or sympathetic activation during and following female sexual arousal [52, 53]. There may also be partner effects such as exposure to an intimate partner’s microbiota [35], or (among women not using condoms) exposure to ejaculate. Several factors in ejaculate may be immunomodulatory, such as inhibin [54] and prostaglandins [55]. However, although there are studies documenting immune effects of repeated exposure to seminal fluid (e.g., inducing tolerance to paternal alloantigens in maternal Treg cells [56]) within the female reproductive tract, is unclear if these effects translate to immunity at other sites (i.e., if these factors are transferred into the general circulation in a bioactive form). It is also possible that sexual intercourse introduces pathogens, irritants, or other agents that activate vaginal (and downstream, general circulating) immune response via exposure to lubricants, powders or spermicides on condoms, sex toys, hands or mouth, and so on. Finally, there may be psychosocial effects of sexual behavior leading to immune differences, such as lower stress due to increased perception of social support from an intimate partner [57]
Shifts between IgA and IgG could occur through class-switching of B-cells, mediated by cytokines released by T-helper cells. Consistent with this idea, several studies have shown a decrease of Th2-type cytokines (which promote class switching to IgA) from menses to ovulation [49], followed by a rise in Th2-type cytokines during the luteal phase [58]. As a B cell can only produce one immunoglobulin type at a time, there could be tradeoffs between IgG and IgA. This may explain why sexually abstinent women showed decreases in IgG concurrently to increases in IgA. Why sexually abstinent women showed changes in either immunoglobulin, however, is still unknown.
Another potential mechanism for shifts between IgA and IgG is immunoredistribution; namely, B cells that produce IgA or IgG may be selectively released at various points of the cycle [23]. Much as the endocrine system keeps most circulating hormones bound to carrier proteins to allow rapid increases on demand, the immune system may sequester IgA or IgG (or their producing cells) in the tissues [25, 26]. And, much like the endocrine system’s responsiveness to social behaviors, the immune system may be able to respond to reproductive cues such as sexual behavior.
This may partially explain our unexpected finding that women reporting infrequent condom use had lower serum IgG at ovulation: it is possible that for these women, IgG is recruited to the reproductive tract rather than general circulation (where we measured IgG). If so, it would imply the lower levels of salivary IgA observed in sexually active women at ovulation could reflect higher IgA expression in the reproductive tract. Alternatively, it is possible that IgG is more susceptible to recruitment through immunoredistribution than IgA. Again, the differential half-lives of these two antibodies may drive different strategies, as redistribution of a longer-lived antibody (IgG) may be more efficient while down-regulation of production may be more efficient for a shorter-lived antibody (IgA). It is also possible that condom use itself may drive changes in IgG due to vaginal irritation in response to the condom’s material or lowered sexual arousal leading to increased risk of vaginal tearing [59]. Because the mechanisms by which sexual activity may impact women’s immune function are unknown, it is possible that there is some behavioral or environmental difference between condom users and non-users that mediates this effect. For example, women who use intrauterine devices as their primary form of contraception tend to have greater access to gynecologic health care and are less likely to intend to become pregnant in the next year than women using condoms as their primary form of contraception [60]. All of the sexually active women in the present study reported they did not intend to become pregnant and all were (at least nominally) contracepting, and thus their “reproductive investment” is arguably different from women actively trying to conceive. Studies comparing in women who are and are not contracepting (and comparing women using different modes of contraception) are needed to tease apart these effects.
Several limitations to the present study should be noted. Due to the invasiveness of repeated venipuncture, we were limited in the number of serum samples we could collect and were not able to observe potentially important effects in the follicular and luteal phases. Our sample was predominantly White and relatively young; future work will need to extend findings into larger samples with a more diverse population, particularly given the impact of race [61] and age [62] on humoral immunity. Consideration of partner variables, such as the sexual partner’s sex/gender, age, race, and health status will also help develop models of the mechanisms driving these effects. To improve interpretability of results, we recruited women with regular cycles who were not taking hormonal contraceptives; however, this excluded the ~15% of women reporting irregular cycles [63] and 17% of reproductive-aged women taking hormonal contraceptives [64]. Further specification of sexual events (e.g., separating out vaginal penetration by a penis vs. dildo) may also improve future investigations regarding the mechanisms of the reported immune effects of sexual activity. Finally, total IgG and IgA levels cannot tell us if an individual has “better” or “worse” immune function, as high antibody count could reflect either immune readiness or current (asymptomatic) infection.
Nevertheless, the changes in humoral immune parameters reported here do reflect how the immune system reacts to sexual activity over time, and as such have important implications. Specifically, these findings suggest that tradeoffs between reproduction and women’s humoral immunity, previously documented in the context of pregnancy and lactation [65], may also be relevant in the context of sexual activity. These findings add to the emerging literature suggesting humoral immunity is sensitive to sociosexual environment in humans [30, 66], which in turn are informed by the broader literature on reproduction-immune tradeoffs in non-human animals [67]. While shifts in humoral immunity across the menstrual cycle may promote reproduction, they may also produce a window of opportunity for infection, particularly sexually transmitted infections, in vulnerable individuals [17]. The findings from the present study suggest that such windows may be differentially active in individuals who are and are not sexually active – and thus, may respond differentially to interventions to prevent or treat infectious disease. For example, vaccine response is dependent on the humoral immune system. Women who are vaccinated during their follicular phase have greater disease-specific IgA and IgG production than those vaccinated during their luteal phase [68]; the present study findings suggest that sexual activity status may further moderate vaccine response. At minimum, the data presented here call for further work investigating the complex interactions of sexual behavior, menstrual cycle phase, and humoral immunity.
Highlights.
Sexually active women had higher IgG at ovulation than sexually abstinent women.
Women reporting high frequency of sexual activity had decreased IgA at ovulation.
Women reporting low frequency or no sexual activity had increased IgA at ovulation.
Condom use was associated with an increase in IgG at ovulation.
Acknowledgements
This work was partially funded by the Office of the Vice Provost of Research at Indiana University-Bloomington through the Collaborative Research and Creative Activity Funding Award, and partially by the American Psychological Foundation’s Henry P. David Award for Research in Human Reproductive Behavior and Population Studies. Dr. Lorenz is supported by grant T32HD049336-09 from the National Institute of Child Health and Human Development. None of the funding bodies had involvement in the conduct of the research of preparation of the manuscript.
Footnotes
Menstrual cycle phase did not predict condom use (χ2(6) = 7.01, p = .320), indicating women were not selectively using condoms only during fertile windows.
Conflicts of interest: The authors have no conflict of interest to declare.
Contributor Information
Gregory E. Demas, Email: gdemas@indiana.edu.
Julia R. Heiman, Email: jheiman@indiana.edu.
References
- 1.Safaeian M, Falk RT, Rodriguez AC, Hildesheim A, Kemp T, Williams M, et al. Factors associated with fluctuations in IgA and IgG levels at the cervix during the menstrual cycle. J. Infect. Dis. 2009;199:455–463. doi: 10.1086/596060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usala S, Usala F, Haciski R, Holt J, Schumacher G. IgG and IgA content of vaginal fluid during the menstrual cycle. The Journal of reproductive medicine. 1989;34:292–294. [PubMed] [Google Scholar]
- 3.Gomez E, Ortiz V, Saint-Martin B, Boeck L, Diaz-Sanchez V, Bourges H. Hormonal Regulation of the Secretory IgA (sIgA) System: Estradiol- and Progesterone-induced Changes in sIgA in Parotid Saliva Along the Menstrual Cycle. Am. J. Reprod. Immunol. 1993;29:219–223. doi: 10.1111/j.1600-0897.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis KP, Maciulla GJ, Yannone ME, Gooch GT, Lox CD, Whetstone MR. Cervical mucus immunoglobulins as an indicator of ovulation. Obstet. Gynecol. 1983;62:388–392. doi: 10.1097/00006250-198309000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Keller MJ, Guzman E, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein S, et al. PRO 2000 elicits a decline in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS. 2007;21:467–476. doi: 10.1097/QAD.0b013e328013d9b5. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher G, Yang S. Cyclic changes of immunoglobulins and specific antibodies in human and rhesus monkey cervical mucus. The Uterine Cervix in Reproduction. 1977:187. [Google Scholar]
- 7.Kutteh W, Prince S, Hammond K, Kutteh C, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin. Exp. Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horne CHW, Mallinson AC, Ferguson J, Goudie RB. Effects of oestrogen and progestogen on serum levels of α2-macroglobulin, transferrin, albumin, and IgG. J. Clin. Pathol. 1971;24:464–466. doi: 10.1136/jcp.24.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widerstrom L, Bratthall D. Increased IgA levels in saliva during pregnancy. Eur. J. Oral Sci. 1984;92:33–37. doi: 10.1111/j.1600-0722.1984.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 10.Lü FX, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller CJ. Immunoglobulin Concentrations and Antigen-Specific Antibody Levels in Cervicovaginal Lavages of Rhesus Macaques Are Influenced by the Stage of the Menstrual Cycle. Infect. Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang N, Hammarström L. IgA deficiency: what is new? Current Opinion in Allergy and Clinical Immunology. 2012;12:602–608. doi: 10.1097/ACI.0b013e3283594219. [DOI] [PubMed] [Google Scholar]
- 12.Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M. Stress and the Menstrual Cycle: Relevance of Cycle Quality in the Short-and Long-Term Response to a 5-Day Endotoxin Challenge during the Follicular Phase in the Rhesus Monkey 1. The Journal of Clinical Endocrinology & Metabolism. 1998;83:2454–2460. doi: 10.1210/jcem.83.7.4926. [DOI] [PubMed] [Google Scholar]
- 13.Parr MB, Parr EL. Immunohistochemical localization of immunoglobulins A, G and M in the mouse female genital tract. J. Reprod. Fertil. 1985;74:361–370. doi: 10.1530/jrf.0.0740361. [DOI] [PubMed] [Google Scholar]
- 14.Bernard O, Rachman F, Bennett D. Immunoglobulins in the mouse uterus before implantation. J. Reprod. Fertil. 1981;63:237–240. doi: 10.1530/jrf.0.0630237. [DOI] [PubMed] [Google Scholar]
- 15.Fahrbach KM, Malykhina O, Stieh DJ, Hope TJ. Differential binding of IgG and IgA to mucus of the female reproductive tract. PloS one. 2013;8:e76176. doi: 10.1371/journal.pone.0076176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui D, Han G, Shang Y, Liu C, Xia L, Li L, et al. Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta-analysis. Clin. Chim. Acta. 2015;444:29–36. doi: 10.1016/j.cca.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nature Reviews Immunology. 2015 doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak J, Kwak FM, Gilman-Sachs A, Beaman KD, Cho DD, Beer AE. Immunoglobulin G infusion treatment for women with recurrent spontaneous abortions and elevated CD56+ natural killer cells. Early pregnancy (Online) 2000;4:154–164. [PubMed] [Google Scholar]
- 19.Gundlapalli AV, Scalchunes C, Boyle M, Hill HR. Fertility, Pregnancies and Outcomes Reported by Females with Common Variable Immune Deficiency and Hypogammaglobulinemia: Results from an Internet-Based Survey. J. Clin. Immunol. 2015;35:125–134. doi: 10.1007/s10875-014-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fialova L, Malbohan I, Kalousova M, Soukupova J, Krofta L, Štipek S, et al. Oxidative stress and inflammation in pregnancy. Scand. J. Clin. Lab. Invest. 2006;66:121–128. doi: 10.1080/00365510500375230. [DOI] [PubMed] [Google Scholar]
- 21.Schaefer K, Brown N, Kaye PM, Lacey CJ. Cervico-Vaginal Immunoglobulin G Levels Increase Post-Ovulation Independently of Neutrophils. PLoS ONE. 2014;9:e114824. doi: 10.1371/journal.pone.0114824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, et al. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin. Exp. Immunol. 2002;128:10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braude S, Tang-Martinez Z, Taylor GT. Stress, testosterone, and the immunoredistribution hypothesis. Behavioral Ecology. 1999;10:345–350. [Google Scholar]
- 24.Dhabhar FS, Mcewen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunityin vivo: A potential role for leukocyte trafficking. Brain. Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5808–5813. doi: 10.1073/pnas.0501650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhabhar FS. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain. Behav. Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 27.Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr. Biol. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 28.McKean KA, Nunney L. BATEMAN'S PRINCIPLE AND IMMUNITY: PHENOTYPICALLY PLASTIC REPRODUCTIVE STRATEGIES PREDICT CHANGES IN IMMUNOLOGICAL SEX DIFFERENCES. Evolution. 2005;59:1510–1517. [PubMed] [Google Scholar]
- 29.Horton RE, Ball TB, Wachichi C, Jaoko W, Rutherford WJ, Mckinnon L, et al. Cervical HIV-specific IgA in a population of commercial sex workers correlates with repeated exposure but not resistance to HIV. AIDS Res. Hum. Retroviruses. 2009;25:83–92. doi: 10.1089/aid.2008.0207. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz T, van Anders S. Interactions of Sexual Activity, Gender, and Depression with Immunity. The Journal of Sexual Medicine. 2014;11:966–979. doi: 10.1111/jsm.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown SG, Morrison LA, Calibuso MJ, Christiansen TM. The menstrual cycle and sexual behavior: relationship to eating, exercise, sleep, and health patterns. Women Health. 2008;48:429–444. doi: 10.1080/03630240802575179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charnetski CJ, Brennan FX. Sexual frequency and Immunoglobulin A (IgA) Psychol. Rep. 2004;94:839–844. doi: 10.2466/pr0.94.3.839-844. [DOI] [PubMed] [Google Scholar]
- 33.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown NR, Sinclair RC. Estimating number of lifetime sexual partners: Men and women do it differently. J. Sex Res. 1999;36:292–297. [Google Scholar]
- 35.Kort R, Caspers M, van de Graaf A, van Egmond W, Keijser B, Roeselers G. Shaping the oral microbiota through intimate kissing. Microbiome. 2014;2:41. doi: 10.1186/2049-2618-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meston CM, Lorenz T. Physiological stress responses predict sexual functioning and satisfaction differently in women who have and have not been sexually abused in childhood. Psychological Trauma: Theory, Research, Practice, and Policy. 2013;5:350–358. doi: 10.1037/a0027706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Anders SM, Goldey KL, Bell SN. Measurement of testosterone in human sexuality research: Methodological considerations. Arch. Sex. Behav. 2014;43:231–250. doi: 10.1007/s10508-013-0123-z. [DOI] [PubMed] [Google Scholar]
- 38.Bishop N, Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. 2009 doi: 10.2741/3540. [DOI] [PubMed] [Google Scholar]
- 39.Greenwald A, Gonzalez R, Harris RJ, Guthrie D. Effect sizes and p values: What should be reported and what should be replicated? Psychophysiology. 1996;33:175–183. doi: 10.1111/j.1469-8986.1996.tb02121.x. [DOI] [PubMed] [Google Scholar]
- 40.Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation–a role for the immune system. Clin. Endocrinol. (Oxf) 2001;55:701–710. doi: 10.1046/j.1365-2265.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- 41.Johnson SR, Petzold CR, Galask RP. Qualitative and Quantitative Changes of the Vaginal Microbial Flora During the Menstrual Cycle. Am. J. Reprod. Immunol. Microbiol. 1985;9:1–5. doi: 10.1111/j.1600-0897.1985.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz T, Demas GE, Heiman JR. Partnered sexual activity moderates midcycle decreases in inflammation in healthy women. doi: 10.1016/j.fertnstert.2016.11.010. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol. Rev. 2014;36:71–82. doi: 10.1093/epirev/mxt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 1970;49:673. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mestecky J. Saliva as a Manifestation of the Common Mucosal Immune Systema. Ann. N. Y. Acad. Sci. 1993;694:184–194. doi: 10.1111/j.1749-6632.1993.tb18352.x. [DOI] [PubMed] [Google Scholar]
- 46.Prasad A, Mumford SL, Louis GMB, Ahrens KA, Sjaarda LA, Schliep KC, et al. Sexual activity, endogenous reproductive hormones and ovulation in premenopausal women. Horm. Behav. 2014;66:330–338. doi: 10.1016/j.yhbeh.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilcox A, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Hum. Reprod. 2004;19:1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- 48.Lorenz T, Worthman C, Vitzthum VJ. Links between inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications. doi: 10.1093/emph/eov029. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lorenz T, Heiman JR, Demas GE. Sexual activity modulates shifts in Th1/Th2 cytokine profile across the ovarian cycle. doi: 10.1016/j.fertnstert.2015.09.001. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krüger TH, Haake P, Hartmann U, Schedlowski M, Exton MS. Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci. Biobehav. Rev. 2002;26:31–44. doi: 10.1016/s0149-7634(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 51.Brody S, Krüger TH. The post-orgasmic prolactin increase following intercourse is greater than following masturbation and suggests greater satiety. Biol. Psychol. 2006;71:312–315. doi: 10.1016/j.biopsycho.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Lorenz TA, Harte CB, Hamilton LD, Meston CM. Evidence for a curvilinear relationship between sympathetic nervous system activation and women's physiological sexual arousal. Psychophysiology. 2012;49:111–117. doi: 10.1111/j.1469-8986.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Exton N, Chau Truong T, Exton M, Wingenfeld S, Leygraf N, Saller B, et al. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrino. 2000;25:187–199. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 54.Aleman-Muench GR, Soldevila G. When versatility matters: activins/inhibins as key regulators of immunity. Immunol. Cell Biol. 2012;90:137–148. doi: 10.1038/icb.2011.32. [DOI] [PubMed] [Google Scholar]
- 55.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlström AC, Care AS. Seminal fluid drives expansion of the CD4+ CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol. Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morokoff PJ, Gillilland R. Stress, sexual functioning, and marital satisfaction. J. Sex Res. 1993;30:43–53. [Google Scholar]
- 58.Faas M, Bouman A, Moesa H, Heineman MJ, de Leij L, Schuiling G. The immune response during the luteal phase of the ovarian cycle: a Th2-type response? Fertil. Steril. 2000;74:1008–1013. doi: 10.1016/s0015-0282(00)01553-3. [DOI] [PubMed] [Google Scholar]
- 59.Crosby R, Yarber WL, Sanders SA, Graham CA. Condom discomfort and associated problems with their use among university students. J. Am. Coll. Health. 2005;54:143–147. doi: 10.3200/JACH.54.3.143-148. [DOI] [PubMed] [Google Scholar]
- 60.Frost JJ, Darroch JE. Factors Associated with Contraceptive Choice and Inconsistent Method Use, United States, 2004. Perspectives on Sexual and Reproductive Health. 2008;40:94–104. doi: 10.1363/4009408. [DOI] [PubMed] [Google Scholar]
- 61.Lucey DR, Hendrix CW, Andrzejewski C, Melcher GP, Butzin CA, Henry R, et al. Comparison by race of total serum IgG, IgA, and IgM with CD4+ T-cell counts in North American persons infected with the human immunodeficiency virus type 1. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1992;5:325–332. [PubMed] [Google Scholar]
- 62.Arranz E, O'mahony S, Barton J, Ferguson A. Immunosenescence and mucosal immunity: significant effects of old age on secretory IgA concentrations and intraepithelial lymphocyte counts. Gut. 1992;33:882–886. doi: 10.1136/gut.33.7.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 64.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010 and changes in patterns of use since 1995. In: Control CfD, editor. National health statistics reports. 2012. pp. 1–25. [PubMed] [Google Scholar]
- 65.Abrams ET, Miller EM. The roles of the immune system in women's reproduction: Evolutionary constraints and life history trade-offs. Am. J. Phys. Anthropol. 2011;146:134–154. doi: 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- 66.Gettler LT, McDade TW, Agustin SS, Feranil AB, Kuzawa CW. Testosterone, immune function, and life history transitions in filipino males (Homo sapiens) International Journal of Primatology. 2014;35:787–804. [Google Scholar]
- 67.Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends in Ecology & Evolution. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Kozlowski PA, Williams SB, Lynch RM, Flanigan TP, Patterson RR, Cu-Uvin S, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. The Journal of Immunology. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]