Abstract
Treatment of Parkinson’s disease (PD) with dopamine replacement relieves symptoms of poverty of movement, but often causes drug-induced dyskinesias. Accumulating clinical and pre-clinical evidence suggests that the primary motor cortex (M1) is involved in the pathophysiology of PD and that modulating cortical activity may be a therapeutic target in PD and dyskinesia. However, surprisingly little is known about how M1 neurotransmitter tone or gene expression are altered in PD, dyskinesia or associated animal models. The present study utilized the rat unilateral 6-hydroxydopamine (6-OHDA) model of PD / dyskinesia to characterize structural and functional changes taking place in M1 monoamine innervation and gene expression. 6-OHDA caused dopamine pathology in M1, although the lesion was less severe than in the striatum. Rats with 6-OHDA lesions showed a PD motor impairment and developed dyskinesia when given L-DOPA or the D1 receptor agonist, SKF81297. M1 expression of two immediate-early genes (c-Fos and ARC) was strongly enhanced by either L-DOPA or SKF81297. At the same time, expression of genes specifically involved in glutamate and GABA signaling were either modestly affected or unchanged by lesion and/or treatment. We conclude that M1 neurotransmission and signal transduction in the rat 6-OHDA model of PD / dyskinesia mirror features of human PD, supporting the utility of the model to study M1 dysfunction in PD and the elucidation of novel pathophysiological mechanisms and therapeutic targets.
Keywords: Parkinson’s Disease, Dyskinesia, L-DOPA, D1 Agonist, Primary Motor Cortex, Immediate-early Gene
Parkinson’s disease (PD) is principally caused by the loss of dopamine (DA) cells in the substantia nigra, leading to poverty of movement (Dauer and Przedborski, 2003; Jankovic, 2008). Treatment with L-DOPA relieves PD symptoms, but long-term use typically causes L-DOPA-induced dyskinesias (LID) that are in part due to supersensitization of DA D1 receptors (Cenci et al., 2011; Feyder et al., 2011). An alternative strategy to treating PD has involved the use of primary motor cortex (M1) transcranial magnetic stimulation, which has shown promise in two meta-analyses (Elahi et al., 2009; Fregni et al., 2005).
Even though conventional anti-PD therapies modulate M1 activity and the region can be directly targeted for symptomatic relief, relatively little is known about how M1 monoamine transmission and gene expression are altered in human PD patients or in associated animal models. In the lone post-mortem study of M1 catecholamine fibers in PD patients, axons staining positively for tyrosine hydroxylase (TH; predominantly DA neurons: Hokfelt et al., 1977; Miner et al., 2006) were reduced by 24–74% compared to controls, depending on the cortical layer (Gaspar et al., 1991). In the popular 6-hydroxydopamine (6-OHDA) rat model of PD, reductions in M1 TH-positive fibers have been reported using optical density (Halje et al., 2012) or qualitative histology (Debeir et al., 2005), but there have been no attempts to rigorously quantify the extent of fiber loss. Changes in M1 monoamine tissue concentrations have not been assessed in humans or rat models of PD. Studies in Parkinsonian primates have sometimes reported reductions in M1 DA, norepinephrine (NE) and serotonin (5-HT) levels, while others studies have found M1 monoamines to be equal to controls despite severe subcortical monoamine pathology (Engeln et al., 2015; Pifl et al., 1991). It is unclear how these changes in monoamine innervation effect cellular physiology in M1, although, at least in the prefrontal cortex, DA receptors modulate both glutamate and GABA currents (Lewis and O’Donnell, 2000; Seamans et al., 2001a,b). A similar pattern may be occurring in M1, as animal models of PD typically show abnormal firing patterns of M1 glutamatergic and GABAergic cells (Brazhnik et al., 2012; Halje et al., 2012; Parr-Brownlie and Hyland, 2005; Pasquereau and Turner, 2011; Watts and Mandir, 1992).
The influence of DA depletion and exogenous DA replacement on local M1 gene expression is unclear, although a key role for M1 DA is to facilitate motor learning, likely through promoting plasticity in M1 (Floel et al., 2005; Hosp and Luft, 2013). Under normal circumstances, motor learning in M1 is associated with DA-dependent induction of the immediate-early gene c-Fos: expression levels rise while learning a motor task and decline nearly to control levels with repeated performance of the task (Hosp et al., 2011; Kleim et al., 1996). Since LID is often viewed as a pathological form of motor learning that is coincident with striatal c-Fos induction, it is possible that M1 c-Fos is involved in abnormal motor learning during LID (Calabresi et al., 2000, 2015; Mura et al., 2002). While multiple laboratories have reported that M1 c-Fos is induced by L-DOPA during dyskinesia (Halje et al., 2012; Ostock et al., 2011), these studies have been performed only in animals with multiple exposures to L-DOPA and the contribution of D1 receptors to this effect is unclear. Whereas c-Fos is critical for affecting transcriptional activity, another immediate-early gene, activity-regulated cytoskeletal-associated protein (ARC), is important for promoting synaptic plasticity in part through AMPA receptor trafficking, and may identify unique aspects of cortical plasticity (Bramham et al., 2008; Korb and Finkbeiner, 2011; Perez-Cadahia et al., 2011). Indeed, ARC protein was recently shown to be preferentially enhanced by L-DOPA among rats that displayed significant LID as opposed to more stable L-DOPA responders (Bastide et al., 2014).
The goal of the present study was to characterize structural and functional changes occurring in M1 in a widely used rat model of PD / LID, in order to spur further research and highlight therapeutic approaches. First, 6-OHDA-induced changes in M1 TH-fiber innervation and monoamine tissue concentrations were quantified using immunohistochemistry and high performance liquid chromatography (HPLC). Next, real-time reverse-transcriptase polymerase chain reaction (PCR) was used to examine changes in M1 gene expression after DA lesion and treatment with L-DOPA or the D1 receptor agonist SKF81297 (SKF). Our hypothesis was that 6-OHDA would reduce DA and NE innervation of M1, while DA replacement would pathologically enhance expression of M1 immediate-early genes and other genes involved in glutamate signaling, coincident with the induction of dyskinetic behavior.
Experimental Procedures
2.1 Animals
All experiments used male Sprague-Dawley rats (Taconic Farms, Hudson, NY, USA) that were 9–11 weeks old at the start of the experiment (N = 86). Rats were pair-housed in plastic cages and given free access to water and standard laboratory rat food. The colony room was maintained at 22–23 °C on a 12 h light/dark cycle, with experiments taking place during the light cycle. Throughout the study, animals were cared for in full accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the most-current (2011) National Institutes of Health “Guide for the Care and Use of Laboratory Animals”.
2.2 Drugs
All drugs were delivered at a volume of 1 ml/kg. Buprenorphine hydrochloride (Hospira Inc., Lake Forest, IL, USA) was dissolved in saline. (±)SKF81297 hydrobromide (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline with 20% dimethyl sulfoxide. 6-OHDA hydrobromide and L-DOPA methyl ester hydrochloride (Sigma-Aldrich) were dissolved in saline with 0.1% ascorbic acid. Multiple doses of L-DOPA were used, but the peripheral decarboxylase inhibitor benserazide hydrochloride (Sigma-Aldrich) was always co-administered at a constant dose of 15 mg/kg. Sodium pentobarbital (Fort Dodge Animal Health, Fort Dodge, IA, USA) was suspended in an alcohol/dH2O mixture by the manufacturer. Desipramine hydrochloride and quinpirole hydrochloride (Sigma-Aldrich) were dissolved in dH2O.
2.3 Lesion Surgeries
All rats received unilateral lesions to the medial forebrain bundle (MFB). In different experiments, sham or active lesions were created by infusing vehicle (Veh) or 6-OHDA, respectively. For analgesic purposes, rats were given buprenorphine (0.03 mg/kg; i.p.) immediately prior to surgery and 24 h after surgery. Animals were anaesthetized with isoflurane (2–3% for 30–45 min; Baxter Healthcare, Deerfield, IL, USA) mixed with oxygen (1 L/min). Since PD patients show significant NE pathology in M1 (Gaspar et al., 1991), NE neurons were not protected from pathology using the NE transport blocker desipramine. The exception to this is experiment 3B, where rats were treated with desipramine (25 mg/kg, ip) 30 min prior to 6-OHDA infusion, since these rats were used as part of a different study (see experiment 1 of Dupre et al., 2013). The following coordinates relative to bregma were used to target the MFB according to the rat brain atlas of Paxinos and Watson (1998): AP −1.8 mm; ML −2.0 mm; DV −8.6 mm, with the incisor bar 5 mm below the interaural line. A syringe with 26 gauge needle (Hamilton, Reno, NV, USA) was lowered into the target site and 6-OHDA (12 µg) or Veh was injected at a constant flow rate of 2 µl/min for 2 min. The needle was withdrawn 5 min later.
2.4 Forepaw Adjusting Steps Test
The forepaw adjusting steps (FAS) test is a measure of akinesia, a cardinal symptom of PD (Jankovic, 2008). Rats with >80% unilateral striatal DA depletion take fewer steps with the lesioned side of the body (Chang et al., 1999). To perform the test, an experimenter blind to treatment condition held the rat’s hindlimbs and one forelimb such that the free forelimb was forced to bear the rat’s body weight. Rats were moved laterally for 90 cm over 10 s while another experimenter counted the number of steps taken. Each FAS test consisted of three trials for each forelimb in each direction (toward or away from a rat’s midline), for a total of 12 trials per rat. Total steps with the lesioned forelimb were divided by steps with the non-lesioned forelimb to derive a “percent intact” score: lower scores indicate greater motor impairment. FAS scores were the primary inclusion criteria for the present research: rats with a 6-OHDA lesion were only considered Parkinsonian (and included in the data set) if they manifested <60% intact stepping.
2.5 Abnormal Involuntary Movements Scale
The abnormal involuntary movements (AIMs) test is a metric of dyskinesia. Rats were monitored for AIMs using a procedure modified from Cenci and Lundblad (2007) and described in detail in Lindenbach et al. (2011). Rats were observed in clear-plastic cylinders and were rated by a trained observer (≥95% reliability), every 10 min for 60–180 min (depending on the experiment). During each rating period, individual dyskinesia severity scores ranging from 0 (not present) to 4 (severe and not interruptible) were given for axial, limb and orolingual dyskinesias. The three AIMs subtypes were summed to create a single AIMs score for data analysis.
2.6 Statistical Analysis
Statistical analysis was performed using SPSS (IBM, Chicago, IL, USA) with alpha set at 0.05. Since the AIMs scale has ordinal intervals, medians are used as the measure of central tendency. Such data were analyzed with the non-parametric Friedman test (within-subjects, omnibus), the Wilcoxon signed-rank test (within-subjects, contrasts), the Kruskal-Wallis test (between-subjects, omnibus) and the Mann Whitney U (between-subjects, contrasts).
All other data were analyzed using standard ANOVAs and followed up by t-test contrasts if the relevant omnibus comparison was significant. Effect sizes for F tests are reported as partial eta squared (ηp2), which measures the fraction of variance independently predicted by a single effect (range 0–1). If a given analysis was between-subjects and analyzed with parametric statistics, data more than 2.5 standard deviations from the group mean were considered outliers and discarded. This resulted in the removal of no data from exp 1, 1% of data from exp 2, 2% of data from exp 3A and 3% of data from exp 3B.
2.7 Experiment 1: Effect of Lesion on Striatal and M1 TH Fiber Innervation
2.7.1 Procedure
This experiment determined the effect of a MFB 6-OHDA lesion on striatal and M1 catecholamine innervation (n = 12; 6 per group; see Fig. 1 for diagram). Surgery was performed on rats as described above with animals receiving a unilateral MFB 6-OHDA lesion or Veh (sham) lesion. After 3 weeks of surgical recovery, rats were treated intermittently with multiple doses of L-DOPA (2–12 mg/kg) as well as the D1 agonist SKF (0.08 and 0.8 mg/kg) and the D2 agonist quinpirole (0.05 and 0.5 mg/kg; both injected s.c.). The AIMs scores from this portion of the experiment were used as unpublished pilot data. For subsequent immunohistochemical analyses, after a 10 d drug washout period, rats were transcardially perfused with 4% formaldehyde in phosphate-buffered saline.
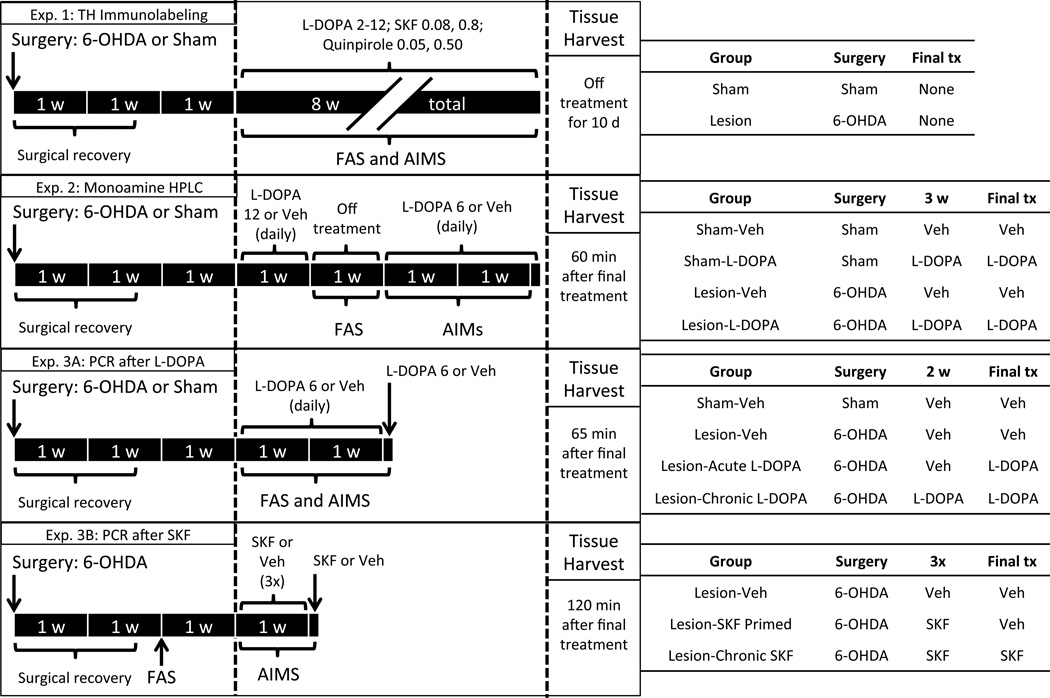
Figure 1.
Schematic illustrating the timing, manipulations, behavioral analyses and treatment groups in the present experiment. All animals underwent surgery with a unilateral intracranial injection of 6-hydroxydopamine (6-OHDA) or vehicle (Veh; “Sham”) into the medial forebrain bundle. Three weeks after surgery, treatment regimens began with animals receiving L-DOPA, the D1 agonist SKF81297 (SKF) or the D2 agonist quinpirole. Animals were periodically assessed for Parkinsonian akinesia with the forepaw adjusting steps (FAS) test or monitored for dyskinesia with the abnormal involuntary movements (AIMs) test. Tissue was harvested after decapitation at the time point noted. Other abbreviations used: HPLC, High performance liquid chromatography; PCR, Polymerase chain reaction; TH, Tyrosine hydroxylase.
2.7.2 Immunohistochemistry
Coronal sections (40 µm) were cut using a sliding microtome (Model SM2000R; Leica Microsystems, Bannockburn, IL, USA). Three sections each 1 mm apart were selected for processing (anterior to bregma, in mm: 2.6, 1.6, and 0.6). M1 was analyzed in all three slices and the striatum was examined in the two posterior sections. Prior to processing and between incubations, all slices were triple-washed with phosphate-buffered saline. Sections were first immersed in 0.3% H2O2 for 30 min. Subsequently, slices were placed in blocking buffer (phosphate-buffered saline with 1% bovine serum albumin, 1% normal goat serum and 0.4% Triton X-100 [Sigma-Aldrich]) for 120 min at room temperature. Sections were then incubated in a mouse anti-TH primary antibody (1:500 dilution in blocking buffer; Millipore, Billerica, MA, USA) at 4° C overnight. Next, slices were bathed in a horse anti-mouse secondary antibody (1:200 dilution in blocking buffer; Vector Laboratories, Burlingame, CA, USA) at 4° C for 120 min. Sections were then incubated for 1 h in a horse radish peroxidase-conjugated avidin-biotin mixture (VectaStain Elite ABC Kit, Vector Laboratories). For the chromagen step, SigmaFast kits (Sigma-Aldrich) containing 3,3’-diaminobenzidine and H2O2 were prepared according to manufacturer’s instructions. After staining, slices were mounted on glass slides, dehydrated, defatted and cover slipped.
2.7.3 Quantification of TH Immunoreactivity
Quantification of striatal TH staining was performed using optical density. Images of the striatum were captured with a photomicroscope at 2× magnification and analyzed with Image J software (NIH, Bethesda, MD, USA). Relative optical density was calibrated using general utility Rodbard function fitted to an NIH step tablet. The primary somatosensory cortex (ipsilateral to each striata) was used as the reference for background staining, since this area receives very little DA innervation relative to the striatum (Pifl et al., 1991) and 6-OHDA did not affect optical density in this cortical region. Reported values are the optical density of the striatum minus the optical density of the primary somatosensory cortex.
In order to quantify M1 fiber density, a stereotaxic microscope (Axioscope 2 plus, Zeiss, Thornwood, NY, USA) was linked to a computer running the “Space Balls” feature of StereoInvestigator software (Version 10, MBF Bioscience, Williston, VT, USA). Space Balls achieves isotropy in three dimensions by superimposing a half-sphere into the tissue while an experimenter counts the number of times a fiber intersects the surface of the half-sphere. After outlining the M1 region, the software selected ~20 sites in each M1 hemisphere of each of the three slices, for a total of ~120 sites per rat. Each site was visualized at 40× magnification and the number of fibers crossing a virtual hemisphere with 15 µm radius was quantified. Length estimates were derived from Mouton et al. (2002), with the computed length divided by the volume of tissue stereologically examined. Data are reported as nm of TH-positive fiber per µm3 of M1 tissue.
2.8 Experiment 2: Effect of Lesion and L-DOPA on M1 Monoamine Concentrations
2.8.1 Procedure
This experiment determined how 6-OHDA lesion and L-DOPA administration alter monoamine concentrations in M1 (n = 28; 7 per group; see Fig. 1 for diagram). Rats, as described above, received either a unilateral MFB 6-OHDA lesion or sham lesion. Three weeks after surgery, rats were given daily L-DOPA (12 mg/kg; s.c.) or Veh for 7 consecutive days. Rats were left untreated for days 8–14, during which time Parkinsonian motor impairment was assessed with the FAS test. Daily L-DOPA (6 mg/kg) or Veh injections resumed on days 15–28, and rats were assessed for dyskinesia using the AIMs scale on day 27. On day 28, rats were rapidly decapitated 60 min after injection with L-DOPA (6 mg/kg) or Veh, a time point that corresponds with peak dyskinesia expression in lesioned animals given L-DOPA. M1 tissue was then processed for HPLC analysis of monoamine content.
2.8.2 Monoamine Tissue Analysis
HPLC coupled to electrochemical detection was performed on M1 tissue according to a protocol for monoamine analysis by Kilpatrick et al. (1986) and described in Dupre et al. (2011). Tissue was homogenized in ice-cold perchloric acid (0.1 M) with 1% ethanol and 0.02% EDTA. The homogenates were spun for 30 min at 14,000 g with the temperature maintained at 4° C. Aliquots of supernatant were analyzed for abundance of DA, the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), NE, 5-HT and the 5-HT metabolite 5-hydroxyindolacetic acid (5-HIAA). Samples were separated using a mobile phase composed of 90 mM NaH2PO4, 50 µM EDTA, 1.7 mM octane sulphonic acid and 10% acetonitrile, adjusted to pH 3.0 with σ-phosphoric acid. The guard cell was set to +300 mV; the first electrode in the analytic cell was set to −100 mV and the second analytic electrode set to +250 mV. Standards of known concentrations were run across a range of 10−6 to 10−9 M and samples were plotted on the corresponding regression line. Values were adjusted to wet tissue weights and expressed as pg (of monoamine) per mg (of tissue).
2.9 Experiment 3A: Effect of Lesion and L-DOPA on mRNA Expression in M1
2.9.1 Procedure
This experiment determined how 6-OHDA lesions and L-DOPA alter the transcription of genes associated with cortical plasticity as well as glutamate and GABA signaling (n = 26; 6–7 per group; see Fig. 1 for diagram). Two weeks after surgery, rats with sham or 6-OHDA lesions were examined with the FAS test to verify Parkinsonian motor impairment in 6-OHDA-lesioned animals and create equivalently Parkinsonian treatment groups. One week later (“day 1”), rats were given daily injections of L-DOPA (6 mg/kg; s.c.) or Veh for 14 d. During this time, AIMs were monitored on days 1, 8 and 14 for 180 min. The FAS test was performed 60 min after drug injection on days 2 and 13. On day 15, rats in the “Acute L-DOPA” and “Chronic L-DOPA” groups received L-DOPA (6 mg/kg), while others received Veh. Subsequently, rats were monitored for AIMs for 60 min (six total ratings per rat); at 65 min post-injection, rats were decapitated for M1 mRNA analysis. M1 tissue was examined bilaterally for transcription levels of seven genes: 1. β-actin (as housekeeper); 2. c-Fos; 3. ARC; 4. the NMDA receptor subunit NR2A; 5. the NMDA receptor subunit NR2B; 6. vesicular glutamate transporter type I (VGLUT1); 7. glutamic acid decarboxylase 67 kDA (GAD67).
2.9.2 Real-time Reverse-Transcriptase PCR
Tissue was lysed with TriZol (Life Technologies, Grand Island, NY, USA), mixed with phenol/chloroform and centrifuged at 12,000 g for 15 min. mRNA was precipitated with 70% ethanol and purified using RNeasy columns (Qiagen, Valencia, CA, USA). Concentration of mRNA was determined via spectrophotometry using a Nanodrop 2000 (Thermo-Fischer Scientific, Waltham, MA, USA). cDNA was amplified with the IQ SYBR Green Supermix kit (BioRad, Hercules, CA, USA). A reaction master mix of volume 40 µl consisted of 20 µl SYBR Green, 17.6 µl RNase-free water, 0.4 µl cDNA template, and 2 µl of sample. From each master mix, 10 µl was pipetted in triplicate into a 384-well plate and analyzed using a CFX1000 Thermal Cycler (BioRad). Each gene was amplified for 40 cycles lasting 90 sec each: 30 sec at 95° C to denature cDNA, 30 sec at 57–60° C (depending on primer sequence) to allow primers to bind and 30 sec at 72° C to extend fragments. Relative gene expression was quantified using the 2−ΔΔCT method, with expression levels for transcripts of interest normalized to observed β-actin levels for each sample. Relative expression was then normalized to 100% of ultimate control values.
2.9.3 Primer Sequences
Gene sequences were obtained from GenBank at the National Center for Biotechnology Information and primer specificity was verified by the Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/). The primer sequences used (forward / reverse) were as follows: β-actin (5’-AGC ATC ACC CCA TTT GAT GT-3’ / 5’-GTC GTA CCA CTG GCA TTG TG-3’); c-Fos (5’-CCA AGC GGA GAC AGA TCA AC -3’ / 5’-AAG TCC AGG GAG GTC ACA GA-3’); ARC (5’-TCA GAC CAT CAC AGA ACA CCT TT-3’ / 5’-CCC TTG GGT TTG GTG CCT AC -3’); NR2A (5’-GCT ACA CAC CCT GCA CCA ATT-3’ / 5’-CAC CTG GTA ACC TTC CTC AGT GA-3’); NR2B (5’-CCC AAC ATG CTC TCT CCC TTA A-3’ / 5’-CAG CTA GTC GGC TCT CTT GGT T-3’); GAD67 (5’-CAC AAA CTC AGC GGC ATA GA-3’ / 5’-GAC CAG GAT GGC AGA ACA CT-3’); VGLUT1 (5’-GCC TTT TGC GGT TCC TAT GC-3’ / 5’- GGA GAT GCT GGG GTG TAG TG-3’).
2.10 Experiment 3B: Effect of D1 Activation on mRNA Expression in M1
2.10.1 Procedure
All rats received a unilateral MFB 6-OHDA lesion (n = 20; 6–7 per group; see Fig. 1 for diagram). Two weeks after surgery, the FAS test was used to assign rats to equivalently Parkinsonian groups. One week later, on three sessions 2–3 d apart, one group of rats received Veh while two groups of rats received the D1 agonist SKF (0.8 mg/kg; s.c.). This treatment regimen has been shown to produce stable dyskinesia (Dupre et al., 2008, 2011). Immediately after injection, rats were monitored for AIMs for 120 min. Two days later, rats were given one final injection: two groups received Veh while one group received SKF (0.8 mg/kg). Rats were rated for AIMs for 120 min and decapitated for M1 mRNA analysis, using the same PCR technique described in sections 2.9.2. and 2.9.3. Additional data from these rats has been previously reported in experiment 1 of Dupre et al. (2013). For the present experiment, only c-Fos and ARC (along with the housekeeper β-actin) were examined, given the modest modulation of other genes by L-DOPA treatment in experiment 3A.
Results
3.1 Experiment 1: Effect of Lesion on Striatal and M1 TH Fiber Innervation
This experiment examined the effect of 6-OHDA lesion on TH fiber innervation in the striatum (using optical density) and in M1 (using stereology). The behavioral phenotype of Parkinsonism was verified with the FAS test; rats with a 6-OHDA lesion averaged 43% intact stepping and took significantly fewer steps with their affected forelimb than sham-lesioned rats (t10 = 8.86, p < .001). Effects on TH-positive fibers were determined with a 2×2 mixed-model ANOVA: Hemisphere (Ipsilateral or Contralateral [to Lesion]) * Lesion (6-OHDA or Sham).
Striatal TH
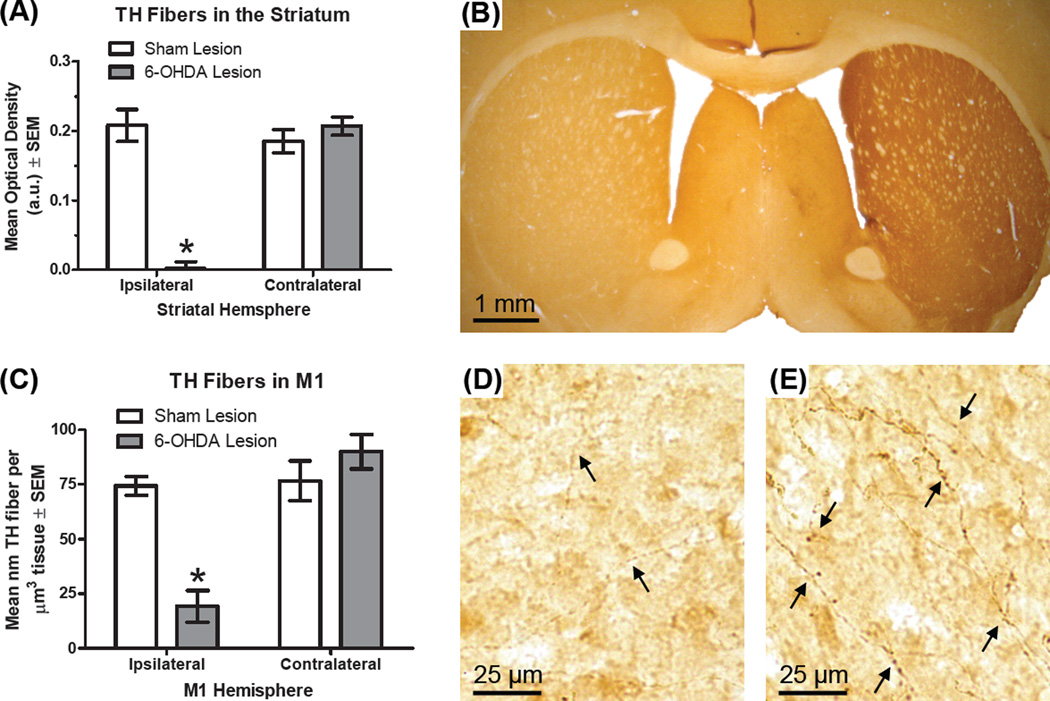
Omnibus ANOVA showed a main effect of hemisphere (F1,10 = 50.13, p < .001, ηp2 = .834) and 6-OHDA lesion reduced striatal optical density (F1,10 = 23.05, p = .001, ηp2 = .697). Importantly, there was a hemisphere*lesion interaction (F1,10 = 77.88, p < .001, ηp2 = .886). 6-OHDA-lesioned rats had a 99% reduction in ipsilateral striata TH optical density compared to the contralateral striata (Fig. 2A–B; t5 = 12.65, p < .001).
Figure 2.
Effect of lesion on tyrosine hydroxylase (TH)-positive fibers in the striatum and primary motor cortex (M1; n = 6 per group). Rats received a unilateral 6- hydroxydopamine (6-OHDA) lesion or sham to the medial forebrain bundle. Rats were later transcardially perfused and tissue was stained with an antibody specific for TH, the rate-limiting enzyme in the synthesis of dopamine and norepinephrine. (A) Optical density (in arbitrary units) of striatal TH fibers. (B) Photomicrograph of a coronal section containing the striatum from a rat with a unilateral 6-OHDA lesion (left side is ipsilateral to lesion). (C) Length-volume estimate of TH fibers in M1. (D) Photomicrograph showing M1 ipsilateral to 6-OHDA lesion. Arrows are used to highlight TH-positive fibers. (E) Photomicrograph of M1 contralateral to 6-OHDA lesion. * p < .05 vs. contralateral hemisphere.
M1 TH
The results of the 2×2 ANOVA showed there were fewer fibers in the ipsilateral hemisphere (F1,10 = 21.55, p = .001, ηp2 = .683), fewer fibers in 6-OHDA-lesioned animals (F1,10 = 9.48, p = .012, ηp2 = .487) and there was a hemisphere*lesion interaction (F1,10 = 18.85, p = .001, ηp2 = .653). Animals with 6-OHDA lesions averaged a 75% reduction in the density of TH-positive fibers ipsilateral vs. contralateral to lesion (Fig. 2C–E; t5 = 5.76, p = .002).
3.2 Experiment 2: Effect of Lesion and L-DOPA on M1 Monoamine Concentrations
As a complement to experiment 1, we next determined how 6-OHDA lesion and L-DOPA impacted monoamine tissue concentrations in M1. Rats in this experiment with a 6-OHDA lesion averaged 44% intact stepping and took fewer steps with their lesioned forelimb than sham-lesioned animals with the same forelimb (t26 = 6.76, p < .001). After chronic L-DOPA (6 mg/kg) exposure, 6-OHDA-lesioned animals that were given L-DOPA showed significant dyskinesia (as measured by the AIMs test) compared with 6-OHDA-lesioned animals treated with Veh (Z = 3.13, p = .002).
M1 tissue was collected 60 min after final treatment and concentrations of monoamines and their metabolites in M1 tissue were determined via HPLC (Table 1). Changes in monoamine concentration due to experimental manipulation were determined by using a 3-way mixed-model ANOVA: Hemisphere (Ipsilateral or Contralateral [to Lesion]) * Lesion (6-OHDA or Sham) * Treatment (L-DOPA or Veh). Pending results of omnibus comparisons, four contrasts were planned for each neurochemical, examining the effect of 6-OHDA (compared to Sham) and L-DOPA (compared to Veh; both ipsilateral to lesion only).
Table 1.
Effect of 6-OHDA and L-DOPA on M1 monoamines and their metabolites
| Hemisphere | Lesion | Treatment | DA | DOPAC | NE | 5-HT | 5-HIAA |
|---|---|---|---|---|---|---|---|
| Ipsilateral | Sham | Vehicle | 33 ± 2 | 43 ± 3 | 279 ± 22 | 327 ± 11 | 3513 ± 234 |
| Contralateral | -- | 35 ± 3 | 48 ± 4 | 305 ± 12 | 258 ± 13 | 3551 ± 199 | |
| Ipsilateral | 6-OHDA | Vehicle | 29 ± 5 | 32 ± 7 | * 126 ± 50 | 291 ± 25 | 3679 ± 289 |
| Contralateral | -- | 33 ± 2 | 52 ± 9 | 343 ± 13 | 346 ± 79 | 4212 ± 292 | |
| Ipsilateral | Sham | L-DOPA (6 mg/kg) |
* 94 ± 9 | * 85 ± 10 | 243 ± 14 | 239 ± 22 | 3187 ± 135 |
| Contralateral | -- | 88 ± 4 | 83 ± 2 | 270 ± 5 | 240 ± 16 | 3484 ± 177 | |
| Ipsilateral | 6-OHDA | L-DOPA (6 mg/kg) |
# § 66 ± 5 | # 54 ± 5 | § 36 ± 18 | 664 ± 305 | 3651 ± 146 |
| Contralateral | -- | 76 ± 6 | 99 ± 16 | 284 ± 20 | 231 ± 20 | 3846 ± 242 |
Rats received unilateral injections of 6-hydroxydopamine (6-OHDA) or sham into the medial forebrain bundle (n = 7 per group). After recovery, rats were treated with daily L-DOPA 6 mg/kg or vehicle (Veh) and decapitated 60 min after final injection. Primary motor cortex tissue was processed via high performance liquid chromatography for concentrations of dopamine (DA), the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), norepinephrine (NE), serotonin (5-HT) or the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA). Reported values are mean pg (of monoamine) per mg (of tissue) ± SEM. Individual planned contrasts were only performed on the ipsilateral hemisphere.
p < .05 vs. Sham + Veh;
p < .05 vs. 6-OHDA + Veh;
p < .05 vs. Sham + L-DOPA.
DA
Main effects of lesion and treatment showed that 6-OHDA reduced DA (F1,24 = 9.80, p = .005, ηp2 = .290), treatment with L-DOPA increased DA (F1,24 = 168.40, p < .001, ηp2 = .875) and there was a lesion*treatment interaction (F1,24 = 5.33, p = .030, ηp2 = .182). Specifically within the ipsilateral hemisphere, when animals were given Veh, DA levels were the same between sham animals and those with a 6-OHDA lesion. Systemic L-DOPA increased DA among animals with a sham lesion (t10 = 6.64, p < .001) and among those with a 6-OHDA lesion (t14 = 5.51, p < .001). However, sham animals given L-DOPA displayed greater M1 DA content than 6-OHDA-lesioned animals given LDOPA (t12 = 3.05, p = .010), suggesting greater synthesis of DA from L-DOPA in shams.
DOPAC
Overall analysis revealed main effects of hemisphere (F1,23 = 8.42, p = .008, ηp2 = .268) and treatment (F1,23 = 29.54, p < .001, ηp2 = .562). Importantly, there was a hemisphere*lesion interaction (F1,23 = 6.23, p = .023, ηp2 = .213). In Veh-treated rats, DOPAC levels did not differ between 6-OHDA-lesioned animals and shams. L-DOPA increased DOPAC concentrations among sham animals (t9 = 4.34, p = .002) and 6-OHDA-lesioned animals (t14 = 2.66, p = .019).
NE
Omnibus ANOVA results showed a main effect of hemisphere (F1,24 = 64.08, p < .001, ηp2 = .728). An effect of lesion showed that 6-OHDA reduced NE (F1,24 = 15.13, p = .001, ηp2 = .387) with a further reduction in NE by treatment with L-DOPA (F1,24 = 7.64, p = .011, ηp2 = .242). There was also a hemisphere*lesion interaction (F1,24 = 40.37, p < .001, ηp2 = .627). In Veh-treated animals, NE levels were lower among animals given 6-OHDA than among shams (t12 = 2.49, p = .028). Similarly, among animals given LDOPA, 6-OHDA-lesioned animals had significantly less NE than sham animals (t12 = 8.69, p < .001).
5-HT and 5-HIAA
Analysis of 5-HT with a 3-way ANOVA yielded no significant effects. Analyzing the 5-HT metabolite 5-HIAA, there was only a main effect of lesion showing that 6-OHDA lesion increased 5-HIAA (F1,24 = 4.59, p = .042; ηp2 = .161). No planned contrasts were conducted.
3.3 Experiment 3A: Effect of Lesion and L-DOPA on mRNA Expression in M1
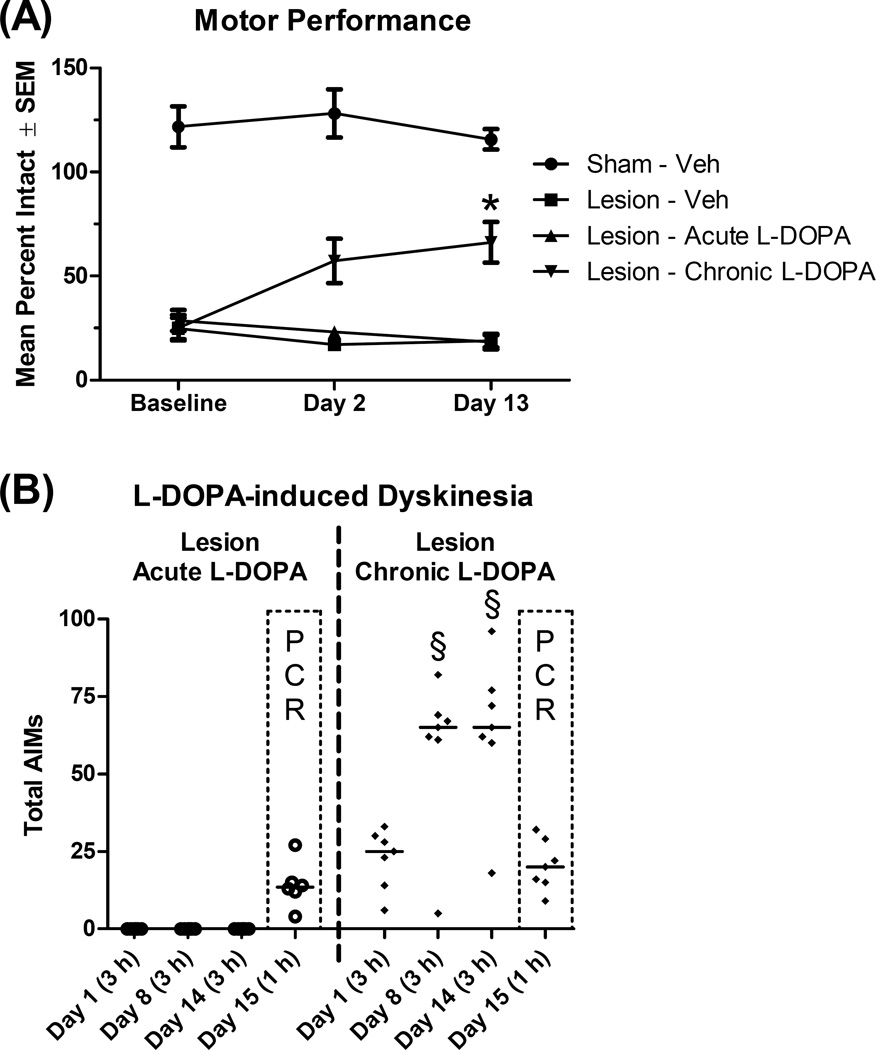
This experiment examined how M1 gene expression is altered by 6-OHDA lesion and/or L-DOPA treatment. In this cohort, rats with a 6-OHDA lesion averaged 26% intact stepping and took fewer steps with their lesioned forelimb than sham rats with their sham-lesioned forelimb (t24 = 21.32, p < .001). Rats were then treated with daily L-DOPA (6 mg/kg) or Veh for 14 d and FAS was performed again on days 2 and 13 of treatment. Change in motor performance across days was analyzed with a 4×3 mixed-model ANOVA: Group (Sham - Veh, Lesion - Veh, Lesion - Acute L-DOPA, Lesion - Chronic L-DOPA) * Day (Baseline, Day 2, Day 13). Results revealed an effect of group (F3,22 = 85.90, p < .001, ηp2 = .921) and a group*day interaction (F6,44 = 4.06, p = .003, ηp2 = .356). Planned comparisons were conducted comparing day 2 and 13 to baseline for each group. As seen in figure 3A, rats receiving chronic L-DOPA showed a trend for increased stepping on day 2 (t6 = 2.14, p = .076) and a significant improvement in stepping on day 13 (t6 = 3.77, p = .009).
Figure 3.
Effect of dopamine lesion and treatment with L-DOPA on motor performance and dyskinesia (n = 6–7 per group). Rats received a unilateral lesion with 6-hydroxydopamine (or sham) and were allowed to recover for 3 weeks. Rats were subsequently treated daily for 14 d with vehicle (Veh), except rats in the “Chronic” condition, which received daily L-DOPA (6 mg/kg). (A) Motor performance was assessed with the forepaw adjusting steps test at baseline (prior to treatment) and on days 2 and 13 of daily L-DOPA or Veh. (B) Dyskinesia was assessed with the abnormal involuntary movements (AIMs) scale for 3 h on day 1, 8 and 14 of daily L-DOPA or Veh. On day 15, rats in the “Acute” and “Chronic” groups received L-DOPA (6 mg/kg), while others received Veh. Rats were assessed for AIMs for 60 min and tissue was harvested at 65 min. Data points show total AIMs scores for individual rats, while the horizontal line indicates the median score. * p < .05 vs. baseline (Lesion - Chronic L-DOPA); § p < .05 vs. Day 1.
As seen in figure 3B, for rats given chronic L-DOPA, dyskinesia scores increased after repeated exposures to L-DOPA (n = 7; χ2 = 8.00, p = .018), with rats showing more AIMs on day 8 (Z = 2.20, p = .028) and day 14 (Z = 2.37, p = .018) relative to day 1 of L-DOPA. After the final injection on day 15, AIMs were measured for 60 min (prior to tissue harvest); rats given chronic L-DOPA showed greater AIMs than rats given acute L-DOPA, although this was not statistically significant (Z = 1.65, p = .100), likely due to the limited sampling window relative to normal AIMs sessions (180 min).
Bilateral M1 tissue was collected on day 15, processed for real-time PCR and analyzed with a 2×4 mixed model ANOVA: Hemisphere (Ipsilateral vs. Contralateral [to Lesion or Sham]) * Group (Sham - Veh, Lesion - Veh, Lesion - Acute L-DOPA, Lesion - Chronic L-DOPA). ANOVA analysis of the housekeeper β-actin revealed no significant effects, demonstrating that levels were equivalent across groups.
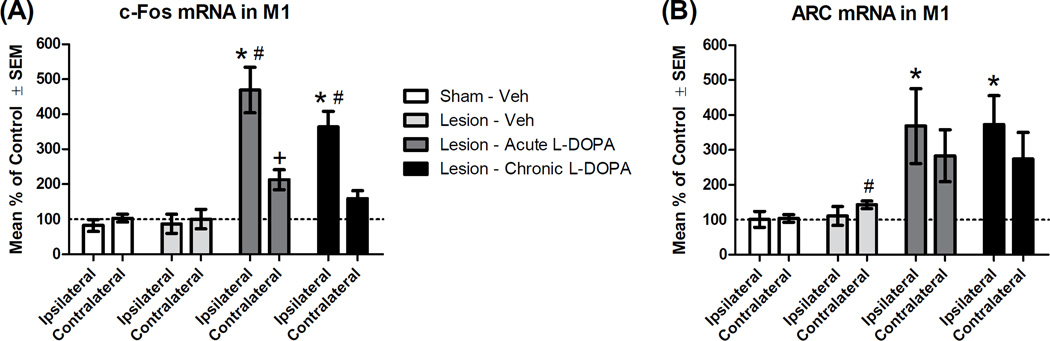
c-Fos
The immediate-early gene c-Fos was examined as it is a general transcription factor and a marker of motor learning in M1 (Hosp and Luft, 2013; Perez-Cadahia et al., 2011; Fig. 4A). The 2×4 ANOVA revealed an effect of hemisphere (F1,22 = 45.50, p < .001, ηp2 = .674), a group effect (F3,22 = 17.36, p < .001, ηp2 = .703) and a hemisphere*group interaction (F3,22 = 20.65, p < .001, ηp2 = .738). We next performed between-subjects comparisons in the ipsilateral hemisphere. Lesion alone did not alter c-Fos expression (t11 = 0.16, p = .879). However, compared to lesion alone, c-Fos was induced ipsilateral to lesion by both acute L-DOPA (t10 = 5.41, p < .001) and chronic L-DOPA (t11 = 5.09, p < .001). In the contralateral hemisphere, c-Fos expression was significantly increased after acute L-DOPA (t10 = 2.81, p = .018). To assess the hemisphere*group interaction further, we determined if L-DOPA was preferentially increasing M1 c-Fos in the ipsilateral hemisphere using a paired-samples t-test. Greater c-Fos mRNA was seen in the ipsilateral vs. contralateral M1 for rats given acute L-DOPA (t5 = 5.25, p = .003) and for rats given chronic L-DOPA (t6 = 6.09, p = .001).
Figure 4.
Effect of L-DOPA on mRNA transcription of the immediate-early genes c-Fos and ARC within the primary motor cortex (M1; n = 6–7 per group). Rats received a unilateral lesion with 6-hydroxydopamine (or sham). After recovery, rats were treated for 14 d with Vehicle (Veh), except rats in the “Chronic” condition, which received daily L-DOPA (6 mg/kg). The next day, rats in the “Acute” and “Chronic” groups received L-DOPA (6 mg/kg), while others received Veh. All rats were decapitated 65 min later and M1 tissue was analyzed via real-time polymerase chain reaction. Hemispheres are denoted as either ipsilateral or contralateral (to lesion or sham). Percent change in mRNA was normalized to control (“Sham - Veh [contralateral]”). (A) c-Fos mRNA. (B) ARC mRNA. * p < .05 vs. Lesion - Veh (ipsilateral); + p < .05 vs. Lesion - Veh (contralateral); # p < .05 vs. own contralateral hemisphere.
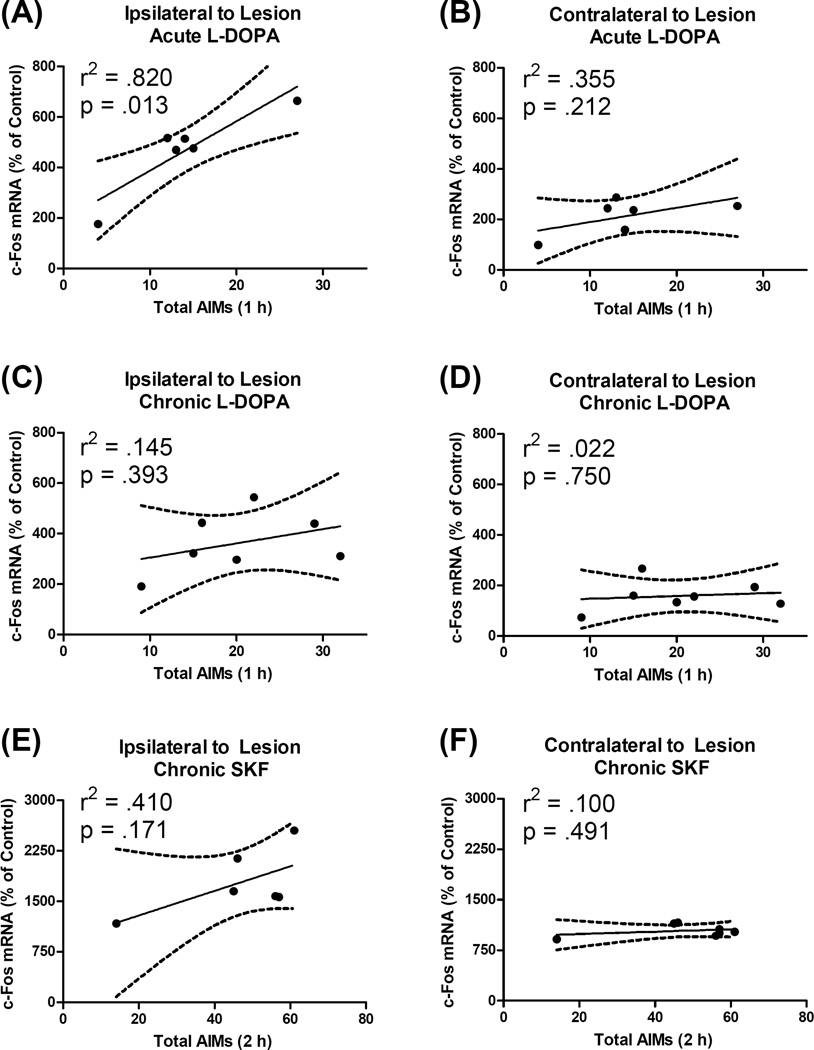
Next, AIMs scores were correlated with percent change in c-Fos in both the ipsilateral and contralateral hemisphere among rats that received acute and chronic L-DOPA. We found a significant positive correlation between total AIMs and c-Fos mRNA ipsilateral to lesion in rats given acute L-DOPA (r2= .820, p = .013; Fig. 7A). Interestingly, expression of c-Fos was not significantly correlated with behavior contralateral to lesion or in rats given chronic L-DOPA (Fig. 7B–D).
Figure 7.
Correlation between dyskinetic behavior and expression of the immediate-early gene c-Fos in the primary motor cortex (M1). Dyskinesia was scored with the abnormal involuntary movements (AIMs) scale while c-Fos mRNA was quantified via polymerase chain reaction. Analyses were performed only on rats that had a (unilateral) 6-hydroxydopamine lesion and received treatment with L-DOPA or the D1 agonist SKF81297 (SKF). Data points reflect individual rat scores. The solid line is the least squared regression line while the dashed line signifies the 95% confidence interval for the slope of the regression line. (A–B) Acute L-DOPA: Previously drug-naïve rats were given their first dose of L-DOPA (6 mg/kg) and tissue was harvested 65 min later. (C–D) Chronic L-DOPA: Rats were treated daily for 15 d with L-DOPA 6 mg/kg and tissue was harvested 65 min after final injections. (E–F) Chronic SKF: Rats were treated four times with SKF 0.8 mg/kg over an 8 d period and tissue was harvested 120 min after final treatment. Note that the axes in panels E–F differ from the axes in panels A–D.
ARC
Another immediate-early gene, ARC, was investigated, since it has been implicated in synaptic plasticity and receptor trafficking (Bramham et al., 2008). ANOVA results revealed an effect of group (F3,22 = 5.63, p = .005), but no other significant effects. As compared to sham, 6-OHDA lesion alone slightly increased ARC expression contralateral to lesion (to 143% of controls; t11 = 2.58, p = .026; Fig. 4B). Ipsilateral to lesion, ARC expression was more strongly increased by both acute L-DOPA (to 368% of controls; t10 = 2.33, p = .042) and chronic L-DOPA (to 373% of controls; t11 = 2.81, p = .017) as compared to Veh-treated animals. Changes in ARC expression were not significantly correlated with AIMs behavior.
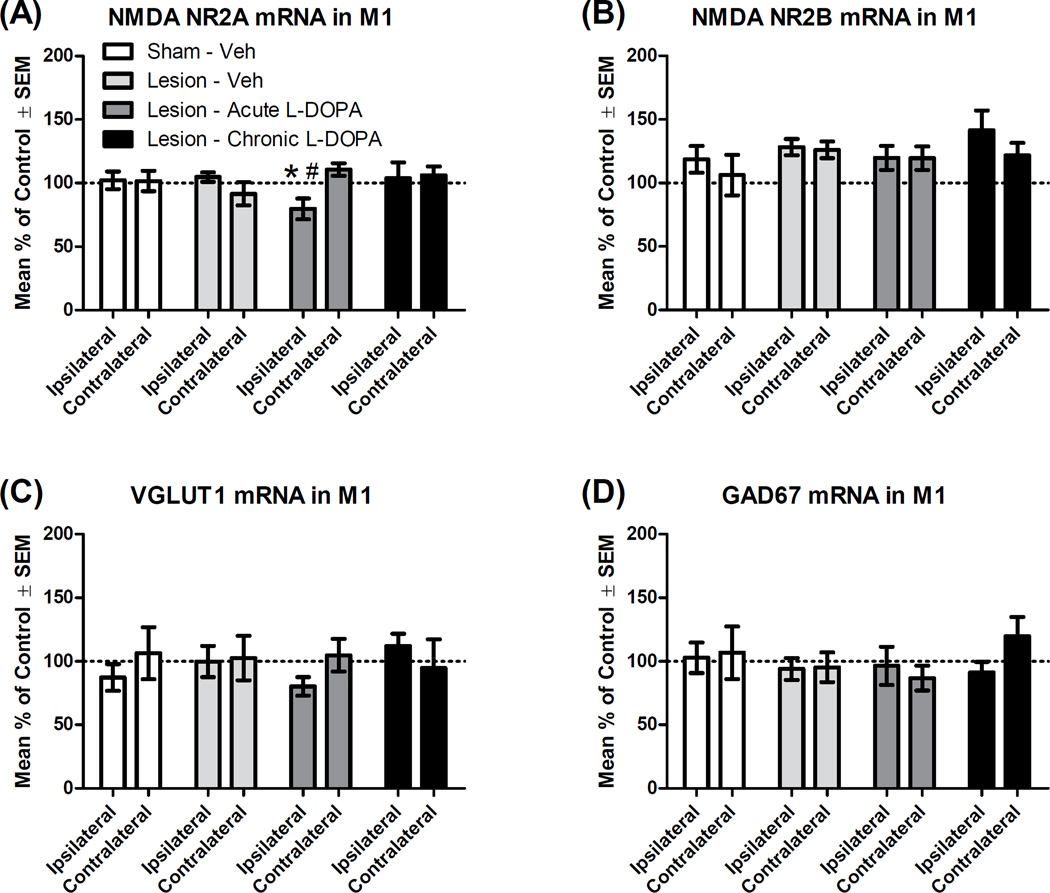
NMDA NR2A and NR2B
The NMDA NR2A subunit, which promotes long-term potentiation, was examined given the role of DA in facilitating M1 plasticity (Fig. 5A; Liu et al., 2004; Hosp and Luft, 2013). Omnibus ANOVA revealed no main effects, but there was a hemisphere*group interaction (F3,22 = 3.10, p = .048, ηp2 = .297). Rats given acute L-DOPA showed less NR2A mRNA in their ipsilateral hemisphere compared to both their own contralateral hemisphere (t5 = 2.63, p = .047) and the ipsilateral side of lesioned rats treated with Veh (t10 = 2.77, p = .020). The NR2B subunit, known to promote long-term depression, was also investigated (Liu et al., 2004), but no significant ANOVA effects were observed (Fig. 5B).
Figure 5.
Effect of L-DOPA on mRNA transcription of the genes associated with glutamate and GABA signaling in the primary motor cortex (M1; n = 6–7 per group). Rats received a unilateral lesion with 6-hydroxydopamine (or sham). After recovery, rats were treated for 14 d with Vehicle (Veh), except rats in the “Chronic” condition, which received daily L-DOPA (6 mg/kg). The next day, rats in the “Acute” and “Chronic” groups received L-DOPA (6 mg/kg), while others received Veh. All rats were decapitated 65 min later and M1 tissue was analyzed via real-time polymerase chain reaction. Hemispheres are denoted as either ipsilateral or contralateral (to lesion or sham). Percent change in mRNA was normalized to control (“Sham - Veh [contralateral]”). (A) NMDA subunit NR2A mRNA. (B) NMDA subunit NR2B mRNA. (C) Vesicular glutamate transporter type I (VGLUT1) mRNA. (D) Glutamic acid decarboxylase 67 kDa (GAD67) mRNA. * p < .05 vs. Lesion - Veh (ipsilateral); # p < .05 vs. own contralateral hemisphere.
VGLUT1
VGLUT1 is a key glutamate transporter in cortical neurons and changes in striatal VGLUT1 protein are evident in the 6-OHDA rat model of PD (Massie et al., 2010; Villalba and Smith, 2011). There were no changes in mRNA levels of VGLUT1 in the present study (Fig. 5C).
GAD67
Finally, the activity of GAD67 was used to assay the activity state of cortical GABA interneurons (Lewis et al., 2005), but no significant effects were found (Fig. 5D).
3.4 Experiment 3B: Effect of D1 Activation on mRNA Expression in M1
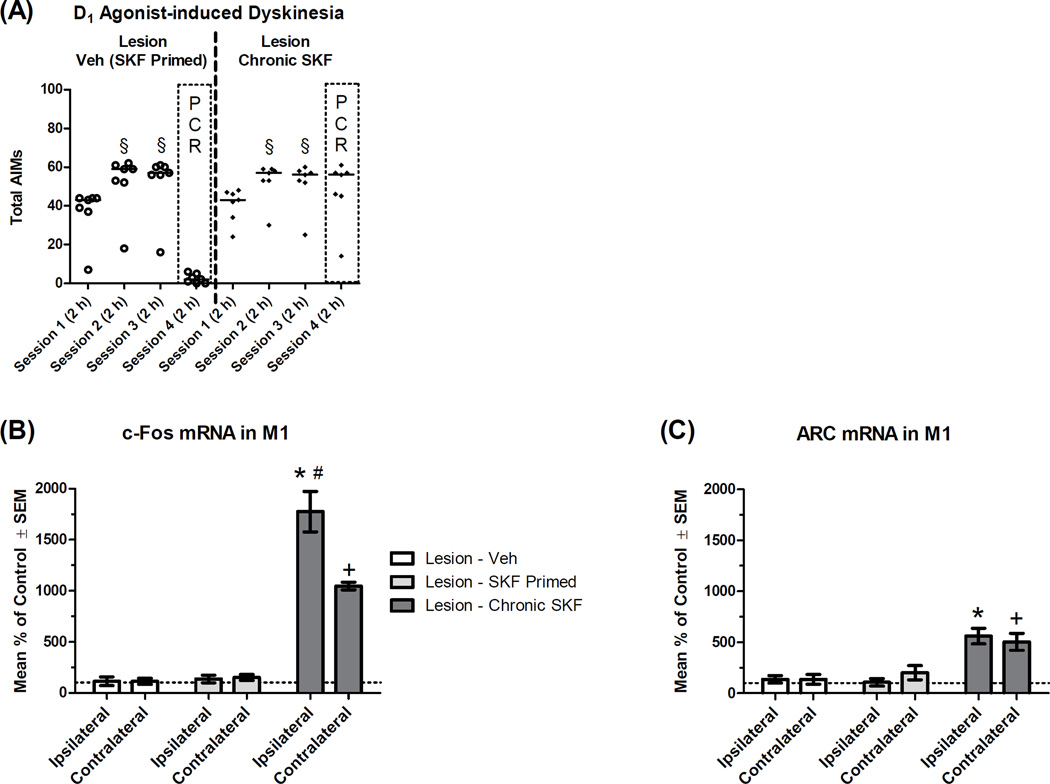
One potential mechanism of LID is the supersensitization of D1 receptors (Cenci et al., 2011; Feyder et al., 2011). Given the data from experiment 3A, suggesting that LID is associated with increases in M1 immediate-early gene expression, we chose to investigate these genes in animals treated with a D1 receptor agonist at a dose that provokes dyskinesia (Dupre et al., 2011, 2013). Parkinsonian status was verified in these rats by showing that rats averaged 36% intact stepping on the FAS test, taking fewer steps with their lesioned forelimb than their intact forelimb (t19 = 16.00, p < .001). Animals were given three injections of the D1 agonist SKF or Veh over a 7 d period and monitored for AIMs after each injection. Friedman test showed that AIMs increased with repeated exposure to SKF (n = 14; χ2 = 21.42, p < .001; Fig. 6A). Further analyses showed that AIMs increased on session 2 of SKF relative to session 1 (Z = 3.30, p = .001), but did not change on session 3 relative to session 2 (Z = 0.49, p = .624), suggesting animals were fully sensitized to SKF (Dupre et al., 2011, 2013).
Figure 6.
Effect of the D1 receptor agonist SKF81297 (SKF) on mRNA transcription of the immediate-early genes c-Fos and ARC within the primary motor cortex (M1; n = 6–7 per group). Rats received a unilateral lesion with 6-hydroxydopamine. (A) After surgical recovery, rats were given a total of three injections of the D1 agonist SKF81297 (SKF) or Veh over a 7 d period (“Sessions 1–3”). Two days later (“Session 4”), rats were given SKF or Veh and rated for AIMs for 120 min. Rats received the same treatment on all four sessions, except the “Lesion - Veh (SKF Primed)” group, which received SKF on sessions 1–3 and Veh on session 4. Data points show total AIMs scores for individual rats, while the horizontal line indicates the median score. Two hours after session 4 injections, all rats were decapitated and M1 tissue was analyzed via real-time polymerase chain reaction. Hemispheres are denoted as either ipsilateral or contralateral (to lesion). Percent change in mRNA was normalized to control (“Lesion - Veh [contralateral]”). (B) c-Fos mRNA. (C) ARC mRNA. § p < .05 vs. Session 1; * p < .05 vs. Lesion - Veh (ipsilateral); + p < .05 vs. Lesion - Veh (contralateral); # p < .05 vs. own contralateral hemisphere.
Bilateral M1 tissue was collected on session 4, processed for real-time PCR and analyzed with a 2×3 mixed model ANOVA: Hemisphere (Ipsilateral vs. Contralateral [to Lesion]) * Group (Lesion - Veh, Lesion - Veh (SKF primed), Lesion - Chronic SKF). Omnibus ANOVA for β-actin revealed a main effect of hemisphere (F1,17 = 4.79, p = .043, ηp2 = .220), whereby the ipsilateral hemisphere averaged 16% less β-actin mRNA than the contralateral side. Given the large effect sizes for target genes in this cohort (Fig. 6B–C) and considering that target gene expression was adjusted to housekeeper gene expression, the small difference in housekeeper expression should not alter data interpretation.
c-Fos
Upon analysis of expression of the immediate-early gene c-Fos, there was a main effect of hemisphere (F1,17 = 18.10, p = .001, ηp2 = .516), group (F2,17 = 143.35, p < .001, ηp2 = .944) and a hemisphere*group interaction (F2,17 = 19.76, p < .001, ηp2 = .699). As shown in figure 6B, amongst animals that were given SKF on sessions 1–3, but given Veh prior to tissue harvest (“Lesion - Veh (SKF Primed)”), there were no significant differences in M1 c-Fos compared to animals given exclusively Veh treatments. Relative to SKF primed animals treated with Veh, SKF primed animals given SKF on session 4 (“Lesion - Chronic SKF”) showed increased c-Fos ipsilateral to lesion (t12 = 9.46, p < .001) and contralateral to lesion (t12 = 20.08, p < .001). However, a paired-samples t-test showed that chronic SKF increased c-Fos to a greater extent ipsilaterally than contralaterally (t6 = 4.66, p = .003). c-Fos expression was not significantly correlated with AIMs behavior (Fig. 7E–F).
ARC
Omnibus ANOVA examining ARC expression revealed an effect of group (F2,17 = 21.58, p < .001, ηp2 = .717), but no effect of hemisphere (F1,17 = 0.28, p = .606) or interaction (F2,17 = 1.62, p = .226). As with c-Fos, Veh-treated animals that were primed with SKF did not show increased ARC mRNA. Rats given chronic SKF showed increased ARC ipsilateral to lesion (t12 = 3.79, p = .003) and contralateral to lesion (t12 = 2.75, p = .018; Fig 6C). Unlike c-Fos, ARC induction by SKF was bilaterally equivalent. ARC expression was not significantly correlated with AIMs behavior.
Discussion
4.1 Summary of Findings
For the first time, functional and structural changes in M1 were characterized using the popular unilateral 6-OHDA rat model of PD. Behavioral aspects of the model were validated by showing that rats manifested a PD motor impairment and developed dyskinesia upon exposure to L-DOPA or the D1 agonist SKF81297 (Figs. 3A–B, 6A). 6-OHDA caused significant TH fiber loss in M1, but did so exclusively in the hemisphere ipsilateral to lesion (Fig. 2C–E). In lesioned animals, M1 tissue concentrations of DA and DOPAC were normal off-drug, but synthesis of DA from L-DOPA was reduced in M1 (Table 1). Among the genes examined in M1, expression was relatively normal after 6-OHDA lesion, but both L-DOPA and SKF caused abnormal bilateral activation of immediate-early genes (Figs. 4–6).
4.2 Lesion-induced Monoamine Perturbations
Using fiber-based stereology, we showed that the density of TH-positive fibers in M1 was reduced by 6-OHDA lesion (down 75% compared to contralateral side; Fig. 2CE). The same rats showed a 99% reduction in striatal TH (Fig. 2A–B), although the latter analysis had to be performed with densitometry, so the magnitude of the lesion may not be directly comparable.
Our data on TH fiber loss in M1 is the first to be performed with unbiased stereology (3-dimensional isotropy) and compares favorably with previous findings in PD animal models and patients. Using unilaterally 6-OHDA-lesioned rats, TH optical density in M1 was reduced by 93% when comparing the lesioned and intact hemispheres (Halje et al., 2012). Gaspar and colleagues (1991) examined post-mortem tissue from PD patients and healthy controls, finding reductions in M1 TH density from 24–74%, depending on the cortical layer (analyzed with hemicycloid overlays, achieving 2-dimensional isotropy). From a technical perspective, TH staining appears to be the most exhaustive method of identifying DA fibers in the frontal cortex since TH antisera in the frontal cortex preferentially labels DA fibers; however, TH labeling is not entirely specific since a small percentage of stained axons are likely to be NE fibers (Hokfelt et al., 1977; Lewis et al., 1987; Miner et al., 2003).
The present study is the first to examine M1 monoamines concentrations in the rat 6-OHDA model of PD, one of the most widely-used models of PD (Schober, 2004). In animals not exposed to L-DOPA, lesion with 6-OHDA did not affect DA or DOPAC tissue levels. Given that 6-OHDA lesion reduced M1 TH-positive fibers while DA concentrations remained normal, our data suggest that compensatory upregulation of DA synthesis occurred in remaining M1 DA fibers, as may be occurring in the DA-lesioned striatum (Song and Haber, 2000).
We also observed that lesion reduced M1 NE levels, regardless of whether the rat was given L-DOPA (Table 1). This finding is commensurate with the idea that 6-OHDA is a general catecholamine toxin since it principally enters cells through the DA and NE transporters (Schober, 2004). Injecting 6-OHDA into the MFB in rats has been shown to reduce striatal NE concentrations; however, TH cell counts in the locus coeruleus were not reduced, suggesting NE terminal loss in the absence of apoptosis (Barnum et al., 2012; Fulceri et al., 2007; Lindenbach et al., 2015). Given that we found significant loss of cortical NE after 6-OHDA in the current study, our data suggest that more rigorous investigations of locus coeruleus pathology may be important for understanding aspects of cortical dysfunction in PD and LID.
4.3 L-DOPA-induced Monoamine Perturbations
When M1 tissue was examined 60 min after treatment, L-DOPA increased M1 DA and DOPAC in all animals, but the increase was attenuated in 6-OHDA-lesioned animals relative to sham-lesioned animals (Table 1). Reduced M1 synthesis of DA from L-DOPA is commensurate with human imaging studies showing reduced 18F-DOPA uptake in M1 of PD patients relative to controls (Moore et al., 2008). Because there are fewer TH-positive fibers in M1, there are fewer axons with the capacity to synthesize DA from L-DOPA (this reaction requires the enzyme amino acid decarboxylase). Future studies should investigate if L-DOPA is increasing M1 extracellular DA release or simply increasing vesicular storage of DA, especially in sham animals, which have an intact DA system and did not exhibit LID behaviors.
We did not find any evidence to suggest that NE was being synthesized from LDOPA in M1, despite the obvious biochemical feasibility of such events. Quite the opposite, ANOVA analysis of NE levels showed a main effect of treatment with LDOPA reducing NE levels. This suggests that chronic L-DOPA (22 d total) negatively regulates NE activity in M1 in a manner at least partially independent of 6-OHDA lesion. This could be due to displacement of NE by L-DOPA-derived DA, given that DA is itself a precursor to NE (Rommelfanger and Weinshenker, 2007). Long-term alterations in cortical NE tone by chronic L-DOPA therapy could conceivably modify affective symptoms in PD patients, although a post-mortem study found prefrontal cortex NE levels were similar among PD patients regardless of whether they died ON or OFF LDOPA (Scatton et al., 1983).
4.4 L-DOPA-induced Gene Expression
We also examined the effect of lesion and L-DOPA on the transcription of genes involved in plasticity as well as glutamate and GABA signaling within M1. This was placed in the context of the behavioral phenotype of the model. We showed that lesioned rats displayed forelimb akinesia, a cardinal symptoms of PD, which was partially reversible by L-DOPA (Fig. 3A). The beneficial effects of L-DOPA on motor performance temporally coincided with the expression of LID behaviors (Fig. 3B). Likewise, the D1 agonist SKF81297 caused dyskinesia that was relatively equivalent to L-DOPA (Fig. 6A).
First, we examined the immediate-early gene c-Fos, which has rapid effects on gene transcription and is implicated in pathological drug-induced learning (Perez-Cadahia et al., 2011). Lesion alone did not affect c-Fos transcription, but acute or chronic L-DOPA increased c-Fos to statistically equivalent levels (Fig. 4A). Previous studies have shown that L-DOPA induces c-Fos coincident with LID behavior, but these studies were performed in rats with multiple L-DOPA exposures so it has remained as open question as to whether c-Fos is principally important for the development or for the expression of LID (Halje et al., 2012; Ostock et al., 2011). We found a large correlation (r2 = .820) between LID behavior and c-Fos induction, but only for rats given acute LDOPA and only in M1 ipsilateral to lesion (Fig. 7A–D). If c-Fos was principally associated with the expression of LID, we would expect c-Fos transcription to correlate with LID in rats given chronic L-DOPA (which it did not) and also that M1 c-Fos should be higher after chronic L-DOPA than after acute L-DOPA (which it was not). Therefore, our results suggest that M1 c-Fos predicts the development of LID and may be involved in behavioral sensitization to L-DOPA.
Given the purported role of D1 receptor overactivation during LID (Feyder et al., 2011), we also investigated M1 c-Fos after administration of a D1 agonist. Rats given three doses of SKF and then left untreated for 2 d (SKF Primed group) did not show c-Fos induction compared to drug-naïve animals (Fig. 6B). However, among rats given SKF on all four sessions (Chronic SKF group), c-Fos mRNA in M1 was increased 18 fold in the hemisphere ipsilateral to lesion compared to Veh-treated animals. The fact that c-Fos was not increased in animals primed with SKF, but given Veh on test day demonstrates that acute exposure to SKF—not SKF priming—is responsible for inducing c-Fos. The magnitude of the increase in c-Fos by D1 receptor stimulation appeared to be greater than that evoked by chronic L-DOPA (4 fold) despite a similar behavioral manifestation of dyskinesia. However, it is important to note that c-Fos was measured 65 min after L-DOPA, but 120 min after SKF (cf. behavior in Figs. 3B, 6A and mRNA in Figs. 4A, 6B) and that the onset of dyskinesia is slower with L-DOPA than SKF since catabolism into DA is required for psychoactivity of L-DOPA.
In addition to c-Fos, another immediate-early gene, ARC, was examined since ARC has been implicated in modulating synaptic activity and receptor trafficking whereas c-Fos is more of a transcription modifier (Bramham et al., 2008; Perez-Cadahia et al., 2011). Similar to c-Fos, ARC expression was increased by both L-DOPA and SKF (Fig 4B, 6C). However, unlike c-Fos, the increases in ARC were statistically equivalent bilaterally. This dissociation between c-Fos and ARC expression suggests that while LDOPA promotes bilateral M1 plasticity in unilaterally-lesioned rats, the changes occurring are not entirely equivalent between hemispheres. By extension, our data also provide further evidence that the type of cortical plasticity measured by c-Fos vs. ARC induction are at least partially independent. A recent study in 6-OHDA-lesioned rats found that L-DOPA (3 mg/kg) increased the number of M1 cells expressing ARC protein to a greater extent in rats that displayed LID vs. stable L-DOPA responders and preferentially increased ARC in the hemisphere ipsilateral vs. contralateral to lesion (Bastide et al., 2014). While the dose of L-DOPA, quantification method and dependent variable (mRNA vs. protein) differed between our respective studies, we note that in both experiments, there was mathematically more ARC in the ipsilateral vs. contralateral hemisphere.
Changes occurring in the hemisphere contralateral to lesion may be relevant for translational medicine, since PD patients manifest symptoms unilaterally during early disease-stages and contralateral hemisphere compensation may mask disease symptoms (Jankovic, 2008; Lieu and Subramanian, 2012). Indeed, there is evidence for bilateral changes in corticostriatal activity among unilaterally lesioned rats (Massie et al., 2010). The fact that L-DOPA and a D1 agonist increased c-Fos and ARC bilaterally in M1 while causing only unilateral dyskinesia suggests that some immediate-early gene activity in M1 may contribute to the pro-motor effects of DA replacement therapy.
There were few changes in the expression of genes involved in glutamate and GABA signaling (Fig. 5), which is surprising given widespread evidence for alterations in neuron firing patterns and receptor binding in the M1 glutamate and GABA system in PD and LID (Ahmed et al., 2011; Brazhnik et al., 2012; Halje et al., 2012; Watts and Mandir, 1992). Administration of acute L-DOPA to lesioned animals caused a small decrease in the expression of the NMDA NR2A subunit (Fig. 5A), a direction of effect that has been interpreted as signifying a reduction in long-term potentiation (Liu et al., 2004). PD patients typically show impaired M1 plasticity that is reversible by L-DOPA, but among patients with LID, long-term potentiation may remain deficient after L-DOPA is administered (Doyon, 2008; Morgante et al., 2006). The present data showing immediate-early gene induction by DA replacement are broadly suggestive of abnormal plasticity occurring in M1 (Figs. 4, 6), but these changes appear to be largely independent of gene expression for glutamate and GABA markers (Fig. 5).
4.5 Conclusions
This is the first study to characterize changes in M1 monoamine innervation and gene expression using the 6-OHDA rat model of PD and drug-induced dyskinesia. Results show that 6-OHDA pathologically reduces catecholamine fibers, although compensatory plasticity may be sufficient to maintain relatively normal DA levels even if NE levels are coincidentally suppressed. Administration of L-DOPA or a D1 receptor agonist strongly induced two immediate-early genes in M1 involved in plasticity, suggesting that M1 is hyperactive during dyskinesia and providing pre-clinical support for the notion that stimulation that reduced M1 excitability may reduce LID (see Wagle-Shukla et al., 2007). In sum, M1 of the 6-OHDA rat model of PD demonstrates face validity with M1 of human PD patients, bolstering its utility for studying M1 dysfunction in PD and LID. Exploring treatments that modulate M1 activity may elucidate novel therapeutic avenues for PD and LID.
Highlights.
The neurotoxin 6-hydroxydopamine reduced tyrosine hydroxylase fibers in the primary motor cortex
6-hydroxydopamine reduced basal norepinephrine and synthesis of L-DOPA-derived dopamine in the primary motor cortex
Either L-DOPA or a D1 agonist increased expression of the immediate-early genes c-Fos and ARC in the primary motor cortex
Acknowledgements
The authors wish to thank Jessica A. George and Dr. Karen L. Eskow Jaunarajs for assistance with chromatography and animal testing. Thanks also to Dr. Terrence Deak, Dr. Lisa M. Savage and Dr. Caryl E. Sortwell for providing critical commentary on the manuscript.
This work was supported by National Institutes of Health grants R01-NS059600 (CB) and F31-NS066684 (KBD) as well as the Center for Development and Behavioral Neuroscience at Binghamton University.
Abbreviations
- 5-HT
Serotonin
- 6-OHDA
6-hydroxydopamine
- AIMs
Abnormal involuntary movements
- ARC
Activity-regulated cytoskeletal-associated protein
- DA
Dopamine
- LID
L-DOPA-induced dyskinesia
- FAS
Forepaw adjusting steps
- GAD67
Glutamic acid decarboxylase 67 kDA
- HPLC
High-performance liquid chromatography
- M1
Primary motor cortex
- MFB
Medial forebrain bundle
- NE
Norepinephrine
- PCR
Polymerase chain reaction
- PD
Parkinson’s disease
- SKF
- TH
Tyrosine hydroxylase
- VEH
Vehicle
- VGLUT1
Vesicular glutamate transporter type I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing financial interests.
Author Contributions
Designed Research: DL, MMC, CYO, KBD, CB
Performed Research: DL, MMC, CYO, KBD
Analyzed Data: DL, MMC, CYO, KBD, CB
Wrote Manuscript: DL, CB
Provided Funding: KBD, CB
References
- Ahmed I, Bose SK, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ. Glutamate NMDA receptor dysregulation in parkinson's disease with dyskinesias. Brain. 2011;134:979–986. doi: 10.1093/brain/awr028. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Bhide N, Lindenbach D, Surrena MA, Goldenberg AA, Tignor S, Klioueva A, Walters H, Bishop C. Effects of noradrenergic denervation on L-DOPA-induced dyskinesia and its treatment by alpha- and beta-adrenergic receptor antagonists in hemiparkinsonian rats. Pharmacol Biochem Behav. 2012;100:607–615. doi: 10.1016/j.pbb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide MF, Dovero S, Charron G, Porras G, Gross CE, Fernagut PO, Bezard E. Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis. 2014;62:179–192. doi: 10.1016/j.nbd.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik E, Cruz AV, Avila I, Wahba MI, Novikov N, Ilieva NM, McCoy AJ, Gerber C, Walters JR. State-dependent spike and local field synchronization between motor cortex and substantia nigra in hemiparkinsonian rats. J Neurosci. 2012;32:7869–7880. doi: 10.1523/JNEUROSCI.0943-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Ghiglieri V, Mazzocchetti P, Corbelli I, Picconi B. Levodopa-induced plasticity: a double-edged sword in Parkinson's disease? Philos Trans B. 2015;370:20140184. doi: 10.1098/rstb.2014.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Giacomini P, Centonze D, Bernardi G. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol. 2000;47:S60–S68. [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE, Odin P. Current options and future possibilities for the treatment of dyskinesia and motor fluctuations in parkinson's disease. CNS Neurol Disord Drug Targets. 2011;10:670–684. doi: 10.2174/187152711797247885. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Debeir T, Ginestet L, Francois C, Laurens S, Martel JC, Chopin P, Marien M, Colpaert F, Raisman-Vozari R. Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol. 2005;193:444–454. doi: 10.1016/j.expneurol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Ostock CY, Eskow Jaunarajs KL, Button T, Savage LM, Wolf W, Bishop C. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre KB, Ostock CY, George JA, Eskow Jaunarajs KL, Hueston CM, Bishop C. Effects of 5-HT receptor stimulation on D1 receptor agonist-induced striatonigral activity and dyskinesia in hemiparkinsonian rats. ACS Chem Neurosci. 2013;4:747–760. doi: 10.1021/cn300234z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on parkinson motor function-Systematic review of controlled clinical trials. Mov Disord. 2009;24:357–363. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- Engeln M, De Deurwaerdere P, Li Q, Bezard E, Fernagut PO. Widespread monoaminergic dysregulation of both motor and non-motor circuits in parkinsonism and dyskinesia. Cereb Cortex. 2015;25:2783–2792. doi: 10.1093/cercor/bhu076. [DOI] [PubMed] [Google Scholar]
- Feyder M, Bonito-Oliva A, Fisone G. L-DOPA-induced dyskinesia and abnormal signaling in striatal medium spiny neurons: Focus on dopamine D1 receptormediated transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Fregni F, Simon DK, Wu A, Pascual-Leone A. Non-invasive brain stimulation for parkinson's disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry. 2005;76:1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F. Abnormal involuntary movements (AIMs) following pulsatile dopaminergic stimulation: Severe deterioration and morphological correlates following the loss of locus coeruleus neurons. Brain Res. 2007;1135:219–229. doi: 10.1016/j.brainres.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Duyckaerts C, Alvarez C, Javoy-Agid F, Berger B. Alterations of dopaminergic and noradrenergic innervations in motor cortex in parkinson's disease. Ann Neurol. 1991;30:365–374. doi: 10.1002/ana.410300308. [DOI] [PubMed] [Google Scholar]
- Halje P, Tamte M, Richter U, Mohammed M, Cenci MA, Petersson P. Levodopa-induced dyskinesia is strongly associated with resonant cortical oscillations. J Neurosci. 2012;32:16541–16551. doi: 10.1523/JNEUROSCI.3047-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol. 1977;55:21–40. [PubMed] [Google Scholar]
- Hosp JA, Luft AR. Dopaminergic meso-cortical projections to m1: role in motor learning and motor cortex plasticity. Front Neurol. 2013;4:145. doi: 10.3389/fneur.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiat. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’states in pyramidal neurons via D1 dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lieu CA, Subramanian T. The interhemispheric connections of the striatum: Implications for parkinson's disease and drug-induced dyskinesias. Brain Res Bull. 2012;87:1–9. doi: 10.1016/j.brainresbull.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach D, Bishop C. Critical involvement of the motor cortex in the pathophysiology and treatment of parkinson's disease. Neurosci Biobehav Rev. 2013;37:2737–2750. doi: 10.1016/j.neubiorev.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach D, Ostock CY, Eskow Jaunarajs KL, Dupre KB, Barnum CJ, Bhide N, Bishop C. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by β-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther. 2011;337:755–765. doi: 10.1124/jpet.111.179416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach D, Palumbo N, Ostock CY, Vilceus N, Conti MM, Bishop C. Side effect profile of 5-HT treatments for parkinson's disease and L-DOPA-induced dyskinesia in rats. Br J Pharmacol. 2015;172:119–130. doi: 10.1111/bph.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Massie A, Schallier A, Vermoesen K, Arckens L, Michotte Y. Biphasic and bilateral changes in striatal VGLUT1 and 2 protein expression in hemi-parkinson rats. Neurochem Int. 2010;57:111–118. doi: 10.1016/j.neuint.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J Comp Neurol. 2003;466:478–494. doi: 10.1002/cne.10898. [DOI] [PubMed] [Google Scholar]
- Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in parkinson's disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29:381–390. doi: 10.1016/j.nbd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in parkinson's disease and levodopa-induced dyskinesias. Brain. 2006;129:1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Mura A, Mintz M, Feldon J. Behavioral and anatomical effects of long-term L-dihydroxyphenylalanine (L-DOPA) administration in rats with unilateral lesions of the nigrostriatal system. Exp Neurol. 2002;177:252–264. doi: 10.1006/exnr.2002.7976. [DOI] [PubMed] [Google Scholar]
- Ostock CY, Dupre KB, Eskow Jaunarajs KL, Walters H, George J, Krolewski D, Walker PD, Bishop C. Role of the primary motor cortex in l-DOPA-induced dyskinesia and its modulation by 5-HT1A receptor stimulation. Neuropharmacology. 2011;61:753–760. doi: 10.1016/j.neuropharm.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Hyland BI. Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat. J Neurosci. 2005;25:5700–5709. doi: 10.1523/JNEUROSCI.0523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex. 2010;21:1372–1378. doi: 10.1093/cercor/bhq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The rat brain in stereotaxic coordinates. fourth ed. San Diego: Academic Press; 1998. [Google Scholar]
- Perez-Cadahia B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- Pifl C, Schingnitz G, Hornykiewicz O. Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience. 1991;44:591–605. doi: 10.1016/0306-4522(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Rommelfanger K, Weinshenker D. Norepinephrine: The redheaded stepchild of parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. PNAS. 2001a;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001b;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Valzania F, Nassetti SA, Tropeani A, Bisulli A, Santangelo M, Tassinari CA. Effects of chronic levodopa and pergolide treatment on cortical excitability in patients with parkinson's disease: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1198–1202. doi: 10.1016/s1388-2457(00)00316-3. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated parkinsonian monkeys. J Comp Neurol. 2011;519:989–1005. doi: 10.1002/cne.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle-Shukla A, Angel MJ, Zadikoff C, Enjati M, Gunraj C, Lang AE, Chen R. Low-frequency repetitive transcranial magnetic stimulation for treatment of levodopa-induced dyskinesias. Neurology. 2007;68:704–705. doi: 10.1212/01.wnl.0000256036.20927.a5. [DOI] [PubMed] [Google Scholar]
- Watts RL, Mandir AS. The role of motor cortex in the pathophysiology of voluntary movement deficits associated with parkinsonism. Neurol Clin. 1992;10:451–469. [PubMed] [Google Scholar]