Abstract
Objective
To examine associations of baseline insulin dynamics with changes in body composition and resting energy expenditure (REE) following weight loss.
Methods
Twenty-one participants with overweight or obesity achieved 10-15% weight loss and then received 3 weight-loss maintenance diets (high-carbohydrate, moderate-carbohydrate, low-carbohydrate) in random order, each for 4 weeks. Body composition was measured at baseline and after weight loss. Insulin 30 minutes after glucose consumption (insulin-30; insulin response), C-peptide deconvolution analysis, HOMA, hepatic insulin sensitivity (IS), and REE were assessed at baseline and after each maintenance diet.
Results
Insulin-30, but not maximal insulin secretion, hepatic IS or HOMA, predicted changes in fat mass (standardized β=0.385, 1.7 kg difference between 10th-90th centile of insulin-30, P=0.04) after weight loss. Insulin-30 (β=−0.341, −312 kcal/d, P=0.008), maximal insulin secretion (β=−0.216, −95 kcal/d, P=0.0002), HOMA (β=−0.394,−350 kcal/d, P=0.002) and hepatic IS (β=0.217, 225 kcal/d, P=0.0003) predicted change in REE during weight-loss maintenance, independent of changes in body composition. The inverse relationship between insulin-30 and REE was substantially attenuated when the low-carbohydrate diet was consumed first.
Conclusions
These findings distinguish a novel phenotype, characterized by high insulin response, at risk for weight regain, and identify a dietary approach to ameliorate this risk.
Keywords: Insulin, body composition and energy expenditure
Introduction
Individual physiological responses to weight loss may vary by phenotype, specifically by variations in insulin dynamics. Conventional measures of insulin sensitivity include the glucose and insulin response to oral glucose load and the homeostatic model assessment of insulin resistance (HOMA). While traditional models suggest that impaired insulin sensitivity leads to compensatory hyperinsulinemia, primary hypersecretion of insulin may also lead to development of obesity (1), insulin resistance (2) and type 2 diabetes (3).
The insulin concentration 30 minutes following oral glucose load (insulin-30) is a novel, reliable proxy measure of insulin secretion (4-6). In the Quebec Family Study, baseline insulin-30 strongly predicted changes in weight over a 6-year period, such that those with highest baseline insulin secretion gained the most weight (7). This effect was especially pronounced in the setting of a low-fat/high carbohydrate diet (7), which increases insulin secretion more than comparison diets controlled for total calories (8). In animal models, high insulin secretion is associated with increased weight gain when consuming a high, but not low, glycemic index diet (9). In human weight loss trials, subjects with high baseline insulin secretion lost more weight on a low-glycemic load diet (10,11).
As an anabolic hormone, insulin mediates post-prandial conversion of glucose and lipids into storage forms (12). Increased insulin action promotes body fat gain (13), as demonstrated by chronic insulin administration in animals (14), initiation of insulin treatment in type 2 diabetes (15) and excessive insulin treatment in type 1 diabetes (16) – an effect that appears to be at least partially independent of energy intake (17). Moreover, high endogenous insulin secretion arising from genetic variation (18), pancreatic tumor, hypothalamic damage, or other causes (19) is prospectively associated with weight gain, whereas drugs that inhibit insulin secretion attenuate weight gain (20). Indeed, among patients with type 2 diabetes receiving standard treatment, high insulin action may adversely affect energy expenditure (21).
Therefore, we aimed to examine the associations of baseline insulin dynamics with changes in body composition during weight loss, and changes in resting energy expenditure (REE) during weight loss maintenance. In addition, we hypothesized that dietary composition may be an effect modifier of these relationships.
Methods
Study design
Participants with overweight or obesity, and otherwise healthy, consumed a reduced-calorie Run In diet (60% of estimated caloric needs) designed to produce 10-15% weight loss over 12 weeks. Following a 4-week weight stabilization period, participants then received 3 weight-loss maintenance test diets in random order, each for a 4-week period. The composition of the diets were high-carbohydrate (60% of energy from carbohydrate, 20% from fat and 20% from protein), moderate-carbohydrate (40% from carbohydrate, 40% from fat and 20% from protein), and low-carbohydrate (10% from carbohydrate, 60% from fat, 30% from protein). The diets were isocaloric, and designed to maintain weight loss. Meals were prepared in the metabolic kitchen at the Brigham and Women’s Hospital, Boston, MA, and were consumed on site or packaged for consumption at home. Participants were admitted to a research unit for a 4- day hospitalization at baseline and at the end of each weight-loss maintenance diet period. The study protocol was approved by the Boston Children’s Hospital and Brigham and Women’s Hospital institutional review boards. Additional details of the protocol were previously presented (22).
Outcome measures
The independent variables of interest were measures of insulin dynamics, specifically insulin-30, insulin secretion by C-peptide deconvolution analysis, hepatic insulin sensitivity and HOMA insulin resistance index. Following an overnight fast, an oral glucose tolerance test (OGTT) was performed with 75 grams dextrose (Trutol, Thermo Fisher Scientific Inc., Waltham, MA) at baseline and the end of each weight-loss maintenance diet period, with blood sampling at −10, −5, 0, +10, +20, +30, +60, +90, and +120 minutes. Glucose concentrations were measured by enzymatic reference method (LabCorp, 001818), insulin concentrations were measured by chemiluminescent assay (Beckman Coulter, Chaska MN) and C-peptide concentrations were measured by competitive radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The insulin concentration at 30 minutes after glucose consumption was used as a measure of insulin response (insulin-30) (4-6). Basal, maximal and total insulin secretion were analyzed by deconvolution analysis of C-peptide concentrations in response to OGTT using ISEC “I(nsulin-)SEC(retion)” software, provided courtesy of Dr. Roman Hovorka (23). Hepatic insulin sensitivity was calculated as the inverse of the product of the glucose area under the curve (AUC) with insulin AUC during the first 30 minutes of OGTT, based on Abdul-Ghani et al (24). HOMA was calculated as the product of the fasting glucose and fasting insulin levels (25).
Body composition assessment was measured at baseline and the end of the 12-week weight loss run-in period by dual-energy x-ray absorptiometry (DXA, Discovery A, Hologic, Inc., Waltham, MA). REE and total energy expenditure (TEE) were measured prior to weight loss and end of each weight-loss maintenance diet period. REE was measured by indirect calorimetry (VMAX, Encore 29n, Viasys Healthcare, Inc., Yorba Linda, CA). TEE was measured under free-living conditions using doubly-labeled water methodology with stable isotope analysis performed at Baylor College of Medicine, Houston, TX.
Statistics
Relationships between baseline measures of insulin dynamics and study completion were evaluated by logistic regression. Baseline relationships between insulin dynamics and metabolic parameters were evaluated by Pearson correlation for continuous variables or t-test for dichotomous variables.
Relationships between baseline insulin dynamics and change in body composition parameters during the Run In diet were evaluated in two methods. The primary analysis was performed using multivariate linear regression, where the dependent variable was change in body composition and independent variable of interest was the specific measure of insulin dynamics with covariates of age, gender and baseline body mass index (BMI). The secondary analysis was based on a previous observation that fat loss can be predicted by initial fat mass and energy deficit (26). We constructed a model of post-weight loss fat mass with independent variables of pre-weight loss fat mass, change in total mass, age and gender and obtained the model residuals. We then evaluated the relationship between the model residuals and measures of insulin dynamics using linear regression.
Relationships between baseline insulin dynamics and REE were evaluated by linear repeated measures model with covariates of age, gender, baseline BMI, dietary composition, treatment order and order of measurement period. Within this model of REE, insulin-30 and treatment order were also tested for effect modification. Subsequent analyses included respective measures of change in body composition achieved during weight loss (e.g. change in lean mass) as covariates.
Regression coefficients were standardized for presentation. For a subset of outcomes, regression coefficients from this model were converted to differences in outcome measure (e.g. body composition or REE) between the 10th and 90th centiles of baseline insulin dynamics measures to provide a clinically relevant estimate of effect size within the observed range in our cohort The 10th centile of insulin-30 roughly coincided with reasonable approximation of normal insulin response (10). The effects of weight loss on measures of insulin dynamics were assessed using a pooled comparison of data from all three weight-loss maintenance diets to pre-weight loss levels.
Statistical significance was considered to be a 2-sided alpha value of P≤0.05. Results presented are mean ± standard error of the mean unless otherwise specified. Hepatic insulin sensitivity was log-transformed for analysis, and outliers for hepatic insulin sensitivity were excluded using an outlier-deletion algorithm as previously reported (22).
Results
Thirty-two participants entered the weight loss phase, 24 were randomized and 21 completed the protocol. Participant demographics, as previously reported (22), and baseline characteristics including biochemical parameters are presented in Tables 1 and 4. Mean change in BMI during each diet period is reported in the Supplemental Figure and did not materially differ between groups.
Table 1.
Patient demographics and baseline characteristics (N=21)
| Demographics | |
|
| |
| Age (y) | 30.3 ± 5.7 |
| Gender | |
| Male | 13 (62%) |
| Female | 8 (38%) |
| Race | |
| White | 4 (19%) |
| Black | 8 (38%) |
| Asian | 4 (19%) |
| Other | 5 (24%) |
| Hispanic Ethnicity | 4 (19%) |
|
| |
| Body composition | |
|
| |
| BMI (kg/m2) | 34.4 ± 4.9 |
| Total mass (kg) | 104.0 ± 18.5 |
| Lean mass (kg) | 66.4 ± 15.4 |
| Lean mass % of total | 63.6 ± 7.7 |
| Fat mass (kg) | 34.7 ± 9.0 |
| Fat mass % of total | 33.6 ± 7.9 |
| Trunk fat (kg) | 17.5 ± 4.8 |
|
| |
| Energy expenditure | |
|
| |
| Total energy expenditure (kcal/d) | 3248 ± 762 |
| Resting energy expenditure (kcal/d) | 1784 ± 376 |
Table 4.
Effects of weight loss and dietary composition on insulin secretion variables
| Baseline | High carbohydrate |
Moderate carbohydrate |
Low carbohydrate |
P-value for pooled weight loss effect |
P-value for diet effect |
|
|---|---|---|---|---|---|---|
| Insulin-30 (pmol/L) | 761 ± 122 | 553 ± 73 | 483 ± 60 | 470 ± 61 | 0.006 | 0.16 |
| Basal insulin secretion (pmol/kg/min) | 2.69 ± 0.19 | 2.15 ± 0.24 | 1.95 ± 0.12 | 1.85 ± 0.19 | <0.0001 | 0.02 |
| Maximal insulin secretion (pmol/kg/min) | 14.4 ± 1.54 | 14.1 ± 1.47 | 13.8 ± 0.88 | 13.0 ± 0.97 | 0.16 | 0.24 |
| Total insulin secretion (pmol/kg*2h) | 1271 ± 129 | 1049 ± 76 | 1140 ± 85 | 1106 ± 92 | 0.06 | 0.45 |
| Time of maximal insulin secretion (min) | 47 ± 6 | 46 ± 7 | 55 ± 7 | 66 ± 8 | 0.04 | 0.05 |
| Hepatic insulin sensitivitya | 0.72 ± 0.09 | 1.15 ± 0.16 | 1.31 ± 0.19 | 1.54 ± 0.24 | 0.004 | 0.03 |
| HOMA-IR | 2.57 ± 0.34 | 1.30 ± 0.10 | 1.16 ± 0.09 | 1.13 ± 0.12 | <0.0001 | 0.24 |
Mean ± SEM, adjusted for age, gender, baseline BMI, diet composition, time and diet sequence
Two values excluded from analysis. Units calculated per Abdul-Ghani et al. (24)
Participants who did and did not complete the study did not differ by baseline measures of insulin dynamics, including insulin-30 (mean±standard deviation 761±556 vs. 555±225 pmol/L, respectively, P=0.28), hepatic insulin sensitivity (0.82±0.57 vs. 0.90±0.47 unit, P=0.51) or HOMA (2.57±1.55 vs. 2.22±1.06, P=0.51).
Relationship of insulin dynamics with baseline body composition and REE
Baseline insulin-30 was positively associated with baseline BMI (r=0.47, P=0.03) and HOMA (r=0.46, P=0.03, adjusted for BMI P=0.43) and negatively associated with hepatic insulin sensitivity (r=−0.86, P<0.0001, adjusted for BMI P= 0.0002). Insulin-30 was not associated with baseline body composition parameters or REE (Table 2). Insulin-30 correlated strongly with parameters obtained from deconvolution analysis of C-peptide, including insulin secretion at 30 minutes (r=0.76, P=0.0003), maximal insulin secretion (r=0.66, P=0.003), basal insulin secretion (r=0.72, P=0.0008) and total insulin secretion (r=0.71, P=0.001).
Table 2.
Baseline relationships of insulin dynamics with demographics, body composition and energy expenditure
| Insulin-30 | Basal Insulin Secretion | Maximal Insulin Secretion | Total Insulin Secretion | Hepatic insulin sensitivity | HOMA-IR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | r | P-value | |
| Demographics | ||||||||||||
|
| ||||||||||||
| Age | −0.26 | 0.25 | −0.30 | 0.23 | −0.14 | 0.58 | −0.18 | 0.49 | 0.05 | 0.86 | −0.10 | 0.67 |
| Gender | - | 0.56 | - | 0.05 | - | 0.18 | - | 0.12 | - | 0.66 | - | 0.72 |
|
| ||||||||||||
| Body composition | ||||||||||||
|
| ||||||||||||
| BMI | 0.47 | 0.03 | 0.50 | 0.03 | 0.13 | 0.60 | 0.25 | 0.33 | −0.50 | 0.04 | 0.75 | <0.0001 |
| Total mass | 0.23 | 0.31 | 0.23 | 0.35 | −0.23 | 0.36 | −0.08 | 0.76 | −0.32 | 0.22 | 0.52 | 0.02 |
| Lean mass | 0.10 | 0.67 | 0.07 | 0.77 | −0.32 | 0.19 | −0.25 | 0.32 | −0.22 | 0.42 | 0.33 | 0.14 |
| % Lean mass | −0.14 | 0.54 | −0.23 | 0.35 | −0.31 | 0.21 | −0.35 | 0.17 | 0.08 | 0.76 | −0.14 | 0.54 |
| Fat mass | 0.31 | 0.17 | 0.36 | 0.14 | 0.10 | 0.70 | 0.25 | 0.33 | −0.29 | 0.28 | 0.50 | 0.02 |
| % Fat mass | 0.17 | 0.47 | 0.27 | 0.29 | 0.33 | 0.19 | 0.37 | 0.15 | −0.11 | 0.69 | 0.18 | 0.44 |
| Trunk fat | 0.26 | 0.25 | 0.41 | 0.09 | 0.02 | 0.93 | 0.20 | 0.43 | −0.30 | 0.26 | 0.56 | 0.008 |
|
| ||||||||||||
| Energy expenditure | ||||||||||||
|
| ||||||||||||
| Resting energy expenditure | 0.20 | 0.40 | 0.20 | 0.42 | −0.21 | 0.41 | −0.11 | 0.67 | −0.29 | 0.27 | 0.40 | 0.07 |
P-value for gender calculated by Student's t-test; remainder calculated by Pearson correlation
Baseline basal insulin secretion by C-peptide deconvolution analysis (r=0.40, P=0.03), baseline HOMA (r=0.75, P<0.0001) and hepatic insulin sensitivity (r=−0.50, P=0.04), but not maximal (r=0.13, P=0.60) or total (r=0.25, P=0.33) insulin secretion by deconvolution analysis, were significantly associated with baseline BMI. Baseline HOMA was positively associated with body composition parameters including total mass (r=0.52, P=0.02), fat mass (r=0.50, P=0.02) and trunk fat (r=0.56, P=0.008, Table 2). Baseline insulin measures did not predict time to achieve goal weight loss (data not shown).
Insulin dynamics and changes in body composition during weight loss maintenance
In the primary model, baseline insulin-30 was prospectively associated with changes in fat mass (standardized β=0.385, corresponding to 1.7 kg difference between 10th and 90th centile of insulin-30, P=0.04, Figure 1), trunk fat mass (standardized β=0.449, 10th-90th difference 1.6 kg, P=0.006), and % lean mass (standardized β=−0.434, 10th-90th difference −1.7%, P=0.05), after weight loss (Table 3). In contrast, insulin secretion as assessed by deconvolution analysis (basal, maximal and total), hepatic insulin sensitivity and HOMA were not associated with changes in body composition. In the secondary model of post-weight loss fat mass, adjusting for baseline fat mass, change in total mass, age and gender, results were qualitatively similar (insulin-30 standardized β=0.048, P=0.05; basal insulin secretion standardized β=0.013, P=0.66; maximal insulin secretion standardized β=−0.001, P=0.97; total insulin secretion standardized β=0.011, P=0.71).
Figure 1.
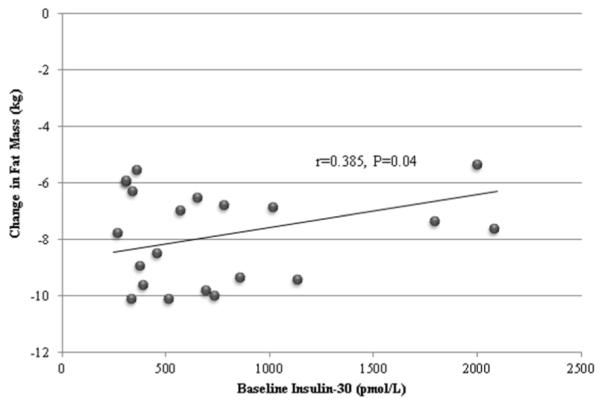
Relationship of baseline insulin-30 with change in fat mass during weight loss.
Table 3.
Relationship between baseline insulin-related measures and changes in body composition and resting energy expenditure (REE)
| Baseline insulin-30 | Baseline basal insulin secretion |
Baseline maximal insulin secretion |
Baseline total insulin secretion |
Baseline hepatic insulin sensitivity |
Baseline HOMA-IR | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Standardized β | P- value |
Standardized β |
P-value | Standardized β |
P- value |
Standardized β |
P-value | Standardized β |
P- value |
Standardized β | P- value |
|
| During weight loss | ||||||||||||
|
| ||||||||||||
| Δ Lean Massa | −0.375 | 0.17 | −0.305 | 0.27 | −0.265 | 0.29 | −0.358 | 0.17 | 0.477 | 0.13 | 0.152 | 0.68 |
| Δ % Leana | −0.434 | 0.05 | −0.299 | 0.23 | −0.067 | 0.77 | −0.206 | 0.41 | 0.351 | 0.53 | −0.084 | 0.78 |
| Δ Fat Massa | 0.385 | 0.04 | 0.137 | 0.53 | 0.023 | 0.91 | 0.051 | 0.81 | −0.100 | 0.71 | 0.197 | 0.45 |
| Δ % Fata | 0.430 | 0.05 | 0.303 | 0.22 | 0.067 | 0.77 | 0.207 | 0.40 | −0.172 | 0.57 | 0.118 | 0.70 |
| Δ T runk Fat Massa | 0.449 | 0.006 | 0.218 | 0.18 | 0.138 | 0.36 | 0.149 | 0.35 | −0.214 | 0.28 | 0.218 | 0.36 |
|
| ||||||||||||
| During weight loss maintenance | ||||||||||||
|
| ||||||||||||
| Δ REEb | −0.341 | 0.008c | −0.274 | <0.0001 c | −0.216 | 0.0002c | −0.212 | 0.002 c | 0.217 | 0.0003c | −0.394 | 0.002c |
Adjusted for age, gender and baseline BMI
Adjusted for age, gender, baseline BMI, diet composition, time and diet sequence
P≤0.05 after adjustment for body composition parameters achieved during weight loss (see Supplemental Table for details)
Insulin dynamics and changes in REE during weight loss maintenance
Higher baseline insulin-30 predicted lower REE during weight loss maintenance (standardized β=−0.341, −312 kcal/d difference between 10th and 90th centile of insulin-30, P=0.008, Table 3). This relationship remained significant after adjustment for changes in body composition during the Run In diet (Supplemental Table). Notably, diet sequence order was a strong effect modifier of the relationship between insulin-30 and REE (standardized β=−1.104, 10th-90th difference −991 kcal/d when starting with high-carbohydrate diet, standardized β=−0.883, 10th-90th difference −793 kcal/d when starting with moderate-carbohydrate diet, and standardized β=−0.269, 10th-90th difference −241 kcal/d when starting with low-carbohydrate diet, P=0.005 for effect modification, Figure 2). That is, consuming the low-carbohydrate diet first strongly attenuated the relationship between insulin-30 and REE.
Figure 2.
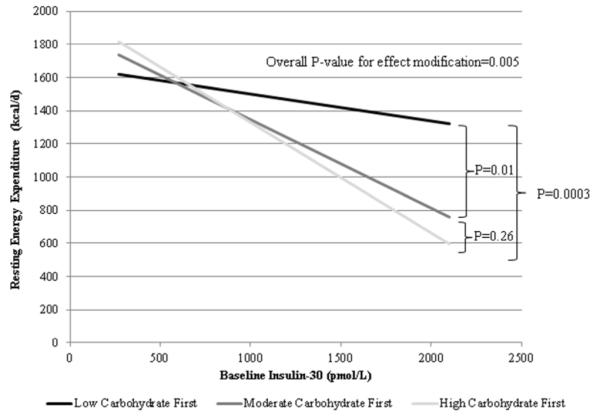
Effect modification by diet of the relationship between baseline insulin-30 and REE during weight loss maintenance. This schematic representation is stratified by first diet in sequence order to demonstrate effect modification.
Higher basal (standardized β=−0.274, 10th-90th difference −133 kcal/d, P<0.0001), maximal (standardized β=−0.216, 10th-90th difference −95 kcal/d, P=0.0002) and total (standardized β=−0.212, 10th-90th difference −116 kcal/d, P=0.002) insulin secretion from deconvolution analysis predicted lower REE during weight loss maintenance (Table 3). These relationships remained significant after adjustment for changes in body composition during Run In diet (Supplemental Table).
Higher HOMA predicted lower REE during weight-loss maintenance (standardized β=−0.394, −350 kcal/d difference between 10th and 90th centile of HOMA, P=0.002) (Table 3). Race modified the relationship between HOMA and REE such that the relationship was strongest in whites (standardized β=−0.532), intermediate in blacks (standardized β=−0.334) and weakest in Asians (standardized β=0.131).
Lower hepatic insulin sensitivity also predicted lower REE during weight-loss maintenance (standardized β=0.217, 225 kcal/d difference between 10th and 90th centile of hepatic insulin sensitivity, P=0.0003) (Table 3). Results were similar with all subjects included in this analysis.
Effects of weight loss on insulin dynamics
Insulin-30 decreased significantly in response to weight loss (P=0.006 for pooled response), without specific effect of dietary composition (baseline 761±122 pmol/L, high-carbohydrate 553±73 pmol/L, moderate-carbohydrate 483±60 pmol/L and low-carbohydrate 470±61 pmol/L, P=0.16 for diet effect, Table 4). In addition, baseline insulin-30 (β =−0.550, P=0.0003) and change in lean mass (β =0.059 pmol/L per g, P=0.05) were independent predictors of change in insulin-30 as an outcome measure, adjusting for age, gender, baseline BMI, diet and treatment order.
Basal insulin secretion, as assessed from C-peptide deconvolution analysis, decreased in response to weight loss (P<0.0001 for pooled response), with differential effect of diet (baseline 2.69±0.19 pmol/kg/min, high-carbohydrate 2.15±0.24 pmol/kg/min, moderate-carbohydrate 1.95±0.12 pmol/kg/min, low-carbohydrate 1.85±0.19 pmol/kg/min, P=0.02, Table 4). Time to maximal insulin secretion after oral glucose varied significantly by diet (baseline 47±6 minutes, high-carbohydrate 46±7 min, moderate-carbohydrate 55±7 min and low-carbohydrate 66±8 min, P=0.04, Table 4).
HOMA improved significantly with weight loss (P<0.0001), without effect of dietary composition (baseline 2.57±0.34, high-carbohydrate 1.30±0.10, moderate-carbohydrate 1.16±0.09, low-carbohydrate 1.13±0.12, P=0.24, Table 4). Hepatic insulin sensitivity improved with weight loss (P=0.004), with differential effect by diet (baseline 0.72±0.09 units, high-carbohydrate 1.15±0.16 units, moderate-carbohydrate 1.31±0.19, low-carbohydrate 1.54±0.24, P=0.03, Table 4).
Conclusion
In this study, we observed that high insulin response at baseline – as assessed by the novel measure insulin-30 – predicts adverse changes in body composition after weight loss in the setting of a rigorously controlled feeding study. At the same amount of weight loss, individuals with high insulin response lose relatively more lean mass and less fat mass than those with low insulin response. This novel result extends prior research in which insulin secretion was shown to modify the effect of dietary composition on total weight loss in ad libitum settings (7,10). While previous data suggest that post-weight-loss insulin sensitivity is associated with greater increases in fat mass (27), our data indicate that pre-weight-loss measures of insulin action (hepatic insulin sensitivity and HOMA) did not predict changes in body composition – underscoring the potential significance of baseline insulin response in body weight regulation. In the pre-weight loss state, insulin response is positively associated with BMI, but not with other body composition parameters. This observation implies that the effects of insulin response on body composition are unmasked or exacerbated by the physiological stress of weight loss. Of note, conventional proxy measures of insulin secretion (C-peptide deconvolution analysis) did not predict changes in body composition
Baseline insulin dynamics, using both novel and conventional measures, were strong, negative predictors of weight-loss induced declines in REE, such that participants with higher insulin response, insulin secretion by deconvolution analysis, and insulin resistance had the largest declines. This relationship persisted despite adjustment for body composition changes and dietary macronutrient content. The mean difference in REE between the lowest and highest centiles of insulin response was approximately 310 kcal/day – a potentially major metabolic effect. By comparison, the difference in TEE when participants consumed the low-carbohydrate diet vs. high-carbohydrate diet (as previously reported) was approximately 325 kcal/day (22).
These adverse effects of insulin response on body composition and resting energy expenditure have potential implications to weight loss maintenance and risk of weight regain. Insulin directs glucose and lipids to storage forms, thereby decreasing the availability of these metabolic fuels in the blood. Especially during negative energy balance, high insulin secretion would tend to restrain release of lipids from adipose tissue, thereby sequestering the primary metabolic fuel in the fasting state. Consequently, the body would necessarily rely to a greater degree on alternative fuels, including amino acids from muscle, to fuel gluconeogenesis. Over time, a shift in substrate utilization in this direction could explain the relatively smaller loss of fat tissue and greater loss in lean tissue observed after weight loss among individuals with high insulin secretion. Reduced availability of circulating metabolic fuels could also explain the lower energy expenditure associated with this phenotype, as previously hypothesized (12).
In addition, we found that the relationship between insulin response and REE was modified by dietary composition during weight loss maintenance. The inverse association between baseline insulin response and change in REE was almost fully attenuated when the low-carbohydrate diet was consumed first. The 20 minute delay in time to peak insulin secretion during OGTT on the low vs. high carbohydrate diet suggests a relevant mechanism. Following a period of diminished stimulation from a low carbohydrate diet, the pancreatic beta-cell becomes less reactive to high glycemic index carbohydrate, and this change persists for at least 1 month after carbohydrate intake is increased. In effect, the relationship between dietary intake and early phase insulin response may be “reset” – at least for a time – after consumption of a low carbohydrate diet. Thus, while major contributors to insulin secretion status are non-modifiable genetics, early life influences), a specific change in dietary composition may confer protection for individuals with high insulin response from the adverse metabolic effects of this phenotype, although the duration of this effect is unknown.
A limitation of this study is small sample size, especially for subgroup analyses, which is likely reflected in the borderline P-values for a subset of our analyses. Insulin dynamics were not associated with study completion, but the 13% post-randomization attrition rate may impact the generalizability of the study. In addition, we employed indirect measures of insulin dynamics, most notably insulin-30. We do not know why insulin-30 better predicted change in body composition then deconvolution analysis. Insulin-30 may be a good proxy for early-phase events related to beta-cell function. However, unlike C-peptide deconvolution analysis, insulin-30 does not address the dynamic balance of insulin release and clearance, and does not capture the late post-prandial response. This discrepancy comprises an important interpretive limitation of the study that warrants exploration in future research. At the same time, insulin-30 is simpler to calculate (without need for a computer program) – an advantage for translation to the clinical setting. In addition, these associations, though prospective in nature, can only suggest causal mechanisms and do not provide proof of causal direction. While we do not have a direct measure of dietary compliance, we did observe a clear differentiation between the diets in other metabolic parameters (e.g. HDL, triglycerides and respiratory quotient) to suggest excellent adherence. Strengths of these analyses, based on the parent study, include a cross-over design to minimize confounding by inter-individual differences, the use of accurate and precise outcome measures, and the ability to conduct diet-phenotype assessment based on important physiological measures.
In conclusion, this study suggests that novel and conventional measures of insulin dynamics influence the metabolic and body composition responses to weight loss, highlighting a phenotype at risk for weight regain. Additional research is needed to confirm these preliminary findings, explore dietary interventions, such as carbohydrate restriction or staged dietary approaches, that may attenuate the adverse effects of high insulin response and improve long-term weight loss maintenance among individuals with this phenotype.
Supplementary Material
What is already known about this subject?
Individual responses to weight loss may vary by phenotype, conceptually providing an opportunity for individualized obesity treatment.
High insulin secretion is associated with excessive weight gain, especially among individuals consuming a high-carbohydrate/low-fat diet.
What does this study add?
We demonstrate that individuals with high insulin response experience changes in body composition and resting energy expenditure during weight loss maintenance that would predispose to weight regain.
The inverse relationship between insulin response and energy expenditure during weight loss maintenance was strongly attenuated by consumption of a low-carbohydrate diet.
This study highlights a novel obesity-related phenotype that may respond especially well to carbohydrate restriction.
Acknowledgments
The authors thank the study participants and nursing staff at the BCH and BWH Clinical and Translational Studies Units.
Funding: R01DK072428 and K24DK082730 from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland; M01RR02172 from the National Center for Research Resources, National Institutes of Health, to the Boston Children’s Hospital General Clinical Research Center; M01RR02635 from the National Center for Research Resources, National Institutes of Health, to the Brigham and Women’s Hospital General Clinical Research Center; UL1RR02575801 from the National Center for Research Resources, National Institutes of Health, to the Harvard Catalyst Clinical and Translational Science Center; New Balance Foundation; T32 DK007477-31 from the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland; and F32HL122080-01 from National Heart, Lung and Blood Institute
Abbreviations
- TEE
Total energy expenditure
- REE
resting energy expenditure
- IS
insulin sensitivity
- insulin-30
insulin concentration at 30 minutes
- BMI
body mass index
- DXA
dual-energy x-ray absorptiometry
Footnotes
Disclosure: DSL received royalties from books about nutrition and obesity. The other authors have no competing interests.
Author contributions: CBE, HAF and DSL designed research; CBE and DSL conducted research; BMH and HAF analyzed data; BMH and DSL wrote the paper; DSL had primary responsibility for final content. All authors read and approved the final manuscript.
Clinical trial registration: NCT00315354
Contributor Information
Bridget M. Hron, Division of Gastroenterology, Hepatology and Nutrition, Boston Children’s Hospital, Boston, MA New Balance Foundation Obesity Prevention Center, Boston Children’s Hospital, Boston, MA.
Cara B. Ebbeling, Division of Endocrinology, Boston Children’s Hospital, Boston, MA New Balance Foundation Obesity Prevention Center, Boston Children’s Hospital, Boston, MA.
Henry A. Feldman, Clinical Research Center, Boston Children’s Hospital, Boston, MA
David S. Ludwig, Division of Endocrinology, Boston Children’s Hospital, Boston, MA New Balance Foundation Obesity Prevention Center, Boston Children’s Hospital, Boston, MA.
References
- 1.Mehran AE, Templeman NM, Brigidi GS, et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012;16:723–37. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37:1025–35. doi: 10.1007/BF00400466. [DOI] [PubMed] [Google Scholar]
- 3.Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE. A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes. 2000;49:2094–101. doi: 10.2337/diabetes.49.12.2094. [DOI] [PubMed] [Google Scholar]
- 4.Chiu KC, Martinez DS, Yoon C, Chuang LM. Relative contribution of insulin sensitivity and beta-cell function to plasma glucose and insulin concentrations during the oral glucose tolerance test. Metabolism. 2002;51:115–20. doi: 10.1053/meta.2002.29027. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki T, Miyake Y, Hagura R, et al. Risk factors for worsening to diabetes in subjects with impaired glucose tolerance. Diabetologia. 1984;26:44–9. doi: 10.1007/BF00252262. [DOI] [PubMed] [Google Scholar]
- 6.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–92. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 7.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87:303–9. doi: 10.1093/ajcn/87.2.303. [DOI] [PubMed] [Google Scholar]
- 8.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728–32. doi: 10.1093/jn/133.9.2728. [DOI] [PubMed] [Google Scholar]
- 9.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364:778–85. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- 10.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 11.Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care. 2005;28:2939–41. doi: 10.2337/diacare.28.12.2939. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311:2167–8. doi: 10.1001/jama.2014.4133. [DOI] [PubMed] [Google Scholar]
- 13.Stolz DJ, Martin RJ. Role of insulin in food intake, weight gain and lipid deposition in the Zucker obese rat. J Nutr. 1982;112:997–1002. doi: 10.1093/jn/112.5.997. [DOI] [PubMed] [Google Scholar]
- 14.Cusin I, Rohner-Jeanrenaud F, Terrettaz J, Jeanrenaud B. Hyperinsulinemia and its impact on obesity and insulin resistance. Int J Obes Relat Metab Disord. 1992;16(Suppl 4):S1–11. [PubMed] [Google Scholar]
- 15.Sinha A, Formica C, Tsalamandris C, et al. Effects of insulin on body composition in patients with insulin-dependent and non-insulin-dependent diabetes. Diabet Med. 1996;13:40–6. doi: 10.1002/(SICI)1096-9136(199601)13:1<40::AID-DIA991>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 16.Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care. 2001;24:1711–21. doi: 10.2337/diacare.24.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torbay N, Bracco EF, Geliebter A, Stewart IM, Hashim SA. Insulin increases body fat despite control of food intake and physical activity. Am J Physiol. 1985;248:R120–4. doi: 10.1152/ajpregu.1985.248.1.R120. [DOI] [PubMed] [Google Scholar]
- 18.Le Stunff C, Fallin D, Schork NJ, Bougneres P. The insulin gene VNTR is associated with fasting insulin levels and development of juvenile obesity. Nat Genet. 2000;26:444–6. doi: 10.1038/82579. [DOI] [PubMed] [Google Scholar]
- 19.Sigal RJ, El-Hashimy M, Martin BC, Soeldner JS, Krolewski AS, Warram JH. Acute postchallenge hyperinsulinemia predicts weight gain: a prospective study. Diabetes. 1997;46:1025–9. doi: 10.2337/diab.46.6.1025. [DOI] [PubMed] [Google Scholar]
- 20.Lustig RH, Greenway F, Velasquez-Mieyer P, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-finding trial of a long-acting formulation of octreotide in promoting weight loss in obese adults with insulin hypersecretion. Int J Obes (Lond) 2006;30:331–41. doi: 10.1038/sj.ijo.0803074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda K, Fujimoto S, Goto M, et al. Impact of endogenous and exogenous insulin on basal energy expenditure in patients with type 2 diabetes under standard treatment. Am J Clin Nutr. 2011;94:1513–8. doi: 10.3945/ajcn.111.017889. [DOI] [PubMed] [Google Scholar]
- 22.Ebbeling CB, Swain JF, Feldman HA, et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–34. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50:253–64. doi: 10.1016/0169-2607(96)01755-5. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Hall KD. Predicting metabolic adaptation, body weight change, and energy intake in humans. Am J Physiol Endocrinol Metab. 2010;298:E449–66. doi: 10.1152/ajpendo.00559.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gower BA, Hunter GR, Chandler-Laney PC, Alvarez JA, Bush NC. Glucose metabolism and diet predict changes in adiposity and fat distribution in weight-reduced women. Obesity (Silver Spring) 2010;18:1532–7. doi: 10.1038/oby.2009.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


