Abstract
Background
Ketamine, an N-Methyl-D-aspartate receptor antagonist, is used as a pediatric anesthetic because of its favorable safety profile. It is also being investigated as an antidepressant. Unfortunately, ketamine causes adverse reactions including hallucinations and is associated with a high prevalence of abuse among adolescents. Although chronic ketamine use has been shown to produce cognitive impairments even years following cessation, little is known about its long-term consequences on adolescents. The beta-lactam ceftriaxone has been shown to attenuate alcohol withdrawal, and alleviate early brain injury and memory impairments following subarachnoid hemorrhage. However, its ability to reverse the effects of adolescent ketamine exposure is not known. Previous data indicate that ketamine causes a reduction in the number of Excitatory Amino Acid Transporter Type 2 (EAAT2)-containing astrocytes. Additionally, the beta lactam antibiotic ceftriaxone increased expression of EAAT2. As EAAT2 is a principal mechanism of glutamate clearance from the synapse, the current study tests the hypothesis that ceftriaxone may reverse functional consequences of ketamine exposure.
Methods
We examined the effects of chronic ketamine in juvenile mice as well as reversal by ceftriaxone using electroencephalography (EEG). Subsequently, we assessed the effects of these treatments on markers of astrocyte proliferation, using Glial Fibrillary Acidic Protein (GFAP), and function, as evidenced by EAAT2.
Results
Juvenile mice exposed to chronic ketamine showed lasting alterations in EEG measurements as well as markers of astrocyte number and function. These alterations were reversed by ceftriaxone.
Conclusions
Data suggest that ceftriaxone may be able to ameliorate ketamine-induced long-term disturbances in adolescent brains.
Keywords: ketamine, mouse, EEG, glia, EAAT2
1. INTRODUCTION
Ketamine, a non-competitive antagonist at the N-Methyl-D-aspartate (NMDA) receptor, was first developed for its anesthetic and analgesic properties. Despite serious emergence reactions including hallucinations and vivid dreams, the drug is still widely used as an anesthetic in pediatric and veterinary medicine because of its favorable safety profile (Reich and Silvay, 1989). In addition, it is currently being investigated for its anti-depressant properties (Berman et al., 2000; Maeng et al., 2008). Unfortunately, ketamine is also associated with a high prevalence of abuse, particularly among adolescents. It is most commonly abused as a “club drug” because of its powerful hallucinogenic effects and ability to produce amplified sensations and an escape from reality (Abanades et al., 2004). A 2010 teen survey suggested that 1% of 8th graders and approximately 2% of 12th graders in the United States used ketamine within the previous year (NIDA Monitoring the Future, 2011). The drug is even more widely abused in Southeast Asian countries. In fact, it is overtaking 3,4-methylenedioxy-methamphetamine (MDMA) as the most popular drug of abuse among school-aged adolescents in Taiwan (Chen et al., 2009). Therefore, the drug’s widespread abuse presents a significant public health issue that must be addressed.
Chronic use of ketamine has been shown to produce significant cognitive impairment, however relatively little is known about its long-term consequences on adolescents. Frequent adult users show impairment of spatial working memory and pattern recognition memory tasks as well as worse psychological wellbeing and increased rates of depression (Morgan et al., 2010). Interestingly, chronic users show persistence of impairments in areas such as episodic memory and attentional functioning even 3–4 years following significant reduction or cessation of use (Morgan et al., 2004). Studies point to the adolescent period as a time of increased vulnerability to drug-induced cognitive impairment or psychotic-like symptoms in adulthood (Pope et al., 2003). However, the few studies conducted in adolescent animals to date have suggested fewer acute deficits after ketamine administration relative to adult animals (Wiley et al., 2008). Interestingly, recent work in mice suggests that when exposure occurs during early developmental time points, i.e. 4–6 weeks old, analogous to human adolescence, deficits do emerge in these animals during adulthood (12 weeks old; Featherstone et al., 2014). This suggests that adolescent ketamine abuse may have significant lasting negative effects despite an apparent lack of impairments during acute exposure. As such, there may be a window of opportunity to intervene prior to the onset of disability. However, there are currently no interventions available to individuals seeking treatment for these effects after people have stopped using.
A large body of literature indicate that the beta lactam antibiotic ceftriaxone increases expression of EAAT2 across multiple disease states and models including Amyotrophic Lateral Sclerosis (ALS), Huntington’s disease, brain injury, subarachnoid hemorrhage, ischemia, and neuropathic pain, as well as following cocaine or ethanol exposure (Abulseoud et al., 2014, 2012; Amin et al., 2014, 2012; Feng et al., 2014; Jelenkovic et al., 2008; Ji et al., 2005; Kim and Jones, 2013; Lai et al., 2011; Mao, 2005; Miller et al., 2012, 2008; Nizzardo et al., 2011; Rothstein et al., 2005; Sari et al., 2011, 2009; Thone-Reineke et al., 2008). These disease states are all characterized by excess glutamate, which is also seen after ketamine administration (Anand et al., 2000). Because EAAT2 (a.k.a. GLT1 in rodents) is a principal mechanism of glutamate clearance from the synapse, the current study tests the hypothesis that ceftriaxone may reverse the functional consequences of ketamine exposure (Halassa et al., 2007). Specifically, one proposed mechanism for the hyperglutamatergic tone following ketamine treatment is that the chronic chemical insult leaves the brain’s astrocytes with a decreased ability to take up excess of the neurotransmitter. Supporting this theory, mice treated with chronic ketamine show decreased hippocampal expression of the glial specific glutamate transporter Excitatory Amino Acid Transporter type 2 (EAAT2) alongside alterations in EEG measures and memory (Featherstone et al., 2012). One proposed mechanism for the down-regulation of EAAT2 is as follows. Ketamine is known to block NMDA receptors via pore blockade, which then leads to a reduction of PP2A and PI3K-mediated AKT phosphorylation. This in turn would reduce NF-kB phosphorylation/activation, ultimately reducing EAAT2 transcription (Li et al., 2006). In addition to changes in EAAT2, chronic ketamine treated mice have shown an increased number of Glial Fibrillary Acidic Protein (GFAP)-positive astrocytes 6 months after ketamine cessation (Featherstone et al., 2012). Similarly, abuse of other illicit drugs such as cocaine, methamphetamine and opiates can also lead to astrogliogenesis, suggesting that this change may be a common response following chronic exposure to drugs of abuse (Bowers and Kalivas, 2003; Narita et al., 2006, 2005). The current study assesses molecular and functional changes in brain following ketamine administration alone or when followed by ceftriaxone. Measures include both protein expression levels for EAAT2 and GFAP as well as changes in EEG activity. A large body of literature indicates that there are highly consistent changes in EEG following manipulations of NMDA receptor-mediated glutamate neurotransmission via pharmacological exposure to ketamine, MK801 and/or PCP in humans, non-human primates and rodents as well as genetic manipulations of NMDA receptors in mice (Amann et al., 2009; Ehrlichman et al., 2008; Featherstone et al., 2012, 2014; Gil-da-Costa et al., 2013; Gunduz-Bruce et al., 2012; Mathalon et al., 2014; Maxwell et al., 2006; Oranje et al., 2000; Schall et al., 2015; Siegel et al., 2003; Umbricht et al., 2002, 2000; Billingslea et al., 2014; Gandal et al., 2012; Lazarewicz et al., 2010; Saunders et al., 2012a, 2012b; Tatard-Leitman et al., 2015). Furthermore, EEG measures are particularly useful for characterizing drug-induced changes in brain function as they have been well characterized following ketamine administration and are relatively unaffected by confounding concurrent changes in motor or non-auditory sensory development (Amann et al., 2009; Featherstone et al., 2012; Maxwell et al., 2006; Siegel et al., 2003; Uhlhaas and Singer, 2011).
Hypotheses
Consistent with previous studies in adults, we anticipated that chronic ketamine exposure during the juvenile period would result in a lasting decrement in theta power without concomitant changes in gamma frequencies (Featherstone et al., 2014).
Consistent with previous studies in adults, we anticipated that chronic ketamine exposure would cause a lasting increase in GFAP and a reduction in the astrocyte selective EAAT2 (Featherstone et al., 2012).
We proposed that ceftriaxone would reverse ketamine-induced alterations in GFAP and EAAT2 as well as corresponding alterations in EEG measures.
2. METHODS
2.1 Subjects
Thirty-two male C57BL/6Hsd (B6) mice were obtained at 3 weeks of age from Jackson Labs (Bar Harbor, ME, USA). Mice were housed four to a cage until implantation of electrodes, following which they were singly housed until completion of the study. All mice were kept in a vivarium on a 12 hour light/dark cycle (lights on at 8:00 AM) with controlled temperature and (22±1°C). Water and standard mouse lab chow were available to all animals ad libitum. Experiments were performed during the light phase between 9:00 AM and 4:00 PM. All protocols were conducted in accordance with University Laboratory Animal Resources (ULAR) guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
2.2 Injections
Intraperitoneal injections of 20 mg/kg ketamine or 0.9% normal saline vehicle were given daily for 14 days from weeks 3–5 of age. Intraperitoneal injections of 200 mg/kg ceftriaxone or 0.9% normal saline followed for an additional 14 days from weeks 5–7 of age. All injections were administered in a 10 ml/kg volume. The result was four groups of 8 subjects each: ketamine/saline, ketamine/ceftriaxone, saline/saline and saline/ceftriaxone.
2.3 Electrode Implantation
Stereotaxic implantation of electrodes for EEG analysis was done following the last injection, at 7 weeks of age. Animals were anesthetized under isoflurane anesthesia. Three holes were then drilled into the skull at −1.8, −0.8 and +0.2 mm AP, 2.65 mm lateral, and 2.75 mm deep relative to bregma. A three channel recording electrode (Plastics One, Roanoke, VA) was then lowered into the hippocampal region of the brain. This approach allows for integration across electrodes, yielding a single differential recording that is sensitive to vectors generated across the entire auditory pathway (for review please see (Jutzeler et al., 2011)). Ethyl cyanoacrylate (Loctite, Henkel KGaA, Duesseldorf, Germany) and dental cement (Ortho Jet, Lang Dental, Wheeling IL, USA) were used to secure the electrodes to the skull. Animals were given a one-week recovery period before EEG testing.
2.4 EEG Recording and Analysis
EEG recording took place at 8 weeks of age. Animals with broken or loose head caps were excluded yielding a final test number of 8/8/6/6 for ketamine/saline, ketamine/ceftriaxone, saline/saline and saline/ceftriaxone. Each mouse was placed into a cage fitted with an individual auditory speaker that was then placed inside a Faraday cage. After a fifteen-minute acclimation period auditory stimuli were generated by Micro1401 hardware and Spike2, version 6.0 software (Cambridge Electronic Design, Cambridge, UK). ERPs were recorded during a single click paradigm with presentations of a 9 KHz tone (10 ms, 85 dB) at an 8 second inter-stimulus interval. A total of 300 clicks were delivered.
ERPs were analyzed using Spike 2 software (CED, Cambridge, UK). Event related potential (ERP) amplitude was calculated as the change in amplitude relative to the previous point of inflection. The P20 was defined as the maximum value between 15 and 35 ms, the N40 was the minimum value between 25 and 60 ms, and the P80 as the maximum value between 50 and 200 ms. Latency for each component was calculated as the time at which the maximum or minimum deflection occurred within each time interval.
2.4.1 Baseline Power
Raw EEG was recorded for a 60 sec period prior to the start of auditory stimuli. The fast Fourier transformation function native to Spike2 was used to decompose power into 0.81 Hz bins (Hanning window). Absolute Gamma was quantified as the average of EEG power between 30 and 80 Hz. Absolute theta was quantified as average of EEG power 4 to 12 Hz.
2.4.2 Event-related Power
Data were processed using EEGLAB (Schwartz Center for Computational Neuroscience) to create a time-frequency measure for power. Three hundred single-trial epochs, ranging from −1 to 2 sec relative to click onset, were extracted from the continuous EEG and analyzed further. Power was calculated using Morlet wavelets in 116 logarithmically spaced frequency bins between 4 and 120 Hz, with wavelet cycle numbers ranging from 2 to 10 (Delorme and Makeig 2004). Power was expressed in decibels (dB) as logμv10. The frequency band between 4 and 12 Hz was defined as theta, 30 to 80 Hz was defined as gamma, and 80 to 120 Hz as high gamma. Theta was quantified as the average power between 0 and 200 ms, gamma and high gamma power were the average from 0 to 60 ms.
2.5 Western Blots
Mice were sacrificed at 20 weeks of age and their hippocampi were removed and immediately frozen on dry ice before being stored at −80°C. Hippocampal tissue was homogenized using 10–15 strokes of a micro tissue grinder in 100μL of TES Buffer (Tris EDTA Sucrose buffer: 25mM Tris-HCl, pH 7.4, 5mM NaF, 1mM Na3VO4, 1mM EDTA, 1mM EGTA, and 320mM Sucrose). The homogenate was centrifuged at 1000g for 20 minutes, and the supernatant was collected in 2mL tubes. This supernatant was centrifuged again at 14000 rpm for 30 minutes, and the resulting supernatant was taken as a cytoplasmic fraction. The pellets were treated with TES buffer without sucrose and centrifuged at 14000 rpm for 10 minutes. The resulting supernatant was taken as a membranous fraction.
Protein content for cytoplasmic and membrane fractions were quantified by Bio-Rad DC Protein Assay, using a SpectraMax microplate reader and SoftMax Pro 6.3. The protein (10ug/uL) were then separated by electrophoresis using Novex 10% Tris Glycine gel (Invitrogen) and 1X Novex Tris-Glycine SDS Running Buffer under 140V and 50mA over 60 minutes at room temperature. Proteins were then transferred from the gel to PVDF membranes (Millipore) using 1X Novex Tris-Glycine Transfer Buffer under 50V and 500mA for 60 minutes at 4°C, after which the membranes were washed with distilled water. The membranes were incubated with Odyssey Blocking Buffer (Li-Cor) for 60 minutes at room temperature, followed by three 10 minute washes in 1X PBS 0.05% Tween 20 (PBS-T). The membranes were then incubated with primary antibodies at a concentration of 1:500 in Odyssey buffer overnight at 4°C. The primary antibodies used were rabbit anti-EAAT2 antibody (aback, ab41621) or mouse anti-GFAP antibody (Millpore, MAB360). After incubation, the membranes were washed with PBS-T buffer and incubated again with respective secondary antibody, goat anti-rabbit (IRDye 680RD, Li-Cor) or goat anti-mouse (IRDye 888CW, Li-Cor), both at a concentration of 1:1000 in Odyssey buffer for 60 minutes at room temperature. Secondary antibody incubation was followed by two 10-minute washes in 1X PBS-T and one 10-minute wash in 1X PBS. For visualization, Odyssey infrared imaging system (Li-Cor) was used and Odyssey V3.0 was used for quantitative analysis. Samples were standardized using anti-α-tubulin antibodies (TU-02) (sc-8035, Santa Cruz Biotechnology) or (2144S, Cell Signaling Technology), both at a concentration of 1:1000, and then incubated with goat anti-rabbit (IRDye 680RD, Li-Cor) or goat anti-mouse (IRDye 888CW, Li-Cor) at a concentration of 1:10000, following the same procedure described above. Quantification of GFAP or EAAT2 was calculated relative to tubulin.
2.6 Statistical Analysis
All statistical analyses were performed using STATISTICA (StatSoft, Inc., 2002, version 6). ERP Amplitude and Latency: Data were analyzed using a two-way ANOVA with ketamine and ceftriaxone treatment as the independent variables and either amplitude or latency as the dependent variable. Event Related Power: Data were analyzed separately for each frequency band using a two-way ANOVA with ketamine and ceftriaxone treatment as the independent variables and power as the dependent variable. Post-hoc comparisons were made with Fisher LSD test. Western blots: The overall drug effect across all experimental groups was compared by two-way ANOVA with ketamine and ceftriaxone treatment as the independent variables. Post-hoc comparisons were made with Fisher LSD test to control for multiple comparisons. The level of significance was set at p < 0.05.
3. RESULTS
One animal was removed from the ketamine/saline group for EEG analyses due to poor quality data.
3.1 ERP Amplitude and Latency
There were no significant main effects of ketamine or ceftriaxone and no significant interaction of the two observed for P20, N40 or P80 amplitude or latency. However, there was a trend towards increased N40 latency in the ketamine treated animals compared to ceftriaxone treated animals [F(1,25)=3.31, p=.081) (data not shown).
3.2 Event Related Power
Theta
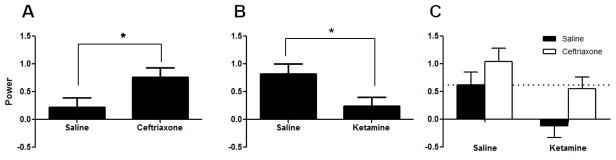
There were no observed changes in baseline theta power. A significant increase in induced theta power was observed in ceftriaxone-treated animals compared to saline-treated animals [F(1,23)=5.97, p=.023] (Figure 1a). Additionally, a significant reduction in induced theta power was observed in ketamine-treated animals when compared to saline-treated animals [F(1,23)=7.40, p =.012] (Figure 1b). Although there was no significant interaction between the two drugs, ceftriaxone fully reversed the effects of ketamine such that there was no significant difference between saline/saline and ketamine/ceftriaxone groups (MS=0.334, df=23, p=0.845) (Figure 1c). Additionally, ceftriaxone significantly increased induced theta activity among ketamine-treated animals (ketamine/ceftriaxone vs. ketamine/saline, p=0.035).
Figure 1.
Induced Theta Power Figure 1A. Saline vs. ceftriaxone. A significant increase in induced theta power was observed in ceftriaxone-treated animals compared to saline-treated animals [F(1,23)=5.97, p=.023]
Figure 1B. Saline vs. ketamine. A significant reduction in induced theta power was observed in ketamine-treated animals when compared to saline-treated animals [F(1,23)=7.40, p =.012]. Figure 1C. Ceftriaxone fully reversed the effects of ketamine such that there was no significant difference between saline/saline and ketamine/ceftriaxone groups (MS=0.334, df=23, p=0.845). Ceftriaxone significantly increased induced theta activity among ketamine-treated animals (ketamine/ceftriaxone vs. ketamine/saline, p=0.035).
3.2.1 Gamma
There were no observed changes in baseline gamma power. There were no significant effects of ketamine or ceftriaxone, and no interaction of the two on any aspect of event related gamma power.
3.2.2 High Gamma
There were no observed changes in baseline high gamma power. There was a significant interaction of ketamine and ceftriaxone on evoked high gamma [F(1,23)=4.71, p=.041) (Figure 2). Post-hoc analysis revealed that ketamine/saline animals had significantly higher evoked gamma power than saline/saline animals (MS=0.212, df=23, p=0.010). Additionally, ketamine/saline animals had significantly higher evoked gamma than ketamine/ceftriaxone animals (p=0.033). High gamma evoked power of saline/ceftriaxone animals did not deviate significantly from that of saline/saline animals (p=0.392).
Figure 2.
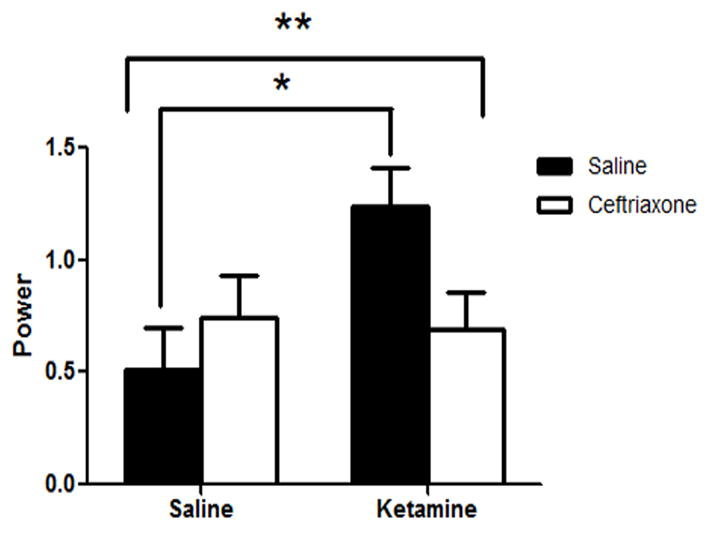
Evoked High Gamma Power. There was a significant interaction of ketamine and ceftriaxone on evoked high gamma activity [F(1,23)=4.71, p=.041). Post-hoc analysis revealed that ketamine/saline animals had significantly higher evoked gamma power than saline/saline animals (MS=0.212, df=23, p=0.010). Additionally, ketamine/saline animals had significantly higher evoked gamma than ketamine/ceftriaxone animals (p=0.033).
3.3 Western blots
3.3.1 GFAP
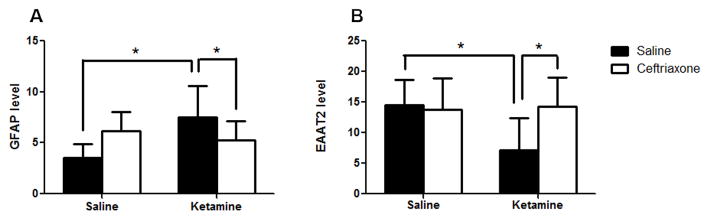
There was no significant main effect of ketamine [F(1,24)=3.16, p = .088] and no significant main effect of ceftriaxone [F(1,24)=0.033, p=.860]. However, there was a significant interaction between ketamine and ceftriaxone [F(1,24)=8.45, p=0.01] (Figure 3a). Post hoc analysis revealed a significant increase in GFAP in ketamine/saline animals as compared to saline/saline animals (MS=4.88, df=24, p=0.003). However, ceftriaxone caused a normalization of GFAP such that there was no significant difference between the ketamine/ceftriaxone-treated animals and saline/saline animals (p=0.180). Additionally, there was a significant decrease in GFAP among the ketamine/ceftriaxone group relative to ketamine/saline group (p=0.048). There was also a trend towards an increase in the saline/ceftriaxone group relative to the saline/saline group (p=.052). Interestingly there was no significant difference between the saline/ceftriaxone and ketamine/ceftriaxone groups (p=0.430).
Figure 3.
Effect of ketamine and ceftriaxone on brain levels of GFAP and EAAT2. Saline-treated animals are shown in black while ceftriaxone treated animals are shown in white. Figure 3A. GFAP. There was a significant interaction between ketamine and ceftriaxone on GFAP expression [F(1,24)=8.45, p=0.01]. Ketamine-exposed animals showed a significant increase in GFAP compared to controls (ketamine/saline vs. saline/saline, MS=4.88, df=24, p=0.003). However, ceftriaxone caused a normalization of GFAP such that there was no significant difference between the ketamine/ceftriaxone group and the saline/saline controls (p=0.180). Among the ketamine-exposed animals, there was a significant decrease in GFAP in those subsequently treated with ceftriaxone (ketamine/ceftriaxone vs. ketamine/saline, p=0.048). Figure 3B. EAAT2. There was a significant interaction between ketamine and ceftriaxone for EAAT2 expression [F(1,24) = 4.3501, p=0.048]. There was a significant reduction in EAAT2 among the ketamine-exposed animals compared to the controls (ketamine/saline vs. saline/saline, MS=23.80, df=24, p=0.010). Ceftriaxone caused a significant increase among ketamine-treated animals such that the EAAT2 level in the ketamine/ceftriaxone group was significantly higher than in the ketamine/saline group (p=0.008). There was no significant difference between saline/saline and ketamine/ceftriaxone (p=0.932), indicating that ceftriaxone fully reversed the effects of ketamine on EAAT2.
3.3.2 EAAT2
There were no significant main effects of ketamine [F(1,24)=3.43, p=0.076] or ceftriaxone [F(1,24)=3.001, p=0.096] on EAAT2 expression. However, there was a significant interaction between ketamine and ceftriaxone [F(1,24) = 4.3501, p=0.048] (Figure 3b). Post-hoc analysis revealed that that there was a significant reduction in EAAT2 among the ketamine/saline group relative to the saline/saline group (MS=23.80, df=24, p=0.010). However, there was no significant difference between saline/saline and either saline/ceftriaxone (p=0.817) or ketamine/ceftriaxone (p=0.932), indicating that ceftriaxone fully reversed the effects of ketamine on EAAT2. Ceftriaxone caused a significant increase among ketamine-treated animals such that ketamine/ceftriaxone was significantly higher than ketamine/saline (p=0.008). Additionally, there was no significant difference between ketamine/ceftriaxone and saline/ceftriaxone groups (p=0.871).
4. DISCUSSION
In the present study, juvenile mice exposed to chronic ketamine showed lasting alterations in neuronal functioning as assessed by EEG measurements as well as molecular markers of astrocyte number and function. Interestingly, these alterations were reversed when ketamine exposed animals were subsequently treated with ceftriaxone. These results suggest that ceftriaxone may be able to ameliorate drug-induced disturbances in adolescent brains, even after cessation of ketamine use.
First, we proposed that juvenile mice exposed to chronic ketamine would show reduced theta power without associated changes in other components of the EEG. A significant lasting reduction in event related theta power was observed in the ketamine-treated mice, consistent with previously published reports in both adults and juveniles (Featherstone et al., 2012, 2014). Also consistent with other work in juvenile mice, there was no evidence of a significant effect of ketamine on the P20, N40 or P80 peak amplitudes or latencies, or on any elements of background or event related gamma oscillations at this time point (Featherstone et al., 2014). Despite being consistent with work done in juveniles, these results are in direct contrast to the many alterations in the EEG found in adult mice (Featherstone et al., 2012; Maxwell et al., 2006). It is interesting to note the contrasting effect of ketamine on juvenile and adult EEG, given that ketamine shows a much lower prevalence of emergence reactions when used as an anesthetic in a pediatric population (Bergman, 1999; White and Ryan, 1996). This work diverges from others when looking at the effects of ketamine exposure on gamma oscillations. Here, an increase in evoked high gamma power was seen in ketamine treated animals. Others have conversely found decreased gamma power in rodents exposed to chronic ketamine, however previous results were found in adult animals and high gamma activity was not evaluated (Featherstone et al., 2014). Many prior studies have shown that there is a functional distinction between low gamma and high gamma oscillations and therefore there may be utility in looking at the two separately (Carlson et al., 2011; Crone et al., 1998). Although the full significance of high gamma oscillations has yet to be fully elucidated, they have been shown to play a distinct role in neuronal synchrony, selective attention, working memory, visual grouping of objects, and in motor and language processing (Crone et al., 2001, 1998; Ray et al., 2008; Vidal et al., 2006; Yamamoto et al., 2014). High frequency oscillations are also thought to be important in cross-frequency coupling, a phenomenon in which of subsets of neurons within different synaptic networks of the hippocampus communicate for higher order functioning (Canolty et al., 2006; Draguhn et al., 1998). Cross-frequency coupling has been implicated in attention, learning, detection of sensory signals, reward processing and working memory (Canolty and Knight, 2010). The disturbance seen here in evoked high gamma activity in response to juvenile chronic ketamine exposure may represent a disruption in higher order functions as is seen in humans exposed to the drug.
In addition to changes in EEG, we proposed that chronic ketamine exposure would cause lasting changes in astrocyte number and function, consistent with previous studies in adults (Featherstone et al., 2012). We found that mice exposed to ketamine showed a significant increase in GFAP levels and a significant reduction in EAAT2 expression compared to ketamine naïve animals. The current findings extend previous studies by demonstrating that the brains of juvenile animals, like adults, experience a change in astrocyte number and function following chronic ketamine exposure. Interestingly, this pathological hallmark of ketamine yields different effects with regard to EEG alterations at different ages of exposure. As previous studies have shown cognitive deficits associated with lasting changes in astrocyte number and function, these data suggest that astrocytes may mediate some of the lasting sequelae of ketamine abuse. Therefore, targeting therapeutic approaches that directly modulate astrocytes may yield a novel approach to treating the lasting cognitive deficits from juvenile drug abuse.
Finally, we proposed that ceftriaxone would reverse ketamine-induced alterations in EEG measures, GFAP levels and EAAT2 expression. As previously mentioned, the ketamine-exposed animals in this study showed a significant reduction in induced theta power. However, when ketamine was followed by ceftriaxone, the induced theta power returned to a similar level as that seen among drug naïve controls (Figure 1c). Ceftriaxone also significantly reversed the changes in evoked high frequency gamma power seen in ketamine-exposed mice. Specifically, mice that received ketamine followed by ceftriaxone displayed a subsequent normalization in evoked high gamma activity while ketamine naïve animals given ceftriaxone showed a slight qualitative increase. Therefore, ceftriaxone appears to have a homeostatic effect on high gamma power among ketamine-treated animals, rather than a simple reduction across all animals.
Ceftriaxone also appeared to ameliorate the observed astrocyte-specific molecular changes in ketamine-exposed animals. Mice given ceftriaxone following ketamine treatment showed significantly higher EAAT2 expression and significantly lower GFAP levels than those given ketamine alone. These changes brought the ketamine-exposed animals closer to the control levels, suggesting that ceftriaxone administration is able to reverse disturbances in astrocyte number and function induced by ketamine. Taken together, EEG and protein expression data indicate that treatments directed at modifying astrocyte-specific molecular pathways have the potential to reverse functional deficits in people who abuse drugs such as ketamine during adolescence.
This study was limited by a small sample size, particularly after several animals were lost following implantation of electrodes. Therefore, negative findings may be limited in power. Alternatively, significant findings among this relatively small sample suggest that changes are likely to be robust. Another limitation was that EEG testing was only performed at one time point, approximately 3 weeks after last ketamine exposure. In order to confirm a lasting alteration in brain function, additional time points may be necessary. Furthermore, this study did not include behavioral measures to further characterize the disturbances in brain function caused by juvenile exposure to ketamine. Lastly, astrogliogenesis and reduction of EAAT2 has also been observed in the brains of animals exposed to other drugs of abuse including cocaine (Bowers and Kalivas, 2003). Therefore, future studies will both extend the time course as well as behavioral manifestations of ceftriaxone after juvenile exposure to a variety of drugs of abuse.
In conclusion, the current work suggests that juvenile mice exposed to chronic ketamine display significant disturbances in EEG as well as alterations in number and functionality of astrocytes. These disturbances can be mitigated with administration of ceftriaxone after cessation of ketamine administration. For this reason, ceftriaxone appears to be a promising agent in its ability to reverse ketamine induced alterations in brain function. Therefore, ceftriaxone may have a role to play in the treatment of adolescent drug users, but may also serve to protect patients treated with ketamine for its anesthetic and anti-depressant properties. Future studies should examine the delayed effects of ceftriaxone on EEG measures into adulthood to determine whether it has a sustained protective effect. In addition, it will be very important to correlate the ceftriaxone effect on EEG measures reported here with behavioral measures of brain function and development.
Highlights (for review).
System asks for this file even though it is not needed for non-reviews
Footnotes
Author Disclosures
The Authors have no financial conflicts of interest disclosures relevant to the content of the current manuscript. SJS has received grant support and consulted to Merck, Astellas and Boehringer Ingleheim.
Contributors
Steve Siegel obtained funding, designed experiments, did analyses and contributed to writing and editing of manuscript.
Keisha Dodman performed experiments, did analyses and wrote the manuscript
Rob Featherstone assisted with experimental design and analyses and provided feedback on writing of manuscript.
Jaicey Bang performed experiments and analyses and wrote portions of the manuscript.
Yuling Liang assisted with performance of experiments and analyses of data.
Conflict of interest statement
Dr. Siegel has consulted for or received grant support from Teva, Merck, Astellas within the past 2 years. No other authors have any conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abanades S, Peiro AM, Farre M. Club drugs: old medicines as new party drugs. Med Clin (Barc) 2004;123:305–311. doi: 10.1016/s0025-7753(04)74499-1. [DOI] [PubMed] [Google Scholar]
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann LC, Halene TB, Ehrlichman RS, Luminais SN, Ma N, Abel T, Siegel SJ. Chronic ketamine impairs fear conditioning and produces long-lasting reductions in auditory evoked potentials. Neurobiol Dis. 2009;35:311–317. doi: 10.1016/j.nbd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin B, Hajhashemi V, Abnous K, Hosseinzadeh H. Ceftriaxone, a beta-lactam antibiotic, modulates apoptosis pathways and oxidative stress in a rat model of neuropathic pain. Biomed Res Int. 2014;2014:937568. doi: 10.1155/2014/937568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin B, Hajhashemi V, Hosseinzadeh H, Abnous K. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 2012;224:15–25. doi: 10.1016/j.neuroscience.2012.07.058. [DOI] [PubMed] [Google Scholar]
- Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Bergman SA. Ketamine: review of its pharmacology and its use in pediatric anesthesia. Anesth Prog. 1999;46:10–20. [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A. 2011;108:E962–970. doi: 10.1073/pnas.1109625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Fu TC, Ting TT, Huang WL, Tang GM, Hsiao CK, Chen CY. Use of ecstasy and other psychoactive substances among school-attending adolescents in Taiwan: national surveys 2004–2006. BMC Public Health. 2009;9:27. doi: 10.1186/1471-2458-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II Event-related synchronization in the gamma band. Brain. 1998;121(Pt. 12):2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D, Jefferys JG. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394:189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J Cogn Neurosci. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Liang Y, Saunders JA, Tatard-Leitman VM, Ehrlichman RS, Siegel SJ. Subchronic ketamine treatment leads to permanent changes in EEG, cognition and the astrocytic glutamate transporter EAAT2 in mice. Neurobiol Dis. 2012;47:338–346. doi: 10.1016/j.nbd.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Nagy LR, Hahn CG, Siegel SJ. Juvenile exposure to ketamine causes delayed emergence of EEG abnormalities during adulthood in mice. Drug Alcohol Depend. 2014;134:123–127. doi: 10.1016/j.drugalcdep.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Wang W, Dong Y, Wu L, Huang J, Ma Y, Zhang Z, Wu S, Gao G, Qin H. Ceftriaxone alleviates early brain injury after subarachnoid hemorrhage by increasing excitatory amino acid transporter 2 expression via the PI3K/Akt/NF-kappaB signaling pathway. Neuroscience. 2014;268:21–32. doi: 10.1016/j.neuroscience.2014.02.053. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, Ortinski PI, Leitman V, Liang Y, Thieu T, Anderson R, Pierce RC, Jonak G, Gur RE, Carlson G, Siegel SJ. GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Transl Psychiatry. 2012;2:e142. doi: 10.1038/tp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-da-Costa R, Stoner GR, Fung R, Albright TD. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci U S A. 2013;110:15425–15430. doi: 10.1073/pnas.1312264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D’Souza DC, Ford JM, Krystal JH, Mathalon DH. Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry. 2012;71:969–977. doi: 10.1016/j.biopsych.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS. Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med (Maywood) 2008;233:1389–1394. doi: 10.3181/0803-RM-83. [DOI] [PubMed] [Google Scholar]
- Ji HF, Shen L, Zhang HY. Beta-lactam antibiotics are multipotent agents to combat neurological diseases. Biochem Biophys Res Commun. 2005;333:661–663. doi: 10.1016/j.bbrc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Jutzeler C, McMullen ME, Featherstone RF, Tatard-Leitman VM, Gandal MJ, Carlson GC, Siegel SJ. Electrophysiological deficits in schizophrenia: models and mechanisms. In: Uehara T, editor. Psychiatric Disorders. InTech; Rijeka: 2011. [Google Scholar]
- Kim SY, Jones TA. The effects of ceftriaxone on skill learning and motor functional outcome after ischemic cortical damage in rats. Restor Neurol Neurosci. 2013;31:87–97. doi: 10.3233/RNN-2012-120245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai PC, Huang YT, Wu CC, Lai CJ, Wang PJ, Chiu TH. Ceftriaxone attenuates hypoxic-ischemic brain injury in neonatal rats. J Biomed Sci. 2011;18:69. doi: 10.1186/1423-0127-18-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- Li LB, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, Robinson MB. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mao J. Glutamate transporter: an unexpected target for some antibiotics. Mol Pain. 2005;1:5. doi: 10.1186/1744-8069-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ahn KH, Perry EB, Jr, Cho HS, Roach BJ, Blais RK, Bhakta S, Ranganathan M, Ford JM, D’Souza DC. Effects of nicotine on the neurophysiological and behavioral effects of ketamine in humans. Front Psychiatry. 2014;5:3. doi: 10.3389/fpsyt.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharmacol Exp Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Bunner KD, Gaither TW, Klein EL, Barton SJ, Rebec GV. Up-regulation of GLT1 reverses the deficit in cortically evoked striatal ascorbate efflux in the R6/2 mouse model of Huntington’s disease. J Neurochem. 2012;121:629–638. doi: 10.1111/j.1471-4159.2012.07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Monaghan L, Curran HV. Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction. 2004;99:1450–1461. doi: 10.1111/j.1360-0443.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Tsuda M, Koizumi S, Narita M, Yajima Y, Inoue K, Suzuki T. Long-lasting change in brain dynamics induced by methamphetamine: enhancement of protein kinase C-dependent astrocytic response and behavioral sensitization. J Neurochem. 2005;93:1383–1392. doi: 10.1111/j.1471-4159.2005.03097.x. [DOI] [PubMed] [Google Scholar]
- Nizzardo M, Nardini M, Ronchi D, Salani S, Donadoni C, Fortunato F, Colciago G, Falcone M, Simone C, Riboldi G, Govoni A, Bresolin N, Comi GP, Corti S. Beta-lactam antibiotic offers neuroprotection in a spinal muscular atrophy model by multiple mechanisms. Exp Neurol. 2011;229:214–225. doi: 10.1016/j.expneurol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clin Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Gandal MJ, Roberts TP, Siegel SJ. NMDA antagonist MK801 recreates auditory electrophysiology disruption present in autism and other neurodevelopmental disorders. Behav Brain Res. 2012a;234:233–237. doi: 10.1016/j.bbr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA, Gandal MJ, Siegel SJ. NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol Dis. 2012b;46:93–100. doi: 10.1016/j.nbd.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall U, Muller BW, Kargel C, Gunturkun O. Electrophysiological mismatch response recorded in awake pigeons from the avian functional equivalent of the primary auditory cortex. Neuroreport. 2015;26:239–244. doi: 10.1097/WNR.0000000000000323. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S, White R, Featherstone RE, Ray R, Ortinski PI, Banerjee A, Gandal MJ, Lin R, Alexandrescu A, Liang Y, Gur RE, Borgmann-Winter KE, Carlson GC, Hahn CG, Siegel SJ. Pyramidal cell selective ablation of N-methyl-d-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry. 2015;77:556–568. doi: 10.1016/j.biopsych.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thone-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hortnagl H, Godes M, Muller S, Rumschussel K, Funke-Kaiser H, Villringer A, Steckelings UM, Unger T. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26:2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- White JM, Ryan CF. Pharmacological properties of ketamine. Drug Alcohol Rev. 1996;15:145–155. doi: 10.1080/09595239600185801. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. Age-dependent differences in sensitivity and sensitization to cannabinoids and ‘club drugs’ in male adolescent and adult rats. Addict Biol. 2008;13:277–286. doi: 10.1111/j.1369-1600.2007.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–857. doi: 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]