Abstract
The purpose of the present study was to characterize the properties of GABAA receptor currents in human sensory neurons. Neurons were obtained from adult organ donors. GABAA currents were recorded in isolated neurons. Both large inactivating low affinity currents and smaller persistent high affinity currents were present in all of the 129 neurons studied from 15 donors. The kinetics of human GABAA currents were slower than those in rat sensory neurons. GABA currents were completely blocked by bicuculline (10 µM), and persistent currents were activated by the δ-subunit preferring agonist, THIP. The GABA current equilibrium potential was ~20 mV more hyperpolarized than in rat neurons. Both low and high affinity currents were increased by inflammatory mediators but via different second messenger pathways. These results highlight potentially important species differences in the properties of ion channels present in their native environment and suggest the use of human sensory neurons may be a valuable tool to test compounds prior to use in humans.
Keywords: dorsal root ganglion, intracellular Cl- concentration, patch clamp, protein kinase A, protein kinase C, tyrosine kinase
Introduction
Behavioral pharmacological data from rodents indicate that GABAA receptor signaling is critically involved in the modulation of nociception at sites throughout the central nervous system (CNS) (Price et al., 2009). Particularly important is the gating of afferent input to the spinal cord. While data from rodent models also suggest that post-synaptic inhibitory circuitry in the spinal cord dorsal horn is likely to be important for regulation of nociceptive threshold, particularly in the presence of injury, available electrophysiological, pharmacological and morphological data suggest that pre-synaptic inhibition of afferent input is the dominant mechanism for inhibition of somatosensory input into the CNS (Eccles et al., 1962, Eccles et al., 1963, Nishi et al., 1974, Mokha et al., 1983, Hiura et al., 1998, Reeve et al., 1998, Rudomin and Schmidt, 1999, Bae et al., 2000, Olave et al., 2002, Sutherland et al., 2002, Sokal and Chapman, 2003, Vesselkin et al., 2003, Weng and Dougherty, 2005). Virtually all dorsal root ganglion (DRG) neurons from rat respond to GABA with a rapidly activating, bicuculline-sensitive anion current (Oyelese et al., 1995, Zhu et al., 2012a). However, in contrast to neurons in the CNS, activation of GABAA receptors on primary afferents results in membrane depolarization. This paradoxical response is thought to be due to a relatively high concentration of intracellular Cl− as a result of the persistent expression of the Na+-K+-Cl−-co-transporter, NKCC1 into adulthood.
Despite the wealth of knowledge of GABAA signaling in rodents and the growing interest in the use of GABAA receptor ligands for the treatment of pain, there is only one report of GABAA currents in human sensory neurons (Maddox et al., 2004). Interestingly, the currents from adult human DRG neurons described in this study were resistant to the classical GABAA receptor antagonists bicuculline and picrotoxin (Maddox et al., 2004). And while there appear to be limited differences between human and rodent GABAA receptor homologs in heterologous expression systems, evidence of unique pharmacological properties of the GABAA currents in human sensory neurons raises the possibility that the differences are due to processes unique to the native environment. Thus, given the therapeutic potential of GABAA receptor ligands suggested by preclinical data (Witschi et al., 2011), and in light of growing concerns over the extent to which preclinical data translate to human clinical conditions (Seok et al., 2013), we sought to further characterize GABAA currents in human sensory neurons.
Our results suggest that while there are many similarities between the GABAA currents present in rodent and human sensory neurons, there are marked differences. These included biophysical properties of the evoked currents, which were more slowly activating and inactivating in human DRG neurons, as well as the response to inflammatory mediators. That is, in contrast to the selective potentiation of high affinity GABAA currents previously observed in rat DRG neurons (Lee and Gold, 2012), both low and high affinity currents were potentiated in human sensory neurons via what appeared to be distinct second messenger pathways. These results highlight potentially important species differences in the properties of ion channels present in their native environment and suggest the use of human sensory neurons may be a valuable tool with which to test compounds developed in heterologous expression systems and validated in rodent models prior to the use in humans.
Experimental Procedures
Human Subjects
L4 and L5 DRG were collected from organ donors with the consent of family members for the use of their loved one’s tissue for research purposes.
DRG collection
Following the collection of tissue needed for transplantation purposes, L4 and L5 DRG were accessed via a ventral approach. Briefly, the lumbosacral trunk was found running medially to the psoas major. Blunt dissection was used to follow the spinal nerves of the L4 and L5 ganglia to their respective foramen in the vertebral column. An oscillating autopsy saw (Mopec, Oak Park MI) was used to cut through the vertebral bodies above and below the L4 and L5 ganglia, respectively. Cuts were then made through the vertebral pedicals, with care to keep the saw blade angled above the ganglia. A Virchow skull breaker was then used to lift the ventral surface of the spinal column off en bloc, exposing the ganglia and cauda equina. Ganglia were carefully freed of connective tissue, the central and peripheral processes were cut and the ganglia were placed in ice cold collection media (Table 1) of the following composition: 124.5 mM NaCl, 5 mM KCl, 1.2 mM MgSO4, 1 mM CaCl2, and 30 mM HEPES. The pH of the collection media was adjusted to 7.35 with NaOH and filter sterilized for storage at 4°C for no more than two weeks.
Table 1.
Solutions for collection dissociation and culturing of human DRG neurons
| Collection Media |
Dissociation Media |
Broth | Basal Media | Complete Media |
|
|---|---|---|---|---|---|
| Purpose | Collection and transfer to DRG | Enzymatic treatment and quenching enzymatic treatment | Added to dissociation media | Percoll spins | Plating and culture |
| Composition | 129.5 mM NaCl, 5 mM KCl, 1.2 mM MgS042, 1 mM CaCl2, 30 mM HEPES | Collection media + Broth at 25 ml/l | 200 mM glucose, 400 mM NaCl, 0.9% phosphoric acid (w/w) | L-15 media containing: 60 mg imidizole, 15 mg aspartic acid, 15 mg glutamic acid, 15 mg cystine, 5 mg β-alanine, 10 mg myoinositol, 10 mg cholineCl, 5 mg p-aminobenzoic acid, 25 mg fumaric acid, 2 mg vitamin B12 and 5 mg of lipoic acid (which was first dissolved in methanol at a concentration of 1g/2.5 ml) | Basal Media containing: Heat inactivated fetal bovine serum (1:10 with basal media), to which is added (to a final concentration): 50 ng/ml nerve growth factor (NGF 2.5S), 0.3 mg/ml glutamine, 0.225 mg/ml glucose, 2.55 mg/ml ascorbic acid, and 0.12 mg/ml glutathione, and 0.2% (w/v) NaHCO3 |
| Notes | Adjust pH to 7.35 with NaOH, sterile filter, store at 4°C for < 2wks | Made fresh every time | Diluted in double distilled H20. Sterile filtered. Stored at − 20°C. | Components added to 500 ml bottle of L-15. Stored at −4°C for < 1wk | Made fresh every time |
With this approach, it was possible to recover all four ganglia in well under 15 minutes. The time between cross clamp and the start of the DRG isolation was usually between 45 min and an hour, and as a result, we were generally able to have the DRG in collection media in a little over an hour.
In an initial set of pilot experiments, we sought to determine how long the DRG could sit in collection media prior to dissociation before the overall health of the isolated DRG neurons began to deteriorate, as manifest by a poor yield of neurons, high resting intracellular Ca2+ concentrations and/or depolarized (>−40 mV) resting membrane potential. This was to determine the maximal travel time, and therefore the farthest hospital we could use for the recovery of tissue. Results from this analysis suggested that ~3 hrs was the outside limit.
Isolation of DRG neurons
The protocol employed was adapted from that developed by Baumann and colleagues (Baumann et al., 2004). Ganglia were transferred to fresh collection media and carefully freed of connective tissue and fat under a dissecting microscope. “Cleaned” tissue was then cut into small (~2–5 mm3) pieces and these pieces were transferred to dissociation media (Table 1) containing 1 mg/ml of collagenase P (Roche Bioscience), 1 mg/ml of Trypsin (TRL, Worthington) and 0.1 mg/ml DNAse 1 (Roche Bioscience). in a siliconized glass petri dish (35 mm diameter), which was placed on a shaking tray in a 37°C water bath. Fresh enzyme solution was added every 90 minutes, where the enzymatic treatment was terminated when pieces of ganglia began to visibly fall apart in the dissociation media. The enzymatic treatment time varied as a function of the age of the donor, where older donors required considerably more time (up to 3.5 hrs) to obtain isolated neurons. At the end of the enzymatic treatment, pieces of ganglia were transferred to a 15 ml tube and mechanically dissociated in the enzyme solution via gentle passage through a fire polished pasture pipette. Enzymatic activity was quenched with the addition of 7 ml of collection media supplemented with 2 mg/ml soybean trypsin inhibitor (type II, Sigma-Aldrich), 1 mg/ml bovine serum albumin (Sigma-Aldrich), fetal bovine serum (to a final concentration of 10%, Hyclone), and 2 mM CaCl2 (as a final concentration), and dispersed cells were centrifuged at 450G for 4 minutes. The supernatant was gently removed and the pellet was re-suspended in a Leibovitz L-15 (Invitrogen) based media (basal media, Table 1) containing (per 500 ml): 60 mg imidazole, 15 mg aspartic acid, 15 mg glutamic acid, 15 mg cystine, 5 mg β-alanine, 10 mg myo-inositol, 10 mg cholineCl, 5 mg p-aminobenzoic acid, 25 mg fumaric acid, 2 mg vitamin B12 and 5 mg of lipoic acid (which was first dissolved in methanol at a concentration of 1g/2.5 ml). All chemicals used to supplement the L-15 media were obtained from Sigma-Aldrich. Cells were spun again for 4 minutes at 450G. The supernatant was removed and the pellet resuspended in 1 ml of basal media. This was loaded on a Percoll (Sigma-Aldrich) gradient consisting of a “heavy layer” (1.1 part Percoll to 2.9 parts basal media) below a “light layer” (0.5 part Percoll to 3.5 parts basal media), which was spun at 1800G for 10 min. The Percoll was removed and the pellet was resuspended in complete media which was composed of basal media diluted 1:10 with fetal bovine serum and then supplemented to yield a final concentration of 50 ng/ml nerve growth factor (NGF 2.5S, Invitrogen), 0.3 mg/ml glutamine (Invitrogen), 0.225 mg/ml glucose (Sigma-Aldrich), 2.55 mg/ml ascorbic acid (Sigma-Aldrich), 0.12 mg/ml glutathione (Invitrogen), and 0.2% (w/v) NaHCO3 (Sigma-Aldrich). Cells were plated onto poly-L-lysine coated glass coverslips (Invitrogen) placed in 35 mm culture dishes and stored in a CO2 (5%) incubator at 37°C for 4 hour prior to flooding the culture dishes with Complete Media. Neurons were studied within 12 (acute) or 30 hrs (overnight) in culture.
Patch clamp electrophysiology
Whole-cell patch-clamp recordings, conventional and perforated patch, were performed with a HEKA EPC10 amplifier controlled with Pulse software (HEKA Eletronik GmbH, Lambrecht, Germany) or an Axopatch 200B controlled with pClamp (v 10.2) software (Molecular Devices, Carlsbad, CA) used in combination with a Digidata 1320A A/D converter (Molecular Devices). Unless otherwise noted, data were acquired at 10 kHz and filtered at 2 kHz. Borosilicate glass (WPI, Sarasota, FL) electrodes were 1–2 MΩ when filled with one of two electrode solutions used in this study. For conventional whole-cell patch clamp recording, the electrode solution contained (in mM): CsCl 140, MgCl2 1, EGTA 11, HEPES 10, Mg-ATP 2, and GTP 1; pH was adjusted to 7.2 with Tris-base and osmolality was adjusted to 310 mOsm with sucrose. For perforated patch recording, the electrode solution contained (in mM) K-Methansulphonate 100 mM, KCl 40, MgCl2.6H2O 2, CaCl2 1, EGTA 11, HEPES 10 and NaCl 5; pH was adjusted to 7.2 with Tris Base, and osmolality was adjusted to 317 mOsm with sucrose (all salts from Sigma-Aldrich). Gramicidin (Sigma-Aldrich) was used for perforated patch recording in order to obtain electrical access with a minimal impact on resting intracellular Cl− concentration (Akaike, 1996). A stock solution of gramicidin (1.5 mg/100 µl) was prepared in DMSO. This was diluted with electrode solution in a 1:300 ratio to give a final concentration of 50 µg/ml. The gramicidin containing electrode solution was vortexed for 15s. No filtering was applied. The tip of the electrode was loaded with a small volume of gramicidin free electrode solution in order to avoid interference of the antibiotic with seal formation. Gramicidin containing electrode solution was back loaded. The progress of perforation was monitored with the capacitative transient to a 5 mV step. Experiments were not started until access resistance was less than 7 MΩ.
The bath solution contained the following (in mM): choline-Cl 130, KCl 3, CaCl2 2.5, MgCl2 0.6, HEPES 10, and glucose 10; pH was adjusted to 7.4 with Tris-base and osmolality was adjusted to 320 mOsm with sucrose. In some experiments, choline-Cl was replaced with NaCl to more accurately estimate resting membrane potential. Neurons were held at −60 mV. Currents were evoked by 4 or 60 seconds of focal (Fast-Step, Warner Instruments) application of GABA or GABAA receptor agonists at different concentrations.
Test agents
Drugs were diluted to final concentrations in bath solution from stock solutions at least 1000 times greater than the highest concentration employed. Drugs used were GABA, δ subunit containing GABAA receptor agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP), and GABAA receptor antagonist bicuculline. Inflammatory mediators consisted of a combination of histamine (1µM, dissolved in water, 100mM stock concentration), bradykinin (10 µM, dissolved in 1% acetic acid, 23.58 mM stock concentration), and prostaglandin E2 (1 µM, dissolved in 100% ethanol, 10 mM stock concentration). All drugs were obtained from Sigma-Aldrich and prepared as stock solution stored at −20°C, then diluted in bath solution before use. Data analysis: Data are expressed as mean ± SEM. Differences between groups were assessed with a one- or two-way ANOVA or a Students t-test, where p < 0.05 was considered statistically significant.
Study Approval
Written consent for the use of donor tissue for research purposes was obtained from family members. All procedures were approved by the University of Pittsburgh Committee for Oversight of Research and Clinical Training Involving Decedents and the Center for Organ Recovery and Education, Pittsburgh, PA (http://www.core.org).
Results
The 129 DRG neurons studied were obtained from 15 donors that included 5 men and 10 women. The average age of the donors was 51.7 ± 3.1 yrs with a spread from 26 to 73 yrs. All donors were of white/non-hispanic origin. The breakdown of sex by age is summarized in Table 2.
Table 2.
GABAA currents in human DRG
| Sex | Age (yrs) | Current Density 10 µM (pA/pF |
Current Density 1 mM (pA/pF) |
|---|---|---|---|
| Male (n = 5) | 48.8 ± 3.9 | 0.76 ± 0.16 (n = 14) | 17.2 ± 4.8 (n = 16) |
| Female (n = 10) | 53.2 ± 4.3 | 0.74 ± 0.10 (n = 46) | 16.1 ± 1.5 (n = 60) |
Data from a total of 129 neurons studied.
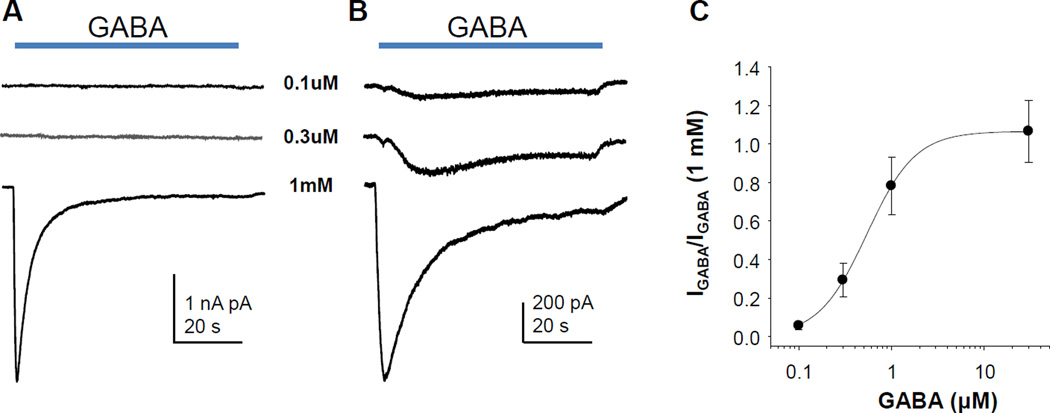
While there was considerable variability in the current amplitude, 1 mM GABA evoked a clearly detectable current in every neuron tested (n = 76). The average peak amplitude was 2.7 ± 0.2 nA and average peak current density of 16.3 ± 1.2 pA/pF. While the currents were still large, this current density is significantly lower than that previously described in rat DRG neurons recorded under identical conditions (Lee et al., 2012). In acutely dissociated neurons, the vast majority of evoked current was rapidly activating and rapidly inactivating, with little if any non-inactivating current (Figure 1A). Consistent with recent results from rat (Lee et al., 2012), there was an increase in persistent current with time in culture (Figure 1B). The persistent current had a high affinity for activation, with detectable current activated with 100 nM GABA in some neurons (Figure 1B). Concentration-response data indicated that the EC50 for current activation was 540 nM (Figure 1C).
Figure 1.
High and low affinity GABAA currents in human sensory neurons. A. In acutely dissociated neurons, a low affinity transient current predominates. GABA was applied at the time indicated. B. After 24hr or more in culture, a high affinity persistent current was detectable. C. Concentration-response data from 10 neurons were normalized with respect to the amplitude of the current evoked with 1 mM GABA. Pooled data were fitted with a Hill equation to yield an EC50 of 540 nM.
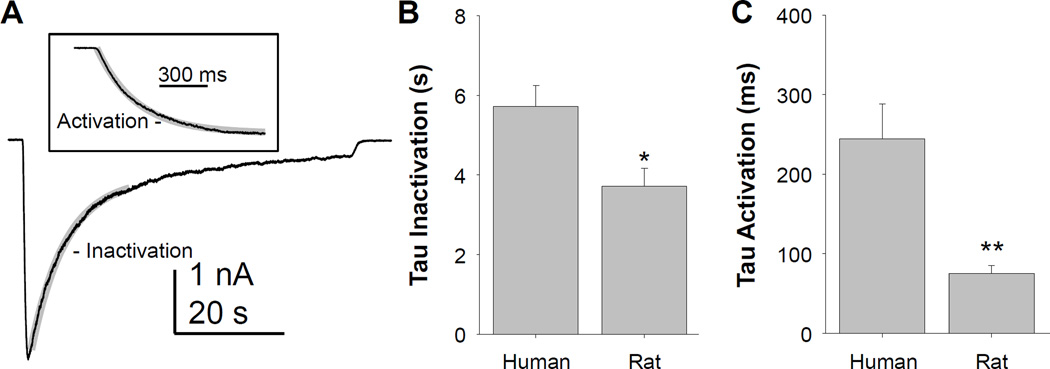
To further characterize the biophysical properties of GABA evoked currents in human sensory neurons, we determined the time constant (tau) of current inactivation in response to a prolonged application of 1 mM GABA (Figure 2A). A shorter application of GABA was used to define the time constant of current activation (Figure 2A inset). Compared to GABAA currents evoked with identical protocols in small diameter rat DRG neurons, GABAA currents in human DRG neurons inactivated (Figure 2B) and activated (Figure 2C) more slowly as indicated by significantly larger time constants of inactivation and activation.
Figure 2.
The kinetics of low affinity GABA (1 mM) evoked currents in human sensory neurons are slower than those observed in rat sensory neurons. A. The fast component of current inactivation was well fitted with a single exponential equation (gray trace) used to estimate the inactivation time constant. Inset: A single exponential function was also used to determine the time constant of current activation. B. Pooled inactivation time constant (tau) data from 11 human and 9 rat neurons were analyzed with a t-test which confirmed that the difference between groups was significant. C. Pooled activation time constant (tau) data from human and rat neurons was also analyzed with a t-test confirming that activation of GABAA currents in rat sensory neurons was faster than in human sensory neurons. * is p < 0.05 and ** is p < 0.01.
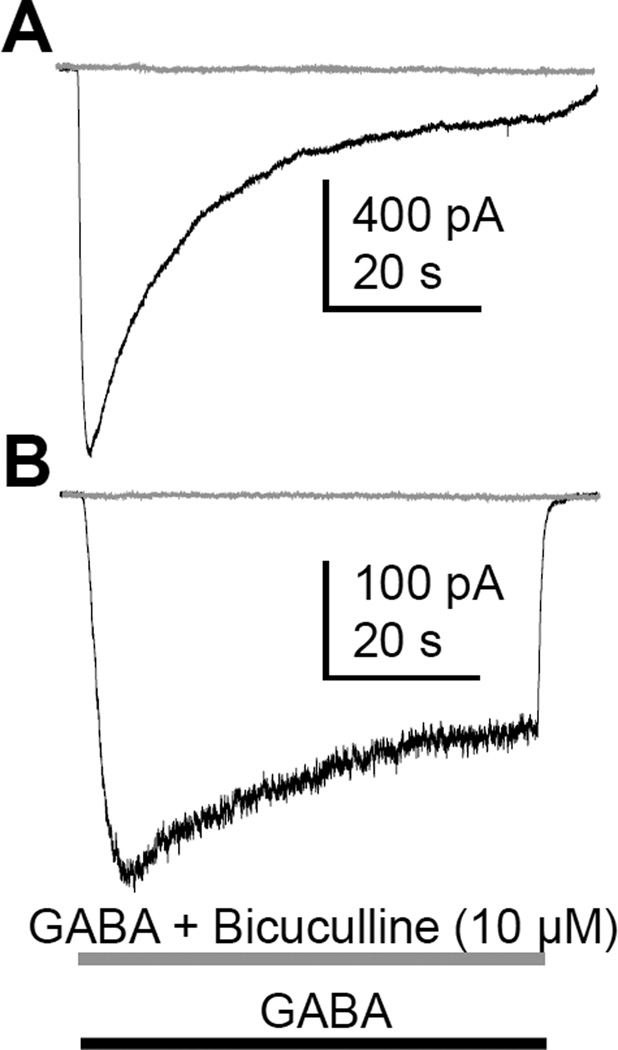
Given previous data from human DRG neurons indicating that GABA evoked currents are resistant to the GABAA receptor antagonist bicuculline (Maddox et al., 2004), we next assessed the sensitivity of the GABA evoked currents in human neurons to classical GABAA receptor antagonists. Both transient low affinity and persistent high affinity current was completely blocked by bicuculline (10 µM, Figure 3), and picrotoxin (3 µM, not shown).
Figure 3.
GABAA currents from adult human sensory neurons were blocked by bicuculline. GABA was applied as indicated alone (black trace) and then 5 minutes later in combination with bicuculline (gray trace). Both low (A) and high (B) affinity currents were completely blocked by bicuculline. Comparable results were obtained in all 5 of the other neurons tested.
Previous data from rat DRG neurons suggest the high affinity persistent current is carried by δ-subunit containing GABAA receptors (Lee et al., 2012). To begin to assess the extent to which a comparable receptor contributes to the high affinity persistent GABA current in human DRG neurons, we assessed the actions of the δ-subunit preferring agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol (THIP) (Adkins et al., 2001, Brown et al., 2002). In 8 of 8 neurons tested with 1 mM THIP, a persistent current of 2.34 ± 0.45 pA/pF was clearly detectable. Preliminary results (n = 2) suggest a persistent current could be evoked with 10 µM THIP (data not shown).
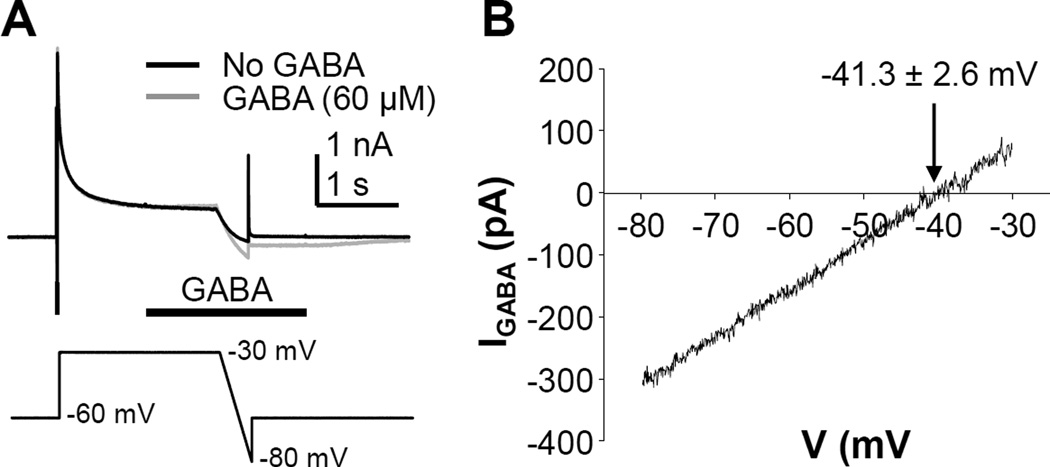
Data from mouse (Sung et al., 2000), rat (Mao et al., 2012, Zhu et al., 2012b), and cat (Gallagher et al., 1978) suggest that the intracellular concentration of Cl− in primary sensory neurons is high enough that GABAA receptor activation results in membrane depolarization. Because the direction of current flow may have a profound impact on the functional consequences of GABAA receptor activation, we determined the equilibrium potential of the GABA evoked current in human sensory neurons studied with whole cell access obtained with gramicidin-perforated patch (Akaike, 1996). A ramp protocol was used to determine the reversal potential of GABA evoked currents (Figure 4A and B). The average reversal potential observed in 6 neurons from 2 donors was −41.3 ± 2.6 mV (Figure 4B). Because HEPES-buffered recording solutions were used to minimize the contribution of HCO3− to the GABA current reversal potential, it was possible to estimate the concentration of intracellular Cl− in human sensory neurons with the Nernst equation. Results of this analysis suggested that the intracellular concentration of Cl− in human sensory neurons was 27.7 ± 2.8 mM.
Figure 4.
The GABAA current equilibrium potential in human sensory neurons is depolarized relative to normal resting membrane potentials. A. A ramp protocol was used to determine the equilibrium potential for the GABA evoked currents. A step-and ramp voltage clamp protocol (bottom trace) was used to evoke current in the presence (gray trace) and absence (black trace) of GABA (applied half way through the step to −30 mV). B. The difference current was plotted relative to the ramp voltage, and the point of 0 current was considered the equilibrium potential. The mean (± SEM) of the equilibrium potential determined from 6 neurons from two different donors is indicated.
To determine the impact of GABA on resting membrane potential, 6 neurons were studied in current clamp with a NaCl-based bath solution. The average resting membrane potential in these neurons was −57.6 ± 1.2 mV (range −54 to −60 mV). The GABA (1 mM, 4 sec) induced depolarization was 7.1 ± 1.4 mV (range 4 to 13 mV). This depolarization was not sufficient to evoke an action potential in any of the neurons tested.
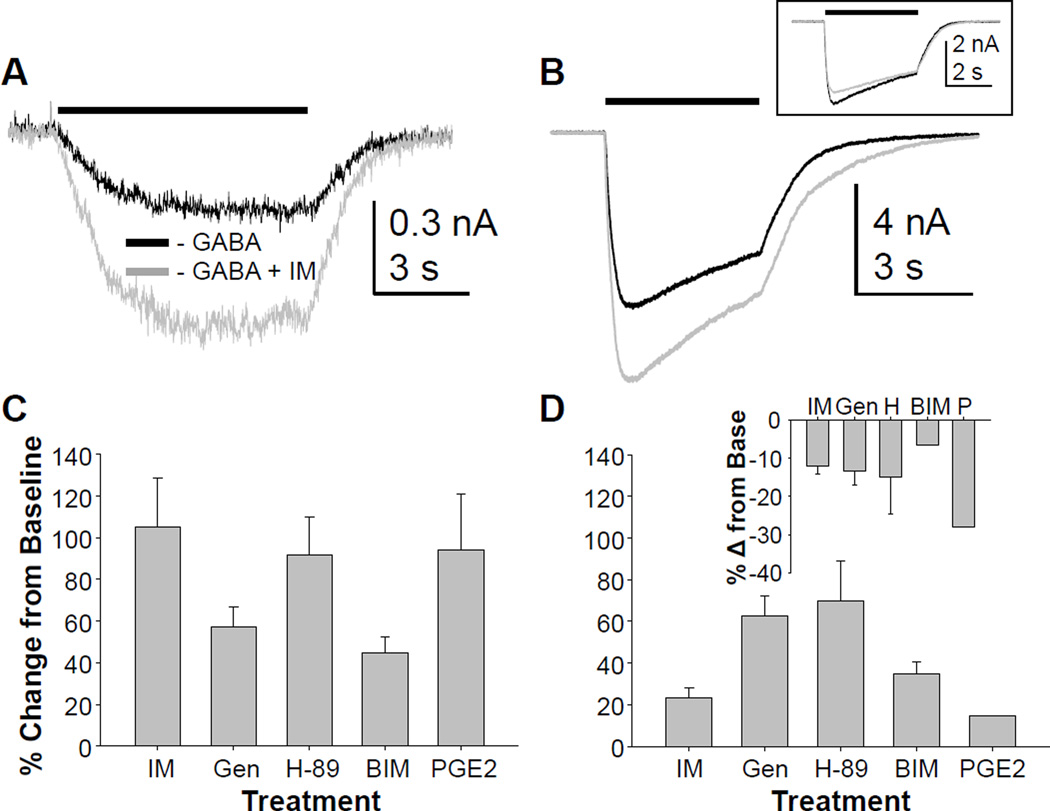
To further determine the extent to which the properties of GABAA currents observed in rodent sensory neurons described in previous studies (Lee and Gold, 2012) reflect those from humans, we determined the impact of inflammatory mediators on GABAA currents in human DRG neurons. In rat DRG, an inflammatory soup composed of PGE2 (1 µM), bradykinin (1 µM) and histamine (10 µM), results in the potentiation of high affinity currents in the absence of a detectable change in low affinity currents (Lee and Gold, 2012). Application of the same combination of inflammatory mediators to human DRG neurons resulted in an increase in high affinity currents (evoked with 10 µM) GABA in 14 of 15 neurons tested (Figure 5A). The increase in current was 105 ± 23.5% in neurons in which an increase in current was detected. Application of vehicle (n = 6), resulted in no significant change (~3%) in response to either 10 µM or 1 mM GABA. Both the proportion of neurons responding with an increase in high affinity current (p < 0.01) and the magnitude of the change (p < 0.01) were significantly greater in neurons treated with inflammatory mediators than in vehicle treated neurons (not shown). Interestingly, there was no apparent increase in the potency with which GABA activated the high affinity current in human sensory neurons, as there was no evidence of currents activated by 30 nM GABA in the presence of inflammatory mediators. This is in marked contrast to the inflammatory mediator-induced increase in GABA potency observed in rat sensory neurons (Lee and Gold, 2012).
Figure 5.
High and low affinity GABAA currents in human sensory neurons are modulated by inflammatory mediators. A. GABA was applied before and then 3 minutes after the application of a combination of inflammatory mediators (IM). In the majority of neurons tested (15 of 16), the high affinity current (evoked with 10 µM) GABA was increased following IM application. B. Changes in low affinity currents were studied in the same neurons, and in half of these (6 of 13), the low affinity current was also increased by IM. Inset: In a minority of neurons (3 of 13), however, a decrease in low-affinity current was observed. Separate groups of neurons were pretreated with the TK kinase inhibitor, genistein (Gen, n = 11), the PKA inhibitor H-89 (n = 8), or the PKC inhibitor BIM (n = 5) prior to the application of GABA and then GABA with inflammatory mediators. Pooled high (C) and low (D) affinity current data were analyzed as a percent change from the baseline GABA current evoked from neurons treated with IM alone or pre-treated with kinase inhibitors. A two-way ANOVA indicated a significant interaction between current type (high vs low affinity) and treatment. Of note, only the neurons in which low affinity current increased by > 10% were included in this analysis, and plotted in panel D. The IM-induced decrease in low affinity current (D inset) was observed in a subpopulation of neurons pre-incubated with each inhibitor and no significant impact of inhibitor was detected in these neurons. Finally, a group of neurons (n = 5) was treated with PGE2 (P) alone, rather than in combination with bradykinin and histamine. Pooled high (C) and low (D) affinity current data were included with plots of pooled IM data for comparison.
Inflammatory mediator-induced changes in low affinity currents were more complex than those observed for high affinity current. That is, in 6 of 13 neurons, there was an increase in low affinity current (Figure 5B), in 3 of 13 neurons there was a decrease in low affinity current (Figure 5B inset), while in the remainder, there was no change (< ±10%). As the majority (12 of 13) of neurons were challenged with both 10 µM and 1 mM GABA before and after IM application, the variable changes in low affinity current was observed in neurons in which there was a clear increase in high affinity current.
The remaining experiments were focused on second messenger pathways underlying the actions of inflammatory mediators. Inflammatory mediators were applied following a pre-incubation with inhibitors of tyrosine kinase (genistein, 10 µM for up to 20 min), protein kinase A (PKA, H89, 10 µM, 3–5 min), or protein kinase C (PKC, BIM, 1 µM, 3–5 min). None of the inhibitors had a detectable influence on the magnitude of the high or low affinity current prior to the application of inflammatory mediators, but had a differential impact on the actions of IM on the high (Figure 5C) and low (Figure 5D) affinity GABA currents. Data were analyzed with a two-way ANOVA, which revealed a significant (p < 0.01) interaction between current type (high vs low affinity) and kinase inhibitor. This was due to the suppression of the IM-induced potentiation of high affinity current with genistein and BIM, and the increase in IM-induced potentiation of low affinity current with genistein and H-89. While only neurons with a low affinity current potentiated by IM were included in this two-way ANOVA, there was no detectable influence of kinase inhibitors on either the proportion of neurons in which IM-induced a decrease in low affinity current, or the magnitude of the decrease in current (Figure 5D inset).
Finally, to determine whether the combination of inflammatory mediators was needed to drive the changes in GABA currents, PGE2 was tested alone on seven neurons. Changes in high affinity currents were assessed in six of these, where an increase in 10 µM evoked current was observed in five of the six neurons tested (Figure 5C). No change in current was observed in the sixth neuron. These changes in high affinity current were not significantly different from those evoked with the combination of inflammatory mediators (p > 0.05). PGE2-induced changes in low affinity currents were assessed in four of these neurons: currents were increased in one of these (by 16%, Figure 5D) and decreased in two (by 24.6 and 31.5%, Figure 5D inset).
Discussion
The purpose of the present study was to characterize the properties of GABAA currents in human sensory neurons. Toward that end, we optimized a protocol for the generation of isolated sensory neurons obtained from organ donors that enabled us to maintain viable neurons in culture for days after dissociation and plating. GABA-evoked currents were present in every neuron studied. While low affinity transient currents were predominant in acutely dissociated neurons, high affinity persistent currents emerged with time in culture. Both high and low affinity currents were easily detectable, but current density was significantly (p < 0.01, n = 76 for human and 21 for rat) lower than that previous observed in rat DRG neurons recorded under identical conditions (Lee et al., 2012). Furthermore, the activation and inactivation of GABAA currents in human neurons was significantly slower than those in rat sensory neurons (Figure 2). Currents were completely blocked with the GABAA antagonists bicuculline and picrotoxin. Persistent currents could be evoked with the δ-subunit preferring agonist THIP. The reversal potential of GABAA currents was depolarized relative to resting membrane potential of ~−58 mV recorded in human sensory neurons. High affinity currents were potentiated by inflammatory mediators, an action attenuated by inhibitors of TK and PKC. Finally, the impact of inflammatory mediators on low affinity currents was more complex: currents were potentiated in the majority of neurons studied, but inhibited in others, and the potentiation was further augmented by inhibitors of TK and PKA. Taken together, these data indicate that while there are similarities between the properties of GABAA currents in rodent and human sensory neurons, there are important differences as well.
There were clearly similarities between human and rat with respect to the properties of GABAA currents present in sensory neurons. Transient low affinity and persistent high affinity GABAA currents are present in sensory neurons from both species. Persistent currents in both species have the pharmacology of δ-subunit containing receptors. And in both species, high affinity GABAA currents are potentiated by inflammatory mediators. However, there were also a number of interesting, and potentially important differences. The kinetics of the low affinity currents were different between the two species, which may have important implications for strategies based on leveraging the state-dependent properties of the channel, such as those thought to account for the unique therapeutic profile of the NMDA receptor antagonist, memantine (Johnson et al., 2015). While the intracellular Cl− concentration is still high in human sensory neurons relative to estimates of this property in CNS neurons (Price et al., 2009), there is a ~20 mV difference between human (−40 mV) and rat (−20 mV) (Zhu et al., 2012b) sensory neurons with respect to the Cl− equilibrium potential. If HCO3− has the same impact on the reversal potential of GABAA currents in human neurons as it does in rat neurons, the GABAA reversal potential in human sensory neurons may be even more hyperpolarized than the ~−41 mV observed in the absence of HCO3− in the present study. This would suggest that the mechanisms of GABA induced inhibition of human sensory neurons may be very different than those thought to account for the presynaptic inhibition of rat sensory neurons. In rat sensory neurons, GABA-induced depolarization has been suggested to promote inactivation of voltage-gated Na+ channels (Price et al., 2009), activation of low threshold K+ channels (Zhu et al., 2012b), and membrane shunting (Price et al., 2009).
With a more hyperpolarized GABAA reversal potential, neither of these depolarization-dependent (Na+ channel inactivation and K+ channels activation) mechanisms would be as substantively engaged. More importantly, the rationale for reducing the intracellular Cl− concentration, such as via an increase in KCC2 and/or a decrease in NKCC1 activity, as a therapeutic strategy to increase the inhibitory actions of GABA (Price et al., 2005, Gagnon et al., 2013) would be far less compelling.
Finally, while the evidence of inflammatory mediator-induced modulation of GABAA currents in human sensory neurons provides further support for the utility of this model system for the study of nociceptive signaling, the inflammatory mediator-induced modulation of GABAA currents in human sensory neurons was qualitatively and quantitatively different than that previously described in rat sensory neurons (Lee and Gold, 2012). The increase in high affinity current density in the absence of a shift in potency is consistent with second messenger-induced regulation of receptor turnover (Jurd et al., 2010). Furthermore, the apparent difference in the second messenger-induced regulation of low and high affinity GABAA currents is consistent with the suggestion that different channels underlie these two current types (Vithlani et al., 2011). These results also indicate that at least three different second messenger cascades are engaged by inflammatory mediators in human sensory neurons. Minimally, however, these observations suggest that GABAA currents in human sensory neurons are dynamically regulated, where the net impact of the regulation is going to depend on the combination of second messenger pathways activated, GABAA current reversal potential as well as the properties of the other ion channels present in the membrane.
While differences between GABAA currents in human sensory neurons and those previously described in rodents are interesting and potentially important, it should be appreciated that in addition to the potential role for species differences, experimental factors may contribute to these differences as well. For example, human sensory neurons were necessarily obtained postmortem, while we routinely obtain DRG from deeply anesthetized rodents prior to euthanasia (Lee et al., 2012, Lee and Gold, 2012, Zhu et al., 2012a, Zhu et al., 2012b, Zhu et al., 2013). And while it is possible to obtain healthy dissociated sensory neurons from rodents with a relatively short postmortem interval (we have had reasonable success up to 20 minutes postmortem in rats), we have been unable to obtain healthy rodent sensory neurons with the ~1 hr postmortem interval required for the collection of human tissue used in the present study. Similarly, our previous rodent studies involved exclusively young adult male rats, while human neurons were from both males and females who were, on average, middle age. However, we obtained evidence arguing against a potential role for either sex or age in the species differences observed: there was detectable influence of sex on the properties of GABAA currents observed in human sensory neurons (i.e., Table 2); and the trends toward differences in density (9.1 ± 3.9 pA/pF n = 8 vs 18.2 ± 2.3 pA/pF n = 8, p = 0.06) and kinetics (i.e., tau of activation was 435 ± 140 ms and 195 ± 20 ms, p = 0.57) of the currents in neurons from the youngest (23 yrs) and oldest (73 yrs) donor studied, respectively, were in the opposite direction to that which could account for the apparent species differences. Finally, it should also be noted that because of evidence for heterogeneity in the regulation of intracellular Cl− within a neuron (Wright et al., 2011), conclusions drawn from the Cl− equilibrium potential measured at the neuron cell body with respect to the afferent terminals, should be made with caution.
With increased understanding of the complexity of GABAA receptor signaling, there is growing interest in targeting GABAA receptors for the treatment of pain (Zeilhofer et al., 2012). Recent evidence also suggests it may be possible to target GABAA receptors on primary afferent neurons for the treatment of pain (Naik et al., 2008), which would enable the use of peripherally restricted ligands as a means of avoiding many of the adverse effects associated with centrally acting GABAA receptor ligands. However, the vast majority of what is known about GABAA receptors is derived from the study of human receptors heterologously expressed in an array of different cell types (Vithlani et al., 2011), or native receptors in neurons obtained from other species, most commonly rats and mice. Given the relatively dramatic impact that heterologous expression systems and species differences can have on the biophysical and pharmacological properties of ion channels, in the face of the relatively high cost of clinical trials, we suggest that confirming the potency and selectivity of a ligand for its intended target in its native environment and most relevant species, should become an integral step in drug development. Our data indicate that human DRG neurons may be particularly valuable in this regard.
Highlights.
-
-
Bicuculline sensitive GABAA currents are present in all human DRG neurons.
-
-
The kinetics of GABAA currents in rat DRG neurons are faster than human currents.
-
-
The GABA current equilibrium potential was ~20 mV more hyperpolarized than in rat neurons.
-
-
Low and high affinity human GABAA currents were increased by inflammatory mediators.
-
-
Human sensory neurons may be a valuable tool to test compounds prior to use in humans.
Acknowledgements
We thank Dr. Rajesh Bhattacharjee for his expert technical assistance in preparing the neurons for study. We also thank Dr. Tom Baumann for his advice on the dissociation and culture of human DRG neurons. This study was supported by the National Institutes of Health Grant 1R01NS063010 (MSG) and a Collaborative Research Grant from Eli Lilly.
Abbreviations
- DRG
dorsal root ganglion
- GABA
γ-aminobuteric acid
- GABAA receptor
A-type GABA receptor
- THIP
agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridine-3-ol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Participated in research design: Zhang, Lee, Priest, Belfer and Gold.
Conducted experiments: Zhang, Lee, and Gold
Performed data analysis: Zhang, Lee, and Gold.
Wrote or contributed to the writing of the manuscript: Zhang, Priest, Belfer and Gold.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- Akaike N. Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Prog Biophys Mol Biol. 1996;65:251–264. doi: 10.1016/s0079-6107(96)00013-2. [DOI] [PubMed] [Google Scholar]
- Bae YC, Ihn HJ, Park MJ, Ottersen OP, Moritani M, Yoshida A, Shigenaga Y. Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J Comp Neurol. 2000;418:299–309. [PubMed] [Google Scholar]
- Baumann TK, Chaudhary P, Martenson ME. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur J Neurosci. 2004;19:1343–1351. doi: 10.1111/j.1460-9568.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962;160:62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, Willis WD. Pharmacological Studies On Presynaptic Inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Higashi H, Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol (Lond) 1978;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiura A, Nasu F, Ishizuka H. Relationship of substance P- and CGRP-immunoreactive central endings of the primary afferent neurons to GABAergic interneurons in the guinea pig substantia gelatinosa. Okajimas Folia Anat Jpn. 1998;74:231–235. doi: 10.2535/ofaj1936.74.6_231. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Glasgow NG, Povysheva NV. Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol. 2015;20:54–63. doi: 10.1016/j.coph.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Tretter V, Walker J, Brandon NJ, Moss SJ. Fyn kinase contributes to tyrosine phosphorylation of the GABA(A) receptor gamma2 subunit. Mol Cell Neurosci. 2010;44:129–134. doi: 10.1016/j.mcn.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Charbonnet M, Gold MS. Upregulation of high-affinity GABA(A) receptors in cultured rat dorsal root ganglion neurons. Neuroscience. 2012;208:133–142. doi: 10.1016/j.neuroscience.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Gold MS. Inflammatory mediators potentiate high affinity GABA(A) currents in rat dorsal root ganglion neurons. Neurosci Lett. 2012;518:128–132. doi: 10.1016/j.neulet.2012.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Mao S, Garzon-Muvdi T, Di Fulvio M, Chen Y, Delpire E, Alvarez FJ, Alvarez-Leefmans FJ. Molecular and functional expression of cation-chloride cotransporters in dorsal root ganglion neurons during postnatal maturation. J Neurophysiol. 2012;108:834–852. doi: 10.1152/jn.00970.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokha SS, McMillan JA, Iggo A. Dorsal root potentials in the cat: effects of bicuculline. Brain Res. 1983;259:313–318. doi: 10.1016/0006-8993(83)91265-9. [DOI] [PubMed] [Google Scholar]
- Naik AK, Pathirathna S, Jevtovic-Todorovic V. GABA(A) receptor modulation in dorsal root ganglia in vivo affects chronic pain after nerve injury. Neuroscience. 2008;154:1539–1553. doi: 10.1016/j.neuroscience.2008.04.061. [DOI] [PubMed] [Google Scholar]
- Nishi S, Minota S, Karczmar AG. Primary afferent neurones: the ionic mechanism of GABA-mediated depolarization. Neuropharmacology. 1974;13:215–219. doi: 10.1016/0028-3908(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Olave MJ, Puri N, Kerr R, Maxwell DJ. Myelinated and unmyelinated primary afferent axons form contacts with cholinergic interneurons in the spinal dorsal horn. Exp Brain Res. 2002;145:448–456. doi: 10.1007/s00221-002-1142-5. [DOI] [PubMed] [Google Scholar]
- Oyelese AA, Eng DL, Richerson GB, Kocsis JD. Enhancement of GABAA receptor-mediated conductances induced by nerve injury in a subclass of sensory neurons. J Neurophysiol. 1995;74:673–683. doi: 10.1152/jn.1995.74.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–555. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AJ, Dickenson AH, Kerr NC. Spinal effects of bicuculline: modulation of an allodynia-like state by an A1-receptor agonist, morphine, and an NMDA-receptor antagonist. J Neurophysiol. 1998;79:1494–1507. doi: 10.1152/jn.1998.79.3.1494. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal DM, Chapman V. Effects of spinal administration of muscimol on C- and A-fibre evoked neuronal responses of spinal dorsal horn neurones in control and nerve injured rats. Brain Res. 2003;962:213–220. doi: 10.1016/s0006-8993(02)04057-x. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland FI, Bannatyne BA, Kerr R, Riddell JS, Maxwell DJ. Inhibitory amino acid transmitters associated with axons in presynaptic apposition to cutaneous primary afferent axons in the cat spinal cord. J Comp Neurol. 2002;452:154–162. doi: 10.1002/cne.10374. [DOI] [PubMed] [Google Scholar]
- Vesselkin NP, Adanina VO, Rio JP, Reperant J. Ultrastructural study of glutamate- and GABA-immunoreactive terminals contacting the primary afferent fibers in frog spinal cord. A double postembedding immunocytochemical study. Brain Res. 2003;960:267–272. doi: 10.1016/s0006-8993(02)03795-2. [DOI] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–1022. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HR, Dougherty PM. Response properties of dorsal root reflexes in cutaneous C fibers before and after intradermal capsaicin injection in rats. Neuroscience. 2005;132:823–831. doi: 10.1016/j.neuroscience.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31:8134–8142. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R, Raimondo JV, Akerman CJ. Spatial and temporal dynamics in the ionic driving force for GABA(A) receptors. Neural plasticity. 2011;2011:728395. doi: 10.1155/2011/728395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Dua S, Gold MS. Inflammation-induced shift in spinal GABA(A) signaling is associated with a tyrosine kinase-dependent increase in GABA(A) current density in nociceptive afferents. J Neurophysiol. 2012a;108:2581–2593. doi: 10.1152/jn.00590.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Lu SG, Gold MS. Persistent inflammation increases GABA-induced depolarization of rat cutaneous dorsal root ganglion neurons in vitro. Neuroscience. 2012b;220:330–340. doi: 10.1016/j.neuroscience.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang XL, Gold MS. Activity-dependent hyperpolarization of EGABA is absent in cutaneous DRG neurons from inflamed rats. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]