Abstract
Objectives
Prescription opioid (PO) use by people who inject drugs (PWID) is a growing public health concern. Non-fatal overdose remains a leading source of morbidity among PWID, however, little is known about the relationship between PO injection and non-fatal overdose in this population. In this study we sought to examine the impact of PO injection on non-fatal overdose among PWID from Vancouver, Canada.
Methods
Data were derived from two open prospective cohorts of PWID for the period of December, 2005 to May, 2014. Multivariable generalized estimating equations were used to examine the odds of overdose among those who injected: POs; heroin; and POs and heroin.
Results
In total, 1660 PWID (33.7% women) participated in this study. In multivariable analyses, in comparison to those who were injecting non-opioid drugs, exclusive PO injection was not significantly associated with non-fatal overdose (adjusted odds ratio [AOR]: 1.17, 95% confidence interval [CI]: 0.74 – 1.86). The odds of non-fatal overdose were elevated for heroin injection (AOR: 1.72, 95% CI: 1.31 – 2.27), but were greatest for those who injected both heroin and POs (AOR: 2.46, 95% CI: 1.83 – 3.30).
Discussion
Compared to injecting non-opioids, injecting POs exclusively did not increase risk of non-fatal overdose; however, injecting both POs and heroin doubled the risk. This may reflect consistencies in drug potency and composition when POs are used, as well as unique characteristics of exclusive PO injectors. Our findings call for the continued scale-up of evidence-based overdose prevention interventions for people who inject opioids, including POs.
Keywords: Prescription opioids, non-fatal overdose, opioid overdose, illicit drug use, injection drug use
1. INTRODUCTION
An epidemic of opioid-related overdose deaths in North America has followed from substantial increases in the use of prescription opioid analgesics (POs; Centers for Disease Control and Prevention, 2012; Cerda et al., 2013; Manchikanti and Singh, 2008). The use of diverted POs among high-risk substance-using populations, including people who inject drugs (PWID), has also increased (Bruneau et al., 2012; Fischer et al., 2008). While alarmingly high rates of PO injection have been documented in rural areas where access to a range of illicit drugs may be limited (Havens et al., 2007), accounts from urban drug centers have also demonstrated that despite high accessibility of heroin, the availability of diverted POs has also increased (Nosyk et al., 2012). While the association between PO use and accidental overdose in the general public has been well-established (Bohnert et al., 2011; Dart et al., 2015; Fischer et al., 2014), the impact of illicit PO use on overdose among long-term drug users, such as PWID, is not known.
Overdose remains a leading cause of morbidity and mortality among PWID (Degenhardt and Hall, 2012; Miller et al., 2007; Warner-Smith et al., 2002), and is prevalent with roughly 30-45% of PWID having experienced at least one non-fatal overdose in their lifetime (Havens et al., 2011; Kerr et al., 2007; Ochoa et al., 2005; Pollini et al., 2006; Sherman et al., 2007), and as many as 20% reporting a non-fatal overdose in the previous year (Ochoa et al., 2005). The health consequences of non-fatal overdose can be severe and include a range of injuries such as acute hypoxia including neurological (e.g., stroke) and solid organ (e.g., renal failure) concerns (Warner-Smith et al., 2002, 2001). Furthermore, those who have survived an overdose are at a heightened risk for future overdose (Darke et al., 2005; Kinner et al., 2012), including fatal overdose (Stoove et al., 2009). Non-fatal overdose also poses a major burden on the health care system as a leading reason for emergency department presentation (Palepu et al., 2001), which can result in long term institutionalization among those with severe neurological injuries.
Past research has identified a significant positive association between PO use (i.e., oral, intranasal, or intravenous administration) and non-fatal overdose (Lake et al., 2015), and has also found that among PO-using young adults, non-fatal overdose is more common among those who inject POs (Silva et al., 2013b). However, few studies have explored a potential independent association between PO injection and non-fatal overdose among PWID. While many PWID have long-term experience with heroin injection, the harms associated with PO injection may be perceived as comparatively less threatening: despite similarities in pharmacological effect (Trescot et al., 2008), key differences in the composition and preparation of these two types of drugs may produce different overdose risks. For example, whereas POs are manufactured for licit use and maintain consistent doses and purities, these characteristics may be unpredictable in heroin due to it being unregulated (Werb et al., 2013).
As PO injection becomes more prevalent among high-risk drug using populations, understanding the effect of PO injection is critical to overdose prevention efforts. The present study therefore aims to investigate the effect of PO injection on non-fatal overdose among PWID.
2. MATERIALS AND METHODS
2.1 Study Sample
The Vancouver Injection Drug Users Study (VIDUS) and the AIDS Care Cohort to Evaluate Exposure to Survival Services (ACCESS) are ongoing open prospective cohorts of adults who use illicit drugs recruited through self-referral and street outreach in Vancouver, Canada. The cohorts have been described in detail previously (Strathdee et al., 1998). Briefly, VIDUS enrolls HIV-negative people who report injecting an illicit drug at least once in the previous month; ACCESS enrolls HIV-positive people who report using an illicit drug other than cannabis in the previous month. For both cohorts, other eligibility requirements include being aged 18 years or older, residing in the greater Vancouver region and providing written informed consent. All study instruments and follow-up procedures are harmonized, allowing data from both cohorts to be combined into one dataset for analysis.
At baseline and semi-annually thereafter, participants complete an interviewer-administered questionnaire eliciting socio-demographic data as well as information pertaining to drug use, risk behaviours, and health care utilization. Nurses collect blood samples for HIV testing (VIDUS) or disease monitoring (ACCESS), and hepatitis C serology, and also provide basic medical care and referrals to appropriate health care services. Participants received a $30 (CDN) honorarium for each study visit. The University of British Columbia/Providence Health Care Research Ethics Board has provided ethical approval for the study.
2.2 Measures
The present analysis was restricted to participants who completed a baseline questionnaire and at least one follow-up questionnaire between December, 2005 and May, 2014, and who reported active (i.e., previous six month) injection drug use. The outcome of interest was self-reporting experiencing an overdose in the previous six months, which was assessed and recorded at baseline and every follow-up interview over the study period. Consistent with previous work (Kerr et al., 2007; Mitra et al., 2015) participants who responded ‘yes’ to the question “In the previous six months, have you overdosed by accident (i.e., where you had a negative reaction from using too much drugs)?” were considered recent survivors of non-fatal overdose. PO injection was assessed through the question: “In the last six months, which of the following drugs did you inject?”, to which participants were provided a list and pictures of common POs. The list underwent yearly modifications in order to reflect up-to-date trends in the types of illicit POs used. The most recent questionnaire included oxycodone (OxyNeo, OxyContin, Percocet), hydrocodone (Dilaudid), morphine, meperidine (Demerol), methadone, fentanyl, and pentazocine (Talwin). Participants were also given the option of specifying other POs that were not on the list. We then built a four-category primary exposure variable: no opioid injection (i.e., people who inject non-opioid drugs including cocaine or methamphetamine; reference category); PO injection exclusive of heroin injection (category 2); heroin injection exclusive of PO injection (category 3); and co-occurring heroin and PO injection (category 4).
We examined various potential sociodemographic and substance use-related confounders based on previously established associations with non-fatal overdose. Sociodemographic confounders were: age (per year older); gender (male vs. female); ethnicity (Caucasian vs. other); recent homelessness (yes vs. no); and recent incarceration (yes vs. no). Behavioural/drug-related confounders were: length of injection drug use in year (per year longer); binge drug use, defined as using more drugs than usual (yes vs. no); injecting in public (yes vs. no); requiring help injecting (yes vs. no); enrolled in a methadone maintenance program (yes vs. no); heavy alcohol use, defined as >14 drinks per week or >4 drinks on one occasion for men, and >7 drinks per week or >3 drinks on one occasion for women (National Institute on Alcohol Abuse and Alcoholism; yes vs. no); cocaine injection (yes vs. no); methamphetamine use (yes vs. no); benzodiazepine use (yes vs. no); crack smoking (yes vs. no); and non-injection PO use (yes vs. no). Unless otherwise specified, all behavioural and substance use-related variables as well as recent incarceration and recent homelessness refer to events or behaviours that occurred at least once in the previous six months.
2.3 Analysis
First, we examined the univariable relationships between each independent variable and non-fatal overdose using generalized estimating equations (GEEs) with logit link for correlated data (Lee et al., 2007). This method uses an exchangeable correlation structure to provide standard errors adjusted by multiple observations per individual (i.e., multiple follow-up data for each participant), and was chosen given the repeated binary outcome measure. This method has been used successfully in previous non-fatal overdose studies (Kinner et al., 2012; Milloy et al., 2008). Next, we built a full multivariable GEE model that included all variables significantly association with the outcome at p<0.10 in the univariable model. Using a conservative stepwise backward selection approach, we fit a series of reduced models comparing the coefficient value associated with the main explanatory variable in the full model to its corresponding value in each of the reduced models, and dropped the secondary variable associated with the smallest relative change. We continued this iterative process until the minimum change exceeded 5%. All analyses were performed in SAS software version 9.3 (SAS Institute Inc., Cary, NC). All p-values are two-sided.
3. RESULTS
Between December, 2005 and May, 2014, 1,660 actively injecting study participants, including 559 (33.7%) women, completed a total 10,919 study visits. Of these 10,919 observations, 233 (2.1%) were removed from the analysis due to invalid or missing values, yielding a total of 10686 analytic observations. The median number of follow up visits was 5 (interquartile range [IQR]: 2 – 10). The baseline median age of the sample was 42.1 years (IQR: 35.5 – 48.0). The proportion of PWID reporting injection of POs during the previous six months ranged from 16.3% to 35.1% (median: 24.5%), with rates peaking between June and November, 2007. Heroin was the most commonly injected opioid, with 30.1% – 49.3% (median: 40.5%) of PWID reporting exclusive heroin injection in the previous six months and 10.9% - 29.7% (median: 19.4%) reporting injection of both heroin and POs. A small proportion of PWID (2.7% - 6.6%) injected POs exclusive of heroin at each follow-up. The median prevalence of non-fatal overdose during each follow-up period was 6.0% (IQR: 4.9% – 7.1%), with the highest rate being reported at 8.8% between December, 2012 and May, 2013. By the end of the study period, a total of 413 (24.9%) individuals experienced a total of 670 non-fatal overdoses. Table 1 summarizes sample baseline characteristics of the sample, stratified by opioid injection status.
Table 1.
Baseline characteristics of 1660 PWID in Vancouver, stratified by opioid injection status
| Characteristic | Opioid Injection† |
||||
|---|---|---|---|---|---|
| None n (%) (n = 582) | PO Only n (%) (n = 51) | Heroin Only n (%) (n = 590) | Both n (%) (n = 437) | p-value | |
| Socio-demographic factors | |||||
| Age (Med, IQR) | 43.7 (37.1 – 49.3) | 41.7 (33.9 – 47.7) | 41.0 (34.3 – 47.7) | 41.1 (34.5 – 46.7) | <0.001 |
| Male | 397 (68.2) | 35 (68.6) | 373 (63.2) | 296 (67.7) | 0.263 |
| Caucasian | 306 (52.6) | 33 (64.7) | 357 (60.5) | 296 (67.7) | <0.001 |
| Homeless† | 144 (24.7) | 15 (29.4) | 202 (34.2) | 203 (46.5) | <0.001 |
| Incarcerated†§ | 54 (9.3) | 5 (9.8) | 103 (17.5) | 120 (27.5) | <0.001 |
| Substance use-related factors | |||||
| Years of injection (Med, IQR) | 20.4 (13.0 – 28.3) | 20.8 (12.1 – 27.5) | 18.4 (11.6 – 28.1) | 19.4 (11.6 – 28.6) | 0.587 |
| Binge drug use† | 223 (38.3) | 21 (41.2) | 223 (37.8) | 206 (47.1) | 0.013 |
| Injected in public† | 90 (15.5) | 12 (23.5) | 236 (40.0) | 279 (63.8) | <0.001 |
| Needed help injecting† | 107 (18.4) | 13 (25.5) | 136 (23.1) | 140 (32.0) | <0.001 |
| In a methadone program† | 193 (33.2) | 18 (35.3) | 313 (53.1) | 186 (42.6) | <0.001 |
| Heavy alcohol use† | 109 (18.7) | 6 (11.8) | 80 (13.6) | 76 (17.4) | 0.075 |
| Any cocaine injection† | 308 (52.9) | 15 (29.4) | 259 (43.9) | 277 (63.4) | <0.001 |
| Any crystal meth use† | 110 (18.9) | 17 (33.3) | 109 (18.5) | 161 (36.8) | <0.001 |
| Any benzodiazepine use†§ | 5 (0.9) | 3 (5.9) | 7 (1.2) | 13 (3.0) | 0.005 |
| Any crack smoking† | 407 (70.0) | 35 (68.6) | 474 (80.3) | 387 (88.6) | <0.001 |
| Any PO non-injection†§ | 12 (2.1) | 5 (9.8) | 26 (4.4) | 67 (15.3) | <0.001 |
| Non-fatal overdose†§ | 23 (4.0) | 2 (3.9) | 39 (6.6) | 49 (11.2) | <0.001 |
In the previous six months
p-value was generated with Fisher's Test
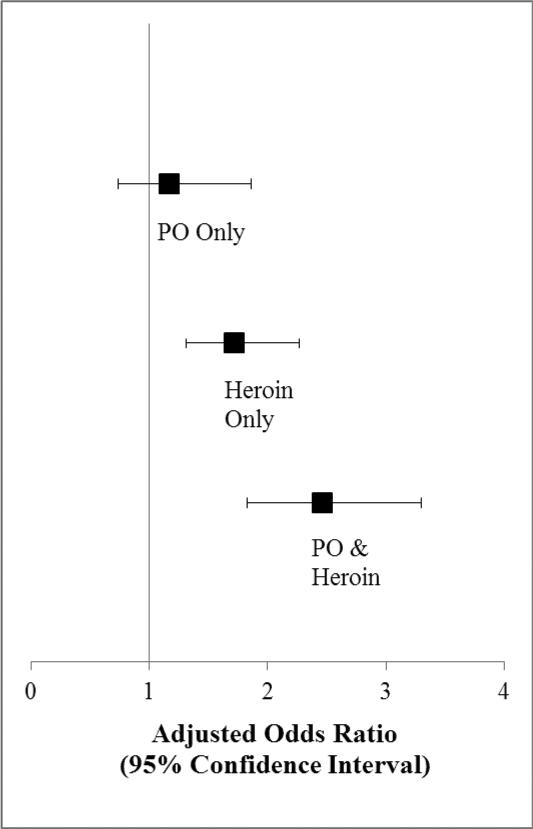
In the final multivariable GEE model adjusted for incarceration, public injecting, assisted injecting, methadone maintenance treatment, heavy alcohol use, and PO non-injection (Table 2, Figure 1), exclusive PO injection was not independently associated with non-fatal overdose (adjusted odds ratio [AOR]: 1.17, 95% confidence interval [CI]: 0.74 – 1.86); however, participants who injected heroin but not POs had significantly increased odds of overdosing (AOR: 1.72, 95% CI: 1.31 – 2.27), and those who injected both heroin and POs exhibited the highest odds of overdosing (AOR: 2.46, 95% CI: 1.83 – 3.30), compared to those who did not recently inject an opioid. Other factors positively associated with non-fatal overdose in the multivariable model included incarceration (AOR: 1.73, 95%CI: 1.40 – 2.13), public injecting (AOR: 1.46, 95% CI: 1.20 – 1.79), requiring help injecting (AOR: 1.51, 95% CI: 1.22 – 1.88), heavy alcohol use (AOR: 1.30, 95% CI: 1.04 – 1.62), and non-injection PO use (AOR: 1.44, 95% CI: 1.14 – 1.84). Methadone maintenance treatment was negatively associated with non-fatal overdose (AOR: 0.72, 95% CI: 0.59 – 0.86).
Table 2.
Univariable and multivariable GEE¥ analyses of factors associated with recent non-fatal overdose among 1660 PWID in Vancouver, Canada
| Characteristic | Odds Ratio |
|||
|---|---|---|---|---|
| Unadjusted | p-value | Adjusted | p-value | |
| Type of opioid injected£ | ||||
| None | 1.00 | 1.00 | ||
| PO only | 1.34 (0.86 - 2.09) | 0.190 | 1.17 (0.74 - 1.86) | 0.505 |
| Heroin only | 1.92 (1.48 - 2.48) | <0.001 | 1.72 (1.31 - 2.27) | <0.001 |
| Both PO and heroin | 3.40 (2.60 - 4.43) | <0.001 | 2.46 (1.83 - 3.30) | <0.001 |
| Age | ||||
| Per year older | 0.99 (0.98 - 1.00) | 0.110 | - | - |
| Gender | ||||
| Female | 1.00 | - | - | |
| Male | 1.03 (0.83 - 1.28) | 0.800 | - | - |
| Ethnicity | ||||
| Other | 1.00 | - | - | |
| Caucasian | 1.13 (0.91 - 1.41) | 0.277 | - | - |
| Homeless£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.64 (1.36 - 1.97) | <0.001 | - | - |
| Incarcerated£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.20 (1.78 - 2.71) | <0.001 | 1.73 (1.40 - 2.13) | <0.001 |
| Years of injection | ||||
| Per year more | 1.00 (0.99 - 1.01) | 0.797 | - | - |
| Binge drug use£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.42 (1.20 - 1.67) | <0.001 | - | - |
| Injected in public£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 2.07 (1.72 - 2.50) | <0.001 | 1.46 (1.20 - 1.79) | <0.001 |
| Needed help injecting£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.79 (1.46 - 2.18) | <0.001 | 1.51 (1.22 - 1.88) | <0.001 |
| Methadone program£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.67 (0.56 - 0.80) | <0.001 | 0.72 (0.59 - 0.86) | <0.001 |
| Heavy alcohol use£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.32 (1.07 - 1.63) | 0.010 | 1.30 (1.04 - 1.62) | 0.022 |
| Cocaine injection£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.10 (0.92 - 1.31) | 0.310 | - | - |
| Crystal meth use£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.82 (1.50 - 2.21) | <0.001 | - | - |
| Benzodiazepine use£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.32 (0.79 - 2.21) | 0.292 | - | - |
| Crack smoking£ | ||||
| No | 1.00 | - | - | |
| Yes | 1.13 (0.93 - 1.37) | 0.220 | - | - |
| PO non-injection£ | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.89 (1.50 - 2.39) | <0.001 | 1.44 (1.14 - 1.84) | 0.003 |
GEE: Generalized Estimating Equation
§ 95% CI = 95% Confidence Interval
In the previous six months
Figure 1.
Association between opioid injection status and non-fatal overdose among 1660 PWID (adjusted for incarceration, public injecting, methadone maintenance treatment, heavy alcohol use, and PO non-injection)
4. DISCUSSION
Over the study period, approximately one-quarter of participants reported recent injection of POs, which is comparatively lower than the roughly one-half (Leclerc et al., 2011) and three-quarters (Bruneau et al., 2012) of PWID who report injecting POs in other Canadian settings. Our comparatively lower PO injection rates may be due, in part, to the presence of a controlled prescription program in British Columbia designed to educate physicians about proper opioid prescribing practices (Richard et al., 2012). Our lower rates are also a reflection of the well-established local heroin market, which likely reduces the demand for POs compared to cities with low heroin availability. The prevalence of PO injection reached its peak at 35% in 2007, coinciding with a significant increase in PO availability recorded in this setting between 2006 and 2007 (Nosyk et al., 2012). The past six month prevalence of non-fatal overdose (median: 4.2%) was also low compared with drug-using populations in other settings (Coffin et al., 2007; Fischer et al., 2004).
A small number of studies have examined the relationship between PO injection and non-fatal overdose among PWID, including two that found no association (Fischer et al., 2004; Jenkins et al., 2011), and one that found a moderately significant association (Havens et al., 2011). However, the present study appears to be the first to consider the unique effect of PO injection in comparison to heroin injection, as well as the combined impact of both heroin and PO injection on the risk of non-fatal overdose. In doing so, we identified a small group of PWID (3 – 7%) who injected POs but not heroin, a consistently large group (30 – 50%) who injected heroin but not POs, and a moderate-to-large sized group (11 – 30%) who injected both types of opioids. We also found positive associations with non-fatal overdose for participants who were recently incarcerated, had injected in public, required help injecting, engaged in heavy alcohol drinking, and used POs through non-injection routes of administration. These secondary findings are consistent with a well-established body of literature establishing clear associations with non-fatal overdose for these socio-structural and substance use patterns in the current setting (Kerr et al., 2007), and similar settings across North America (Coffin et al., 2007; Jenkins et al., 2011).
Our main finding is interesting in light of widespread concern related to opioid overdoses in the general population, as PO-related overdoses are a leading cause of injury-related death in the North America (Warner et al., 2011). It appears that exclusive PO injection may not pose a major threat for non-fatal overdose, compared to PWID who do not inject opioids. This finding might be partially explained by simply the avoidance of heroin, which is known to undergo fluctuations in potency and composition (Darke et al., 1999; Schneider and Meys, 2011; Smithson et al., 2004), and the fact that POs provide a reliable dose and composition. In fact, there have been several documented periods of overdoses (fatal and non-fatal) in the study region linked with unusually highly potent and/or fentanyl-tainted heroin (BC Coroners Service, 2011). Between 2012 and 2013, there was almost a 4-fold increase in province-wide fentanyl-related overdose deaths alongside various anecdotal reports of users’ mistaking fentanyl for another substance (e.g., heroin, oxycodone [Canadian Community Epidemiology Network on Drug Use (CCENDU; 2015)]. Over this same time period, the rate of non-fatal overdose in the present study peaked at 9%, suggesting the possible influence of illicit fentanyl. It has been argued, however, that opioid concentration alone may only play a minor – and sometimes non-significant – role in predicting overdose (Darke and Farrell, 2014; Risser et al., 2007). The small group of PO only-using PWID in our study might also differ from concurrent PO and heroin users in various ways that comparatively minimize risk for non-fatal overdose. For example, since heroin is readily available in this setting, people who inject POs may have a preference for POs for a specific purpose, such as self-management of pain or as a means of reducing use of heroin (“self-treating” opioid dependence or withdrawal) – both of which have been previously documented among people who use POs illicitly (Davis and Johnson, 2008; Hakansson et al., 2007; Monte et al., 2009; Voon et al., 2014). Furthermore, qualitative exploration in this environment has revealed a small subset of PWID who choose to inject POs rather than heroin as a harm reduction method, as the consistent purity and dosage of POs allows for the ability to more accurately monitor drug intake (Kerr T 2015, personal communication, May 27). This type of “harm reduction” through PO use has also been noted among younger recreational drug users, who commonly cite the ability to more accurately predict and manage dosing and the ability substitute other risky substance use (e.g., excessive alcohol consumption) as reasons for using POs (Quintero, 2009; Silva et al., 2013a). As research continues to characterize motives for engaging in nonmedical PO use and abuse among more generalized populations (McCabe et al., 2007; Young et al., 2012; Zacny and Lichtor, 2008), future qualitative studies may help to uncover motive-specific PO use among PWID.
Despite the absence of an association between exclusive PO use and non-fatal overdose when compared with non-opioid-using PWID, the majority of those who injected POs in this study also reported injecting heroin, and we have demonstrated that these individuals were at a much greater risk of non-fatal overdose. A previous study among these two cohorts noted a significant and positive relationship between PO use (i.e., injection and non-injection administration) and non-fatal overdose (Lake et al., 2015). In the current study, which controlled for non-injection PO use, we were able to identify some underlying trends in this previously noted association; namely, those who are using POs via non-injection and those who are injecting POs in addition to heroin may have accounted for the bulk of this group's risk for non-fatal overdose. PWID who use both types of opioids may be those who predominantly use heroin but will use POs when more easily available, as has been documented through qualitative exploration (Lankenau et al., 2012). Based on previous work characterizing PO-using PWID, these individuals might also be more likely to engage in higher-risk drug use, including poly-substance use (Bruneau et al., 2012; Khosla et al., 2011; Leclerc et al., 2011; Ompad et al., 2008). This interpretation is also in line with descriptive trends observed at baseline in the present study (Table 1). Finally, the strong association with non-fatal overdose identified among this sub-sample of poly-opioid users is consistent with reports pointing to the detection of other illicit substances in a large portion of PO-attributable overdose deaths (Hall et al., 2008; Jones et al., 2013; Roxburgh et al., 2011).
The findings of the present study suggest several implications for policy and programming aimed at reducing harms among people who use illicit opioids. In an attempt to curb rates of abuse, certain POs have switched to so-called abuse-deterrent formulations (Schneider et al., 2010). Previous research in this type of setting demonstrates that users may still attempt injection regardless of this formulation, or substitute with easier-to-inject opioids, such as heroin (Cassidy et al., 2014; Cicero et al., 2012; Coplan et al., 2013). As heroin injection – with and without concurrent PO injection – was found to be associated with an increased risk of non-fatal overdose, research should continue to explore strategies that facilitate safe preparation and injection of POs (e.g., single-use syringe filters, such as Sterifilt® (Roux et al., 2011)), including abuse-deterrent formulations, in order to prevent transitions to potentially more dangerous opioid injection. Particularly as the rate of non-fatal overdose has not decreased significantly in this study sample over time, our results support the scale-up of structural interventions that have been shown to reduce non-fatal overdose rates and prevent fatal overdoses. Take-home naloxone programs have shown great life-saving results locally (Oluwajenyo et al., 2014) and elsewhere (Strang et al., 2008). Yet, many opioid users in British Columbia do not carry naloxone or know how to use it (Sorge et al., 2015). Thus, increased inter-community collaboration between primary physicians, naloxone prescribers and distributors, and social service providers is urgently needed. People who inject POs as well as those who inject heroin should be targeted in these outreach and training efforts. This study exemplifies the high vulnerability of people who inject both types of opioids. While Vancouver is home to the sole supervised injection facility in North America, PO injection has been documented – often alongside other intensive illicit drug use – throughout Canada and the US (Firestone and Fischer, 2008; Johnson et al., 2013; Zibbell et al., 2014). Our findings strongly support the need to expand such facilities to other regions where opioid injection is prevalent. Finally, while various research – including secondary findings from the present study – has demonstrated the protective effect of methadone maintenance treatment on overdose (Brugal et al., 2005; Caplehorn et al., 1996; Kerr et al., 2007; Schwartz et al., 2013), retention in methadone treatment has long been a challenge among opioid users in British Columbia (Anderson and Warren, 2004). While methadone is the dominant opioid substitution therapy (OST) in this setting, promising second-line therapies such as diacetylmorphine (Oviedo-Joekes et al., 2009; Strang et al., 2010), hydromorphone (Oviedo-Joekes et al., 2010), and morphine (Hammig et al., 2014) have the potential to reduce overdose rates through improving treatment engagement and retention; however, widespread implementation of these programs has not received adequate political attention (Nosyk et al., 2013). Further research is required to understand how engagement and retention rates for different OST programs may differ across groups of people who inject POs (both primarily and occasionally) and those who use heroin in this type of population.
This study is subject to some limitations. First, despite extensive street outreach and snowball sampling recruitment efforts, VIDUS and ACCESS are not random samples, and our results may not be generalizable to other illicit drug-using populations. Secondly, we relied on self-reported data for this study, which may have invited recall inaccuracies and socially-desirable responses. Thus, while self-reported measures from PWID, including substance use behaviours have been shown to be reasonably valid and reliable (Darke, 1998), we cannot guarantee biased-free estimates. We used a broad definition of non-fatal overdose, which may have introduced bias to our outcome measure. Specifically, our definition is not exclusive to opioid overdose symptoms and may capture non-fatal overdoses related to other drugs (e.g., stimulants); however, we believe this broad definition of non-fatal overdose is advantageous in the current setting, where poly-drug use is the norm. This study was unable to capture data related to fatal overdoses, and may have missed any non-fatal overdoses associated with loss-to-follow-up (e.g., a non-fatal overdose leading to an extended hospital stay). While the majority of diverted POs are regulated pharmaceuticals (Fischer et al., 2010; Inciardi et al., 2007), we acknowledge there may have been cases where an illicitly manufactured PO was injected inadvertently (e.g., illicit fentanyl). Our study was not able to identify these cases. Finally, both the main explanatory and outcome measures referred to events in the previous six months, and we were unable to ascertain a temporal relationship between the two within the same six-month periods.
In conclusion, the present study demonstrated that, after controlling for co-occurring non-opioid illicit drug use, people who inject both POs and heroin, and those who inject heroin but not POs, are both at a significantly increased risk of non-fatal overdose, with the former group exhibiting the highest risk. Those who injected POs but not heroin were not at an increased risk of non-fatal overdose compared with non-opioid users. These findings should be interpreted in light of the current study population, which has an extensive history of poly-substance use. Our findings highlight the need for increased access to structural-level overdose prevention interventions for people who inject POs (and particularly those who also inject heroin). This includes widespread implementation of alternative evidence-based opioid substitution treatments programs, scale-up of take-home naloxone distribution and training, and expansion of supervised injection facilities.
Article Highlights.
This study assessed the independent association between prescription opioid (PO) injection and non-fatal overdose.
Data were obtained from two prospective cohorts of people who inject drugs in Vancouver, Canada.
The odds of non-fatal overdose were significantly increased for heroin-only injectors but not PO-only injectors.
The odds of non-fatal overdose were highest for participants who injected both heroin and POs.
Acknowledgments
We extend our gratitude to the participants in both the VIDUS and ACCESS studies for their contribution to this research, as well as current and past study researchers and staff. We would also like to thank the staff of the British Columbia Centre for Excellence in HIV/AIDS – specifically Tricia Collingham, Carmen Rock, Kristie Starr, Sabina Dobrer, Deborah Graham, Peter Vann, Jennifer Matthews, Steve Kain, and Calvin Lai for their research and administrative assistance.
Role of funding source: This study was supported by the US National Institutes of Health (R01DA021525 and U01DA038886), and was undertaken, in part, from funding through a Tier 1 Canada Research Chair in Inner City Medicine, which supports Dr. Evan Wood. Dr. M-J Milloy is supported in part by the United States National Institutes of Health (R01-DA021525). Dr. Kanna Hayashi is supported by a fellowship from the Canadian Institutes of Health Research. Beyond providing financial support, the funders did not contribute to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Julio Montaner has received educational grants from, served an ad hoc advisor to, or spoken at various events sponsored by: Abbott Laboratories, Agouron Pharmaceuticals Inc., Boehringer Ingelheim Pharmaceuticals Inc., Borean Pharma AS, Bristol-Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffman-La Roche, Immune Response Corporation, Incyte, Janssen-Ortho Inc., Kucera Pharmaceutical Company, Merck Frosst Laboratories, Pfizer Canada Inc., Sanofi Pasteur, Shire Biochem Inc., Tibotec Pharmaceuticals Ltd., and Trimeris Inc.
REFERENCES
- Anderson JF, Warren LD. Client retention in the British Columbia Methadone Program, 1996-1999. Can. J. Public Health. 2004;95:104–109. doi: 10.1007/BF03405776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BC Coroners Service [April 27, 2015];BC Coroners Service warns of rise in heroin-related deaths. 2011 http://www2.news.gov.bc.ca/news_releases_2009-2013/2011PSSG0059-000493.pdf.
- Bohnert AS, Valenstein M, Bair M, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- Brugal MT, Domingo-Salvany A, Puig R, Barrio G, García de Olalla P, De La Fuente L. Evaluating the impact of methadone maintenance programmes on mortality due to overdose and AIDS in a cohort of heroin users in Spain. Addiction. 2005;100:981–989. doi: 10.1111/j.1360-0443.2005.01089.x. [DOI] [PubMed] [Google Scholar]
- Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107:1318–1327. doi: 10.1111/j.1360-0443.2012.03803.x. [DOI] [PubMed] [Google Scholar]
- Canadian Community Epidemiology Network on Drug Use (CCENDU) CCENDU Bulletin: Deaths Involving Fentanyl in Canada, 2009-2014. 2015.
- Caplehorn JRM, Dalton MYNS, Haldar F, Petrenas A-M, Nisbet JG. Methadone maintenance and addicts' risk of fatal heroin overdose. Subst. Use Misuse. 1996;31:177–196. doi: 10.3109/10826089609045806. [DOI] [PubMed] [Google Scholar]
- Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 2014;15:440–451. doi: 10.1111/pme.12295. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention CDC grand rounds: prescription drug overdoses -a U.S. epidemic. MMWR. 2012;61:10–13. [PubMed] [Google Scholar]
- Cerda M, Ransome Y, Keyes KM, Koenen KC, Tracy M, Tardiff KJ, Vlahov D, Galea S. Prescription opioid mortality trends in New York City, 1990-2006: examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132:53–62. doi: 10.1016/j.drugalcdep.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N. Engl. J. Med. 2012;367:187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Tracy M, Bucciarelli A, Ompad D, Vlahov D, Galea S. Identifying injection drug users at risk of nonfatal overdose. Acad. Emerg. Med. 2007;14:616–623. doi: 10.1197/j.aem.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Coplan PM, Kale H, Sandstrom L, Landau C, Chilcoat HD. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol. Drug Saf. 2013;22:1274–1282. doi: 10.1002/pds.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–263. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
- Darke S, Farrell M. Would legalizing illicit opioids reduce overdose fatalities? Implications from a natural experiment. Addiction. 2014;109:1237–1242. doi: 10.1111/add.12456. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W, Weatherburn D, Lind B. Fluctuations in heroin purity and the incidence of fatal heroin overdose. Drug Alcohol Depend. 1999;54:155–161. doi: 10.1016/s0376-8716(98)00159-8. [DOI] [PubMed] [Google Scholar]
- Darke S, Williamson A, Ross J, Teesson M. Non-fatal heroin overdose, treatment exposure and client characteristics: findings from the Australian treatment outcome study (ATOS). Drug Alcohol Rev. 2005;24:425–432. doi: 10.1080/09595230500286005. [DOI] [PubMed] [Google Scholar]
- Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, Green JL. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- Davis WR, Johnson BD. Prescription opioid use, misuse, and diversion among street drug users in New York City. Drug Alcohol Depend. 2008;92:267–276. doi: 10.1016/j.drugalcdep.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Firestone M, Fischer B. A qualitative exploration of prescription opioid injection among street-based drug users in Toronto: behaviours, preferences and drug availability. Harm Reduct. J. 2008;5:30. doi: 10.1186/1477-7517-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Bibby M, Bouchard M. The global diversion of pharmaceutical drugs non-medical use and diversion of psychotropic prescription drugs in North America: a review of sourcing routes and control measures. Addiction. 2010;105:2062–2070. doi: 10.1111/j.1360-0443.2010.03092.x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Brissette S, Brochu S, Bruneau J, El-Gabalawy R, Noel L, Rehm J, Tyndall M, Wild C, Mun P, Haydon E, Baliunas D. Determinants of overdose incidents among illicit opioid users in 5 Canadian cities. CMAJ. 2004;171:235–239. doi: 10.1503/cmaj.1031416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Jones W, Urbanoski K, Skinner R, Rehm J. Correlations between prescription opioid analgesic dispensing levels and related mortality and morbidity in Ontario, Canada, 2005-2011. Drug Alcohol Rev. 2014;33:19–26. doi: 10.1111/dar.12089. [DOI] [PubMed] [Google Scholar]
- Fischer B, Patra J, Firestone Cruz M, Gittins J, Rehm J. Comparing heroin users and prescription opioid users in a Canadian multi-site population of illicit opioid users. Drug Alcohol Rev. 2008;27:625–632. doi: 10.1080/09595230801956124. [DOI] [PubMed] [Google Scholar]
- Hakansson A, Medvedeo A, Andersson M, Berglund M. Buprenorphine misuse among heroin and amphetamine users in Malmo, Sweden: purpose of misuse and route of administration. Eur. Addict. Res. 2007;13:207–215. doi: 10.1159/000104883. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, Crosby AE, Paulozzi LJ. Patterns of abuse among unintentional pharmaceutical opioid overdose fatalities. JAMA. 2008;300:2613–2620. doi: 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- Hammig R, Kohler W, Bonorden-Kleij K, Weber B, Lebentrau K, Berthel T, Babic-Hohnjec L, Vollmert C, Hopner D, Gholami N, Verthein U, Haasen C, Reimer J, Ruckes C. Safety and tolerability of slow-release oral morphine versus methadone in the treatment of opioid dependence. J. Subst. Abuse Treat. 2014;47:275–281. doi: 10.1016/j.jsat.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Havens JR, Oser CB, Knudsen HK, Lofwall M, Stoops WW, Walsh SL, Leukefeld CG, Kral AH. Individual and network factors associated with non-fatal overdose among rural Appalachian drug users. Drug Alcohol Depend. 2011;115:107–112. doi: 10.1016/j.drugalcdep.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens JR, Walker R, Leukefeld CG. Prevalence of opioid analgesic injection among rural nonmedical opioid analgesic users. Drug Alcohol Depend. 2007;87:98–102. doi: 10.1016/j.drugalcdep.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Inciardi JA, Surratt HL, Kurtz SP, Cicero TJ. Mechanisms of prescription drug diversion among drug-involved club- and street-based populations. Pain Med. 2007;8:171–183. doi: 10.1111/j.1526-4637.2006.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Banta-Green CJ, Maynard C, Kingston S, Hanrahan M, Merrill JO, Coffin PO. Risk factors for nonfatal overdose at Seattle-area syringe exchanges. J. Urban Health. 2011;88:118–128. doi: 10.1007/s11524-010-9525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Fibbi M, Langer D, Silva K, Lankenau SE. Prescription drug misuse and risk behaviors among young injection drug users. J. Psychoactive Drugs. 2013;45:112–121. doi: 10.1080/02791072.2013.785811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E. Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend. 2007;87:39–45. doi: 10.1016/j.drugalcdep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Khosla N, Juon HS, Kirk GD, Astemborski J, Mehta SH. Correlates of non-medical prescription drug use among a cohort of injection drug users in Baltimore City. Addict. Behav. 2011;36:1282–1287. doi: 10.1016/j.addbeh.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner SA, Milloy MJ, Wood E, Qi J, Zhang R, Kerr T. Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addict. Behav. 2012;37:691–696. doi: 10.1016/j.addbeh.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake S, Wood E, Buxton J, Dong H, Montaner J, Kerr T. Prescription opioid use and non-fatal overdose in a cohort of injection drug users. Am J. Drug Alcohol Ab. 2015;41:257–263. doi: 10.3109/00952990.2014.998366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankenau S, Teti M, Silva K, Bloom J, Harocopos A, Treese M. Patterns of prescription drug misuse among young injection drug users. J. Urban Health. 2012;89:1004–1016. doi: 10.1007/s11524-012-9691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc P, Roy E, Morissette C, Michel A, Parent R, SurvUDI Working Group Extent And Correlates Of Injection Of Prescription Opioids For Non-Medical Purposes In The Survudi Network. 20th Annual Canadian Conference on HIV/AIDS Research. Can. J. Infect. Dis. Med. Microbiol., Toronto, Ontario. 2011:80b. [Google Scholar]
- Lee JH, Herzog TA, Meade CD, Webb MS, Brandon TH. The use of GEE for analyzing longitudinal binomial data: a primer using data from a tobacco intervention. Addict. Behav. 2007;32:187–193. doi: 10.1016/j.addbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician. 2008;11:S63–88. [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Boyd CJ, Teter CJ. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict. Behav. 2007;32:562–575. doi: 10.1016/j.addbeh.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C, Kerr T, Strathdee S, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm Reduct. J. 2007;4:1–7. doi: 10.1186/1477-7517-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Kerr T, Mathias R, Zhang R, Montaner JS, Tyndall M, Wood E. Non-fatal overdose among a cohort of active injection drug users recruited from a supervised injection facility. Am. J. Drug Alcohol Abuse. 2008;34:499–509. doi: 10.1080/00952990802122457. [DOI] [PubMed] [Google Scholar]
- Mitra G, Wood E, Nguyen P, Kerr T, DeBeck K. Drug use patterns predict risk of non-fatal overdose among street-involved youth in a Canadian setting. Drug Alcohol Depend. 2015;153:135–139. doi: 10.1016/j.drugalcdep.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte AA, Mandell T, Wilford BB, Tennyson J, Boyer EW. Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J. Addict. Dis. 2009;28:226–231. doi: 10.1080/10550880903014767. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism [September 1, 2015];Drinking Levels Defined. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, Wood E, Montaner JS. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff. (Millwood) 2013;32:1462–1469. doi: 10.1377/hlthaff.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Marshall BD, Fischer B, Montaner JS, Wood E, Kerr T. Increases in the availability of prescribed opioids in a Canadian setting. Drug Alcohol Depend. 2012;126:7–12. doi: 10.1016/j.drugalcdep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa KC, Davidson PJ, Evans JL, Hahn JA, Page-Shafer K, Moss AR. Heroin overdose among young injection drug users in San Francisco. Drug Alcohol Depend. 2005;80:297–302. doi: 10.1016/j.drugalcdep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Oluwajenyo B, Tzemis D, Al-Qutub D, Amlani A, Kesselring S, Buxton JA. A quantitative and qualitative evaluation of the British Columbia Take Home Naloxone program. CMAJ Open. 2014;2:E153–E161. doi: 10.9778/cmajo.20140008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ompad DC, Fuller CM, Chan CA, Frye V, Vlahov D, Galea S. Correlates of illicit methadone use in New York City: a cross-sectional study. BMC Public Health. 2008;8:375. doi: 10.1186/1471-2458-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, Schechter MT. Diacetylmorphine versus methadone for the treatment of opioid addiction. N. Engl. J. Med. 2009;361:777–786. doi: 10.1056/NEJMoa0810635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Joekes E, Guh D, Brissette S, Marsh DC, Nosyk B, Krausz M, Anis A, Schechter MT. Double-blind injectable hydromorphone versus diacetylmorphine for the treatment of opioid dependence: a pilot study. J. Subst. Abuse Treat. 2010;38:408–411. doi: 10.1016/j.jsat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Palepu A, Tyndall M, Leon H, Muller J, O'Shaughnessy MV, Schechter MT, Anis A. Hospital utilization and costs in a cohort of injection drug users. CMAJ. 2001;165:415–420. [PMC free article] [PubMed] [Google Scholar]
- Pollini RA, McCall L, Mehta SH, Vlahov D, Strathdee SA. Non-fatal overdose and subsequent drug treatment among injection drug users. Drug Alcohol Depend. 2006;83:104–110. doi: 10.1016/j.drugalcdep.2005.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero G. Rx for a party: a qualitative analysis of recreational pharmaceutical use in a collegiate setting. J. Am. Coll. Health. 2009;58:64–70. doi: 10.3200/JACH.58.1.64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, Ojala V, Ojala A, Bowles SK, Banh HL. Monitoring programs for drugs with potential for abuse or misuse in Canada. Can. Pharm. J. 2012;145:168–171. doi: 10.3821/145.4.cpj168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser D, Uhl A, Oberndorfer F, Hönigschnabl S, Stichenwirth M, Hirz R, Sebald D. Is there a relationship between street heroin purity and drug-related emergencies and/or drug-related deaths? An analysis from Vienna, Austria. J. Forensic Sci. 2007;52:1171–1176. doi: 10.1111/j.1556-4029.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Roux P, Carrieri MP, Keijzer L, Dasgupta N. Reducing harm from injecting pharmaceutical tablet or capsule material by injecting drug users. Drug Alcohol Rev. 2011;30:287–290. doi: 10.1111/j.1465-3362.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- Roxburgh A, Ritter A, Grech K, Slade T, Burns L. Trends in Drug Use and Related Harms in Australia, 2001 to 2011. National Drug and Alcohol Research Centre, University of New South Wales; Sydney: 2011. [Google Scholar]
- Schneider J, Matthews M, Jamison R. Abuse-deterrent and tamper-resistant opioid formulations. CNS Drugs. 2010;24:805–810. doi: 10.2165/11584260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schneider S, Meys F. Analysis of illicit cocaine and heroin samples seized in Luxembourg from 2005–2010. Forensic Sci. Int. 2011;212:242–246. doi: 10.1016/j.forsciint.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, O'Grady KE, Sharfstein JM, Warren G, Olsen Y, Mitchell SG, Jaffe JH. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am. J. Public Health. 2013;103:917–922. doi: 10.2105/AJPH.2012.301049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Cheng Y, Kral AH. Prevalence and correlates of opiate overdose among young injection drug users in a large U.S. city. Drug Alcohol Depend. 2007;88:182–187. doi: 10.1016/j.drugalcdep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K, Kecojevic A, Lankenau SE. Perceived drug use functions and risk reduction practices among high-risk nonmedical users of prescription drugs. J. Drug Issues. 2013a;43:483–496. doi: 10.1177/0022042613491099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K, Schrager SM, Kecojevic A, Lankenau SE. Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs. Drug Alcohol Depend. 2013b;128:104–110. doi: 10.1016/j.drugalcdep.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson M, McFadden M, Mwesigye S-E, Casey T. The impact of illicit drug supply reduction on health and social outcomes: the heroin shortage in the Australian Capital Territory. Addiction. 2004;99:340–348. doi: 10.1046/j.1360-0443.2003.00603.x. [DOI] [PubMed] [Google Scholar]
- Sorge J, Buxton J, Amlani A, Ishiguro S. Substance Use Trends in BC: A Survey of Harm Reduction Clients. BC Centre for Disease Control; Vancouver: 2015. [Google Scholar]
- Stoove MA, Dietze PM, Jolley D. Overdose deaths following previous non-fatal heroin overdose: record linkage of ambulance attendance and death registry data. Drug Alcohol Rev. 2009;28:347–352. doi: 10.1111/j.1465-3362.2009.00057.x. [DOI] [PubMed] [Google Scholar]
- Strang J, Manning V, Mayet S, Best D, Titherington E, Santana L, Offor E, Semmler C. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction. 2008;103:1648–1657. doi: 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, Williams H, Zador D, Evers R, Groshkova T, Charles V, Martin A, Forzisi L. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet. 2010;375:1885–1895. doi: 10.1016/S0140-6736(10)60349-2. [DOI] [PubMed] [Google Scholar]
- Strathdee S, Palepu A, Cornelisse PGA, Yip B, O'Shaughnessy MV, Montaner J, Schechter MT, Hogg R. Barriers to use of free antiretroviral therapy in injection drug users. JAMA. 1998;280:547–549. doi: 10.1001/jama.280.6.547. [DOI] [PubMed] [Google Scholar]
- Trescot A, Datta S, Lee M, Hansen H. Opioid pharmacology. Pain Physician. 2008;11:S133–S153. [PubMed] [Google Scholar]
- Voon P, Callon C, Nguyen P, Dobrer S, Montaner J, Wood E, Kerr T. Self-management of pain among people who inject drugs in Vancouver. Pain Manag. 2014;4:27–35. doi: 10.2217/pmt.13.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. Drug poisoning deaths in the United States, 1980-2008. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- Warner-Smith M, Darke S, Day C. Morbidity associated with non-fatal heroin overdose. Addiction. 2002;97:963–967. doi: 10.1046/j.1360-0443.2002.00132.x. [DOI] [PubMed] [Google Scholar]
- Warner-Smith M, Darke S, Lynskey M, Hall W. Heroin overdose: causes and consequences. Addiction. 2001;96:1113–1125. doi: 10.1046/j.1360-0443.2001.96811135.x. [DOI] [PubMed] [Google Scholar]
- Werb D, Kerr T, Nosyk B, Strathdee S, Montaner J, Wood E. The temporal relationship between drug supply indicators: an audit of international government surveillance systems. BMJ Open. 2013;3:e003077. doi: 10.1136/bmjopen-2013-003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, McCabe SE, Cranford JA, Ross-Durow P, Boyd CJ. Nonmedical use of prescription opioids among adolescents: subtypes based on motivation for use. J. Addict. Dis. 2012;31:332–341. doi: 10.1080/10550887.2012.735564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Nonmedical use of prescription opioids: motive and ubiquity issues. J. Pain. 2008;9:473–486. doi: 10.1016/j.jpain.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Hart-Malloy R, Barry JL, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am. J. Public Health. 2014;104:2226–2232. doi: 10.2105/AJPH.2014.302142. [DOI] [PMC free article] [PubMed] [Google Scholar]