Abstract
Brain-derived neurotrophic factor (BDNF) is abundantly expressed by both developing and adult rat visceral sensory neurons from the nodose ganglion (NG) in vivo and in vitro. We have previously shown that BDNF is released from neonatal NG neurons by activity and regulates dendritic development in their postsynaptic targets in the brainstem. The current study was carried out to examine the cellular and molecular mechanisms of activity-dependent BDNF expression in neonatal rat NG neurons, using our established in vitro model of neuronal activation by electrical field stimulation with patterns that mimic neuronal activity in vivo.
We show that BDNF mRNA (transcript 4) increases over 3-fold in response to a 4-h tonic or bursting pattern delivered at the frequency of 6 Hz, which corresponds to the normal heart rate of a newborn rat. No significant increase in BDNF expression was observed following stimulation at 1 Hz. The latter effect suggests a frequency-dependent mechanism of regulated BDNF expression. In addition to BDNF transcript 4, which is known to be regulated by activity, transcript 1 also showed significant upregulation. The increases in BDNF mRNA were followed by BDNF protein upregulation of a similar magnitude after 24 h of stimulation at 6 Hz. Electrical stimulation-evoked BDNF expression was inhibited by pretreating neurons with the blocker of voltage-gated sodium channels tetrodotoxin and by removing extracellular calcium. Moreover, our data show that repetitive stimulation-evoked BDNF expression requires calcium influx through N-, but not L-type, channels.
Together, our study reveals novel mechanisms through which electrical activity stimulates de novo synthesis of BDNF in sensory neurons, and points to the role of N-type calcium channels in regulating BDNF expression in sensory neurons in response to repetitive stimulation.
Keywords: Baroreceptor, Calcium channels, Electrical field stimulation, Nodose ganglion
Graphical Abstract

Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of neuronal growth factors, is a key player in activity-dependent mechanisms of neuronal maturation and synaptic plasticity (Huang and Reichardt, 2001; Poo, 2001), including somatosensory pathways (Malcangio and Lessmann, 2003). Studies from our and other laboratories indicate that BDNF is also a mediator of activity-dependent plasticity in visceral sensory pathways with first-order neurons in the nodose ganglion (NG; Balkowiec et al., 2000; Clark et al., 2011; Martin et al., 2012; Martin et al., 2009).
BDNF is abundantly expressed in rat NG neurons in vivo throughout the postnatal development and into adulthood, and the magnitude of BDNF release from these neurons is regulated in vitro by physiological patterns of electrical stimulation (Balkowiec and Katz, 2000; Martin et al., 2009). BDNF is expressed not only in cell bodies, but also central terminals of NG neurons in the brainstem nucleus tractus solitarius (NTS), where NG neurons form first-order synapses of cardiorespiratory control pathways. Consistent with BDNF release from central terminals of NG afferents in the NTS, BDNF regulates dendritic morphology of developing NTS neurons (Martin et al., 2012). In addition, hypertension, a natural stimulus for NG afferents (Jones and Thoren, 1977), is associated with increased BDNF expression in both the NG and the NTS in vivo (Vermehren-Schmaedick et al., 2013). These data suggest that BDNF expression is regulated in NG neurons in an activity-dependent manner, but the underlying mechanisms are unknown.
BDNF expression in CNS neurons depends on calcium influx through voltage-gated calcium channels. Previous studies in which neurons were activated by chronic depolarization indicate that the channel involved in regulation of BDNF expression is the L-type channel (Finkbeiner and Greenberg, 1998). On the other hand, when NG neurons are depolarized in a phasic rather than tonic manner, expression of the enzyme tyrosine hydroxylase is not dependent on L-type, but instead on N-type channels (Brosenitsch and Katz, 2001). Yet, the role of voltage-gated calcium channels in gene expression evoked by repetitive stimulation has not been described for the BDNF gene. Also, the cellular and molecular mechanisms of BDNF expression in NG neurons, such as the role of stimulation frequency and contribution of individual transcripts, have not been addressed.
The present study was designed to examine the effects of repetitive electrical field stimulation at physiologically-relevant frequencies on BDNF mRNA and protein expression in neurons, using dissociated cultures of the neonatal rat NG as a model. We have characterized the frequency dependence of BDNF expression, the contribution of individual transcripts of the BDNF gene, and the role of voltage-gated sodium and calcium channels. Portions of this work have previously been published in abstract form (Vermehren-Schmaedick and Balkowiec, 2009).
EXPERIMENTAL PROCEDURES
Animals
Postnatal day 1–3 Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study. All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University, and conformed to the Policies on the Use of Animals and Humans in Neuroscience Research approved by the Society for Neuroscience.
Preparation of NG cultures
Animals were euthanized by intraperitoneal injection of Euthasol (0.1 mg/kg) and decapitated. Nodose ganglia were dissected and dissociated as previously described by our laboratory (Hsieh et al., 2010; Martin et al., 2009). In order to obtain neuron-enriched cultures, 12–16 nodose ganglia dissociated in 0.5 ml of plating medium were layered on top of a two-layer gradient of Percoll (Sigma, St. Louis, MO; 35%-60%; 1 ml each) and centrifuged for 10 min at 800 x g at 4 °C. A vast majority of non-neuronal cells and cell debris formed a visible thin layer at the ‘plating medium-35%’ interface and, therefore, could be easily separated from neurons accumulated at the ‘35%–60%’ interface. The neuron-enriched interface was collected and rinsed twice with culture medium before plating in 48-well plates (Nunclon Surface; Nalge Nunc Int.) pre-coated with poly-D-lysine (0.1 mg/ml; Sigma) and laminin (0.4 μg/ml; Sigma). Two to three dispersed nodose ganglia were plated per one culture well, and an average experiment consisted of 16 wells (8 treatment conditions in duplicates), including electrode-fitted but not stimulated controls, each treated with either a vehicle or a drug. The cultures were grown in Neurobasal-A medium (Invitrogen) supplemented with B-27 serum-free supplement (Invitrogen), 0.5 mM L-glutamine (Invitrogen), 2.5% fetal bovine serum (HyClone, Logan, UT), and 1% Penicillin-Streptomycin-Neomycin antibiotic mixture (Invitrogen), for 5–7 days at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The medium was replaced with fresh medium every 3 days, and 24 h before the beginning of electrical field stimulation.
Electrical field stimulation
Following the initial incubation, NG cultures were stimulated in 48-well plates. The wells were fitted with paired platinum electrodes (0.25 mm wire diameter; one pair per well), connected in parallel (four wells per set) to the stimulator (MultiStim System; Digitimer; Welwyn Garden City, Hertfordshire, UK). The stimulation pattern delivered independently by each output of the stimulator was controlled by the 8-channel programmable pulse generator Master-8-cp (AMPI, Jerusalem, Israel). Two additional sets of four wells were fitted with pairs of electrodes, but not connected to the stimulator (non-stimulated controls). Our previous studies showed that the presence of electrodes by itself does not affect activity-dependent regulation of BDNF (Hsieh et al., 2010). The plate was placed in the incubator, and the neurons were stimulated with biphasic rectangular pulses of 0.2 ms duration and amplitude of 100 mA per well, delivered at various patterns (see Results) for 1, 2, 4 h (BDNF mRNA) or 24 h (BDNF protein).
Pharmacological treatment
The treatment began 30 min prior to electrical stimulation, and continued throughout the stimulation. Each drug was added to both stimulated and unstimulated cultures. Tetrodotoxin (TTX; 3 mM stock in citrate buffer, pH=4.8; Tocris Bioscience, Ellisville, MO), ω-conotoxin GVIA (50 μM stock in PBS; Sigma), and nimodipine (10 mM stock in ethanol; Sigma), were used at final concentrations of 1.5 μM, 1 μM, and 2 μM, respectively.
Measurement of BDNF cell content
Immediately following removal of the culture medium, NG cultures were treated with pre-chilled lysis buffer (20 mM Tris buffer, pH 7.4, 137 mM NaCl, 1% Nonidet-P40, 10% glycerol, 1 mM PMSF, 0.5 mM sodium vanadate, 10 μM aprotinin, 10 μM actinonin, and 100 μM leupeptin; 75 μl/well). Next, the cells were scraped off and the entire well content was transferred to a siliconized (Sigmacote®; Sigma) and pre-chilled 1.5-ml microcentifuge tube. Each well was then rinsed with 150 μl of pre-chilled Block & Sample buffer (1x; BDNF Emax™ ImmunoAssay System, Promega), and the well content was transferred into the microcentrifuge tube to combine with the cell lysate. The content of two identically treated sister culture wells was combined in one microcentrifuge tube, and each sample (the total volume of 450 μl) was sonicated on ice using a microprobe sonicator (2 × 1.5 W, 5 s each; Sonicator 3000, Misonix, Inc., Farmingdale, NY). Subsequently, the samples were transferred to an anti-BDNF-coated 96-well ELISA plate (4 wells/sample). BDNF ELISA was performed according to the manufacturer’s instructions (BDNF Emax™ ImmunoAssay System, Promega). We have previously confirmed the specificity of the BDNF ELISA kit in our model using RNA interference (Hsieh et al., 2010).
RNA extraction and quantitative RT-PCR
RNA extraction and qPCR were performed as previously described by our laboratory (Balkowiec-Iskra et al., 2011; Hsieh et al., 2010; Vermehren-Schmaedick et al., 2013). Briefly, total RNA was extracted using TRIzol (Invitrogen) and re-suspended in 10 mM Tris pH 8.5. To make cDNA, 1 μg of total RNA was reverse transcribed using Tetro cDNA synthesis kit (Bioline, Taunton, MA) following the manufacturer’s instructions. Quantitative PCR was performed using Sensimix Plus SYBR master mix (Quantace, Taunton, MA) and the MX3000P real time PCR system (Stratagene; Cedar Creek, TX). The forward primers for BDNF were: 5′-aaagccgaacttctcacatgat-′ (BDNF1), 5′-ctcccccttttaactgaagagaa-3′ (BDNF4), ctttggggcagacgagaaag-3′ (BDNF6), with a common reverse primer, 5′-attcacgctctccagagtcc-3′. Primers for the BDNF protein-coding sequence (CDS), designed to amplify all BDNF transcripts, were: 5′-ggtcacagcggcagataaaaagac- 3′ (forward) and 5′-ttcggcattgcgagttccag-3′ (reverse; Fig. 1). The PCR products were 273, 280, 275 and 188 bp, respectively. Data normalization was carried out against the endogenous reference gene transcript hypoxanthine-guanidine phosphoribotransferase (HPRT, forward: 5′-caggccagactttgttggat-3, reverse: 5′-ggctgcctacaggctcatag-3′; PCR product of 328 bp in size). Genbank accession numbers are EF125675.1 (BDNF-I), EF125679.1 (BDNF-IV/formerly known as BDNF-III), EF125680.1 (BDNF-VI/formerly known as BDNF-IV), NM012513 (BDNF-CDS), and NM012583.2 (HPRT).
Figure 1. Multiple promoters of the rat BDNF gene and BDNF transcripts examined in the present study.
(A) A schematic of the BDNF gene structure, as described by Aid et al. (2007), with exons and introns shown as boxes and lines, respectively. Roman numerals I through IX refer to promoter regions within respective untranslated regions (UTRs). The translated protein-coding sequence (CDS) is shown in dark blue. UTRs containing activity-dependent promoters I, IV and VI relevant to this study are shown in yellow, magenta, and green, respectively. (B) A schematic of BDNF transcripts measured in this study, where the 5′ untranslated exons I (yellow), IV (magenta) and VI (green), respectively, are spliced to the common 3′ protein-coding region. Arrows indicate primer sets (F, forward; R, reverse) used for real-time polymerase chain reactions in this study.
Calculations and statistical analysis
Each value of activity-dependent BDNF expression was calculated by subtracting levels of BDNF in unstimulated, but treated with vehicle or drug, cultures from levels of BDNF measured in stimulated, and correspondingly treated, cultures. Consequently, the amount of BDNF expressed over the culture period prior to stimulation was not taken into account. BDNF levels were calculated from the standard curve prepared for each plate, using SOFTmax PRO® vs. 4.3 software (Molecular Devices). The standard curves were linear within the range used (0–500 pg/ml) and the quantities of BDNF in experimental samples were always within the linear range of the standard curve. Data are expressed as mean ± standard error, and the sample size (n) represents the number of individual cultures. Samples were compared using ANOVA followed by Duncan’s or Tukey’s multiple comparison procedure, and P<0.05 was considered significant.
RESULTS
Electrical stimulation-mediated BDNF expression in NG neurons is regulated primarily by bdnf promoter IV
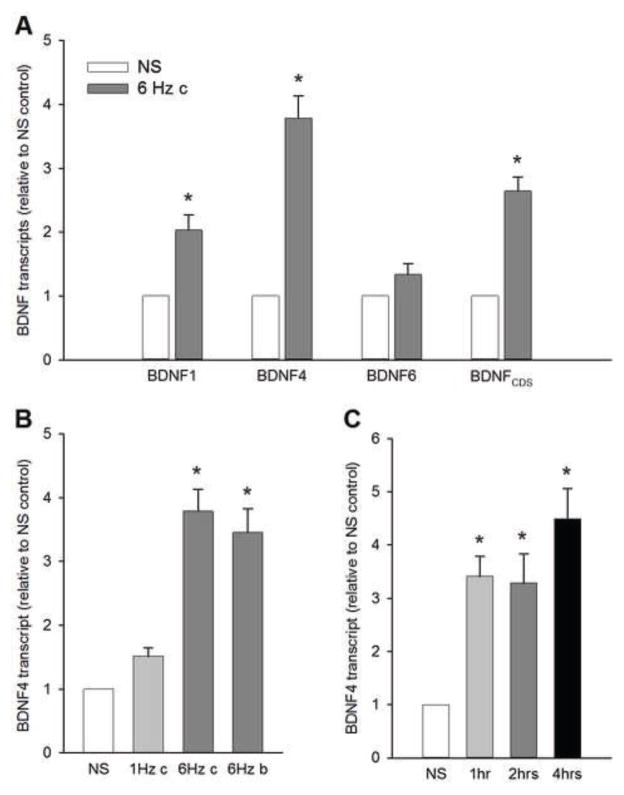
Neuronal activity is known to upregulate BDNF expression (Castren et al., 1992; Ernfors et al., 1991; Isackson et al., 1991) and, in other neuronal systems, the process involves several bdnf transcripts that are differentially generated in response to specific stimuli and their downstream signaling pathways (Cohen and Greenberg, 2008). The best-characterized promoters of the BDNF gene that are regulated by activity include promoter I, IV (formerly known as III), and VI (formerly known as IV; Fig. 1; Aid et al., 2007; Finkbeiner, 2000; Hong et al., 2008; Tabuchi et al., 2000; Tao et al., 1998; West et al., 2001). Therefore, we first compared levels of individual bdnf transcripts in NG neurons activated by electrical field stimulation that is relevant to the physiological activity of cardiovascular afferents whose cell bodies reside in the NG, such as arterial baroreceptors. Arterial baroreceptors express high levels of BDNF during postnatal development and into adulthood (Martin et al., 2009), and their firing pattern is associated with the arterial pulse and, thus, the heart rate. For these studies, we used a tonic stimulation at 6 Hz (the inter-pulse interval of 166.7 ms), which corresponds to the average heart rate of a newborn rat and, thereby, the basic firing frequency of baroreceptor and other cardiovascular afferents that follow blood pressure rises during each cardiac cycle.
A 4-h stimulation of NG neurons at 6 Hz resulted in a significant upregulation of bdnf transcripts specifically from exon I and IV, and the protein-coding region, but not exon VI. Moreover, the magnitude of the increase in bdnf transcript 4 was nearly two times greater than the increase in bdnf transcript 1 (Fig. 2A). This suggests a higher sensitivity of bdnf promoter IV to electrical stimulation-mediated activation compared with promoter I. Together, these data indicate that repetitive electrical stimulation of NG neurons at physiologically-relevant frequencies leads to an upregulation of transcription of the bdnf gene in a promoter-selective manner. In subsequent experiments of this study we focused on bdnf transcript 4. BDNF mRNA expression in NG neurons depends on stimulation frequency, but not pattern.
Figure 2. Electrical stimulation-evoked BDNF mRNA expression in NG neurons is regulated primarily by bdnf promoter IV, and depends on stimulation frequency, but not pattern.
(A) Levels of activity-dependent (BDNF1, BDNF4, and BDNF6) and total protein-coding region (BDNFCDS) transcripts following a 4-h stimulation at 6 Hz (gray bars). N=15 independent cultures. (B) Levels of BDNF 4 transcript following: a continuous stimulation at 1 Hz (1 Hz c), a continuous stimulation at 6 Hz (6 Hz c), and a bursting stimulation at 6 Hz (6 Hz b, 4 pulses per burst), delivered for 4 hrs. N=6 independent cultures. (C) BDNF mRNA expression significantly increases already within an hour of stimulation. Levels of BDNF 4 transcript following a continuous stimulation at 6 Hz delivered for 1 hr, 2 hrs, or 4 hrs. N=4 independent cultures. Data have been normalized to the endogenous reference gene transcript hypoxanthine-guanidine phosphoribotransferase (HPRT), and presented as relative to non-stimulated (NS) control levels (white bars). *, different from NS (P<0.05; ANOVA followed by Tukey’s multiple comparison).
BDNF mRNA expression in NG neurons depends on stimulation frequency, but not pattern
Previous studies from our laboratory indicate that the magnitude of BDNF release from NG neurons is dependent on the stimulation pattern (Martin et al., 2009). Recently, we have also shown that hypertension, which increases activity of cardiovascular NG afferents, leads to upregulation of NG BDNF expression. Therefore, in our quest for understanding the cellular mechanisms of activity-dependent BDNF expression in NG neurons, we compared the effects of 6-Hz stimulation with two other patterns that are also relevant to the physiological activity of cardiovascular afferents: i) stimulation at 1 Hz, which corresponds to a low-level activity in baroreceptor afferents that are not likely to be activated in each cardiac cycle at normal levels of blood pressure, and ii) bursting stimulation at the inter-burst frequency of 6 Hz (4 pulses per burst, delivered at the intra-burst frequency of 72 Hz, i.e. the inter-pulse interval of 13.89 ms; the average stimulation frequency of 24 Hz), which corresponds to the activity in baroreceptor afferents that respond to greater blood pressure rises with multiple action potentials (Liu et al., 1998; Martin et al., 2009).
A 4-h stimulation of sister cultures resulted in significant, over 3-fold increases in BDNF mRNA expression (transcript 4) in response to 6-Hz stimulation patterns, but not 1-Hz stimulation. Increasing the duration of stimulation at 1 Hz and 6 Hz from 4 to 6 h did not significantly increase the magnitude of the upregulation (data not shown). Moreover, there was no significant difference in the magnitude of BDNF expression between continuous and bursting pattern of 6-Hz stimulation (Fig. 2B), despite the 4 times greater total number of pulses delivered to NG neurons with the bursting pattern. Together, these data indicate that the mechanisms regulating BDNF expression in NG neurons are stimulation frequency-dependent, but do not discriminate between bursting and tonic stimulation or the total number of delivered pulses. Based on these data, we used 6-Hz continuous stimulation for the remainder of experiments in this study.
Since BDNF expression is mediated by an immediate early gene, we also examined the time course of BDNF upregulation by comparing levels of BDNF transcript 4 among sister cultures of NG neurons stimulated at 6 Hz for 1, 2, and 4 hrs. Our data show that BDNF mRNA expression is significantly upregulated already after 1 and 2 hrs, but a 4-hr stimulation consistently yields significantly higher levels of expression (Fig. 2C).
Electrical stimulation-evoked increase in BDNF mRNA in NG neurons requires tetrodotoxin (TTX)-sensitive sodium currents
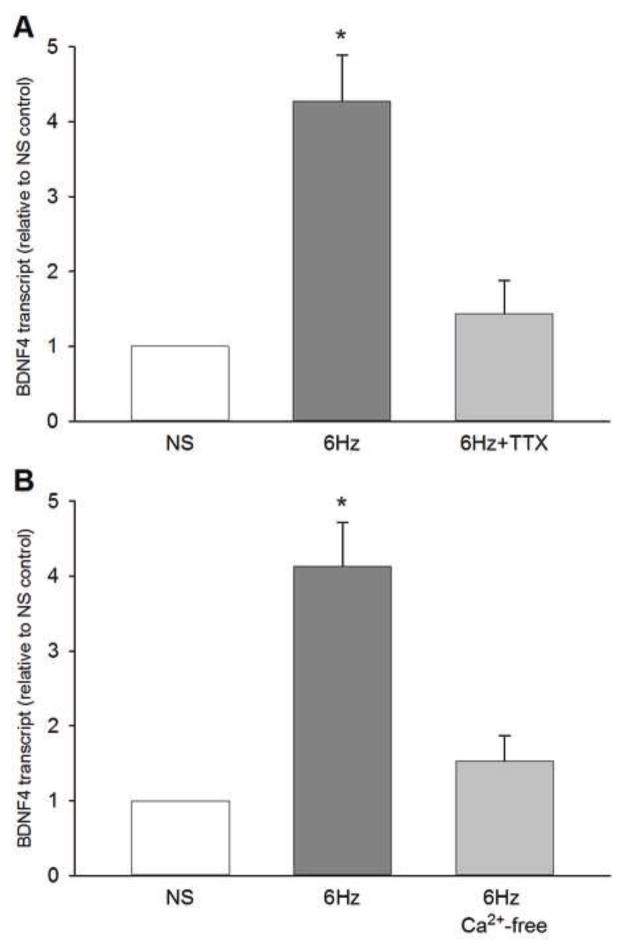
Neuronal action potentials are determined to a large extent by activity of voltage-dependent sodium channels, and primary sensory neurons express both TTX-sensitive and TTX-resistant sodium channels. To determine whether TTX-sensitive sodium channels are involved in the mechanisms of de novo BDNF synthesis in electrically-stimulated NG neurons, we examined the effects of pretreatment of NG cultures with TTX on BDNF mRNA levels.
One half of NG neuron cultures was pre-treated with TTX (1.5 μM) for 30 min prior to the beginning of a 4-h electrical field stimulation at 6 Hz. The sister cultures were treated with vehicle PBS and served as controls. TTX abolished the electrical stimulation-induced upregulation of BDNF mRNA (Fig. 3A). These data show that de novo synthesis of BDNF in NG neurons in response to repetitive electrical stimulation requires TTX-sensitive sodium currents.
Figure 3. Sodium influx through tetrodotoxin (TTX)-sensitive channels and extracellular calcium are required for electrical stimulation-evoked BDNF mRNA expression.
(A) Levels of BDNF 4 transcript following a 4-h continuous stimulation at 6 Hz in the absence (6 Hz) or presence (6 Hz + TTX) of tetrodotoxin (TTX), a blocker of voltage-gated sodium channels. N=5 independent cultures. (B) Levels of BDNF 4 transcript following a 4-h continuous stimulation at 6 Hz in normal extracellular calcium (6 Hz) or its relative absence (6 Hz Ca2+-free). N=4 independent cultures. BDNF transcript levels were normalized to the HPRT transcript, and presented as relative to non-stimulated (NS) control levels. *, different from NS (P<0.05; ANOVA followed by Tukey’s multiple comparison).
Electrical stimulation-evoked upregulation of BDNF expression in NG neurons requires calcium influx through N-, but not L-type channels
Studies using chronic depolarization to activate neurons indicate that L-type calcium channels are involved in regulation of BDNF expression (Finkbeiner and Greenberg, 1998). However, the contribution of specific subtypes of voltage-gated calcium channels to regulation of BDNF expression evoked by repetitive stimulation at physiologically-relevant frequencies has not been determined. In addition, our previous studies revealed a neuron type specificity in the contribution of different calcium channels to activity-dependent BDNF release (Balkowiec and Katz, 2002; Buldyrev et al., 2006; Martin et al., 2009). Therefore, we next sought to characterize dependence of BDNF expression in NG neurons on calcium influx.
Similar to BDNF release (Balkowiec and Katz, 2002; Buldyrev et al., 2006), the electrical stimulation-evoked BDNF mRNA expression in NG neurons was abolished in the absence of extracellular calcium (Fig. 3B). This result is consistent with the previously described requirement for calcium influx in regulation of BDNF expression in CNS neurons (Finkbeiner, 2000; West et al., 2001).
Pretreatment of NG cultures with the L-type channel antagonist Nimodipine (2 μM) did not have any significant effect on the electrical stimulation-evoked increase in BDNF mRNA (Fig. 4A). Nifedipine (2 μM), another L-type channel antagonist, was similarly ineffective (data not shown). On the other hand, an increase in BDNF mRNA evoked by the same stimulation protocol (6 Hz) was significantly inhibited by pretreatment with 1 μM ω-Conotoxin GVIA, an N-type calcium channel antagonist (Fig. 4A). The effect of a combined application of both Nimodipine (2 μM) and ω-Conotoxin GVIA (1 μM) was consistent with the effects of these antagonists applied individually, resulting in abolition of BDNF mRNA expression (Fig. 4A). These data indicate that BDNF expression in NG neurons evoked by repetitive electrical stimulation is controlled by calcium entry through N- rather than L-type channels.
Figure 4. Electrical stimulation-evoked BDNF expression requires calcium influx through N-, but not L-, type channels.
(A) BDNF transcript 4 (normalized to HPRT) and (B) BDNF protein levels, following a 4-h continuous stimulation at 6 Hz in the absence (Control) or presence of Nimodipine (Nim, L-type channel antagonist), ω-Conotoxin (ωCtx, N-type channel antagonist), or a combination of both drugs (Nim/ωCtx), relative to non-stimulated control levels (NS; white bars). N= (A) 9 and (B) 7 independent cultures. *, different from NS (P<0.05; ANOVA followed by (A) Tukey’s or (B) Duncan’s multiple comparison).
Since previous studies of activity-dependent BDNF expression in chronic depolarization models indicated a prominent role of L-type calcium channels (Finkbeiner and Greenberg, 1998; Wheeler et al., 2012), we next compared the effects of L- and N-type channel blockers on levels of BDNF transcript 4 in NG neurons stimulated with 40 mM KCl, a chronic depolarization model. First, the magnitude of upregulation of BDNF transcript 4 during a 4-h KCl-depolarization (4.49 ± 0.61, n=10 independent cultures; P<0.05) was similar to the upregulation evoked by a 4-h electrical stimulation at 6 Hz (Figs. 2–4). However, in sharp contrast to the effects of electrical stimulation, the increase in BDNF mRNA in response to chronic KCl-evoked depolarization was unaffected by TTX (1.5 μM; 4.27 ± 0.56, n=3 independent cultures) and by the N-type calcium channel antagonist ω-Conotoxin GVIA (1 μM; 4.05 ± 0.73, n=3 independent cultures). Consistent with previous studies in other neuronal populations, BDNF transcript 4 expression evoked by chronic depolarization was significantly inhibited by the L-type channel antagonist Nimodipine (2 μM; 2.38 ± 0.66, n=3 independent cultures). This latter result indicates that both N- and L-type currents are capable of increasing BDNF expression in NG neurons, and the most influential route of calcium entry is determined by the type of stimulation that evokes BDNF expression.
The increase in BDNF mRNA is accompanied by upregulation of BDNF protein
It has previously been shown that activity-dependent expression of BDNF mRNA can be regulated independently of protein, such that increases in BDNF transcripts are not followed by increases in BDNF protein (Nanda and Mack, 2000; Tropea et al., 2001). Therefore, we next examined whether the increased transcription of BDNF in NG neurons in response to repetitive electrical stimulation results in an increase in BDNF protein. Consistent with the increases in BDNF transcripts, BDNF protein was also significantly upregulated in response to electrical stimulation at 6 Hz (Fig. 4B). Moreover, the effects of voltage-activated calcium channel antagonists on BDNF protein expression (Fig. 4B) mirrored the effects observed for BDNF transcript 4 (Fig. 4A). This result indicates that BDNF translation faithfully follows changes in transcription of the BDNF gene in NG neurons.
DISCUSSION
The present study provides the first evidence that repetitive electrical stimulation promotes neuronal BDNF expression through cellular mechanisms that are distinct from those described in chronic depolarization models of neuronal activation. Our study shows that electrical stimulation of NG neurons at frequencies that are relevant to physiological activity of NG cardiovascular afferents promotes de novo synthesis of BDNF, acting at the transcriptional level in a promoter-specific manner. The increase in BDNF transcripts is largely mediated through bdnf promoter IV, known to be regulated by neuronal activity. Unlike BDNF expression induced by chronic depolarization, electrical stimulation-evoked BDNF expression requires TTX-sensitive sodium currents and calcium influx specifically through N-type voltage-gated channels.
To stimulate BDNF expression, we applied electrical impulses to cultured NG neurons in order to mimic neuronal activity in vivo. This approach unveiled novel mechanisms that regulate transcription of the bdnf gene. Our data indicate that electrical stimulation leads to a selective upregulation of BDNF transcripts 1 and 4, whereas transcript 6 is unaffected. Intriguingly, BDNF transcript 6 has previously been shown to be regulated by activity in other models of neuronal activation. Since specific BDNF transcripts could regulate the dependence of BDNF synthesis on individual stimuli, or unique functions of the resultant BDNF protein (Cohen and Greenberg, 2008), the current findings underscore the complexity of the regulatory mechanisms that determine BDNF transcription and the role of distinct BDNF transcripts in determining the functional specificity of BDNF.
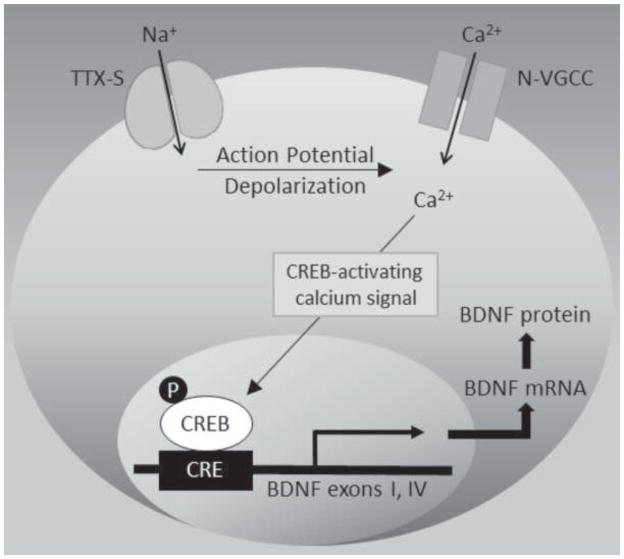
Using the same in vitro model as in the current study, we have previously shown that repetitive electrical stimulation of NG neurons leads to phosphorylation of cAMP-response element (CRE)-binding protein (CREB; Balkowiec and Katz, 2000), consistent with a central role of CREB in calcium-dependent regulation of the BDNF gene (West et al., 2001). Since CREB can be phosphorylated in response to calcium influx specifically through N-type channels (Wheeler et al., 2012; Zhao et al., 2007), these previous findings together with our current data indicate that repetitive electrical stimulation increases BDNF expression in sensory neurons by activating CREB in response to calcium influx through N-type channels (Fig. 5).
Figure 5. Proposed model of electrical stimulation-mediated BDNF expression.
Electrical stimulation opens voltage-gated, tetrodotoxin-sensitive (TTX-S) sodium channels, resulting in action potential-dependent depolarization, which, unlike chronic depolarization, preferentially opens N-type voltage-gated calcium channels (N-VGCC). Calcium entering through N-VGCC activates signaling cascades (e.g., calcium/calmodulin-dependent kinases) that, upon translocation to the nucleus, increase phosphorylation (P) of cAMP response element (CRE)-binding protein (CREB), a transcription factor known to regulate the BDNF gene. CREB phosphorylation leads to activation of bdnf exons I and IV, and subsequent BDNF mRNA and protein synthesis.
Cardiovascular afferents differ in their discharge characteristics, with some firing a single action potential in response to a normal pulse pressure rise, and others being activated only at higher pressures. The discharge pattern of the latter is usually characterized by bursts of action potentials generated with each pressure pulse (Chapleau, 1991; Chapleau and Abboud, 1987; Seagard et al., 1993; Seagard et al., 1990; van Brederode et al., 1990). Our present results indicate that even single discharge characteristics, i.e. relatively low levels of resting activity in NG neurons, drive BDNF transcription of the magnitude that is indistinguishable from that evoked by bursting patterns of stimulation. Thus, unlike BDNF release from these neurons (Balkowiec and Katz, 2000; Martin et al., 2009), BDNF transcription seems to be insensitive to the stimulation pattern or the total number of the delivered pulses, as long as the duration of stimulation remains the same. This feature of action potential-dependent BDNF expression in NG neurons suggests that BDNF is not limited to highly active neurons, and that activity-dependent BDNF effects are regulated by the magnitude of BDNF release rather than BDNF expression.
One likely possibility is that NG-derived BDNF provides trophic support at developing NG afferent synapses in the brainstem NTS. We have previously shown that BDNF is present in central terminals of NG afferents in the NTS and released from these neurons in an activity-dependent manner (Martin et al., 2009). Moreover, BDNF regulates dendritic branching of developing NTS neurons (Martin et al., 2012). Abnormalities of NTS neuron development result in disturbed cardiorespiratory homeostasis and have been linked to such disorders as Rett syndrome (Katz et al., 2009), and Sudden Infant Death Syndrome (SIDS; Kinney, 2009). Both conditions are associated with BDNF deficits in the brainstem, including the NTS (Kline et al., 2010; Schmid et al., 2012; Tang et al., 2012). Our current data are likely to contribute to a better understanding of the underlying mechanisms of these conditions, and may also prove useful for designing new therapeutic interventions to restore normal levels of endogenous BDNF.
Our previous studies have also demonstrated that BDNF continues to be expressed by NG afferents throughout adulthood (Martin et al., 2009). The latter observation together with the current data suggests a role for BDNF in the maintenance of active synapses in the NTS. In fact, the selective strengthening of active connections by BDNF could be applicable to both maturing and adult synapses. In support, the role of BDNF in activity-dependent synaptic plasticity is well documented in other systems (Huang and Reichardt, 2001; Poo, 2001).
A previous study from our laboratory has identified hypertension as a natural stimulus that leads to increased BDNF levels in NG visceral primary sensory neurons and their central targets in the dorsal medulla in vivo (Vermehren-Schmaedick et al., 2013). Our current data in neonatal NG neurons in vitro showing regulation of BDNF expression primarily by bdnf promoter IV are consistent with in vivo data in hypertensive rats. On the other hand, the fact that the stronger, ‘bursting’ electrical stimulation of NG neurons fails to evoke a greater magnitude of BDNF expression is unexpected. While the latter result may seem inconsistent with our findings in hypertensive animals, it can also help to understand the mechanisms of NG BDNF upregulation in hypertension. Namely, the present data suggest that the hypertension-induced NG BDNF upregulation is a result of recruitment of previously inactive, or relatively inactive, NG afferents whose activity significantly increases as a direct, or indirect, response to hypertension. In other words, based on our current data, it is unlikely that the hypertension-induced NG BDNF upregulation is a result of the transition to ‘bursting’ firing patterns among previously active subpopulations of cardiovascular afferents. In support, our data in hypertensive animals provide evidence for recruitment of NG neurons, i.e. the numbers of BDNF-immunoreactive cells are significantly higher in hypertensive, compared to normotensive, animals (Vermehren-Schmaedick et al., 2013). The lack of stimulation pattern-dependence of BDNF expression also suggests that a potential BDNF involvement in the hypertension-induced synaptic remodeling that counteracts baroreflex resetting only at selected NTS synapses would more likely be regulated by increased BDNF release from highly active NG afferents than by BDNF expression. However, it is important to note that the current data have been obtained using a newborn NG neuron model and, as such, may not be fully relevant to NG neurons from mature, hypertensive animals.
Our results show that the action potential-evoked BDNF expression in NG neurons is largely dependent on N-type calcium channels. This is in sharp contrast to virtually all previous studies of the cellular mechanisms of activity-dependent BDNF expression that indicate its dependence on L-, rather than N-type channels (Finkbeiner and Greenberg, 1998). However, these studies used chronic depolarization models to stimulate the cells, and under those conditions N-type channels get quickly inactivated. Thus, the most likely explanation of the discrepancy is the type of neuronal activation leading to BDNF expression. It is worth noting that, under physiological conditions, all neurons experience the action potential-dependent activation that has been mimicked in our model.
Although nodose ganglia contain functionally distinct populations of visceral sensory neurons, cardiovascular afferents retain BDNF expression in vitro (Martin et al., 2009) and, therefore, most likely contribute to the detected BDNF expression. Moreover, previous studies strongly suggest that cardiovascular afferent pathways do not differ from other NG pathways with respect to stimulus-response characteristics at first-order synapses in the NTS (Mifflin 1997; Bailey et al. 2006). Also, our previous studies indicate that BDNF is not expressed by ganglionic non-neuronal cells (Buldyrev et al., 2006; Martin et al., 2009), thus, the increase in BDNF expression is unlikely to be attributed to a non-neuronal source.
In conclusion, the current data offer new insights into the cellular mechanisms of activity-dependent BDNF expression in neurons. Together with our previous studies, they identify BDNF as a candidate trophic factor responsible for activity-dependent maturation and maintenance of primary afferent synapses in the brainstem NTS, with implications for understanding mechanisms of development and activity-dependent plasticity in visceral sensory pathways in health and disease.
HIGHLIGHTS.
BDNF mRNA expression in sensory neurons is regulated by stimulation frequency.
Repetitive stimulation-evoked BDNF expression requires voltage-gated sodium channels.
Repetitive stimulation-evoked BDNF expression requires N-type calcium channels.
Electrical stimulation at 6 Hz activates bdnf promoters I and IV.
Acknowledgments
The authors express their gratitude to Ms. Carly C. Hernandez for the expert technical assistance. This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (award number R01 HL076113) and OHSU School of Dentistry Dean’s Research Award to A.B.
LIST OF ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CDS
protein-coding sequence
- CNS
central nervous system
- HPRT
hypoxanthine-guanidine phosphoribotransferase
- NS
non-stimulated neurons NG, nodose ganglion
- NTS
nucleus tractus solitarius
- N-VGCC
N-type voltage-gated calcium channels
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- qPCR
quantitative real-time polymerase chain reaction
- TTX
tetrodotoxin
- UTR
untranslated region
Footnotes
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
A.V.S. and A.B. designed the study. A.V.S. and R.A.K. conducted the experiments. A.V.S., R.A.K., and A.B. analyzed the data. A.B. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. J Neurosci. 2000;20:1904–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec-Iskra E, Vermehren-Schmaedick A, Balkowiec A. Tumor necrosis factor-alpha increases brain-derived neurotrophic factor expression in trigeminal ganglion neurons in an activity-dependent manner. Neuroscience. 2011;180:322–333. doi: 10.1016/j.neuroscience.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain–derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapleau MW. Types of baroreceptor afferent neurons. Circ Res. 1991;68:619–620. doi: 10.1161/01.res.68.2.619. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circ Res. 1987;61:648–658. doi: 10.1161/01.res.61.5.648. [DOI] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J Neurosci. 2011;31:12318–12329. doi: 10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Calcium regulation of the brain-derived neurotrophic factor gene. Cel Mol Life Sci. 2000;57:394–401. doi: 10.1007/PL00000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HY, Robertson CL, Vermehren-Schmaedick A, Balkowiec A. Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J Neurosci Res. 2010;88:1285–1297. doi: 10.1002/jnr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jones JV, Thoren PN. Characteristics of aortic baroreceptors with non-medullated afferents arising from the aortic arch of rabbits with chronic renovascular hypertension. Acta Physiologica Scandinavica. 1977;101:286–293. doi: 10.1111/j.1748-1716.1977.tb06010.x. [DOI] [PubMed] [Google Scholar]
- Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen CY, Bonham AC. Metabotropic glutamate receptors depress vagal and aortic baroreceptor signal transmission in the NTS. Am J Physiol. 1988;275:H1682–H1694. doi: 10.1152/ajpheart.1998.275.5.H1682. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol Sci. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Martin JL, Brown AL, Balkowiec A. Glia determine the course of brain-derived neurotrophic factor-mediated dendritogenesis and provide a soluble inhibitory cue to dendritic growth in the brainstem. Neuroscience. 2012;207:333–346. doi: 10.1016/j.neuroscience.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem. 2009;108:450–464. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Short-term potentation of carotid sinus nerve inputs to neurons in the nucleus of the solitary tract. Respir Physiol. 1997;110:229–236. doi: 10.1016/s0034-5687(97)00087-x. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Mack KJ. Seizures and sensory stimulation result in different patterns of brain derived neurotrophic factor protein expression in the barrel cortex and hippocampus. Brain Res Mol Brain Res. 2000;78:1–14. doi: 10.1016/s0169-328x(00)00054-1. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, Massa SM, Longo FM, Katz DM. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagard JL, Hopp FA, Drummond HA, Van Wynsberghe DM. Selective contribution of two types of carotid sinus baroreceptors to the control of blood pressure. Circ Res. 1993;72:1011–1022. doi: 10.1161/01.res.72.5.1011. [DOI] [PubMed] [Google Scholar]
- Seagard JL, van Brederode JF, Dean C, Hopp FA, Gallenberg LA, Kampine JP. Firing characteristics of single-fiber carotid sinus baroreceptors. Circ Res. 1990;66:1499–1509. doi: 10.1161/01.res.66.6.1499. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Tang S, Machaalani R, Waters KA. Expression of brain-derived neurotrophic factor and TrkB receptor in the sudden infant death syndrome brainstem. Respir Physiol Neurobiol. 2012;180:25–33. doi: 10.1016/j.resp.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tropea D, Capsoni S, Tongiorgi E, Giannotta S, Cattaneo A, Domenici L. Mismatch between BDNF mRNA and protein expression in the developing visual cortex: the role of visual experience. Eur J Neurosci. 2001;13:709–721. doi: 10.1046/j.0953-816x.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- van Brederode JF, Seagard JL, Dean C, Hopp FA, Kampine JP. Experimental and modeling study of the excitability of carotid sinus baroreceptors. Circ Res. 1990;66:1510–1525. doi: 10.1161/01.res.66.6.1510. [DOI] [PubMed] [Google Scholar]
- Vermehren-Schmaedick A, Balkowiec A. Pattern- and promoter-specific regulation of BDNF mRNA expression in nodose ganglion neurons stimulated with physiological patterns of baroreceptor activity. Soc Neurosci Abstr. 2009:710.3. [Google Scholar]
- Vermehren-Schmaedick A, Jenkins VK, Hsieh HY, Brown AL, Page MP, Brooks VL, Balkowiec A. Upregulation of brain-derived neurotrophic factor expression in nodose ganglia and the lower brainstem of hypertensive rats. J Neurosci Res. 2013;91:220–229. doi: 10.1002/jnr.23158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DG, Groth RD, Ma H, Barrett CF, Owen SF, Safa P, Tsien RW. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell. 2012;149:1112–1124. doi: 10.1016/j.cell.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Liu L, Rittenhouse AR. Ca2+ influx through both L- and N-type Ca2+ channels increases c-fos expression by electrical stimulation of sympathetic neurons. Eur J Neurosci. 2007;25:1127–1135. doi: 10.1111/j.1460-9568.2007.05359.x. [DOI] [PubMed] [Google Scholar]