Abstract
Ginsenoside Rb1 (Rb1) reduces food intake in both lean and high-fat diet induced-obese rats; however, the sites and/or mediation of the eating-suppressive effect of Rb1 have not previously been identified. We hypothesized that intraperitoneally (ip) administered Rb1 exerts its anorectic action by enhancing sensitivity to satiation signals, such as cholecystokinin (CCK), and/or that it acts through vagal afferent nerves that relay the satiating signaling to the hindbrain. To test these hypotheses, we gave ip bolus doses of Rb1 (2.5-10.0 mg/kg) and CCK-8 (0.125-4.0 μg/kg) alone or in combination and assessed food intake in rats. Low doses of Rb1 (2.5 mg/kg) or CCK-8 (0.125 μg/kg) alone had no effect on food intake whereas higher doses did. When these subthreshold doses of Rb1 and CCK-8 were co-administered, the combination significantly reduced food intake relative to saline controls, and this effect was attenuated by lorglumide, a selective CCK1-receptor antagonist. Interestingly, lorglumide blocked food intake induced by an effective dose of CCK-8 alone, but not by Rb1 alone, suggesting that Rb1's anorectic effect is independent of the CCK1 receptor. To determine whether peripherally administered Rb1 suppresses feeding via abdominal vagal nerves, we evaluated the effect of ip Rb1 injection in subdiaphragmatic vagal deafferentation (SDA) and control rats. Rb1's effect on food intake was significantly attenuated in SDA rats, compared with that in SHAM controls. These data indicate that the vagal afferent system is the major pathway conveying peripherally administered Rb1's satiation signal.
Keywords: Ginsenoside, food intake, CCK, vagus nerve
1. Introduction
Human obesity has serious consequences on health, including increased risks for non-insulin dependent diabetes mellitus, cancer, hypertension and heart disease. Although the negative impact of obesity on health and well-being is widely recognized, the incidence of obesity continues to increase, and no effective pharmacological therapies are currently available. Therefore, developing safe and effective natural agents or supplements with the property of controlling food intake and body weight has enormous therapeutic potential.
One well-established risk factor for becoming obese is food consumption in excess of the caloric need of the body. Obesity develops when caloric intake exceeds energy expenditure over time with the excess energy eventually being stored as fat [1]. Recently, we found that peripherally administered ginsenoside Rb1 (Rb1), the most abundant and biologically-active component of ginseng, reduces food intake in both lean and high-fat diet induced-obese rats [2]. However, the sites and/or mediation of the eating-suppressive effect of Rb1 are not known. One possibility is that intraperitoneally (ip) administered Rb1 exerts its anorectic action by enhancing the potency of other peripheral satiation signals that are involved in meal termination, such as cholecystokinin (CCK), and/or by acting through vagal afferent nerves, which then relay satiation signaling to the brain.
There were two main goals of the present studies. The first was to identify a potential interaction between Rb1 and CCK. CCK is secreted from duodenal I-cells in response to food ingestion and functions as a within-meal satiation signal [3,4]. Peripheral injection of the octapeptide of CCK (CCK-8) induces short-term satiation by reducing meal size [3,4], and this anorectic effect is attenuated by the administration of CCK1 receptor antagonists and abolished by vagal deafferentation [5,6]. Many studies with CCK receptor agonists and antagonists have extended these findings in several species, including mice, monkeys and humans [7–9]. It is generally accepted that the inhibitory action of peripheral CCK on food intake is mediated via CCK1 receptors on vagal afferent nerves passing from the wall of the intestine to the nucleus of the solitary tract (NTS) and other brain areas [6]. There is also evidence of synergistic interaction between peripheral CCK and other hormones released during digestion. For example, in male rats, treatment with leptin [10], insulin [11] or glucagon [12] increases exogenous CCK's satiating potency. Since both Rb1 and CCK suppress meal size, we hypothesized that they may interact in this process. In the present experiments, we compared the ip administration of Rb1 and CCK-8 alone and in combination on short-term food intake in rats. We also used the CCK1 receptor antagonist, lorglumide, to determine if Rb1's eating-inhibitory effect is dependent on the activity at the CCK-1 receptor.
The second goal was to determine a possible role of the vagus nerves in mediating the eating inhibitory action of ip Rb1. The afferent fibers of the vagus nerve are the major neuroanatomic linkage between the alimentary tract and the nucleus of the solitary tract in the hindbrain, where afferent input is integrated with descending hypothalamic input and where ascending output to the hypothalamus is generated [13]. Importantly, many meal-related metabolites, monoamines, and peptides transmit their satiating signals to the NTS via afferent vagal fibers [14]. We consequently hypothesized that Rb1's satiation signaling may also be conveyed to the brain via vagal afferent nerves. To test this possibility, we investigated the effect of eating-inhibition induced by ip Rb1 in normal rats and in rats with selective subdiaphragmatic vagal deafferentation (SDA).
2. Materials and Methods
2.1. Animals
Male Long-Evans rats (225-250 g, Harlan, Indianapolis, IN) were individually housed in a temperature-controlled vivarium on a 12/12-h light/dark cycle (illuminated from 0400 h to 1600 h) with ad libitum access to pelleted rodent chow (Harlan Teklad, Madison, WI) and water. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati.
2.2. Chemicals
Rb1 purified from ginseng roots by high-performance liquid chromatography (HPLC) was purchased from Jilin University in China. High-performance liquid chromatography (Shimadzu, Kyoto, Japan) analysis was performed in our laboratory and confirmed that the Rb1 had a purity of ≥ 98% using an Rb1 standard obtained from LKT laboratories (St. Paul, MN) [2]. CCK-8, Lorglumide and other chemicals were purchased from Sigma (St. Louis, MO).
2.3.Effects of Rb1 and CCK-8 on energy intake
The food hoppers were removed at 1000 h (for fasting 6 h prior to lights off), and the rats were accustomed to receiving twice-daily ip saline (1 ml/kg) injections. The 1st injection occurred at 1000 h and the 2nd one just before dark (1600 h). Glucose solution (12.5%) was provided immediately after the 2nd injection, and glucose intake was measured at 30 min later. A glucose solution was used instead of chow for accurate assessment of intake over the short observational period (30 min). We administered the first injection 6 h before the second because this duration results in ip Rb1's maximal satiating action [2]. Once the 30-min glucose solution intakes become stable, experimental testing began. At least 5 days were allowed between tests.
To determine the dose-dependent effect of ip Rb1 on energy intake, the rats were divided into different groups (n = 7-10) receiving 0.3 ml of either Rb1 (2.5-10 mg/kg) or equivolume vehicle (saline) at 1000 h, and 0.3 ml of saline at 1600 h. The order of the two conditions was random. Glucose solution was provided immediately after the 2nd injection, and intake was assessed at 30 min later. The largest dose that proved ineffective in suppressing glucose solution was determined to be maximally subthreshold and was subsequently used to examine the effects of co-administration of exogenous Rb1 and CCK-8.
Other groups of rats (n = 7-10) received 0.3 ml of saline ip at 1000 h and equivolume saline or sulfated CCK-8 (0.125, 0.25, 0.5, 1.0, 2.0 or 4.0 μg/kg in saline) just prior to the presentation of the glucose solution. The same basic protocol was used.
2.4. Interaction of Rb1 and CCK-8
The same basic protocol was used. Four groups of rats were used, with each receiving a different combination: saline + saline, Rb1 (2.5 mg/kg) + saline, saline + CCK-8 (0.125 μg/kg), and Rb1 (2.5 mg/kg) + Rb1 (0.125 μg/kg). The 1st injection (saline or Rb1) occurred 6 h, and the 2nd (saline or CCK-8) occurred just prior to the return of glucose solution. Glucose solution intake was measured at 30 min later.
2.5. Effect of a CCK1 receptor antagonist on satiation induced by the co-administration of Rb1 and CCK-8
Four groups of rats were administered three ip injections on the test day. Lorglumide was used as a CCK1 receptor antagonist [15][16]. The injections contained either saline + saline + saline, Rb1 + lorglumide + saline, saline + saline + CCK-8, or Rb1 + lorglumide + CCK-8. The 1st injection (saline or Rb1 at doses of 2.5 or 10 mg/kg) occurred 1000 h, the 2nd injection (saline or lorglumide at a dose of 300 μg/kg [16]) occurred 1500 h, and the 3rd injection (saline or CCK-8 at doses of 0.125 or 2 μg/kg) occurred just prior to glucose solution return at 1600 h. Glucose solution was measured at 30 min later.
2.6. Subdiaphragmatic vagal deafferentation (SDA) surgery
Four days prior to surgery, the rats were provided with a liquid complete nutritional diet (Fortify, Kroger Co., Cincinnati, OH). All rats then underwent either SDA or SHAM surgery, as we described previously [17][18], based on original methods of Norgren and Smith [19]. Briefly, overnight fasted rats were anesthetized, the omo- and sternohyoideus muscles of the rat neck were gently retracted, revealing the base of the left skull. A hole was drilled at the posterior lacerated foramen under microscopic observation to reveal the brain stem and overlying dura. After the dura was broken, the left dorsal vagal rootlets were severed with fine forceps. The skull hole was then packed with gel foam and the neck wound was closed. After that, through a midline laparotomy incision, rat stomach and esophagus were exposed and gently retracted. The dorsal vagal subdiaphragmatic trunk was detached from the esophagus using fine forceps and then cauterized, leaving no intact nerve bundles. The muscle and skin layers of the laparotomy incision were closed. SHAM rats had a similar surgery exposing the vagal rootlets and dorsal subdiaphragmatic vagus, but all afferent and efferent nerves were left intact [17].
After surgeries, rats continued to receive the liquid diet for 2 days and then a semi-liquid diet for 4 d. Since CCK satiating signals are relayed to the brain via its receptor, CCK1, on vagal afferent fibers, functional verification of complete SDA was performed on 4-h fasted rats after a 14-d recovery [17]. Rats received 4 μg/kg CCK-8 ip, and food was returned just before dark onset (1800 h). Food intake was measured after 30 min. The reduction in food intake induced by CCK-8 was 84.0 ± 9.8 % in SHAM rats and 5.8 ± 2.6 % in SDA rats. Based on previous analyses [17], SDA rats with successful elimination of vagal afferent nerves have less than 30% reduction in food intake by CCK-8 administration, which is consistent with the present verification results.
2.7. Food intake in SDA rats
To determine the necessity of vagal afferent nerves to mediate the effect of ip Rb1 on food intake, 4-h fasted SDA or SHAM rats (n = 6-7 rats per group) received ip 0.3 ml of either Rb1 (10 mg/kg) or vehicle (saline) just before dark onset. Food intake was measured at 2, 4, 6, 8, 10 and 24 h after injection [2].
2.8. Statistical analysis
Data were analyzed using GraphPad Prism (version 5.0; San Diego, CA). Two-way ANOVA and two-way repeated-measures ANOVA were used for analysis of food intake followed by the Student-Newman-Keuls test. For other comparisons, t-test was used to compare the difference between two groups, and one-way ANOVA was followed by the Student-Newman-Keuls test. Data are presented as means ± SE. Values of P < 0.05 were considered significant.
3. Results
3.1. Effect of Rb1 on 30-min glucose intake
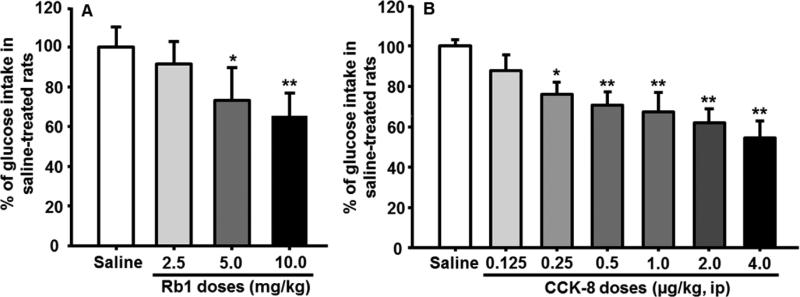
Compared to vehicle-treated controls, the rats given 2.5 mg/kg Rb1 ip consumed comparable glucose solution. At doses of Rb1 of 5 mg/kg and above, glucose solution intake was significantly and dose-dependently reduced relative to vehicle (Fig. 1A). These results suggest that 2.5 mg/kg of Rb1 is maximally subthreshold in this paradigm and that a dose of 5.0 mg/kg is minimally effective for the reduction of glucose intake in rats.
Fig. 1.
Dose-dependent inhibition of glucose consumption induced by Rb1 (A) and CCK-8 (B), respectively, during 30 min. The percentage of inhibiting glucose solution intake was calculated by taking values of saline-treated rats on the same day as 100%. Mean ± S.E., n = 7~12 rats per group. *P < 0.05, **P < 0.01, vs. saline control group.
3.2. Effect of CCK-8 on glucose intake
Glucose solution consumption was significantly reduced by CCK-8 at doses at or above 0.25 μg/kg, compared with vehicle (Fig. 1B). CCK-8 at 0.125 μg/kg had no significant effect on glucose intake, implying that this dose was maximally subthreshold for inhibiting meal size and that the smallest effective dose was 0.25 μg/kg under these experimental conditions. These results are consistent with previous reports [20].
3.3.Effect of combinations of CCK and Rb1 on glucose intake
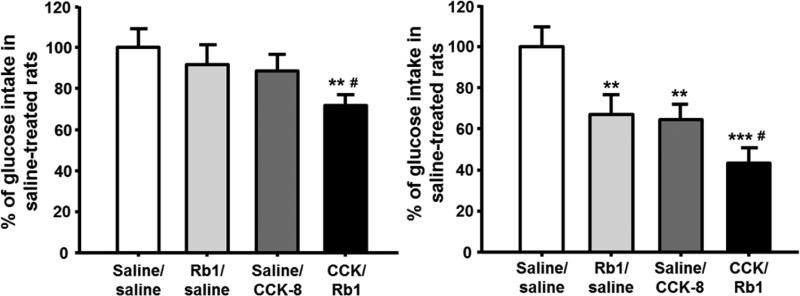
To test the hypothesis that Rb1 functionally interacts with CCK to elicit satiation, the combined subthreshold doses of Rb1 (2.5 mg/kg) and CCK-8 (0.125 μg/kg) significantly inhibited glucose solution intake over 30 min (Fig. 2A). When the dose of Rb1 was increased to 10 mg/kg and the dose of CCK-8 was increased to 2 μg/kg, the inhibitory effect was further enhanced, and comparable to the sum of their individual injections (Fig. 2B). These observations suggest that these two compounds produced an additive but not synergistic effect when given in combination. It cannot be inferred from these data whether CCK-8 and Rb1 both work through a common mechanism.
Fig. 2.
Inhibition of glucose consumption induced by the combination of Rb1 and CCK. A, when administered alone at subthreshold doses, neither Rb1 (2.5 mg/kg) nor CCK-8 (0.125 μg/kg) significantly reduced glucose intake, compared to saline-controls, which was taken as 100%. However, when co-administered, Rb1 and CCK-8 additively reduced glucose intake. B, co-administration of higher effective doses of Rb1 (10 mg/kg) and CCK-8 (2.0 μg/kg) also additively reduced glucose intake, compared to either saline-controls or the rats received Rb1 and CCK-8 individually. Mean ± S.E., n = 7~12, **P < 0.01, ***P < 0.001 vs. saline controls; #P < 0.05, vs. Rb1 or CCK individual injection.
3.4. Effect of the CCK1 receptor antagonist on combined doses of Rb1 and CCK-8
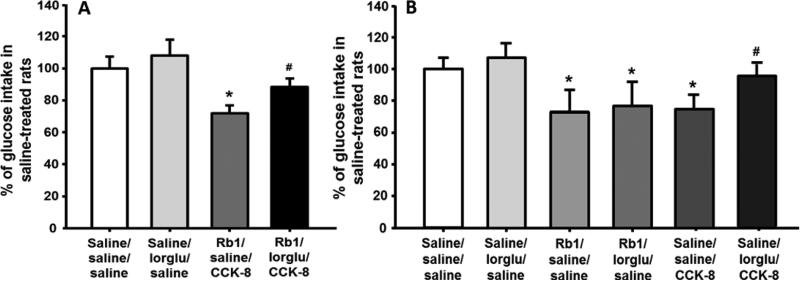
When the selective CCK1 receptor antagonist, lorglumide, was administered in addition to combined subthreshold doses of CCK-8 and Rb1, there was slight but non-significant increase of 30-min glucose intake. Compared with saline controls, rats receiving the combination of subthreshold-doses of Rb1 (2.5 mg/kg), saline and CCK-8 (0.125 μg/kg) had a significant reduction of glucose solution intake, and this reduction was significantly attenuated by lorglumide. The rats with lorglumide plus Rb1 and CCK-8 had comparable food intake as saline-treated rats (Fig. 3A). One possibility is that the pharmacological blockade of the CCK1 receptor diminished only the satiating effect of CCK-8, leaving the satiating effect of Rb1 intact, implying that the satiating effect of Rb1 is CCK1-receptor independent.
Fig. 3.
Blockade of the CCK-1 receptor by lorglumide (lorglu, 300 μg/kg, ip) significantly attenuated the enhanced suppression of glucose intake induced by co-administration of Rb1 (2.5 mg/kg) and CCK-8 (0.125 μg/kg) (A). Lorglumide only blocked glucose intake induced by an effective dose of CCK-8 (0.25 μg/kg) alone, but not by Rb1 (5 mg/kg) alone (B). The percentage of changes of glucose intake was calculated by taking values of vehicle-treated rats on the same day as 100%. Mean ± S.E., n = 7~9. *P < 0.05, vs. saline controls, and #P < 0.05, vs. Rb1 + saline + CCK-8 treatments (A) or saline + saline + CCK-8 (B).
3.5. Effect of the CCK1 receptor antagonist on Rb1-induced satiation
To more directly determine whether Rb1-induced satiation is CCK1-receptor dependent, lorglumide was co-administered with Rb1 and CCK-8, respectively. Treatment with lorglumide almost completely prevented the eating-inhibition induced by an effective dose of CCK-8 (0.25 μg/kg) alone, but not by Rb1 (5 mg/kg) alone (Fig. 3B). These results confirm that Rb1's anorectic effect is independent of the CCK1 receptor.
3.6. Effect of SDA on Rb1-elicited satiation
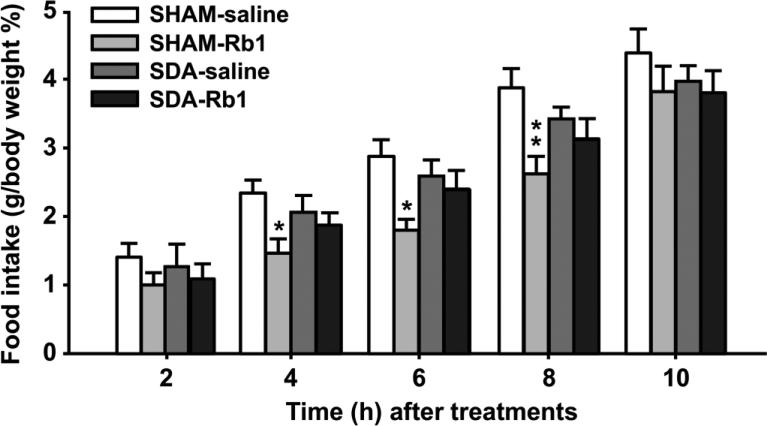
Prior to the test, SDA and SHAM rats maintained on chow had comparable body weights (289.1 ± 27.8 vs. 298.0 ± 29.3, respectively), and this remained the case at the entire time of the experiment. SDA and SHAM rats received either ip saline or Rb1 (10 mg/kg, an effective dose in suppressing food intake in normal rats) [2]. Rb1 significantly reduced food intake at 4, 6, and 8 h in fasted SHAM rats; however, it had no effect on food intake at any time point in SDA rats (Fig. 4). These findings imply that vagal deafferentation attenuates Rb1's action on eating and suggest that Rb1 decreases food intake via activating vagal afferent nerves.
Fig. 4.
Administration of Rb1 significantly reduced food intake in SHAM rats, but not in SDA rats. The rats received ip Rb1 (10 mg/kg) or saline, and food intake was determined at different time points. Mean ± S.E., n = 6-8 rats. *P < 0.05, **P < 0.01, vs. saline controls.
4. Discussion
We previously demonstrated that ip Rb1 dose-dependently suppresses food intake in both ad libitum-fed and 24-h fasted rats, and that this effect is not due to malaise because ip Rb1 does not cause a conditioned taste aversion [2]. More importantly, chronic administration of Rb1 is able to reduce food intake in HFD-induced obese rats, especially during the early period of treatment. In that study, Rb1's anorectic effect was maintained throughout the observation period (4 weeks), such that Rb1's anorectic capacity was still evident with sustained administration. In parallel with the reduction of food intake, Rb1 treatment led to a significant decrease in body weight and an improvement of both glucose tolerance and insulin sensitivity in the HFD-induced obese rats, compared with vehicle controls [2]. However, the sites and/or mediation of the eating-suppressive effect of Rb1 have not been determined. It is possible that ip-administered Rb1 interacts with other peripheral satiating signals, such as CCK, in this regard, and/or that it acts through the vagus nerves, which then relay satiation signaling to the brain.
To assess these possibilities, in our first series of experiments we compared the effects of ip Rb1 and CCK-8 either alone or in combination on 30-min glucose intake in rats. Rb1 at doses of 5 mg/kg or higher significantly reduced glucose intake after a 6-h fast, whereas a lower dose was without effect. Many reports have demonstrated that peripheral CCK-8 decreases food intake with the smallest effective doses between 0.125-0.25 μg/kg [3] depending on experimental conditions. In the present study we found that doses of exogenous CCK-8 above 0.25 μg/kg resulted in inhibition of short-term energy intake within 30 min, and this finding is in agreement with the results reported by other investigators [20].
To test the hypothesis that peripherally administered Rb1 reduces food intake via a CCK-dependent system, subthreshold doses of Rb1 (2.5 mg/kg) and CCK-8 (0.125 μg/kg) were administered in combination. The anorectic effect of CCK-8 was enhanced additively with the co-administration of a subthreshold dose of Rb1, and the total effect of the combined treatment was increased further when a higher dose of Rb1 (10 mg/kg) was co-administered with larger dose of CCK-8 (2 μg/kg). However, at no dose was there a suggestion of a synergistic interaction.
The satiation effect of CCK, when administered alone, has been demonstrated to be vagally mediated through interaction with CCK1 receptors on vagal afferent fibers [6,21,22]. Therefore, we used pharmacological intervention to determine whether Rb1's anorectic action parallels that of CCK-8, and to verify if the CCK1 receptor is involved with Rb1's action on glucose intake. Rats treated with lorglumide alone slightly but not significantly increased glucose intake compared to saline-treated controls, consistent with a previous report [23]. The administration of lorglumide with combined Rb1 and CCK-8 significantly attenuated the total anorectic effect induced by co-administration of Rb1 and CCK-8, resulting in glucose intake approximately half way between lorglumide alone and the combination of CCK-8 and Rb1 (Fig. 3A). These data suggest that the lorglumide successfully reduced the CCK-8 but not the Rb1 contribution to the satiating action. Consistent with this observation, lorglumide did not affect glucose intake induced by an effective dose of Rb1 alone (Fig. 3B). Thus, these findings indicate that Rb1 acts via a non-CCK1 receptor mechanism and that the combination of CCK-8 and Rb1 consequently exerts an additive action to reduce food intake. It is of course possible that the dose of lorglumide (300 μg/kg) used in the present studies was not sufficient to block/attenuate Rb1's satiation effect, although one previous study demonstrated that lorglumide at a dose of 200 μg/kg almost completely reversed CCK-8-induced inhibition of food intake [15]. To confirm lorglumide's effect under our current experimental conditions, we co-administered the same dose of lorglumide with CCK-8 at an effective dose (0.25 μg/kg) and found that the lorglumide was able to completely block CCK-8's anorectic effect. These results therefore strongly imply that Rb1's anorectic effect is independent of the CCK1 receptor. In other words, peripherally administered Rb1 and endogenous CCK-8 may work independently, and eventually reach a common effect to suppress food intake. For example, ip Rb1, like peripheral CCK-8, could act through vagal afferent nerves that relay a satiating signal to the hindbrain.
Multiple peripheral signals, including nutrients, nutrient metabolites, and hormones, regulate short- or long-term food intake and help maintain body weight [24]. Some of these signals transmit their intake-related signals either to the NTS via the vagal afferent nerves and/or to the hypothalamus via the bloodstream. The vagus is a major bidirectional communication conduit between abdominal organs and the brain. It relays information about ingested nutrients and metabolic status in the liver, gut and other abdominal organs to the brain and metabolic control signals from the brain to the periphery [25]. Considering that vagal afferent fibers are the major neuroanatomical structure linking the alimentary tract and the brain, Rb1 signaling may well be conveyed to the hindbrain via the vagal afferent pathway.
To determine whether peripherally administered Rb1 suppresses feeding via the abdominal vagal nerves, we evaluated the effect of SDA on ip Rb1-induced reductions of food intake. SDA is a surgical procedure, which eliminates all neuronal signals mediated via vagal afferent fibers from the upper gut, including the liver, while leaving half of the vagal efferent fibers intact [19][6]. Therefore, different from what occurs with gastric vagotomy, which inevitably blocks gut vagal efferent traffic and often produces gastrointestinal motor and secretory dysfunction, anorexia, and malnutrition in both rats and humans [26][27], SDA has less side effects that might interfere with the normal control of food intake and consequently affect the results of Rb1 treatment. In the present study, we attempted to maximize the validity of our tests by including only data from animals that passed stringent functional criteria for complete lesions.
Consistent with our previous report [17], the SDA rats in the present study had a slight not significantly lower body weight gain, compared to the SHAM rats. A major finding of the present study is that peripherally administered Rb1 significantly decreased food intake in SHAM rats, but not in SDA rats. To our knowledge, this is the first report that intact subdiaphragmatic vagal afferent fibers are necessary for the inhibition of food intake produced by peripherally administered Rb1.
In conclusion, while co-administration of Rb1 and CCK-8 can lead to significant reductions of glucose intake, their pharmacological actions appear to be independent, with only the action of CCK being blocked by lorglumide, a selective CCK1 receptor antagonist. Consistent with this, the blockade of CCK1 receptor using lorglumide did not attenuate Rb1's effect on energy intake, suggesting that Rb1's anorectic effect is independent of the CCK1 receptor. Peripherally administered Rb1 suppressed food intake in SHAM rats. However, this effect was significantly attenuated in SDA rats relative to what occurred in SHAM controls. These results suggest that peripherally administered Rb1 requires intact vagal afferents to reduce food intake.
HIGHLIGHTS.
Co-ip subthreshold doses of Rb1 and CCK significantly reduced energy intake.
Rb1's anorectic effect is independent of CCK1 receptor.
Rb1's effects on feeding was significantly attenuated in SDA rats vs. SHAM rats.
Acknowledgments
This research was supported by the National Center for Research Resources and the National Institute of Diabetes and Digestive and Kidney Diseases through Grant Number DK70992, DK92779, and DK95440. The authors thank Yin Liu at the University of Cincinnati for assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose.
References
- 1.Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 2.Xiong Y, Shen L, Liu KJ, Tso P, Xiong Y, Wang G, et al. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59:2505–12. doi: 10.2337/db10-0315. doi:10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245:323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- 4.Moran TH. Gut peptides in the control of food intake. Int.J Obes.(Lond) 2009;33(Suppl 1):S7–10. doi: 10.1038/ijo.2009.9. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. [February 22, 2015];J. Comp. Physiol. Psychol. 1973 84:488–95. doi: 10.1037/h0034870. http://www.ncbi.nlm.nih.gov/pubmed/4745816. [DOI] [PubMed] [Google Scholar]
- 6.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am.J.Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 7.Silver AJ, Flood JF, Song AM, Morley JE. Evidence for a physiological role for CCK in the regulation of food intake in mice. [May 30, 2015];Am. J. Physiol. 1989 256:R646–52. doi: 10.1152/ajpregu.1989.256.3.R646. http://www.ncbi.nlm.nih.gov/pubmed/2923253. [DOI] [PubMed] [Google Scholar]
- 8.Asin KE, Bednarz L, Nikkel AL, Gore PA, Nadzan AM. A-71623, a selective CCK A receptor agonist, suppresses food intake in the mouse, dog, and monkey. [May 30, 2015];Pharmacol. Biochem. Behav. 1992 42:699–704. doi: 10.1016/0091-3057(92)90017-a. http://www.ncbi.nlm.nih.gov/pubmed/1513850. [DOI] [PubMed] [Google Scholar]
- 9.Drewe J, Gadient A, Rovati LC, Beglinger C. Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. [May 30, 2015];Gastroenterology. 1992 102:1654–9. doi: 10.1016/0016-5085(92)91726-k. http://www.ncbi.nlm.nih.gov/pubmed/1568575. [DOI] [PubMed] [Google Scholar]
- 10.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc.Natl.Acad.Sci.U.S.A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 12.Le Sauter J, Geary N. Pancreatic glucagon and cholecystokinin synergistically inhibit sham feeding in rats. [August 14, 2015];Am. J. Physiol. 1987 253:R719–25. doi: 10.1152/ajpregu.1987.253.5.R719. http://www.ncbi.nlm.nih.gov/pubmed/3688274. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nat. 2000 2000 Apr 6;404404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 14.Grundy D. Signalling the state of the digestive tract. Auton.Neurosci. 2006;125:76–80. doi: 10.1016/j.autneu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Schneider LH, Murphy RB, Smith GP. Two proglumide analogues are equipotent antagonists of the inhibition of food intake by CCK-8. [May 27, 2015];Peptides. 1988 9(Suppl 1):207–14. doi: 10.1016/0196-9781(88)90246-x. http://www.ncbi.nlm.nih.gov/pubmed/2856646. [DOI] [PubMed] [Google Scholar]
- 16.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am.J.Physiol Regul.Integr.Comp Physiol. 2007;293:R1490–R1494. doi: 10.1152/ajpregu.00329.2007. [DOI] [PubMed] [Google Scholar]
- 17.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J.Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo CC, Langhans W, Georgievsky M, Arnold M, Caldwell JL, Cheng S, et al. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology. 2012;153:5857–65. doi: 10.1210/en.2012-1427. doi:10.1210/en.2012-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am.J.Physiol. 1994;267:R1136–R1141. doi: 10.1152/ajpregu.1994.267.4.R1136. [DOI] [PubMed] [Google Scholar]
- 20.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 21.Li BH, Rowland NE. Effects of vagotomy on cholecystokinin- and dexfenfluramine-induced Fos-like immunoreactivity in the rat brain. [May 26, 2015];Brain Res. Bull. 1995 37:589–93. doi: 10.1016/0361-9230(95)00045-g. http://www.ncbi.nlm.nih.gov/pubmed/7670882. [DOI] [PubMed] [Google Scholar]
- 22.Sayegh AI, Ritter RC. Vagus nerve participates in CCK-induced Fos expression in hindbrain but not myenteric plexus. [May 26, 2015];Brain Res. 2000 878:155–62. doi: 10.1016/s0006-8993(00)02731-1. http://www.ncbi.nlm.nih.gov/pubmed/10996146. [DOI] [PubMed] [Google Scholar]
- 23.Kaltwasser MT, Petrack B, Crawley JN. Potency of CR 1409, a new proglumide analog, on cholecystokinin-mediated behaviors and receptor binding. [May 26, 2015];Neurochem. Int. 1987 10:547–53. doi: 10.1016/0197-0186(87)90083-0. http://www.ncbi.nlm.nih.gov/pubmed/20501129. [DOI] [PubMed] [Google Scholar]
- 24.Stubbs RJ. Peripheral signals affecting food intake. [August 14, 2015];Nutrition. 15:614–25. doi: 10.1016/s0899-9007(99)00098-2. http://www.ncbi.nlm.nih.gov/pubmed/10422099. [DOI] [PubMed] [Google Scholar]
- 25.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. doi:10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 26.Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite. 1986;7:1–17. doi: 10.1016/s0195-6663(86)80038-1. [DOI] [PubMed] [Google Scholar]
- 27.King RM, Pairolero PC, Trastek VF, Payne WS, Bernatz PE. Ivor Lewis esophagogastrectomy for carcinoma of the esophagus: early and late functional results. Ann.Thorac.Surg. 1987;44:119–122. doi: 10.1016/s0003-4975(10)62019-x. [DOI] [PubMed] [Google Scholar]