Abstract
Endocrine systems play critical roles in facilitating sexual behavior in seasonally breeding vertebrates. Much of the research exploring this topic has focused on the endocrine correlates of signaling behavior in males and sexual proceptivity in females. What is less understood is how hormones promote the expression of the often complex and highly selective set of stimulus-response behaviors that are observed in naturally breeding animals. In female frogs, phonotaxis is a robust and sensitive bioassay of mate choice and is exhibited by gravid females during the breeding season. In stark contrast, females exhibit low phonotactic responsiveness outside the breeding season, but the administration of hormones can induce sexual proceptivity. Here we test the hypothesis that manipulation of a minimal set of reproductive hormones—progesterone and prostaglandin F2α—are capable of evoking not only proceptive behavior in non-breeding females, but also the patterns of intraspecific selectivity for male sexual displays observed in gravid females tested during the breeding season. Specifically, we investigated whether preferences for faster call rates, longer call durations, and higher call efforts were similar between breeding and hormone-treated females of Cope’s gray treefrog (Hyla chrysoscelis). Hormone injections induced patterns of selective phonotaxis in non-breeding females that were remarkably similar to those observed in breeding females. These results suggest that there may be an important contribution of hormonal pleiotropy in regulating this complex, acoustically-guided sexual behavior. Our findings also support the idea that hormonal induction could be used to evaluate hypotheses about selective mate choice, and its underlying mechanisms, using non-breeding females.
Keywords: Communication, Female choice, Grey treefrog, Mate choice, Sexual selection
1. Introduction
Hormones coordinate the expression of sexual behavior at the onset of favorable environmental conditions in seasonal breeders [1]. The endocrine systems involved in modulating sexual behavior are highly conserved among vertebrate taxa and have been studied in detail in a number of organisms, including teleost fish [2, 3], birds [4, 5] and amphibians [6]. Previous research has principally focused on the role of endocrine systems in promoting the production of sexual displays (typically in males), and to a lesser extent their role in inducing proceptive behavior (typically in females). Recent work, however, suggests that endocrine systems can also have acute effects on mate choice selectivity [7, 8]. Because the choice of a mate is one of the most consequential decisions organisms make in terms of evolutionary fitness [9, 10], and because the act of choosing a mate typically involves the integration of a complex set of sensory (e.g., detection and localization), cognitive (decision-making and integration) and motor (e.g., orientation and movement towards mate) processes, it is conceivable that such behavior involves an equally complex set of physiological regulatory systems. Many hormones, however, are known to simultaneously influence multiple phenotypic traits (i.e., hormonal pleiotropy) [11, 12], due in part to the coordinated expression of a given receptor across multiple target tissues [13, 14]. One goal of evolutionary endocrinology is to experimentally identify the hormonal basis of complex suites of natural behaviors with known fitness implications, such as mate choice. Achieving this goal requires a careful examination of the integrated set of stimulus-response relationships necessary to evoke species-typical mate choice selectivity in wild animals.
In anuran amphibians (frogs and toads), sexual behavior is conspicuously tied to vocal production of advertisement signals (typically in males) and acoustically guided mate choice (typically in females). Female frogs often exhibit robust selectivity for the specific spectral and temporal acoustic properties of conspecific advertisement calls [15-17]. This selectivity functions as a pre-mating species isolation mechanism that ensures females choose conspecific males as mates. Female frogs often also exhibit strong intraspecific selectivity in favor of calls with particular spectral or temporal properties [16-18]. This selectivity extends to preferences for faster calling rates [19] and for calls with lower frequencies [20], longer durations [21], higher amplitudes [22], and greater acoustic complexity [23]. In turn, intraspecific selectivity can benefit females both directly, for example, by reducing time spent searching for a mate [24, 25], and indirectly, for instance, in terms of producing offspring with higher fitness [9].
The most widely used experimental method to investigate mate choice in frogs involves eliciting positive phonotaxis (approach toward sound) in response to broadcasts of real or synthetic models of acoustic signals [26]. Positive phonotaxis by female anurans is a proceptive behavior that reflects sexual motivation because it promotes sexual interaction for the purpose of mating [27-29]. Typically, females are collected in amplexus during their natural breeding seasons, separated from their mates, and placed near a variable number of speakers from which different alternative signals are broadcast. Proceptive females approach sound sources broadcasting calls regarded as those of an acceptable mate. In tests with two or more acoustic alternatives simulating different males, selectivity for preferred sexual partners is revealed when a proportion of females higher than expected by chance approach one of the alternatives. A primary reason for testing females collected in amplexus is that they exhibit patterns of behavioral selectivity similar to those observed when females are tested just prior to choosing an actual mate and entering amplexus in nature [30]. Hence, selective phonotaxis in experimental settings reflect the expression of the same discriminative behavior that females exercise in choosing a mate. Almost immediately after gravid females mate or release their eggs, they become much less responsive, and in some cases, completely unresponsive, to acoustic signals. This dramatic post-mating decline in proceptive behavior in response to acoustic signals almost certainly involves neuroendocrine products, which play important roles in sexual arousal and reproduction in frogs [31]. At a practical level, this remarkable change in sexual motivation imposes a severe limitation on using phonotaxis as a behavioral assay to study mate choice in frogs by limiting experimental studies to occur during a species’ natural breeding season.
In this study of Cope’s gray treefrog (Hyla chrysoscelis), we tested the hypothesis that the combination of progesterone and prostaglandin F2α induces proceptive behavior in females (phonotaxis) that exhibits species-typical patterns of selectivity for male sexual displays. According to this hypothesis, our prediction was that hormonally-induced females would respond similarly to naturally-breeding females in a battery of two-alternative choice tests designed to assess selective preferences for acoustic signals differing in their rate of production, duration, or both. Few previous studies have investigated whether hormonal manipulations can induce the species-typical selective preferences for specific call variants exhibited by gravid females tested during the breeding season [13, 32, 33]. Circulating levels of progesterone increase in female frogs at times when reproduction occurs [34-37], and in combination with estradiol—but not alone—can induce receptive behaviors, such as the adduction of thigh muscles in response to clasping in Xenopus laevis [38]. Prostaglandins play an important role in parturition, ovarian function, and egg laying in vertebrates [39-41], but are relatively unstudied in the context of mate choice behavior [but see 7]. There is some evidence, however, that they may be involved—in concert with other hormones—in regulating phonotaxis and other behaviors related to sexual proceptivity in female frogs [42-45]. Injections of steroid (e.g., estrogen, progesterone), peptide (e.g., human chorionic gonadotropin), and lipid-based hormones (e.g., prostaglandins) can induce phonotaxis in female frogs outside the natural breeding season [6, 31, 46, 47]. However, neither progesterone [38, 48] nor prostaglandin [43, 44, 49] alone is sufficient to induce sexual proceptivity or ovulation in female frogs.
We conducted two experiments. The first experiment evaluated whether injections of progesterone and prostaglandin F2α together elicited higher rates of proceptive behavior (phonotaxis) in non-gravid females compared with negative controls. In a second experiment, we examined whether patterns of selectivity for stimuli varying in call rate, call duration, or call effort (the product of call rate and duration) were similar in gravid females tested during the breeding season and non-gravid, hormone-treated females. Previous work with Cope’s gray treefrogs has established that females prefer displays having faster call rates, longer call durations, and higher call efforts [50-54]. Our direct comparisons of gravid, breeding females and non-gravid, hormone-treated females permitted us to interpret behavioral selectivity with respect to known species-typical patterns.
2. Materials and methods
2.1. Subjects
All subjects were collected as gravid females found in amplexus in wetlands in east-central Minnesota (Carver, Hennepin, Ramsey, and Wright Counties) between 15 May and 30 June in 2008, 2009, 2010, and 2015. Collections were made at night between 2200-0100 hours. All subjects were transported to the lab and maintained at approximately 2°C to prevent egg deposition prior to being used as subjects in phonotaxis tests. We distinguish between four separate groups of subjects in the present study. We use the term “breeding” to refer to the group of females tested during the natural breeding season within 1-3 days of collection and before egg laying. In our laboratory, greater than 98% of females collected and tested during the breeding season exhibit positive phonotaxis in playback experiments (M. A. Bee, unpublished data). All other females were captive frogs housed in the laboratory and tested between June and March after they had oviposited the eggs they carried when collected in amplexus (see the Supplementary Material for details of when specific tests were conducted). Females in the “hormone-treated” group received injections of progesterone and prostaglandin. Females in the “saline-treated” group were treated similarly to females in the hormone-treated group, but received injections of the hormone vehicle only. An “untreated” group of females received no injections. Frogs were housed on a 12L:12D light cycle at approximately 20°C in a rack of custom-modified terraria with sphagnum moss, perches and refugia made of PVC pipes, and flow-through, filtered water. In total, 317 females were collected and used as subjects for this study.
2.2. General testing protocols
We conducted two-alternative choice tests using equipment and procedures described in detail elsewhere [52, 55, 56]. Briefly, tests were conducted under infrared illumination in a 2-m diameter test arena with a carpeted floor and 60-cm high walls that were visually opaque but acoustically transparent. The arena was located inside a custom-built, temperature-controlled (20 ± 1°C), semi-anechoic sound chamber (Industrial Acoustics, Bronx, NY). Two speakers (A/D/S L210, Vista, CA) were positioned on the floor on opposite sides of the arena (180° apart) just outside the arena wall and aimed toward the center of the arena, where an individual subject was remotely released at the start of a choice test. We varied the positions of the speakers each day of testing to eliminate any confounding effects of directional bias. At least 30 min prior to testing, we placed subjects in a temperature-controlled incubator to allow their body temperatures to equilibrate to 20 ± 1°C. Subjects were given up to 8 min to travel the 1-m distance to a speaker and to touch the arena wall within a 15° arc centered in front of a speaker. Frogs that failed to meet this response criterion were scored as “no response.” Subjects tested in multiple tests were returned to the incubator for 10-20 min “timeouts” between consecutive tests. There is little evidence to suggest female frogs experience carry-over effects across separate phonotaxis tests [21, 57, 58]. Tests were typically conducted between 0900 hrs and 0400 hrs the next day.
2.3. Acoustic stimuli
We used custom-written software (courtesy J. J. Schwartz) to generate synthetic stimulus calls (20 kHz, 16 bit) that differed in call rate (calls/min), call duration (pulses/call), or both, but were otherwise identical in all other spectral and temporal properties. Each stimulus was composed of a sequence of identical pulses with values of temporal and spectral properties similar to the average values recorded in our study population (corrected to 20°C) [53] and used in previous studies [52, 55, 56]. Single pulses were created by adding two phase-locked sinusoids with frequencies (and relative amplitudes) of 1.3 kHz (-6 dB) and 2.6 kHz (0 dB). We created calls by concatenating pulses and inter-pulse intervals (50% pulse duty cycle) to achieve the desired number of pulses (Table 1). Sequences of calls were created by inserting appropriate durations of silence between consecutive calls to achieve the desired call rate (Table 1). We shaped the amplitude envelope of each call using a linear rise over the first 60 ms of the call.
Table 1.
Values of call rate, call duration, and call effort in the alternative stimuli used in four test series designed to compare female preferences in breeding and hormone-treated females.
| Test series | Acoustic manipulation | Call rate (calls/min) | Call duration (pulses/call) | Call effort (pulses/min) |
|---|---|---|---|---|
| 1 | Call rate (call effort variable) | 5.3 | 32 | 170 |
| 8.0 | 32 | 256 | ||
| 10.7 | 32 | 342 | ||
| 2 | Call rate (call effort constant) | 5.3 | 48 | 256 |
| 8.0 | 32 | 256 | ||
| 10.7 | 24 | 256 | ||
| 3 | Call duration (call effort variable) | 8.0 | 24 | 192 |
| 8.0 | 28 | 224 | ||
| 8.0 | 32 | 256 | ||
| 8.0 | 36 | 288 | ||
| 8.0 | 40 | 320 | ||
| 4 | Call duration (call effort constant) | 10.7 | 24 | 256 |
| 9.1 | 28 | 256 | ||
| 8.0 | 32 | 256 | ||
| 7.1 | 36 | 256 | ||
| 6.4 | 40 | 256 |
The two alternative stimuli in each test were presented from opposite sides of the arena. Whenever call rate was the same in both alternatives, the two stimuli alternated in time with equal periods of silence preceding and following each call. If call rate differed between the two alternatives, the temporal arrangement of strict alternation between the two alternatives only applied to the first three calls broadcast, and subsequent calls drifted in and out of phase according to their designated call rates. Acoustic stimuli were calibrated using a Brüel & Kjær Type 2250 sound level meter to a playback level of 85 dB SPL (sound pressure level, re 20 μPa, fast RMS, C-weighted) at the central release point in the test arena, 1 m from each speaker. This SPL simulates a naturally calling male at approximately 1 m [59].
2.4. Hormone treatments and controls
Our protocols for hormone injections closely followed those outlined by Gordon and Gerhardt [33] in their study of hormonally-induced phonotaxis in eastern gray treefrogs, Hyla versicolor, which were based on a modification of procedures initially detailed by Schmidt [44] in his study of American toads, Anaxyrus (formerly Bufo) americanus. Though we did not measure circulating levels of hormones in the present study, the dosages and timelines of hormone administration adopted here were previously shown in H. versicolor to yield physiologically relevant circulating concentrations of both progesterone and estradiol that did not differ from wild-caught breeding females [33]. Subjects randomly assigned to the hormone-treated group received an intraperitoneal injection of progesterone 18-24 hours prior to testing and an intramuscular (thigh) injection of prostaglandin F2α 30-60 min prior to testing. Doses depended on body mass according to the following equation:
| (1) |
where W = body mass in grams, and K = 2 mg for progesterone and K = 1200 μg for prostaglandin F2α [33]. The progesterone solution was prepared by dissolving 0.4 g progesterone and 0.04 g tragacanth (both from Sigma-Aldrich Corp., St. Louis, MO) in 100 mL of amphibian Ringer’s solution (Fisher Scientific, Pittsburgh, PA). Tragacanth was used to improve the solubility of progesterone in saline. Prostaglandin F2α was used in the form of Lutalyse® (5 mg/ml dinoprost; Zoetis, Florham Park, NJ). Females assigned to the saline-treated group were treated similarly, but received two mass-specific injections of amphibian Ringer’s solution equivalent in volume to the two mass-specific injections of hormone solutions received by females in the hormone-treated group. For half of the females in the saline-treated group, the Ringer’s solution also included tragacanth in the first injection; for the other half it did not.
2.5. Experiment 1
In the first experiment, we investigated whether hormone injections were necessary to induce phonotaxis in females tested outside the breeding season. Subjects (N = 120 total) in the untreated (N = 30), saline-treated (N = 60), and hormone-treated (N = 30) groups were given a choice between two identical 32-pulse calls with equal call rates of 8 calls/min. The dependent variable was whether or not the subject met our response criterion in response to either stimulus. The untreated and saline-treated groups were considered negative controls for the hormone-treated group. We used pairwise Fisher’s Exact Tests to compare the numbers of subjects meeting our response criterion in the three groups after correcting for multiple comparisons (α = 0.017).
2.6. Experiment 2
In the second experiment, we conducted four series of two-alternative choice tests (Table 1) to evaluate the hypothesis that females in the breeding and hormone-treated groups exhibit similar patterns of preferences for calls differing in call rate (calls/min) and call duration (pulses/call). The product of these two features of calls (call rate × call duration) is termed call effort (pulses/min) and describes the number of pulses produced over time. Females of H. chrysoscelis prefer higher call rates, longer calls, and greater call effort [53]. All of the values of call rate, call duration, and call effort used in the stimulus alternatives of this experiment fell in the range of natural variation for this species (corrected to 20°C) [53].
Test series 1 and 2 examined preferences for call rate (Table 1). In these tests, we gave females a choice between all pairwise tests of call rates of 5.3, 8.0, and 10.7 calls/min. In test series 1, the duration of calls in both alternatives was fixed at 32 pulses/call, which is near the population mean (± standard deviation, SD) of 30 ± 4 pulses/call reported in Ward et al. [53]. Thus, in test series 1, call effort varied directly with call rate (Table 1). In test series 2, we fixed call effort at 256 pulses/min by adjusting call duration accordingly. Consequently, there was a negative relationship between call rate and call duration in this test series (Table 1). Test series 3 and 4 examined preferences for call duration (Table 1). In these tests, we gave females choices between calls having 24, 28, 32, 36, or 40 pulses. In four tests, we paired an approximately average-length call (32 pulses) against alternatives with relative pulse numbers that were -2SD (24 pulses), -1SD (28 pulses), +1SD (36 pulses), or +2SD (40 pulses) relative to the 32-pulse call; a fifth test paired the -1SD and +1SD alternatives against each other; and a sixth test paired the -2SD and +2SD alternatives against each other. In test series 3, call rate was fixed at 8 calls/min; therefore, call effort varied directly with call duration. In test series 4, call effort was fixed at 256 pulses/min by adjusting call rate accordingly, thus creating a negative relationship between call duration and call rate (Table 1). In all choice tests, the presentation order (i.e., which alternative began the sequences of stimulus broadcasts) was counter-balanced across subjects.
Each individual female was used as a subject in one to six two-alternative choice tests, and each test had a sample size between 28 and 30 subjects. Independent groups of subjects were compared in the breeding and hormone-treated groups. As is customary in analyses of two-alternative choice tests with frogs, we used two-tailed binomial tests to evaluate the null hypothesis that equal proportions (0.50) of females chose each alternative (α = 0.05). We also used Generalized Estimating Equations (GEE) [60] to directly compare the proportions of females in the breeding and hormone-treated groups that chose alternatives with faster call rates or longer calls across all choice tests in a particular test series. These analysis included “condition” (i.e., breeding versus hormone-treated) as a fixed main effect. In addition, we included “alternatives” (i.e., which two stimulus alternatives were presented), and “order” (i.e., which alternative began the test) as fixed main effects, though these variables were not of primary interest. Individual subjects were never tested more than once at a given combination of condition, alternative, and order. We selected the most appropriate correlation structure for each model using the Quasi Likelihood Under Independence Model Criterion (QIC) [60, 61]. In preliminary analyses, we included all main effects and interaction terms in the models. We removed non-significant interaction terms prior to final analyses. Fisher’s exact tests were used to compare directly the numbers of breeding and hormone-treated females that chose each of the two alternatives in each choice test. We used pairwise Least Significant Difference (LSD) tests based on marginal means to compare levels of significant factors with more than two levels. A sequential Bonferroni correction was used to control for multiple comparisons [62].
3. Results
3.1. Experiment 1
Hormone injections were necessary to induce phonotaxis. One of 30 subjects (3.3%) in the untreated group, four of 30 subjects (13.3%) in the saline-treated group that also received tragacanth, and five of 30 subjects (16.7%) in the saline-treated group that excluded tragacanth, exhibited positive phonotaxis in response to hearing calls. The numbers of subjects responding in these three control groups did not differ significantly (two-tailed Fisher’s exact test: Ps > 0.200). In contrast, 22 of 30 subjects (73.3%) in the hormone-treated group met our response criterion after exhibiting positive phonotaxis, and this response rate was significantly higher than that of both the untreated group (two-tailed Fisher’s exact test: P < 0.001) and the two saline-treated groups (two-tailed Fisher’s exact test: Ps < 0.009).
3.2. Experiment 2
Overall, breeding and hormone-treated females exhibited similar patterns of selectivity for calls differing in call rate or call duration (Fig. 1; Table 2), although response latencies were slower in hormone-treated females (see Supplementary Material). Across the six tests of differences in call rate, breeding females exhibited significant preferences for higher call rates in all six tests, and hormone-treated females did so in four of six tests (Figs. 1A & 1B; two-tailed binomial tests: Ps < 0.05). In test series 1, when call effort was allowed to vary, 97% to 100% of breeding females, and 82% to 100% of hormone-treated females, chose the faster call rate (Fig. 1A). There was no overall statistical difference between the proportions of breeding and hormone-treated females choosing the alternative with a faster call rate when call effort was allowed to vary (P = 0.155; Table 2, test series 1). There were also no differences between these two groups in direct comparisons made separately for each choice test (two-tailed Fisher’s exact tests: 0.097 < Ps ≤ 1.0). Compared with the variable call effort tests in test series 1, fewer females – 77% to 90% of breeding females and 62% to 76% of hormone-treated females – chose the faster call rate in test series 2, in which call effort was held constant (Fig. 1B). Across all tests in series 2 combined, significantly fewer hormone-treated females chose the alternative with a faster call rate compared with breeding females (P = 0.039; Table 2, test series 2). However, direct comparisons between the breeding and hormone-treated groups in each test failed to reveal significant differences (two-tailed Fisher’s exact tests: 0.057 < Ps < 1.0).
Fig. 1.
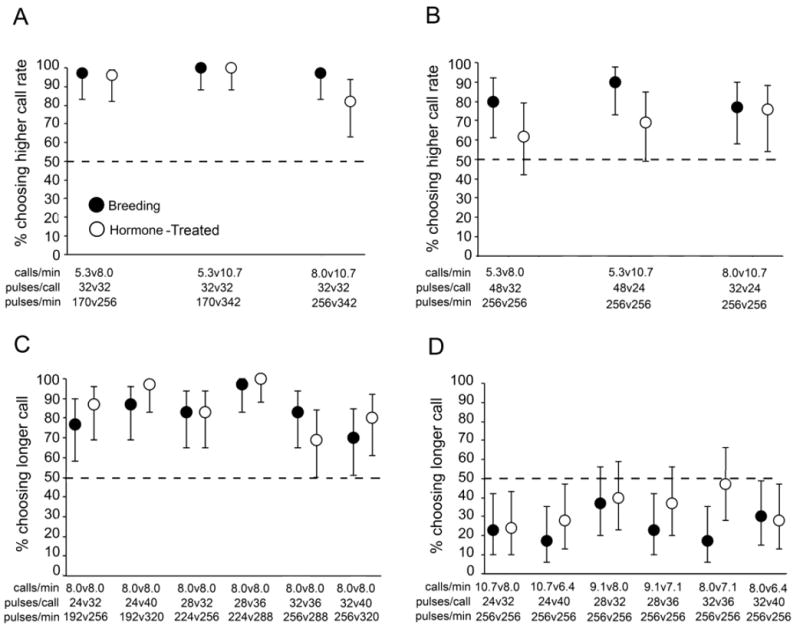
Responses of breeding and hormone-treated females in two-alternative choice tests. Points depict the proportions (±95% exact binomial confidence intervals), expressed as percentages, of breeding females (solid circles) and hormone-treated females (open circles) that chose alternatives with faster call rates or longer call durations. In (A) and (B), call rate was manipulated and call effort was either allowed to vary (A) or was held constant (B). In (C) and (D) call duration was varied and call effort was either allowed to vary (C) or was held constant (D). Values of call properties used in each choice test are depicted along the x-axis (see also Table 1). The horizontal dashed line depicts the null expectation of 0.50; in tests for which the error bars do not overlap the horizontal dashed line, there was a signficant preference in a two-tailed binomial test (P < 0.05). The number of subjects in each two-alternative choice test ranged between N = 28 and N = 30.
Table 2.
Results of GEE analyses examining the effects of condition (i.e., breeding versus hormone-treated), alternatives (i.e., which two alternatives were presented in a particular choice test), and order (i.e., which alternative began the choice test) on choices made in two-alternative choice tests in which call rate or call duration differed between the two alternatives and call effort was allowed to vary freely or was held constant.
| Test series | Acoustic manipulation | Factor | F | df | P |
|---|---|---|---|---|---|
| 1 | Call rate (call effort variable) | Condition | 2.02 | 1 | 0.155 |
| Alternatives | 2.55 | 1 | 0.111 | ||
| Order | 3.01 | 1 | 0.083 | ||
| 2 | Call rate (call effort constant) | Condition | 4.26 | 1 | 0.039 |
| Alternatives | 1.22 | 2 | 0.545 | ||
| Order | 3.13 | 1 | 0.077 | ||
| 3 | Call duration (call effort variable) | Condition | 0.56 | 1 | 0.453 |
| Alternatives | 13.74 | 5 | 0.017 | ||
| Order | 0.13 | 1 | 0.714 | ||
| 4 | Call duration (call effort constant) | Condition | 3.86 | 1 | 0.049 |
| Alternatives | 4.74 | 5 | 0.449 | ||
| Order | 0.06 | 1 | 0.801 |
Across tests comparing call duration, female preferences depended on whether call effort was allowed to vary or held constant (cf Figs. 1C & 1D). In test series 3, in which call effort was variable, significantly more than 50% of females – between 70% and 97% of breeding females and 69% and 100% of hormone-treated females – chose longer calls (Fig. 1C; two-tailed binomial tests: Ps < 0.05). There was no overall difference in the proportions of breeding and hormone-treated subjects choosing the longer call alternative (P = 0.453; Table 2, test series 3). When call effort was held constant in test series 4, however, only 17% to 37% of breeding females, and 24% to 47% of hormone treated females, chose the longer call (Fig. 1D). In fact, significantly fewer than half of females chose the longer call in five of six tests with breeding females and three of six tests with hormone-treated females (two-tailed binomial tests: Ps < 0.05). The remaining tests of breeding and hormone-treated females revealed no significant preferences (two-tailed binomial tests: 0.200 < Ps < 0.856). Overall, hormone-treated females were somewhat less likely to choose longer calls (P = 0.049; Table 2, test series 4), but direct comparisons of the numbers of breeding and hormone-treated females choosing each alternative differed significantly in only one test (two-tailed Fisher’s exact test: P = 0.025) out of six tests (two-tailed Fisher’s exact tests: 0.360 < Ps ≤ 1.0). Recall that when call effort was held constant (test series 4), shorter calls were delivered at relatively faster rates than longer calls. Hence, the preferences of both breeding and hormone-treated females shifted from preferring longer over shorter calls when call rates were equal (test series 3; Fig. 1C) to preferring shorter calls delivered at relatively faster rates when call efforts were equal (test series 4; Fig. 1D). This shift in preference is seen most clearly by comparing the proportions of subjects that chose the longer call in Figure 1C (which are uniformly above 0.50) to those in Figure 1D (which are uniformly below 0.50).
The choices that females made were not dependent upon which alternative began the sequence of alternating calls (Table 2). There was a significant overall effect of alternative only in test series 3 (Table 2). Subjects in test series 3 were more likely to choose the longer call in tests of 28 versus 36 pulses than in tests of 24 versus 32 pulses (LSD test: P = 0.001), 32 versus 36 pulses (LSD test: P < 0.001), and 32 versus 40 pulses (LSD test: P < 0.001) (Fig. 1C).
4. Discussion
Our results are broadly consistent with the hypothesis that the combination of progesterone and prostaglandin F2α induces proceptive behavior in females of Cope’s gray treefrog that is species-typical in its patterns of selectivity for male sexual displays. Breeding and hormone-treated females did not differ in their selectivity for call rate or call duration when call effort varied, and the difference in selectivity for call duration when call effort was constant was just significant (P = 0.049; Table 2). There was considerable overlap in the 95% exact binomial confidence intervals between breeding and hormone-treated groups (Fig. 1), and direct comparisons of outcomes with breeding versus hormone-treated females were non-significant in 15 of 16 comparisons. We interpret this overall pattern of results as demonstrating similar selectivity between breeding and hormone-treated females. This finding is important in light of earlier work on the roles of hormones in the mate choice behaviors of female frogs [6, 31, 46, 47]. Several previous studies have shown that hormone administration can induce sexual proceptivity in female frogs [13, 32, 33, 42-44, 50, 63, 64]. Only three previous studies of only two species (H. versicolor and Physalaemus pustulosus) have shown that hormone administration can induce species-typical patterns of sexual selectivity in the context of intraspecific mate choice [13, 32, 33]. Our findings thus extend a small body of research by empirically demonstrating that behavioral selectivity for male sexual displays is similar in breeding and hormone-treated females. In so doing, these results confirm that hormonal mechanisms that influence proceptive sexual behaviors can also shape selective sexual behaviors in a species-typical fashion.
Cope’s gray treefrog is the diploid member of a cryptic diploid-tetraploid species complex with a remarkable evolutionary history among vertebrates [65, 66]. The tetraploid, H. versicolor, appears to have arisen no fewer than three times independently through pairwise hybridization events between H. chrysoscelis and two other, now-extinct, diploid lineages, making it an allotetraploid. The separate lineages of the tetraploid form a single, interbreeding polyploid species [67]. Previous behavioral studies of the two species confirm that, when call effort is allowed to vary, female prefer faster call rates and longer calls, and these preferences are conserved within the species complex [21, 51-53]. The extent to which the hormonal mechanisms underlying this selectivity may also be conserved is an open question. In the present study, and in earlier work with the tetraploid [33], both breeding females and non-breeding females injected with progesterone and prostaglandin F2α exhibited directional preferences for higher call rates and longer call durations. Our results extend these earlier findings with the tetraploid to a larger number of choice tests pairing a broader range of trait values.
Together, our study and that of Gordon and Gerhardt [33] reveal interesting findings in light of potential differences associated with polyploid speciation. Based on pilot work to determine effective hormone dosages (data not shown), both studies found that injections of the same progesterone quantities worked well for both species. This similarity was somewhat surprising given that difference in ploidy can directly impact endocrine mechanisms [69, 70]. The present study was not intended to investigate these potential species differences. The observation that similar hormone dosages induced broadly similar patterns of proceptive and selective sexual behaviors despite ploidy differences suggests further work on the gray treefrog species complex could help elucidate how hormonal mechanisms evolve following polyploid speciation.
Our results are also broadly consistent with earlier work on a much more distantly related anuran species. Females of the túngara frog (P. pustulosus, Leptodactylidae) injected with human chorionic gonadotropin [32] or estradiol [13] exhibit patterns of mate choice preferences broadly similar to those of females tested shortly after removal from amplexus. Similar selectivity in breeding and hormone-treated females in both túngara frogs and Cope’s gray treefrogs suggests that, in anurans, the response properties of auditory and audio-motor circuits dedicated to processing and responding to conspecific vocalizations are similar between induced and naturally breeding females. Our results indicate that these circuits may be modulated by progesterone and prostaglandin. Comparisons of midbrain audiograms based on multiunit recordings from the auditory midbrain (torus semicircularis, TS) have shown that neural response thresholds increase outside of the natural breeding period [74]. While these changes were examined over seasonal timeframes, it is also possible that more abrupt changes in reproductive behavior, similar to those observed in our study, are the result of these hormones acting on the auditory system. Gonadal steroid hormones are known to influence auditory processing in birds [75, 76], mammals [77, 78], fish [79, 80], and frogs [6]. These effects can arise because of the direct action of hormones on steroid receptors located in the vertebrate inner ear [81]. In anurans, a reduction in the response thresholds of auditory midbrain neurons accompanies a gravid state in female H. cinerea [82], and behavioral receptivity is higher in female P. pustulosus with naturally elevated estradiol levels [32] and a more advanced gravid condition [24].
Although our results demonstrate that progesterone and prostaglandin F2α are sufficient to induce sex- and species-typical phonotaxis in gonadally intact females, they do not demonstrate that they are necessary, nor do they demonstrate an absence of a role for other hormones or interactions among them. As in many studies of wild amphibian behavior, gonadectomies were not performed in the present study, and thus females in the hormone-treated condition likely had low endogenous levels of multiple reproductive hormones, which may interact with exogenous hormones. For instance, treatment with progesterone and prostaglandin F2α elevated endogenous levels of estradiol in intact females of H. versicolor [33]. Likewise, estradiol implants can induce the expression of progesterone receptors in several behaviorally relevant nuclei in the brains of female X. laevis [83]. Similarly, P. pustulosus females injected with human chorionic gonadotropin [32], or estradiol alone [13] exhibit patterns of mate choice preferences broadly similar to breeding females tested shortly after removal from amplexus. Because we did not gonadectomize our frogs, it is possible that our progesterone injections led to increased secretion of estradiol or other gonadal hormones and that these changes facilitated proceptivity and selective phonotaxis. Future studies using gonadectomies and hormone blockade (e.g., fadrazole) [13] will allow for inferences to be drawn regarding the specific set of hormones that are necessary and sufficient for the expression of complex mate choice behavior. Such experimental manipulations will make it possible to further examine how a small set of endocrine products may coordinate and integrate extensive and relatively abrupt changes in sexual behavior. Lesion experiments in H. versicolor have shown that the TS plays a critical role in enabling the audio-motor integration underlying selective phonotaxis [84]. Further, the laminar sub-nucleus of the TS in X. laevis is known to contain both estrogen and progesterone receptors [83], and in females of P. pustulosus, this area exhibits a rapid genomic response following the reception of conspecific advertisement signals, which is then modulated by elevated concentrations of circulating gonadal steroid hormones [85]. Selectively blocking specific receptors across nuclei in the TS, combined with behavioral or neurophysiological testing, could inform our understanding of pleiotropic effects and their timelines of action.
Our data indicate that using hormone induction methods could permit researchers to overcome a major experimental limitation—the ability to evaluate sexual behavior and auditory processing in captive, non-breeding female frogs. For many anuran species, collecting large numbers of amplectant females during what are typically brief breeding seasons can severely limit data collection. Having the ability to pharmacologically induce, at any time of year, the acoustically mediated sexual behaviors observed in wild frogs using a captive population would greatly expand the data collection time window. Further, the option of using captive animals could permit researchers to explore questions previously challenging in this field; for example, performing repeated measures tests across the lifetime of an individual is uncommon in amphibian behavioral studies [but see 86] and almost absent in studies of anuran communication [but see 87], yet this would be feasible with an inducible captive population. Among other things, such work would permit researchers to evaluate which phenotypic traits form constellations by partitioning within- and among-individual variance and co-variance in endocrine levels and endocrine-mediated behavioral traits.
5. Conclusion
Acoustic communication is vital to the social and sexual lives of diverse vertebrate taxa. Anuran amphibians provide excellent systems for examining the hormonal mechanisms that facilitate communication between signalers and receivers. Pursuing this research using anuran amphibians provides some advantages, most importantly that the phonotaxis bioassay provides a sensitive and robust measure of behavioral mate choice, and that hormone manipulation like the kind used in the present study reliably induces species-typical behavior. Understanding the endocrine bases for proceptive behaviors and selectivity in receivers provides an opportunity to reveal the potential role of hormonal pleiotropy as a mechanism for coordinated suites of sexual behavior and thus the underlying structural nature of physiological traits that are under sexual selection. We suggest that future work combine pharmacological manipulations along with behavioral and neurophysiological testing to further elucidate the potential for the pleiotropic effects of a small set of hormones to coordinate the expression of this complex set of sexual behaviors.
Supplementary Material
Highlights.
Female treefrogs prefer male sexual displays that are longer and faster.
Hormone injections elicited proceptive behavior in non-breeding female treefrogs.
Hormone-treated and breeding treefrogs exhibited similar patterns of selectivity.
Hormone administration induces species-typical patterns of sexual selectivity.
Acknowledgments
We thank the Minnesota Department of Natural Resources and the Three Rivers Park District for access to animals and Sandra Tekmen for logistical help in collecting and testing animals. Different aspects of this study were supported by the National Science Foundation in the form of a Graduate Research Fellowship (to EKL) and a CAREER Award (to MAB; IOS 0842759), and by the National Institute on Deafness and Other Communication Disorders (to MAB; R01DC009582). This work was approved by the University of Minnesota Institutional Animal Care and Use Committee (#0809A46721).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wingfield JC, Farner DS. Endocrinology of reproduction in wild species. Avian Biology. 1993;9:163–327. [Google Scholar]
- 2.Stacey NE. Hormonal regulation of female reproductive behavior in fish. Am Zool. 1981;21:305–16. [Google Scholar]
- 3.Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Hormones and Behavior. 2011;59:616–29. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottinger MA, Bakst MR. Endocrinology of the avian reproductive system. Journal of Avian Medicine and Surgery. 1995;9:242–50. [Google Scholar]
- 5.Adkins-Regan E. Hormonal mechanisms of mate choice. Am Zool. 1998;38:166–78. [Google Scholar]
- 6.Arch VS, Narins PM. Sexual hearing: The influence of sex hormones on acoustic communication in frogs. Hearing Research. 2009;252:15–20. doi: 10.1016/j.heares.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidd MR, Dijkstra PD, Alcott C, Lavee D, Ma J, O’Connell LA, et al. Prostaglandin F2 alpha facilitates female mating behavior based on male performance. Behavioral Ecology and Sociobiology. 2013;67:1307–15. [Google Scholar]
- 8.Davis AG, Leary CJ. Elevated stress hormone diminishes the strength of female preferences for acoustic signals in the green treefrog. Hormones and Behavior. 2015;69:119–22. doi: 10.1016/j.yhbeh.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray treefrogs. Science. 1998;280:1928–30. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- 10.Kokko H, Brooks R, Jennions MD, Morley J. The evolution of mate choice and mating biases. Proc R Soc B-Biol Sci. 2003;270:653–64. doi: 10.1098/rspb.2002.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ketterson ED, Nolan V. Adaptation, exaptation, and constraint: A hormonal perspective. American Naturalist. 1999;154:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- 12.Baugh AT, Schaper SV, Hau M, Cockrem JF, de Goede P, van Oers K. Corticosterone responses differ between lines of great tits (Parus major) selected for divergent personalities. General and Comparative Endocrinology. 2012;175:488–94. doi: 10.1016/j.ygcen.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty M, Burmeister SS. Estradiol induces sexual behavior in female túngara frogs. Hormones and Behavior. 2009;55:106–12. doi: 10.1016/j.yhbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Lattin CR, Keniston DE, Reed JM, Romero LM. Are receptor concentrations correlated across tissues within individuals? A case study examining glucocorticoid and mineralocorticoid receptor binding. Endocrinology. 2015;156:1354–61. doi: 10.1210/en.2014-1811. [DOI] [PubMed] [Google Scholar]
- 15.Ryan MJ. Anuran Communication. Washington D.C.: Smithsonian Institution Press; 2001. [Google Scholar]
- 16.Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago: Chicago University Press; 2002. [Google Scholar]
- 17.Wells KD. The Ecology and Behavior of Amphibians. Chicago: University of Chicago Press; 2007. [Google Scholar]
- 18.Ryan MJ, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. American Naturalist. 1992;139:S4–S35. [Google Scholar]
- 19.Sullivan BK. Sexual selection in Woodhouse’s toad (Bufo woodhousei). II. Female choice. Animal Behaviour. 1983;31:1011–7. [Google Scholar]
- 20.Wollerman L. Stabilizing and directional preferences of female Hyla ebraccata for calls differing in static properties. Animal Behaviour. 1998;55:1619–30. doi: 10.1006/anbe.1997.0697. [DOI] [PubMed] [Google Scholar]
- 21.Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. Female preference functions based on call duration in the gray tree frog (Hyla versicolor) Behavioral Ecology. 2000;11:663–9. [Google Scholar]
- 22.Bee MA, Vélez A, Forester JD. Sound level discrimination by gray treefrogs in the presence and absence of chorus-shaped noise. Journal of the Acoustical Society of America. 2012;131:4188–95. doi: 10.1121/1.3699271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MJ. The Túngara Frog: A Study in Sexual Selection and Communication. Chicago: Chicago University Press; 1985. [Google Scholar]
- 24.Baugh AT, Ryan MJ. Female túngara frogs vary in commitment to mate choice. Behavioral Ecology. 2009;20:1153–9. [Google Scholar]
- 25.Baugh AT, Ryan MJ. Mate choice in response to dynamic presentation of male advertisement signals in tungara frogs. Animal Behaviour. 2010;79:145–52. [Google Scholar]
- 26.Gerhardt HC. Phonotaxis in female frogs and toads: Execution and design of experiments. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in Comparative Psychoacoustics. Basel: Birkhäuser Verlag; 1995. pp. 209–20. [Google Scholar]
- 27.Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Hormones and Behavior. 1976;7:105–38. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- 28.Beach FA, Stern B, Carmichael M, Ranson E. Comparisons of sexual receptivity and proceptivity in female hamsters. Behavioral Biology. 1976;18:473–87. doi: 10.1016/s0091-6773(76)92500-1. [DOI] [PubMed] [Google Scholar]
- 29.De Jonge FH, Eerland EMJ, Van De Poll NE. The influence of estrogen, testosterone and progesterone on partner preference, receptivity and proceptivity. Physiology & Behavior. 1986;37:885–91. doi: 10.1016/0022-510x(86)90209-1. [DOI] [PubMed] [Google Scholar]
- 30.Murphy CG, Gerhardt HC. Evaluating the design of mate-choice experiments: The effect of amplexus on mate choice by female barking treefrogs, Hyla gratiosa. Animal Behaviour. 1996;51:881–90. [Google Scholar]
- 31.Wilczynski W, Lynch KS. Female sexual arousal in amphibians. Hormones and Behavior. 2011;59:630–6. doi: 10.1016/j.yhbeh.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch KS, Crews D, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the tungara frog (Physalaemus pustulosus) Hormones and Behavior. 2006;49:450–7. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon NM, Gerhardt HC. Hormonal modulation of phonotaxis and advertisement-call preferences in the gray treefrog (Hyla versicolor) Hormones and Behavior. 2009;55:121–7. doi: 10.1016/j.yhbeh.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad, Bufo japonicus, in association with behavior during the breeding season. General and Comparative Endocrinology. 1990;80:451–64. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- 35.Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spadefoot toad, Scaphiopus couchii. General and Comparative Endocrinology. 1997;105:102–13. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- 36.Medina MF, Ramos I, Crespo CA, González-Calvar S, Fernández SN. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. General and Comparative Endocrinology. 2004;136:143–51. doi: 10.1016/j.ygcen.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Lynch KS, Wilczynski W. Gonadal steroids vary with reproductive stage in a tropically breeding female anuran. General and Comparative Endocrinology. 2005;143:51–6. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Kelley DB. Female sex behaviors in the South African clawed frog, Xenopus laevis: Gonadotropin-releasing, gonadotropic, and steroid hormones. Hormones and Behavior. 1982;16:158–74. doi: 10.1016/0018-506x(82)90016-2. [DOI] [PubMed] [Google Scholar]
- 39.Whittier JM, Crews D. Effects of Prostaglandin F2 α on Sexual Behavior and Ovarian Function in Female Garter Snakes (Thamnophis sirtalis parietalis)*. Endocrinology. 1986;119:787–92. doi: 10.1210/endo-119-2-787. [DOI] [PubMed] [Google Scholar]
- 40.Guillette L, Gaross T, Matter J, Palmer B. Arginne vasotocin-induced prostaglandin synthesis in vitro by the reproductive tract of the viviparous lizard Sceloporus jarrovi. Prostaglandins. 1990;39:39–51. doi: 10.1016/0090-6980(90)90093-b. [DOI] [PubMed] [Google Scholar]
- 41.Slater DM, Zervou S, Thornton S. Prostaglandins and prostanoid receptors in human pregnancy and parturition. Journal of the Society for Gynecologic Investigation. 2002;9:118–24. [PubMed] [Google Scholar]
- 42.Schmidt RS. Mating call phonotaxis in the female American toad: Induction by hormones. General and Comparative Endocrinology. 1984;55:150–6. doi: 10.1016/0016-6480(84)90139-4. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt RS. Mating call phonotaxis in female American toad: Induction by intracerebroventricular prostaglandin. Copeia. 1985;1985:490–2. [Google Scholar]
- 44.Schmidt RS. Prostaglandin-induced mating call phonotaxis in female American toad: Facilitation by progesterone and arginine vasotocin. Journal of Comparative Physiology A. 1985;156:823–9. [Google Scholar]
- 45.Weintraub AS, Kelley DB, Bockman RS. Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Hormones and Behavior. 1985;19:386–99. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- 46.Wilczynski W, Lynch KS, O’Bryant EL. Current research in amphibians: Studies integrating endocrinology, behavior, and neurobiology. Hormones and Behavior. 2005;48:440–50. doi: 10.1016/j.yhbeh.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodley SK. Hormones and reproductive behavior in amphibians. In: Norris DO, Lopez KH, editors. Hormones and Reproduction of Vertebrates: Amphibians. Vol. 2. San Diego: Elsevier Academic Press Inc; 2011. pp. 143–69. [Google Scholar]
- 48.Schmidt RS. Preoptic activation of mating call orientation in female anurans. Behaviour. 1969;35:114–&. doi: 10.1163/156853970x00150. [DOI] [PubMed] [Google Scholar]
- 49.Gobbetti A, Zerani M, Carnevali O, Botte V. Prostaglandin F2α in female water frog, Rana esculenta: Plasma levels during the annual cycle and effects of exogenous PGF2α on circulating sex hormones. General and Comparative Endocrinology. 1990;80:175–80. doi: 10.1016/0016-6480(90)90162-f. [DOI] [PubMed] [Google Scholar]
- 50.Gerhardt HC. Reproductive character displacement of female mate choice in the grey treefrog Hyla chrysoscelis. Animal Behaviour. 1994;47:959–69. [Google Scholar]
- 51.Gerhardt HC, Dyson ML, Tanner SD. Dynamic properties of the advertisement calls of gray tree frogs: Patterns of variability and female choice. Behavioral Ecology. 1996;7:7–18. [Google Scholar]
- 52.Bee MA. Parallel female preferences for call duration in a diploid ancestor of an allotetraploid treefrog. Animal Behaviour. 2008;76:845–53. doi: 10.1016/j.anbehav.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward JL, Love EK, Vélez A, Buerkle NP, O’Bryan LR, Bee MA. Multitasking males and multiplicative females: dynamic signalling and receiver preferences in Cope’s grey treefrog (Hyla chrysoscelis) Animal Behaviour. 2013;86:231–43. [Google Scholar]
- 54.Vélez A, Linehan-Skillings BJ, Gu Y, Sun Y, Bee MA. Pulse number-discrimination by Cope’s gray treefrog (Hyla chrysoscelis) in modulated and unmodulated noise. Journal of the Acoustical Society of America. 2013;134:2079–3089. doi: 10.1121/1.4820883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bee MA. Finding a mate at a cocktail party: Spatial release from masking improves acoustic mate recognition in grey treefrogs. Animal Behaviour. 2008;75:1781–91. doi: 10.1016/j.anbehav.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bee MA, Schwartz JJ. Behavioral measures of signal recognition thresholds in frogs in the presence and absence of chorus-shaped noise. Journal of the Acoustical Society of America. 2009;126:2788–801. doi: 10.1121/1.3224707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akre KL, Ryan MJ. Complexity increases working memory for mating signals. Current Biology. 2010;20:502–5. doi: 10.1016/j.cub.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 58.Kime NM, Rand AS, Kapfer M, Ryan MJ. Consistency of female choice in the tungara frog: a permissive preference for complex characters. Animal Behaviour. 1998;55:641–9. doi: 10.1006/anbe.1997.0752. [DOI] [PubMed] [Google Scholar]
- 59.Gerhardt HC. Sound pressure levels and radiation patterns of vocalizations of some North American frogs and toads. Journal of Comparative Physiology. 1975;102:1–12. [Google Scholar]
- 60.Hardin JW, Hilbe JM. Generalized Estimating Equations. 2. New York: Chapman & Hall/CRC; 2012. [Google Scholar]
- 61.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 62.Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–5. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 63.Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Hormones and Behavior. 1994;28:232–40. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- 64.Tucker MA, Gerhardt H. Parallel changes in mate-attracting calls and female preferences in autotriploid tree frogs. Proceedings of the Royal Society B. Biological Sciences. 2012;279:1583–7. doi: 10.1098/rspb.2011.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holloway AK, Cannatella DC, Gerhardt HC, Hillis DM. Polyploids with different origins and ancestors form a single sexual polyploid species. American Naturalist. 2006;167:E88–E101. doi: 10.1086/501079. [DOI] [PubMed] [Google Scholar]
- 66.Ptacek MB, Gerhardt HC, Sage RD. Speciation by polyploidy in treefrogs: Multiple origins of the tetraploid, Hyla versicolor. Evolution. 1994;48:898–908. doi: 10.1111/j.1558-5646.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 67.Espinoza N, Noor M. Population genetics of a polyploid: is there hybridization between lineages of Hyla versicolor? Journal of Heredity. 2002;93:81–5. doi: 10.1093/jhered/93.2.81. [DOI] [PubMed] [Google Scholar]
- 68.Graham JD, Clarke CL. Physiological Action of Progesterone in Target Tissues 1. Endocrine reviews. 1997;18:502–19. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 69.Benfey TJ, Dye HM, Solar II, Donaldson EM. The growth and reproductive endocrinology of adult triploid Pacific salmonids. Fish Physiology and Biochemistry. 1989;6:113–20. doi: 10.1007/BF01875483. [DOI] [PubMed] [Google Scholar]
- 70.Cayrol C, Garnier DH, Deparis P. Comparative plasma levels of androgens and 17β-estradiol in the diploid and triploid newt, Pleurodeles waltl. General and Comparative Endocrinology. 1985;58:342–6. doi: 10.1016/0016-6480(85)90106-6. [DOI] [PubMed] [Google Scholar]
- 71.Pala I, Coelho MM, Schartl M. Dosage compensation by gene-copy silencing in a triploid hybrid fish. Curr Biol. 2008;18:1344–8. doi: 10.1016/j.cub.2008.07.096. [DOI] [PubMed] [Google Scholar]
- 72.Ohno S. Evolution by Gene Duplication. Heidelberg: Springer-Verlag; 1970. [Google Scholar]
- 73.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–5. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 74.Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia. 1984;1984:844–52. [Google Scholar]
- 75.Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. Journal of Comparative Physiology A. 2010;196:581–99. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maney D, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Frontiers in neuroendocrinology. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miranda JA, Liu RC. Dissecting natural sensory plasticity: hormones and experience in a maternal context. Hearing research. 2009;252:21–8. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Mana D, Ceranic B, Djahanbakhch O, Luxon LM. Alteration in auditory function during the ovarian cycle. Hearing research. 2010;268:114–22. doi: 10.1016/j.heares.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 79.Sisneros JA. Seasonal plasticity of auditory saccular sensitivity in the vocal plainfin midshipman fish, Porichthys notatus. Journal of neurophysiology. 2009;102:1121–31. doi: 10.1152/jn.00236.2009. [DOI] [PubMed] [Google Scholar]
- 80.Rohmann KN, Bass AH. Seasonal plasticity of auditory hair cell frequency sensitivity correlates with plasma steroid levels in vocal fish. The Journal of Experimental Biology. 2011;214:1931–42. doi: 10.1242/jeb.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neuroscience. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. Journal of Comparative Physiology A. 2009;195:341–9. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- 83.Roy EJ, Wilson MA, Kelley DB. Estrogen-induced progestin receptors in the brain and pituitary of the South African clawed frog, Xenopus laevis. Neuroendocrinology. 1986;42:51–6. doi: 10.1159/000124248. [DOI] [PubMed] [Google Scholar]
- 84.Endepols H, Feng AS, Gerhardt HC, Schul J, Walkowiak W. Roles of the auditory midbrain and thalamus in selective phonotaxis in female gray treefrogs (Hyla versicolor) Behav Brain Res. 2003;145:63–77. doi: 10.1016/s0166-4328(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 85.Lynch KS, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav Evol. 2008;71:143–50. doi: 10.1159/000111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson ADM, Krause J. Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behavioral Ecology. 2012;23:1316–23. [Google Scholar]
- 87.Baugh AT, Ryan MJ. The development of sexual behavior in túngara frogs (Physalaemus pustulosus) Journal of Comparative Psychology. 2010;124:66–80. doi: 10.1037/a0017227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


