Abstract
Epidemiological associations suggest populations consuming substantial amounts of dietary soy exhibit a lower risk of prostate cancer. A 20-week randomized, phase II, cross-over trial was conducted in 32 men with asymptomatic prostate cancer. The crossover involved 8 weeks each of soy-bread and soy-almond bread. The primary objective was to investigate isoflavone bioavailability and metabolite profile. Secondary objectives include safety, compliance and assessment of biomarkers linked to prostate carcinogenesis. Two distinct soy breads were formulated to deliver ~60 mg aglycone equivalents of isoflavones/day. The isoflavones were present as aglycones (~78% as aglycones,) in the soy-almond bread (SAB) while in the standard soy bread (SB) predominantly as glucosides (18% total isoflavones as aglycones). Compliance to SB (97% ± 4%) and SAB (92% ± 18%) was excellent, toxicity was rare and limited to grade I gastrointestinal complaints. Pharmacokinetic studies between SB and SAB showed modest differences. Peak serum concentration time (Tmax) was significantly faster with SAB meal compared with SB in some isoflavonoids and AUC0 to 24 hr of dihydrodaidzein and O- desmethylangolensin was significantly greater after a SB meal. An exploratory cluster analysis was used to identify four isoflavone metabolizing phenotypes. Insulin-like growth factor binding protein increased significantly by 41% (p=0.024) with soy intervention. Findings from this study provide the necessary framework to study isoflavone metabolizing phenotypes as a strategy for identification of individuals that might benefit or show resistance to cancer preventive strategies using dietary soy. A standardized soy bread used for future large-scale randomized clinical trials to impact human prostate carcinogenesis is feasible.
Keywords: soy, isoflavones, prostate cancer, dietary intervention, bioavailability
Introduction
The impact of soy consumption on health has been frequently evaluated in both pre-clinical and human studies, yielding provocative results (1). One area of particular interest for applying a soy intervention is in the setting of prostate cancer. Initial epidemiologic observations suggested that populations consuming substantial amounts of dietary soy have a lower risk of prostate cancer (2). It is thought that the impact of soy on health or disease processes may be due to the nature of the soy protein fraction, or the unique array of non-nutrients referred to collectively as bioactive phytochemicals. Phytochemicals of interest include isoflavones, protease inhibitors, inositol hexaphosphate (phytic acid), lignans, phytosterols, and saponins (3). Isoflavones have been studied and demonstrate anti-prostate cancer activity in vitro (4) and in rodent studies of carcinogenesis (5) and tumorigenesis (6). Potential mechanisms of action for soy isoflavones suggest multiple targets in prostate cancer cells that impact growth control and sensitivity to apoptosis. For example, the inhibition of IGF-I associated tyrosine protein kinase activity (4) or processes related to testosterone and androgen receptor activity (7), two of the most critical hormones involved in prostate development, function, and cancer. The tumor-host interaction may also be targeted via modulating angiogenesis (8), inflammation or immune surveillance (9).
Epidemiologic and laboratory studies have stimulated human intervention studies with soy components to impact various biomarkers or physiologic processes relevant to prostate cancer. Several small studies are beginning to provide insight into bioactivity (10, 11). However, the largest human intervention study completed thus far, a study of biochemical failure in 177 post-prostatectomy patients, with randomization to a soy protein powdered drink (41 mg as aglycone equivalents of isoflavones per day for ~2 years) or similar product derived from the milk protein casein showed no impact on risk of biochemical (prostate specific antigen) progression (12). We propose that soy will more likely impact earlier events in prostate carcinogenesis and that metabolism of soy, perhaps via host genetics and/or the microbiome may be a key cofactor.
Soy isoflavone absorption is thought to occur by passive absorption of the aglycones in the small intestine. However, debate continues regarding the bioavailability of isoflavone aglycones compared to their glucosides (13, 14, 15, 16, 17). Studies suggest isoflavones administered in their glucoside forms (predominant in native soybeans) have greater (14), lesser (13, 16), or no difference in absorption as compared to their aglycone forms (15). Thus, understanding isoflavone bioavailability and postprandial pharmacokinetics remains an important question in soy literature.
Adding to the complexity in elucidation biological impact of soy isoflavones, numerous metabolites of soy isoflavones can be formed in the human gut depending on the commensal bacteria in the large intestine (18, 19). Several factors influence the bacterial ecology of an individual’s colon including: genetics (20), gender (21, 22, 23), biological entrapment of isoflavones (24), diet composition (25), food matrix (21, 23, 26), and daidzein degrading phenotype (25). The ability of an individual to produce certain isoflavone metabolites may be important to derive the health benefits from soy consumption (14). The metabolite equol has been of interest because it has selective estrogen receptor modulator activities in vitro (14). Similarly ODMA, another metabolite of daidzein, as well as equol, suppress prostate cancer growth in cell culture (27). Since there are several isoflavone metabolites in urine and plasma, a multi-faceted biological effect is possible. However, few studies have attempted to characterize isoflavone metabolism phenotypes by simultaneously analyzing several metabolites. These profiles could be used to identify individuals most likely to derive benefit from soy consumption.
This study characterizes the absorption and bioavailability of isoflavones from novel, chemically standardized soy (SB) and soy-almond bread (SAB) products. We propose that this is a critical step in defining an optimal and relevant delivery system for the evaluation of soy in human studies. We also hypothesize that bread with greater quantities of bioavailable isoflavones may improve isoflavone absorption and distribution. This may be particularly true for the majority (~60%) of the Western population who appear less capable of metabolizing daidzein conjugates to equol (14, 18). In the present study, we tested bread products in men previously treated for clinically localized prostate cancer but are now experiencing asymptomatic biochemical (PSA failure) recurrence, with no evidence of disease on standard staging studies. This study with standardized food products will also define possibly relevant patterns of absorption and metabolism within a potential target population for future longer term intervention studies.
Materials and Methods
Preparation and isoflavone quantification of standardized soy and soy-almond bread
The standardization and production of soy breads used for this study are detailed in Supplemental Methods S1.
Participants
Study participants were enrolled from the Genitourinary Oncology Clinics at The Ohio State University Medical Center. Men had histologically documented prostate adenocarcinoma with a minimum of two increases in PSA after primary therapy or during active surveillance. Men were asymptomatic with no changes in hormone therapy planned for 5 months. Men were excluded if they had an active malignancy (other than prostate cancer) requiring medical therapy; abnormal kidney or liver function; active digestive illnesses or malabsorptive disorders, metabolic disorders requiring special diet modifications; endocrine disorders requiring hormone administration; known allergy to almonds, wheat, or soy; did not wish to discontinue dietary supplement use, or recent antibiotic use (< 6 months prior to enrollment). All participants were regularly followed by a Medical Oncologist and would be suspended from participation if disease progression required more aggressive medical care. The study protocol was approved by The Ohio State University Comprehensive Cancer Center’s Clinical Scientific Review Committee and the Biomedical Sciences Cancer Institutional Review Board (NCT:01682941). Written and verbal informed consent was obtained from each enrolled participant.
Study Design
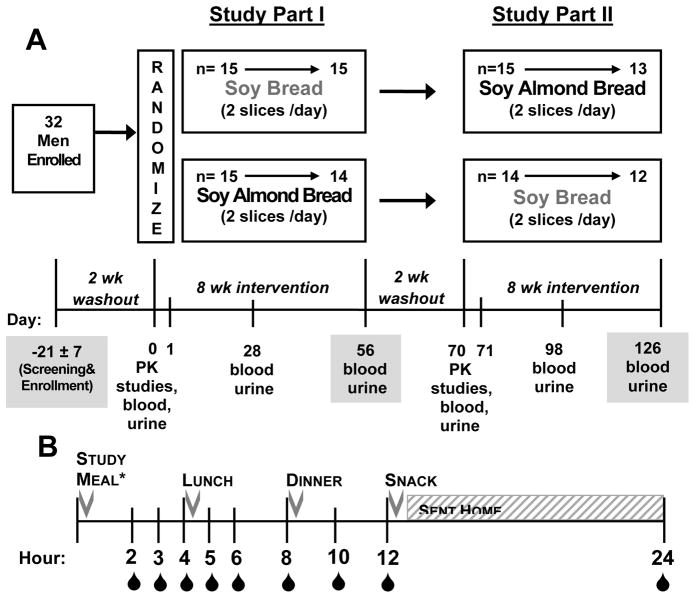
This study was a 20 week, phase II, randomized, crossover design. Study visits were conducted at the Center for Clinical and Translational Science- Clinical Research Center. Each arm of the study was preceded with a 2 week washout (legume-free diet) and intervention commenced with a 24-hour pharmacokinetic study, which was then followed by an 8-week intervention with one of the two study breads. After completion of the first arm, participants then had crossed over to the alternate bread (Figure 1A). A 2 week washout period is typical of feeding trials with soy isoflavone intervention (23). For each of the two pharmacokinetic studies (collected on day 0 and 70), fasted participants arrived at the research center, had an indwelling venous catheter placed and consumed one slice of study bread. Blood was collected immediately before (0 hr) and then at 2, 3, 4, 5, 6, 8, 10, and 12 hours after consumption of the test meal, Figure 1B. Men were sent home after 12 hours and returned 24 hours after the test meal for a blood draw. The test meal consisted of either two slices of SB (167 ± 2 g) or SAB (167±1 g) with water. The two slices of SB provided 404 kcal, while the SAB provided 420 kcal, Supplemental Table S2. Participants received legume-free meals while in the research unit and they were instructed to follow a legume-free diet for the duration of the study. During each 8-week intervention period, men were instructed to supplement their diet with 2 slices of study bread and document consumption in a journal. Frozen loaves of bread were distributed at each study visit with thawing instructions. Condiments were permitted on the bread, but heating was discouraged. Additional blood draws and 24 hour urine collections were completed at days 0, 28, 56, 70, 98, and 126. Men completed a 3 day diet record during each intervention period. All men were also provided a standardized multivitamin/mineral supplement (One Daily Multiple Plus Minerals, CVS Caremark, Woonsocket, RI) and asked to discontinue all other nutritional supplements for the duration of the study.
Figure 1.
A.) Schematic of 20 week randomized crossover design with two standardized soy breads as dietary interventions. Gray boxes represent visits with oncologist. B.) Design of postprandial pharmacokinetic (PK) studies conducted on days 0 and 70. Details of the specific hours when blood was collected are represented with
 and controlled meals were served at the indicated hours.
and controlled meals were served at the indicated hours.
Chemicals
Anhydrous sodium acetate, and diethyl ether (Et2O) as well as HPLC or MS-grade acetonitrile, formic acid (≥98% purity), methanol, and water were purchased from Fisher Scientific (Fairlawn, NJ). Lyophilized glucuronidase/sulfatase from Helix pomatia, dimethyl sulfoxide, and equol were obtained from Sigma-Aldrich (St. Louis, MO). Daidzein, genistein, glycitein, daidzin, genistin, glycitin, acetyl daidzin, malonyl genistin, acetyl genistin were from LC laboratories division (PKC Pharmaceuticals, Inc, Woburn, MA) and malonyl daidzin, and malonyl glycitin from Wako Chemicals USA, Inc (Richmond, VA). Isoflavone metabolites (dihydrodaidzein, dihydrogenistein, ODMA, and 6-OH-ODMA) were from Plantech (Reading, UK).
Isoflavonoid Quantification from Blood
12 hour fasting blood was collected (K3EDTA, Vacutainer, Becton-Dickson, Franklin Lakes, NJ), centrifuged (Sorvall Legend RT Kendro Laboratory Products, Hanau, DE) at 1,000xg at 4°C for 10 minutes and plasma collected. Plasma was aliquoted and stored at −80C until analysis. Extraction of isoflavones from plasma is detailed by Zuniga et al 2013 (28) and a detailed description of the UPLC-MS/MS conditions is provided in the Supplemental Methods S1.
Urine Preparation for Isoflavonoid Analysis
Urine collection (pre-weighed with 05.g/L boric acid) collected for more than 20 hours was considered complete. Urine volume was determined using mass and urine specific gravity. Aliquots of urine were stored at −80°C until HPLC analysis. Extraction of isoflavonoids in urine was adapted as described previously by Zuniga et al. 2013 (28).
Quantification of Isoflavonoids in Urine
HPLC instrumentation for urine analyses was the same as described for soy food analysis (32). Symmetry™ C8 column (4.6 × 75 mm, 3.5 μm, Waters Associated, Milford, MA) affixed to a Symmetry™ C8 guard column (3.0 × 20 mm, 3.5 μm) was used for separation of isoflavonoids extracted from urine. The binary mobile phase was 0.1% aqueous formic acid (solvent A) and 0.1% formic acid in 90% methanol and 10% acetonitrile (solvent B) with a flow rate of 1.3 mL/min. The linear gradient began at 95:5 (solvent A:solvent B), increased to 65:35 in 3 minutes, 42:58 in 12 minutes, 10:90 at 14 minutes, and then returning to 95:5 at 18 minutes, for a total run time of 18 minutes. The inter-assay and intra-assay variability in urine as well as plasma was less than 15%. Urine concentrations less than 46 nmol/L (12.4 ng/mL) to 56 nmol/L (14.2 ng/mL) for the parent (daidzein, genistein, glycitein) isoflavonoids and less than 45 nmol/L (12.3 ng/mL) to 60 nmol (15.6 ng/mL) for intermediate (DHG and DHD) isoflavonoids were considered below the level of quantification. Moreover, urine concentrations less than 125 nmol/L (30 ng/mL) for equol, 85 nmol/L (21.9 ng/mL) for O-DMA, and 137 nmol/L (39.5 ng/mL) for 6′OH-ODMA were considered below the level of quantification
Laboratory and Clinical Measures of Toxicity
Side effects were recorded and scored on the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE v. 4.0) (29) and serum was collected for PSA analysis at all clinic visits. Enrolled men were seen (day 56, and 126) by their attending oncologist for routine assessment of clinically detectable disease and blood (complete blood count with differential, electrolytes, and liver function tests) was collected to evaluate toxicity. All clinical blood samples and PSA were analyzed by OSU’s Wexner Medical Center clinical laboratories. Since soy protein has been associated with altering lipid metabolism, fasting serum lipids (total cholesterol, LDL, HDL, and triglycerides), and endocrine biomarkers (human growth hormone, IGF-1, IGFBP-3, and leptin) were analyzed by the CRC core laboratories using either chemiluminescence or radioimmunoassay methods with a Dimension Xpand Clinical Chemistry analyzer (Siemens Medical Diagnostics, Decatur, GA.). The Intra-assay %CV for lipids and biomarkers were < 2.31% and the inter-assay %CV was <2.35%.
Calculations and Statistical Analysis
Isoflavonoid absorption in plasma during the 24hr pharmacokinetic study was assessed using maximum concentration (Cmax), time to maximum concentration (Tmax), and area under the curve (AUC0 to 24hr) using trapezoidal approximation. Statistical analysis was conducted using SPSS version 19.0 software (IBM, Chicago, IL) and Stata v10.1 (StataCorp LP, College Station, TX). Descriptive analysis were used to determine mean ± SD for food samples and mean ± SE for clinical data, laboratory data, dietary intake of soy breads, and isoflavonoids in biological samples. Independent-t-test determined significant differences in isoflavone composition between SB and SAB. For all analyses, p values ≤ 0.05 were considered significant. A repeated measures analysis of variance was used to assess differences due to treatment, period, and time in plasma and urine isoflavonoids. If significant effects (p ≤ 0.05) were found, then Tukey’s post hoc analysis was performed. An exploratory cluster analysis was performed using a hierarchical agglomerative technique employing Euclidean distances to identify clusters of daidzein metabolizing phenotypes. Due to variation in the total amount of daidzein metabolites across patients, proportions were used in the clustering to identify relative contributions of the constituents. Additionally, the intra-class correlation coefficient (ICC) was used to evaluate the consistency of the daidzein metabolites over time.
Results
Standardized SB and SAB have respectively high glycoside and high aglycone forms of isoflavones
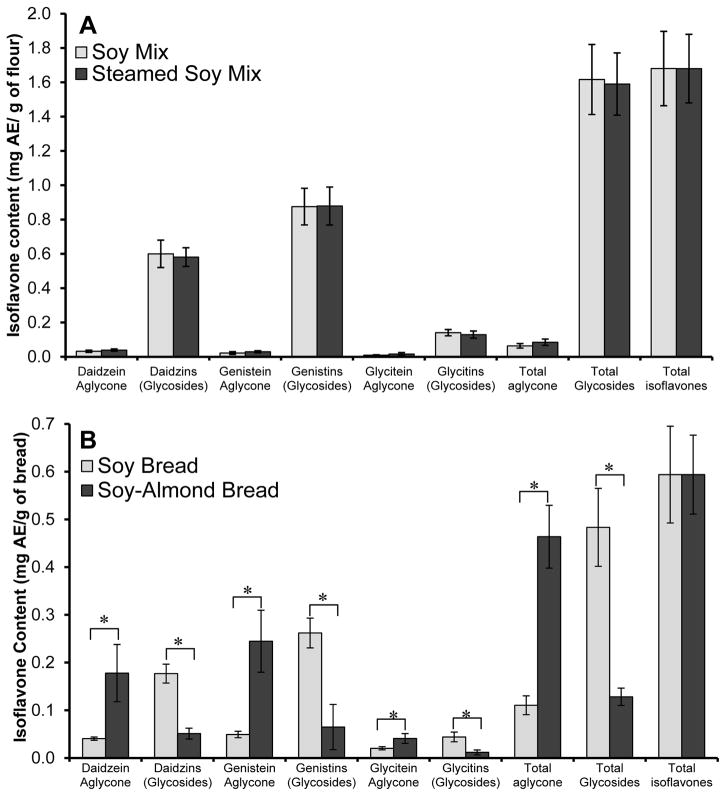
The isoflavone composition in the starting soy mix used for both breads was virtually the same (p>0.80), Figure 2A. As predicted, due to the presence of glucosidase activity from almonds, the bread making process transformed the chemical composition of the isoflavones to be significantly different between SB and SAB yet the total isoflavone content was preserved, Figure 2B. Isoflavones in one slice of SB was 34.9 ± 6.0 mg AE of isoflavones (18% aglycones) and SAB was 34.7 ± 4.8 mg AE isoflavones (78% aglycones). The isoflavone composition and content was found to remain be stable through 2 years of storage (−25°C).
Figure 2.
A.) Isoflavone composition in soy mix and steamed soy mix (n= 27) before it was made into bread. B.) Isoflavone composition in soy breads (dry basis, n=96). Glycosides include acetyl, malonyl-, and the simple β-glucoside for daidzein, genistein, and glycitein. AE, aglycone equivalents. Statistical significance (p≤0.01) was determined using independent t-test and denoted with an asterisks.
Participant Adherence
Of the 32 men (52 to 84 years old) enrolled in this study, 25 completed all study activities, detailed demographics in Supplemental Table S3. Three men withdrew from the study due to undisclosed personal reasons, four men required more aggressive prostate cancer therapy, and one man was diagnosed with a second malignancy requiring therapy. Of the seven men who withdrew from the study, four completed all visits through day 70. Adherence to legume-restricted diet and interventions was excellent (SB = 97 ± 4% and SAB = 92 ± 19%), Supplemental Table S3. Isoflavonoid excretion in 24 hour urine collections were used to verify adherence to the legume-free diet and soy bread interventions. A significant increase (p ≤ 0.001) in total isoflavonoid excretion was observed in urine on days 28 (24.83 ± 2.00 mg/24 hr), 56 (23.01 ± 2.01 mg/24 hr), 98 (23.88 ± 1.53 mg/24 hr), and 126 (22.49 ± 1.35 mg/24 hr) of the intervention compared to the washout days (Day 0 = 0.12 ± 0.03 mg/24 hr and Day 70 = 0.13 ± 0.05 mg/24 hr) confirming that dietary compliance was excellent and soy breads were effective vehicles for isoflavone delivery. Likewise, the total isoflavone concentration in urine among the intervention visits did not significantly differ (p = 0.337). Daidzein (65% of total, 16.9 mg total daidzein isoflavonoids/25.8 mg dietary daidzeins) and genistein (19% of total, 7.0 mg total genistein isoflavonoids/36.3 mg dietary genisteins) was present in urine of all men.
Isoflavone pharmacokinetics after SB and SAB meals show modest differences
Independent of bread type, parent isoflavones (daidzein, genistein, and glycitein) were first detected in plasma at 2 hours after test meals, Supplemental Figure S4. Isoflavone metabolites, dihydrodaidzein and dihydrogenistein, were observed in plasma starting at 3 hours, and microbial metabolites of daidzein, ODMA and equol, were observed in plasma starting at 4 hours after the test meal, Supplemental Figure S4. The time to reach peak serum concentration (Tmax) of daidzein, dihydrodaidzein, genistein, and 6′OH-ODMA was significantly more rapid (p ≤ 0.05) following the meal with SAB compared to the SB meal, Supplemental Figure S4. Moreover, peak absorption (Cmax) of genistein (p=0.025) was significantly higher after a SAB meal compared to SB whereas peak absorption and accumulation (AUC0 to 24 hr) of ODMA (p=0.011) and DHD (p=0.044) was significantly higher after a SB meal compared to SAB, Supplemental Figure S4.
Cluster analysis used to explore individual variability and isoflavone metabolizing phenotypes
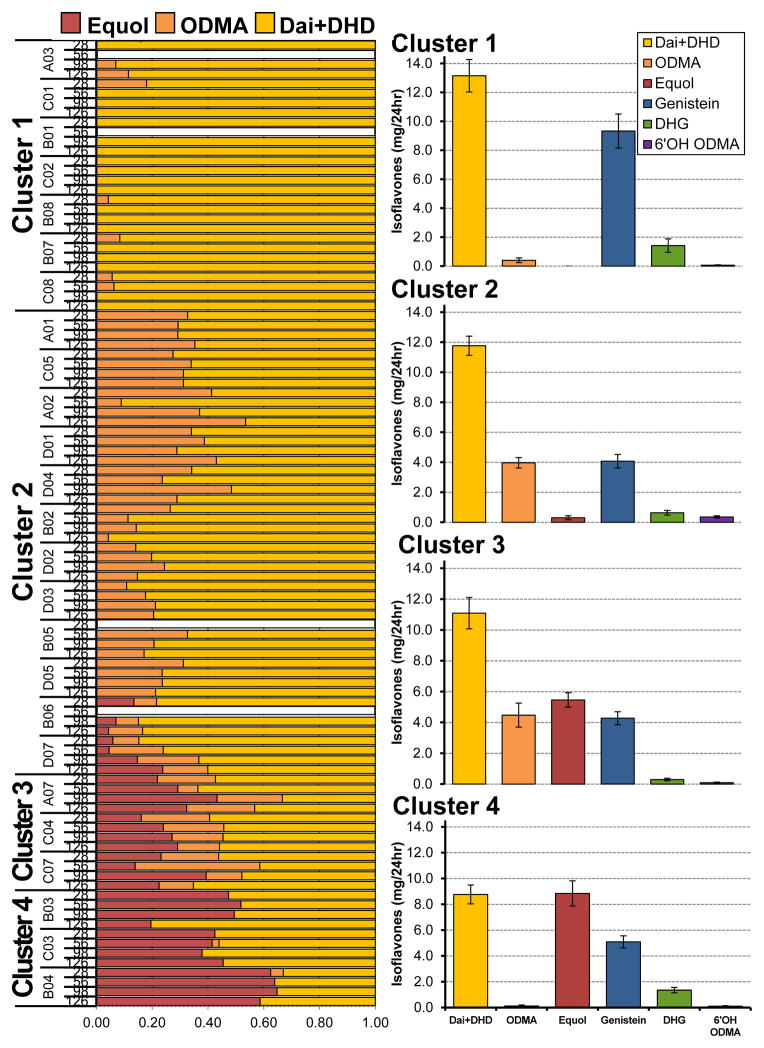
An exploratory cluster analysis was used to characterize isoflavone metabolizing phenotypes in patients. From this analysis using daidzein metabolites from 24 hour urine collections, four clusters emerged consistently over 4 different time points. Relative to the other three clusters, cluster 1 (n=7) as demonstrating the highest excretion of parent (daidzein and genistein) and intermediate (DHD and DHG) isoflavonoids in their urine, Figure 3. ODMA production in cluster 1 was modest and inconsistent and excreted ODMA in relatively low levels (0.40 ± 0.16 mg/24hr). Cluster 2 (n=12) showed the greatest concentration of ODMA in the urine (3.97 ± 0.35 mg/24hr) but very limited amounts of equol (0.31 ± 0.13 mg/24hr) among the equol producers. Men in cluster 3 (n=3) had similar proportions of equol (5.46 ± 0.47 mg/24hr) and ODMA (4.48 ± 0.78 mg/24hr). Cluster 4 (n=3) also has the greatest excretion of equol (8.84 ± 0.98 mg/24hr) and very low levels of ODMA (0.12 ± 0.08 mg/24hr).
Figure 3.
Proportion of daidzein isoflavonoids from 24 hour urine collections (mg/24 hr) taken during 4 time points over 20 weeks (n=25), left. Cluster analysis determined four distinct clusters and their respective isoflavonoid profile, right. White bars indicate urine samples not collected. Significant differences (p≤0.05) between metabolizing phenotype clusters were determined using ANOVA, reported using an asterisks. ODMA, O-desmethylangolensin; DHD, dihydrodaidzein; DHG, dihydrogenistein; 6′OH ODMA, 6′hydroxy-O-desmethylangolesin.
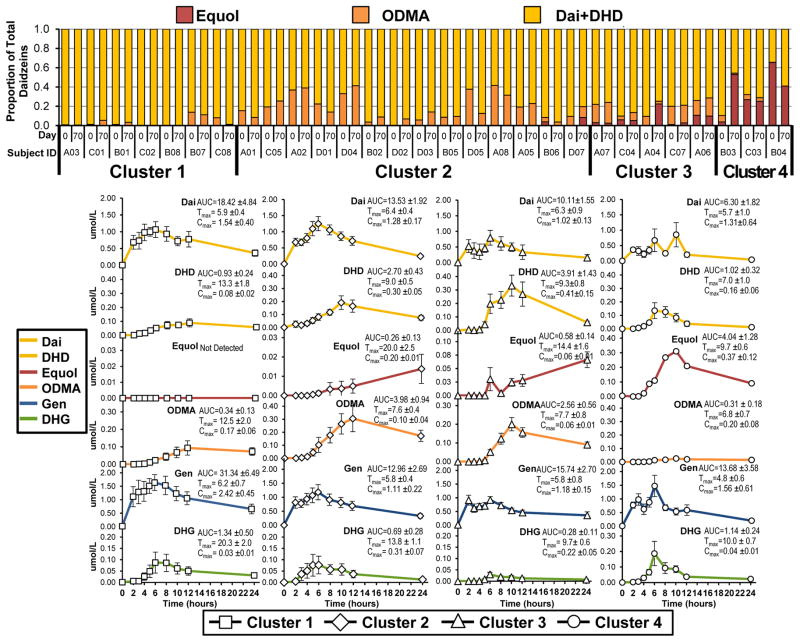
Isoflavonoids from plasma were stratified using the same four clusters generated from the urine isoflavonoid profiles. The plasma isoflavonoid profiles generated from 24 hour AUC showed a similar pattern to that profiled from 24 hour urine, Figure 4. Those in cluster 1 show the highest plasma daidzein (18.42 ± 4.84 μmol/24hr•L) and genistein (31.34 ± 6.49 μmol/24hr•L) AUC. The ODMA producers in cluster 1 reached peak concentrations of ODMA at 12.5 ±2.0 hours, while the other clusters reached peak concentrations of ODMA in ~7 hours. Cluster 2 demonstrates the greatest AUC for ODMA (3.98 ± 0.94 μmol/24hr•L) overall, and lowest AUC of equol (0.26 ± 0.13 μmol/24hr•L) compared to those producing equol but categegorized in other clusters. Men in cluster 3 demonstrate a similar metabolic profile as cluster 2. Yet, the men in cluster 3 compared to those in cluster 2 show a higher DHD AUC (3.91 ± 1.43 μmol/24hr•L), a lower ODMA AUC (2.56 ± 0.94 μmol/24hr•L), and produced equol more consistently and with greater AUC (0.58 ± 0.14 μmol/24hr•L). The plasma isoflavonoid pharmacokinetics revealed that men in cluster 4 had the fastest rate of absorption and production of metabolites with differences ranging between 1 to 10 hours. Cluster 4 reached peak equol concentrations in 9 hours, whereas clusters 2 and 3 began to peak at 24 hours, thus contributing to cluster 4 having the greatest AUC (4.04 ± 1.28 μmol/24hr•L) for equol.
Figure 4.
Proportion of daidzein isoflavonoids from plasma AUC over 24 hr from two visits, top. Plasma isoflavone (μmol/L) pharmacokinetics over a 24 hour period after a meal with soy breads (n=29), reported as mean ± SE within the four phenotype clusters, bottom. DAI, daidzein, DHD, dihydrodaidzein; DHG, dihydrogenistein; Gen, genistein, ODMA, O-desmethylangolensin. AUC, area under the curve (μmol/day•L); Cmax, peak concentration (μmol/L); Tmax, peak time (hours).
SB and SAB intervention was safe with minimal side effects
Using standard National Cancer Institute CTCAE version 4.0, mild (Grade I) gastrointestinal side effects were the most common adverse event in this study. Ten men (40%) complained of bloating and four men (16%) complained of diarrhea while consuming SB or SAB, Supplemental Table S3. The diarrhea resolved when men abstained from consuming study breads, and in all cases men elected to resume consuming soy breads after symptoms resolved. Although bloating returned for some men, mild gastrointestinal symptoms improved by the second treatment period. No toxicity greater than grade 1 was observed. Over the course of this study, a significant decrease in BMI and weight (p < 0.001) was observed. When stratified by bread intervention, weight loss predominantly occurred during the SAB intervention period (2.5 ± 0.3 kg) compared to the SB (0.5 ± 0.7 kg) (p=0.011) intervention, Supplemental Table S3.
Chemistry, liver enzymes, and complete blood count were not affected by dietary intervention. PSA was monitored during this 20 week intervention, Table 1. Although not a primary outcome in this small study, we observed a threefold prolongation in PSADT during the soy intervention period, Table 1 leading to a leveling of PSADT, Supplemental Figure S5. Cholesterol (total, HDL, and LDL) and triglycerides were not significantly impacted over the entire 20 week study, Table 1. However, among men who were hypercholesterolemic at enrollment and who were not on cholesterol lowering agents (n = 7), a significant decrease (p = 0.026) in total cholesterol (day 0, 229 ± 10 mg/dL and day 126, 207 ± 7 mg/dL) and LDL cholesterol (day 0, 150 ± 9 mg/dL and day 126, 132 ± 9 mg/dL) was observed between Day 0 and Day 126. A significant increase in insulin-like growth factor-binding protein 3 (IGFBP3) was observed in our cohort of men, and a significant decrease in pro-inflammatory cytokines which is detailed in a parallel publication (30).
Table 1.
Changes in laboratory measures during the 20 week studya
| Parameters | Day 0 | Day 126 |
|---|---|---|
| Prostate Specific Antigen | ||
| PSA (ng/mL) | 9.36 ± 2.46* | 15.93 ± 3.45* |
| PSA velocity (ng/mL/month)c | 1.64 ± 0.42 | 1.24 ± 0.84 |
| PSADT (months)c | 13.5 ± 2.4 | 36.7 ± 15.5 |
| Lipids | ||
| Overall | ||
| Total Cholesterol (mg/dL) | 172 ± 9 | 169 ± 7 |
| HDL (mg/dL) | 50 ± 4 | 50 ± 4 |
| LDL (mg/dL) | 104 ± 8 | 103 ± 6 |
| Triglycerides (mg/dL) | 130 ± 11 | 139 ± 12 |
| Hypercholesterolemic | ||
| Total Cholesterol (mg/dL) | 229 ± 10* | 207 ± 7* |
| HDL (mg/dL) | 59 ± 9 | 57 ± 8 |
| LDL (mg/dL) | 150 ± 9* | 132 ± 9* |
| Triglycerides (mg/dL) | 147 ± 23 | 153 ± 26 |
| Endocrine | ||
| hGH (ng/mL) | 1.16 ± 0.29 | 1.02 ± 0.31 |
| IGF (ng/mL) | 121.4 ± 6.5 | 125.2 ± 7.2 |
| IGFBP-3 (μg/mL) | 3.36 ± 0.81* | 3.51 ± 0.87* |
| IGF-1:IGFBP-3 Ratio | 36.2 ± 0.9 | 35.5 ± 0.8 |
| Leptin (ng/mL) | 12.96 ± 2.1 | 13.51 ± 2.0 |
Mean ± SD (n=25, men completed study)
PSADT, prostate specific antigen doubling time; HDL, high density lipoprotein; LDL, low density lipoprotein; hGH, human growth hormone; IgF, insulin-like growth hormone; IgFBP-3, insulin-like growth hormone binding protein; VEGF, vascular endothelial growth hormone; IL, interleukin.
Day 0 (PSA values collected prior to but not including Day 0); day 126 (PSA from enrollment to and including Day 126)
Significance (p≤0.05) was determined using ANOVA
DISCUSSION
Future studies testing relevant hypotheses regarding the biological impact of soy in the human diet will be most informative if fully characterized, convenient, and tasteful food products are employed that provide phytochemical exposures similar to that observed in many Asian populations. To this end, we successfully created two soy breads containing a diverse array of bioactive phytochemicals at concentrations typically observed in Asian diets. We also demonstrate that food processing techniques can be utilized to rationally design foods with altered isoflavone chemistry to favor absorption and subsequently, may enhance their bioactivity (31). The two breads tested in this study have complementary compositions of isoflavones, one rich in aglycone forms (SAB) and the other rich in glycoside forms (SB) of isoflavones (32). Most critically, this study demonstrates that characterizing an investigational food product, both chemically and with in vivo pharmacokinetic and safety data, should be a key step towards defining a relevant agent for human intervention trials for cancer, other disease outcomes, and health.
Our study carefully documents the impact of food matrix on isoflavone metabolism, a concept previously addressed in a few studies using liquid and solid forms of soy foods (21, 26, 23). The pharmacokinetic measures (Tmax, Cmax, and AUC) found in our study with SB were similar to those reported by Cassidy and colleagues after a textured vegetable protein (TVP) meal. In that study, the Tmax of daidzein and genistein from soymilk was significantly faster compared to TVP and tempeh yet no significant differences in daidzein and genistein Tmax was observed between the two solid matrices (TVP and tempeh) (21). In our previous study and in Franke and colleagues, isoflavone metabolites in urine were significantly greater with either soy bread (23) or soy nuts (26) compared to a soy-based beverage intervention. Furthermore, aglycone composition is reported to impact isoflavone pharmacokinetics. Aglycone composition among soy foods varies widely, ranging from 12% in soy bars (26) to 50% in tempeh (21) and among the few studies which have engineered high aglycone foods for the purpose of investigating isoflavone bioavailability (15, 16, 17) none have used a food matrix such as bread. Okabe and colleagues report fermented miso (91% aglycone) had significantly faster rates of daidzein, genistein, and equol absorption compared to non-fermented soy (13% aglycone) while the non-fermented soy showed a higher peak absorbance of equol (17). Otherwise identical, the two breads used in this study demonstrated that strategic manipulation of the isoflavone forms will impact the initial absorption of the isoflavone aglycone and affect the transit of parent isoflavones to the lower bowel that brings the colonic microbiota into the dynamic process, adding to the complexity of soy bioactive metabolism and ultimate biological impact.
Few studies have examined single meal pharmacokinetics of isoflavones and metabolites from soy foods. We observed that the absorption of isoflavonoids in plasma displayed a biphasic pharmacokinetic curve which is consistent with earlier reports (15, 16, 17). This pattern may be related to enterohepatic recycling of the isoflavonoids (33, 34) and/or related to the impact of the subsequent second meal on metabolism of components from the earlier test meal (35). Previous studies investigating the bioavailability of isoflavones (aglycones compared to glycosides) using miso soup (17) or soy milk (16) reported peak absorption occurred in the first hour in aglycone-rich products compared to 5 hours with a glycoside-rich food. In our study, a similar biphasic peak pattern was observed however peak absorption occurred after the lunch meal (~5 hours) in both breads with modest differences in peak absorption of isoflavonoids from SAB being only 1 to 3 hours faster than SB. Similar to early studies peak absorption of microbial metabolites was significantly higher in metabolites after SB compared to SAB meal. These modest differences in isoflavonoid absorption resulting after the two different soy bread meals demonstrate that aglycones are readily absorbed in the small intestines. Thus, intestinal beta glucosidase and lactase phlorizin hydrolase activity appear to be proficient in liberating isoflavone aglycones for absorption. Standardized breads used in our clinical trial can in the future be tailored to favor absorption of isoflavone aglycones or metabolites to enhance health outcomes.
A number of soy isoflavone metabolites can be formed by the intestinal microbiota, a variable that is only beginning to be understood. Different proportions of isoflavone metabolites constitute the metabolic phenotype and most likely impacts downstream biological responses. Daidzein can be metabolized in sequence to DHD to equol or ODMA (36). Similarly, genistein has been reported to be metabolized into dihydrogenistein and 6′-hydroxy-O-desmethylangolensin (19, 36). Previous studies suggest that isoflavone metabolites such as equol may have greater antioxidant activity (37) and may be more estrogenic (38) than their parent forms, but the unique role(s) of specific isoflavones, isoflavone patterns, or metabolites in prostate carcinogenesis is very limited and warrants further investigation. A strength of the present study, unlike earlier studies of isoflavone absorption, is the inclusion of a comprehensive investigation of plasma and urine isoflavone metabolites (DHD, DHG, ODMA, equol, and 6′OH-ODMA) over a 24 hour period.
Early studies have characterized and defined daidzein metabolizing phenotypes either by those who produce equol (14) or those who produce ODMA (19) or by their rate of isoflavonoid degradation (25). Moreover, previous studies (39) have identified bacteria responsible for O-DMA production is distinct from those that produce equol (40, 41). In our cohort of men, cluster analysis was used to elucidate similarities and differences among individuals who produced daidzein and genistein metabolites. Using this approach, we discovered that among equol producers (cluster 3 and 4) there are individuals who produce relatively high levels of ODMA and those who produce low amounts. Moreover, we observed a cluster (cluster 1) which did not produce terminal metabolites of daidzein (equol or ODMA) yet they had the highest level of genistein in plasma and urine. Additionally, over the 20 week investigation, we observed a high intraclass correlation (equol 91%, ODMA 83%, daidzein 89%) in these isoflavone metabolizing phenotypes which align with previous studies suggesting a high concordance (>80%) over time in Americans (42) and Japanese (43). Distinguishing these isoflavonoid metabolizing clusters may help to explain inter-individual differences and assist in identifying those individuals that might be more or less responsive to soy intervention which has important implications for designing future cancer prevention trials.
Of the many purported benefits of soy, the most well-established benefit is lowering blood cholesterol, which is mostly attributed to soy protein, and which forms the basis for an approved FDA claim (44). Although no overall changes in blood lipids were observed in our cohort, a subset with untreated hypercholesterolemia at enrollment had a significant decrease in total cholesterol and LDL cholesterol. Our earlier studies with a similar soy bread demonstrated significant decrease in LDL and triglycerides in men with hypercholesterolemia (23). Platz et al. (2008) reported a potential relationship between lower cholesterol or use of statins and inverse lower risk of prostate cancer mortality (45). The significant decrease in lipid parameters in response to soy and the associated prolonged PSADT in this same cohort was an incidental finding and a causal relationship would need extensive additional investigation.
Meta-analyses suggest that high circulating levels of insulin-like growth factor is associated with increased risk of prostate cancer (46) and predictive of advanced disease (47) However, dietary effects on IGF have been mixed. Earlier studies with rat prostate cancer lines treated with genistein demonstrated a decrease in IGF whereas dietary intervention trials with soy isoflavones in men with prostate cancer had shown no significant change in IGF (48) or increase in IGF (49). Although in our cohort there was no significant increase in IGF, there was a significant increase in insulin-binding protein 3 (IGFBP 3) with soy intervention. IGFBP 3 has been shown to inhibit the proliferation of prostate cancer cell lines independent of IGF by behaving as a potent inducer of apoptosis (50). Moreover, there was a concomitant decrease in the ratio of IGF to IGFBP-3 in 64% (16/25) of our patients.
CONCLUSION
In conclusion, food technology can be utilized to produce fully characterized food products to assess dietary hypotheses in cancer. We produced two soy bread products that can be easily incorporated into the diet of men consuming a typical Western diet on a daily basis. We have achieved a dose of soy phytochemicals in two slices of bread per day that will mimic typical soy intake in Asian populations. This study documents the adherence, safety, and bioavailability of isoflavones from two fully characterized soy-bread products; data that is essential to enhance our understanding of potential mechanisms where soy breads may impact biological processes. Evaluation of plasma isoflavonoids and isoflavonoids in urine suggests that differences between the aglycone and glycoside composition in the soy breads had a modest impact on the overall rate of isoflavonoid absorption. This study is the first to provide a comprehensive approach to characterizing isoflavonoid phenotypes using both plasma pharmacokinetics and urine steady states. We have identified four isoflavone metabolite producing phenotypes. Strategies to identify metabolic phenotypes of soy isoflavone intervention are critical to help decipher heterogeneity in biological responses among individuals in clinical studies The tremendous collaborative effort invested in standardizing soy breads for clinical trials have produced two novel soy foods with defined isoflavone profiles and optimized palatability that will serve as an excellent source of soy isoflavones for future large-scale cancer prevention trials.
Supplementary Material
Acknowledgments
Financial support: Supported by NIH grants R21CA125909, 1R01CA169363-01, The Center for Advanced Functional Foods Research and Entrepreneurship (CAFFRE), The OSUCCC Molecular Carcinogenesis and Chemoprevention Program. This work was also supported by the Pelotonia Fellowship Program and the National Center for Advancing Translational Sciences Award 8UL1TR000090-05. Any opinions, findings and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program or of the National Center for Advancing Translational Sciences.
We thank the OSU CCTS Clinical Research Center, OSUCCC Biostatistics, and Nutrient and Phytochemical Shared Resources. We are especially thankful for all the patients who participated in this study.
Footnotes
Conflict of Interest: There are no conflicts of interest related to this work.
References
- 1.Perabo FG, Von Low EC, Ellinger J, von Rucker A, Muller SC, Bastian PJ. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;11:6–12. doi: 10.1038/sj.pcan.4501000. [DOI] [PubMed] [Google Scholar]
- 2.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy AR. Factors that modify radiation-induced carcinogenesis. Health Phys. 2009;97:433–45. doi: 10.1097/HP.0b013e3181ac9262. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–76. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 5.McCormick DL, Johnson WD, Bosland MC, Lubet RA, Steele VE. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr Cancer. 2007;57:184–93. doi: 10.1080/01635580701277478. [DOI] [PubMed] [Google Scholar]
- 6.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–82. [PubMed] [Google Scholar]
- 7.Davis JN, Kucuk O, Sarkar FH. Expression of prostate-specific antigen is transcriptionally regulated by genistein in prostate cancer cells. Mol Carcinog. 2002;34:91–101. doi: 10.1002/mc.10053. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18:408–17. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 9.D’Alessandro T, Prasain J, Benton MR, et al. Polyphenols, inflammatory response, and cancer prevention: Chlorination of isoflavones by human neutrophils. J Nutr. 2003;133:3773S–7S. doi: 10.1093/jn/133.11.3773S. [DOI] [PubMed] [Google Scholar]
- 10.Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132–41. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TWM, Ferketich AK, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60:145–54. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 12.Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. JAMA. 2013;310:170–8. doi: 10.1001/jama.2013.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–9. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 14.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–53. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 15.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002;132:2587–92. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 16.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–6. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 17.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2010;91:658–63. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 18.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 19.Heinonen S, Hoikkala A, Wähälä K, Adlercreutz H. Metabolism of the soy isoflavones daidzein, genistein and glycitein in human subjects: Identification of new metabolites having an intact isoflavonoid skeleton. J Steroid Biochem Mol Biol. 2003;87:285–99. doi: 10.1016/j.jsbmb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Frankenfeld CL, Atkinson C, Thomas WK, Goode EL, Gonzalez A, Jokela T, et al. Familial correlations, segregation analysis, and nongenetic correlates of soy isoflavone-metabolizing phenotypes. Exp Biol Med (Maywood) 2004;229:902–13. doi: 10.1177/153537020422900906. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy A, Brown J, Hawdon A, Faughnan M, King LJ, Millward J, et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Franke AA, Hebshi SM, Pagano I, Kono N, Mack WJ, Hodis HN. Urine accurately reflects circulating isoflavonoids and ascertains compliance during soy intervention. Cancer Epidemiol Biomarkers Prev. 2010;19:1775–83. doi: 10.1158/1055-9965.EPI-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn-Jarvis JH, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food Funct. 2012;3:1051–8. doi: 10.1039/c2fo10284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Lee S, Verbruggen MA, Murphy PA, Hendrich S. The apparent absorptions of isoflavone glucosides and aglucons are similar in women and are increased by rapid gut transit time and low fecal isoflavone degradation. J Nutr. 2004;134:2534–2539. doi: 10.1093/jn/134.10.2534. [DOI] [PubMed] [Google Scholar]
- 26.Franke AA, Ashburn LA, Kakazu K, Suzuki S, Wilkens LR, Halm BM. Apparent bioavailability of isoflavones after intake of liquid and solid soya foods. Br J Nutr. 2009;102:1203–11. doi: 10.1017/S000711450937169X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedlund TE, Bokhoven AV, Johannes WU, Nordeen SK, Ogden LG. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2006;66:557–66. doi: 10.1002/pros.20380. [DOI] [PubMed] [Google Scholar]
- 28.Zuniga KE, Clinton SK, Erdman JW., Jr The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev Res. 2013;6:548–57. doi: 10.1158/1940-6207.CAPR-12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services. NIH NCI. 2009. Common terminology criteria for adverse events (CTCAE) version 4.0. [Google Scholar]
- 30.Lesinski GB, Reville PK, Mace TA, Young GA, Ahn-Jarvis JH, Thomas-Ahner JM, et al. Reduced pro-inflammatory cytokines and immunosuppressive cells with consumption of soy isoflavone enriched bread in men with prostate cancer. 2014. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Pascual-Teresa S, Hallund J, Talbot D, Schroot J, Williams CM, Bugel S, et al. Absorption of isoflavones in humans: Effects of food matrix and processing. J Nutr Biochem. 2006;17:257–64. doi: 10.1016/j.jnutbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Ahn-Jarvis JH, Riedl KM, Schwartz SJ, Vodovotz Y. Design and selection of soy breads used for evaluating isoflavone bioavailability in clinical trials. J Agric Food Chem. 2013;61:3111–20. doi: 10.1021/jf304699k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yueh TL, Chu HY. The metabolic fate of daidzein. Sci Sin. 1977;20(4):513–21. [PubMed] [Google Scholar]
- 34.Watanabe S, Yamaguchi M, Sobue T, Takahashi T, Miura T, Arai Y, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128:1710–15. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 35.Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla ML. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J Agric Food Chem. 2203;51:4603–9. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- 36.Schöefer L, Mohan R, Braune A, Birringer M, Blaut M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol Lett. 2002;208:197–202. doi: 10.1111/j.1574-6968.2002.tb11081.x. [DOI] [PubMed] [Google Scholar]
- 37.Arora A, Nair MG, Strasburg GM. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch Biochem Biophys. 1998;356:133–41. doi: 10.1006/abbi.1998.0783. [DOI] [PubMed] [Google Scholar]
- 38.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J Steroid Biochem Mol Biol. 1993;45:399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 39.Gardana C, Canzi E, Simonetti P. The role of diet in the metabolism of daidzein by human faecal microbiota sampled from Italian volunteers. J Nutr Biochem. 2009;20:940–7. doi: 10.1016/j.jnutbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis) J Nutr. 2003;133:2262–7. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 41.Bowey E, Adlercreutz H, Rowland I. Metabolism of isoflavones and lignans by the gut microflora: A study in germ-free and human flora associated rats. Food Chem Toxicol. 2003;41:631–6. doi: 10.1016/s0278-6915(02)00324-1. [DOI] [PubMed] [Google Scholar]
- 42.Frankenfeld CL, Atkinson C, Thomas WK, Gonzalez A, Jokela T, Wähälä K, et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br J Nutr. 2005;94:873–6. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- 43.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–9. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 44.Food and Drug Administration, Health and Human Services. Food labeling: Health claims; soy protein and coronary heart disease: Final rule. Fed Reg. 1999;64:57699–733. [PubMed] [Google Scholar]
- 45.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–1698. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi R, Berkel HJ, Yu H. Insulin-like growth factor-I and prostate cancer: A meta-analysis. Br J Cancer. 2001 Sep 28;85(7):991–6. doi: 10.1054/bjoc.2001.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 48.Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–7. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 49.Spentzos D, Mantzoros C, Regan MM, Morrissey ME, Duggan S, Flickner-Garvey S, et al. Minimal effect of a low-fat/high soy diet for asymptomatic, hormonally naive prostate cancer patients. Clin Cancer Res. 2003;9:3282–3287. [PubMed] [Google Scholar]
- 50.Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: Local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–76. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.