Abstract
Background
Group 1 mGlu-family proteins (i.e., mGlu) consist of mGlu1 and mGlu5 and their activity may influence voluntary ethanol intake. The present studies sought to examine the influence of these receptors on the development of ethanol dependence using in vitro and in vivo models of chronic, intermittent ethanol (CIE).
Methods
Rat hippocampal explants were exposed to CIE with or without the addition of mGlu1 antagonist (7-hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt; 0.5, 1, and 3 μM) or mGlu5 antagonist (E)-2-methyl-6-styryl-pyridine (SIB-1893; 20, 100, and 200 μM) to assess sparing of withdrawal-induced cytotoxicity. In a separate study, adult male rats were administered CIE with or without the addition of oral administration of group 1 mGlu antagonist 2-Methyl-6-(phenylethynyl)-pyridine (MPEP; 3 mg/kg). Blood ethanol levels (BELs) were determined at 0930 hours on Day 2 of Weeks 1, 2, and 3. Withdrawal behavior was monitored during Day 6 of the third consecutive withdrawal.
Results
CIE produced significant hippocampal cytotoxicity. These effects were attenuated by co-exposure to CPCCOEt (3 μM) with ethanol in the CA3. By contrast, these effects were blocked by SIB-1893 (20 μM) in each primary cell layer. Oral administration of MPEP with ethanol significantly attenuated behavioral effects of subsequent withdrawal and reduced BELs.
Conclusions
These data demonstrate that ethanol activates group 1 mGlu-family proteins to promote withdrawal-associated cytotoxicity in vitro and physical dependence in vivo. These findings suggest that group 1 mGlu-family proteins may be therapeutic targets for treatment of alcohol use disorders.
Keywords: CIE, chronic intermittent ethanol, mGlu, metabotropic glutamate receptor, NeuN, neuron specific nuclear protein
1. INTRODUCTION
Group 1 metabotropic glutamate receptor-family proteins (i.e., mGlu1 and mGlu5) are large guanine nucleotide-binding protein (G-protein)-coupled receptors involved in a myriad of biological processes, such as regulation of second messengers (Schoepp et al., 1994), ion channels (e.g., potassium channels [Charpak et al., 1990]), and neuronal excitability (Davies et al., 1995). Indeed, these receptors are linked to G proteins and phospholipase C and known to stimulate phosphoinositide hydrolysis, as well as interact closely with intracellular scaffolding proteins (e.g., Homer proteins). As an example, prior work using immunofluorescent techniques demonstrated that Homer1b retains group 1 mGlu receptors at the endoplasmic reticulum when these proteins are coexpressed (Roche et al., 1999). Further, immunocytochemical and western blot analyses reveal that Homer proteins can actually bind and activate group 1 mGlu-containing receptors independent of agonist application (for a review, see Spooren et al., 2001). Park and colleagues (2013) demonstrated mGlu5-containing receptors activate N-methyl-D-aspartate (NMDA) receptors allowing cation influx in a Homer-dependent manner to synaptic plasticity.
Ethanol exposure is known to alter group 1 mGlu-family protein expression and signaling, such as increases in group 1 mGlu/Homer2/NR2 expression in the central nucleus and nucleus accumbens core (Obara et al., 2009) and increases in hippocampal Glu5 receptor expression (Cozzoli et al., 2009). Immunohistochemical and western blotting techniques demonstrate that ethanol exposure followed by a single period of withdrawal produces neurotoxicity of the hippocampal pyramidal cornu ammonis (i.e., CA1) cell layer, as well as a ~15–30% increase in mGlu5 and GluN2 polypeptide levels, respectively (Harris et al. 2003). The hippocampal neurotoxicity within the CA1 produced by a single episode of ethanol withdrawal was significantly attenuated via blockade of mGlu5 and NMDA receptors. These data suggest that neurotoxicity produced by ethanol withdrawal involves a cross-talk between mGlu5 and NMDA receptors in organotypic hippocampal slice cultures.
Prior studies unequivocally suggest that the group 1 mGlu regulates alcohol-related behaviors, such as the interoceptive effects of ethanol (Hodge et al., 2006). A prior study utilizing combined behavioral immunohistochemical techniques demonstrated that mGlu5 activity in the nucleus accumbens contributes to the discriminative stimulus effects of ethanol (Besheer et al., 2009). Besheer and Hodge (2005) demonstrated that pretreatment with selective and competitive mGlu5 antagonist mGlu 2-methyl-6-(phenylethyl)-pyridine (MPEP) (30 mg/kg) significantly reduced ethanol appropriate responding in rats trained to discriminate ethanol (1 g/kg/ig). In another study, MPEP (10 mg/kg) administration attenuated the onset and maintenance of ethanol self-administration in inbred mice trained to self-administer ethanol on a fixed ratio 1 schedule of reinforcement while (hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) did not alter ethanol self-administration (Hodge et al. 2006). Similarly, Cozzoli and colleagues (2009) demonstrated that pretreatment with mGlu5 antagonist MPEP dose-dependently reduced binge ethanol consumption while CPCCOEt administration did not attenuate ethanol self-administration. Notably, mGlu5 blockade via MPEP administration reduced cue-induced reinstatement of alcohol-seeking behavior in rats trained to self-administration ethanol and blunted ethanol-dependent increases in extracellular signal-regulated kinase (i.e., ERK1/2) immunofluorescence in the basolateral amygdala and nucleus accumbens shell (Schroeder et al. 2008). In sum, these studies suggest that the reinforcing properties of ethanol are mediated, in part, via activity of the mGlu5.
Collectively, these studies demonstrate that group 1 mGlu-family proteins contribute to hippocampal neurotoxicity produced by a single episode of ethanol withdrawal and influence voluntary intake of ethanol in rodents. However, the functional influence of group 1 mGlu in promoting development of ethanol dependence is not fully understood. Further, the role of activated group 1 mGlu1-family proteins is not fully characterized with regards to ethanol withdrawal-induced neurotoxicity produced by multiple episodes of withdrawal. The present studies sought to examine the functional relationship between ethanol-associated activation of group 1 mGlu-family proteins and subsequent withdrawal-associated cytotoxicity and withdrawal abnormalities following exposure to chronic, intermittent ethanol (CIE) as previously reported using these models in vitro (after Reynolds et al., 2015A) and in vivo (after Reynolds et al., 2015B).
2. MATERIALS AND METHODS
2.1. Organotypic Hippocampal Slice Culture Preparation
Whole brains were harvested from eight-day-old Sprague-Dawley rats (Harlan Laboratories; Indianapolis, IN) and placed in chilled dissecting medium composed of Minimum Essential Medium (MEM; Invitrogen, Carlsbad, CA), 25 mM HEPES (Sigma, St. Louis, MO), and 50 μM streptomycin/penicillin (Invitrogen, Waltham, MA). Midbrain was then removed from each hemisphere. Hippocampi were extracted using glass elbows, cleaned of excess tissue using a dissecting microscope, and sectioned at 200 μM using a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK). Three intact hippocampi were plated onto Millicell-CM 0.4 μM biopore membrane inserts containing 1 mL of pre-incubated culture medium (dissecting medium, distilled water, 36 mM glucose [Fisher, Pittsburg, PA], 25% Hanks’ Balanced Salt Solution [HBSS; Invitrogen], 25% v/v heat-inactivated horse serum [HIHS; Sigma], 1.0% Amphotericin B solution [Sigma], and 0.05% streptomycin/penicillin [Invitrogen]). Hippocampi were maintained in an incubator at 37°C with a gas composition of 5% CO2/95% air for five days before any experiments were conducted.
2.2. Ethanol and Withdrawal Treatments In Vitro
The present report assessed the role of the group 1 mGlu in loss of neuron specific nuclear protein (NeuN; Fox-3) in the pyramidal cell layers of the cornu ammonis (CA1 and CA3) and granule cell layer of the dentate gyrus (after Butler at al., 2010) following binge-like ethanol exposure (50 mM) rat hippocampal explants. NeuN (i.e., Fox-3) is a member of the Fox-1 gene family involved in regulating splicing of pre-mRNA (Kim et al., 2009). This marker is present in nearly all post-mitotic and differentiating neurons, except cerebellar interneurons (Mullen et al., 1992) and is, therefore, used to assess neuronal integrity (Weyer and Schilling, 2003). In the present report, hippocampi were exposed to either ethanol-naïve media or media containing ethanol (50 mM) with or without noncompetitive mGlu1 antagonist CPCCOEt (Tocris Bioscience, Bristol, United Kingdom) or noncompetitive mGlu5 antagonist (E)-2-methyl-6-styryl-pyridine (SIB-1893) for five days in vitro followed by 24-hours of withdrawal; this was repeated three times. The concentration of ethanol (i.e., 50 mM) was selected in order to reflect patterns of binge alcohol use in humans (Eckardt et al., 1998; Jones and Sternebring, 1992). CPCCOEt is a chromene derivative with a twenty-fold selectivity for the mGlu1 over the mGlu5 with an IC50 value of approximately 6.6 μM (Litschig et al., 1999). Prior work demonstrates that SIB-1893 is highly selective for the mGlu5 compared to the mGlu1 (Guarneri et al., 2008) with an IC50 value of approximately 3.7 μM (Litschig et al., 1999). SIB-1893 concentrations (20, 100, and 200 μM) were selected based on prior work showing efficacy in reducing withdrawal-induced neurotoxicity when hippocampi were treated acutely at 200 μM (Harris et al., 2003). Drugs were first dissolved in 100% dimethyl sulfoxide (DMSO; Fisher) to yield a final, working concentration of 0.01% DMSO in the culture medium. Respective control tissue was also treated with 0.01% DMSO. Media changes were conducted every five days with hippocampi maintained inside Ziploc bags filled with 5%CO2/95% air. Water bath solutions consisted of distilled water (50 mL) containing ethanol (50 mM) to prevent evaporation (Prendergast et al., 2004). Hippocampi were fixed by placing 1 mL of 10% formalin solution on the top and bottom of each well for 30 minutes and were washed twice with phosphate buffered saline (PBS). Hippocampi were maintained at 4°C until the conduct of immunohistochemical studies.
2.3. Immunohistochemical Labeling of Neuron Specific Nuclear Protein (NeuN)
Neuronal membranes of fixed hippocampi were permeated using 1 mL of a permeabilization (wash) buffer (200 mL PBS [Invitrogen], 200 μL Triton X-100 [Sigma], 0.010 mg Bovine Serum [Sigma]) for 45 minutes at room temperature. Hippocampi were exposed to primary monoclonal antibody mouse anti-NeuN (Sigma) at a dilution factor of 1:200 for 24 hours at 4°C. Hippocampi were then washed twice with 1x PBS and exposed to goat anti-mouse secondary antibody fluorescein isothiocyanate (FITC; Sigma) at a dilution factor of 1:200 for 24 hours at 4°C. Hippocampi were washed twice with 1x PBS and imaged immediately via SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbaum Inc.; McHenry, IL, USA) through a 5x objective with a Leica DMIRB microscope (W. Nuhsbaum Inc.; McHenry, IL, USA). Images were captured with a SPOT 7.2 color mosaic camera (W. Nuhsbaum). Densitometry using Image J software (National Institutes of Health, Bethesda, MD) was conducted to measure the intensity of FITC fluorescence. FITC fluorescence was detected with a band-pass filter at 495 nm (520 nm emission). Background measurements were recorded for each reading and subtracted from the measurements obtained from each primary cell layer of the hippocampal formation (the granule cell layer of the dentate gyrus and the pyramidal cell layers of the CA3 and CA1 regions).
2.4. Animals
Thirty-two adult, male Sprague-Dawley rats (i.e., weighed in at 300–325 grams upon arrival; Harlan Laboratories, Indianapolis, IN) were housed individually and allowed to acclimate to the animal colony for two days following arrival. Subjects were then handled for two minutes per day for three consecutive days prior to experimentation. Subjects were allowed ad libitum access to food and water throughout the entire duration of the experiment. Food weight data were collected at 0800 hour each week. Each animal was weighed at 0800 hour on days Monday through Friday prior to ethanol administration. A mortality rate of 5% was observed due to complications with the intragastric gavage procedure. Care of all animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and the University of Kentucky’s Institutional Animal Care and Use Committee.
Subjects were administered ethanol (4 g/kg) via intragastric gavage twice daily at 0800 and 1600 for five consecutive days with or without the addition of 3 mg/kg 2-Methyl-6-(phenylethynyl)pyridine (MPEP). MPEP is an mGlu5 antagonist that has been used previously as a neuroprotective compound in several models of drug and alcohol dependence, as well as in several models of neurodegenerative disorders. Given that nasogastric gavage administration of MPEP (10 mg/kg) for one month reduced Parkinsonian symptoms in non-human primates (Morin et al., 2013), we chose to deliver this compound to subjects orally suspended in either ethanol-containing or an isocaloric diet. Ethanol and control diets were composed of 30% v/v Vanilla Ensure® (Abbott Nutrition, Columbus Ohio) and an equal quantity of double-distilled water (after Sharrett-Field et al., 2013; Reynolds et al., 2015B). Ethanol-containing diets were composed of 40% v/v 200 proof ethanol (Sigma-Aldrich, San Diego, CA) whereas control diets were isocalorically matched to ethanol-containing diets via substitution of maltose (Sigma-Aldrich) so as to yield equivalent caloric intake. During periods of withdrawal, subjects received no experimental manipulations; this was repeated for three consecutive cycles. During the third consecutive period of withdrawal, subjects were monitored for 16 hours after the last ethanol administration (i.e., 0900 hours) for physiological manifestations of withdrawal. Withdrawal behavior was monitored in a square Plexiglas chamber for two minutes. During this time, two experimenters, blinded to experimental conditions, rated physical effects of withdrawal using a modified behavioral scale that was described in detail previously in our laboratory (Reynolds et al., 2015B; Self et al., 2009) using a 10-point discrete scale (i.e., all or nothing). This scale measured the following behaviors: rigidity, tremor, stereotypy, retropulsion, dystonic gait, hypoactivity, aggression, splayed paws, vocalization, and seizure.
2.5. Analysis of Blood Ethanol Levels
In order to assess BELs, approximately 200 μL of tail blood was collected into two Fisherbrand heparinized micro-hematocrit capillary tubes (Fisher Scientific) on Day 2 of Weeks 1, 2, and 3. Next, samples were centrifuged for four minutes using an Analox benchtop centrifuge (Analox Instruments) with blood plasma collected and placed into a .65 mL Costar microcentrifuge tube (Fisher Scientific). Samples were stored at −20°C until further analyses of BELs using a colormetric assay (Abcam [Cambridge, United Kingdom]) for Week 1 and the Analox for Weeks 2 and 3. Analox methodology is described in detail in a prior report (Reynolds et al., 2015B). For the ELISA assay, alcohol oxidase oxidized ethanol to generate hydrogen peroxide, which reacts with the probe included in the assay to generate color. All standards, background wells, and sample wells were run in duplicate, per manufacturer’s instructions, and 10 μL of blood plasma were incubated at room temperature for 60 minutes with reaction enzymes in a 96-well plate (Corning, New York). Optical density was measured using a Beckman Coulter DTX 880 Multimodal Detector (Lagerhausstrasse, Austria) using Beckman Coulter Multimode Detection Software (v.20.0.12). Absorbance was detected at 595 nm for standards, background wells, and sample wells. Mean absorbance for each value was determined and then averaged so as to yield one measurement for each sample. Mean absorbance of the blank (i.e., standard with 0 nmol/well ethanol) was subtracted from each standard and sample value to yield the corrected absorbance value, and a standard curve was generated based on values of standards. Concentrations of ethanol in samples were then determined via sample amount from standard curve (i.e., Sa; nmol) divided by the sample volume added into the sample well (i.e., Sv; μL) using this formula: Sa/Sv.
2.6. Statistical Analyses
Statistical analyses were conducted to delineate the influence of group 1 mGlu-family proteins in promoting hippocampal damage using in vitro and in vivo models of ethanol dependence. The current in vitro studies were conducted two times using different rat litters. Data from each litter were converted into percent control values and combined. We conducted a two-way ANOVA (factors: medium and drug) for each primary cell layer of the hippocampal formation (CA1, CA3, and dentate gyrus). This statistical approach is adapted from a prior study conducted in our laboratory suggesting that the CA1 cell layer is selectively sensitive to excitotoxicity (Butler et al., 2010). One-way ANOVA with Dunnett’s post-hocs were conducted to determine which points differed from ethanol or control-treated hippocampi. For in vivo studies, body weight data were analyzed by a three-factor repeated measures ANOVA with week (i.e., Week 1, 2, and 3) as the within-subjects factor and diet (i.e., control and ethanol) and drug (i.e., no drug and MPEP) as the between-subjects factors. Behavioral effects of withdrawal were analyzed by a two-factor factorial ANOVA with diet (i.e., control and ethanol) and drug (i.e., no drug and MPEP) as factors. One-way ANOVA with Tukey’s post-hocs were conducted to determine which points differed from control-treated subjects. BEL data were analyzed by a three-factor repeated measures ANOVA with week (i.e., Week 1, 2, and 3) as the within-subjects factor and diet (i.e., control and ethanol) and drug (i.e., no drug and MPEP) as the between-subjects factors. Follow up analyses were conducted using Bonferroni adjustment for multiple comparisons. Effects were considered significant at p<0.05. For graphical representation and interpretation, data were presented as percent control of the mean +/− the standard error of the mean (SEM).
3. RESULTS
3.1. The mGlu1 Family Antagonist CPCCOEt Reduces CIE-induced Cytotoxicity
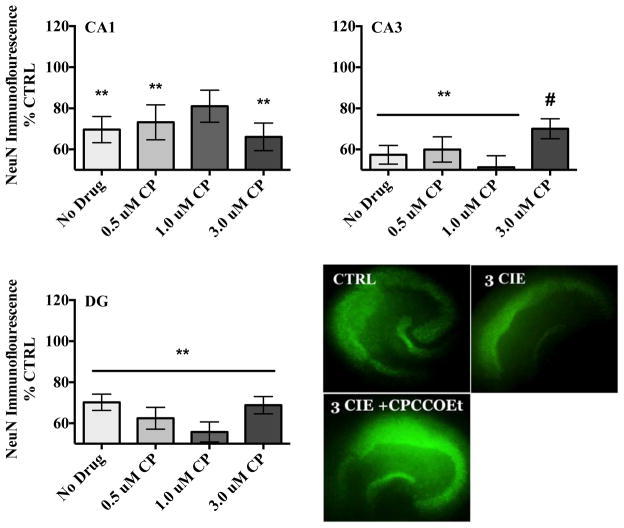
In the pyramidal cell layer of the CA1, ANOVA analyses revealed a significant medium-by-drug interaction (F[3,47] = 4.47, p<0.05). A one-way ANOVA (F[7,40]= 2.98, p= 0.013) with Dunnett’s post-hoc test confirmed that CIE exposure produced a 30% decrease in NeuN immunofluorescence compared to control-treated hippocampi (Figure 1). Co-exposure to ethanol and mGlu1 antagonist CPCCOEt did not alter levels of NeuN immunofluorescence compared to ethanol-treated tissue. Table 1 shows that a 1.0 μM CPCCOEt application alone produced significant reductions of NeuN immunofluorescence in the CA1 in ethanol-naïve tissue.
Figure 1.
Effects of mGlu1 antagonist CPCCOEt (0.5–3 μM) on NeuN immunofluorescence in the rat hippocampus. Data are presented as percent control of the mean +/− the SEM. ** = statistical significance (p <0.05) compared to control hippocampi; # = statistical significance (p <0.05) compared to ethanol hippocampi. N = 12 for control hippocampi; N = 17 for hippocampi exposed to ethanol; N = 13 for hippocampi co-exposed to ethanol and 0.5 μM CPCCOEt; N = 14 for hippocampi co-exposed to ethanol and 1.0 μM CPCCOEt; N = 16 for hippocampi co-exposed to ethanol and 3.0 μM CPCCOEt for each primary cell layer of the hippocampal formation. Representative images depict hippocampi exposed to ethanol-naïve media (control) or ethanol media (50 mM), or hippocampi co-exposed to 3.0 μM CPCCOEt and ethanol (50 mM).
Table 1.
Effects of noncompetitive mGlu1 antagonist CPCCOEt (0.5–3 μM) alone in ethanol-naïve medium. Data are presented as percent control of the mean +/− the SEM. Bold means indicates statistical significance (p <0.05). N = 12 for control hippocampi; N = 12 for hippocampi exposed to 0.5 μM CPCCOEt; N = 14 for hippocampi exposed to 1.0 μM CPCCOEt; N = 14 for hippocampi exposed to 3.0 μM CPCCOEt for each primary cell layer of the hippocampal formation.
| NeuN Immunofluorescence | CA1 ± SEM | CA3 ± SEM | DG ± SEM |
|---|---|---|---|
| Control hippocampi | 100.00 ± 8.54 | 100.00 ± 6.14 | 100.00 ± 5.23 |
| CPCCOEt (0.5 μM) | 79.81 ± 9.55 | 74.26 ± 6.86 | 82.62 ± 5.95 |
| CPCCOEt (1.0 μM) | 64.23 ± 7.79 | 53.53 ± 5.60 | 58.35 ± 4.86 |
| CPCCOEt (3.0 μM) | 98.69 ± 8.54 | 80.04 ± 6.14 | 82.99 ± 5.32 |
In the pyramidal cell layer of the CA3, ANOVA revealed a significant medium-by-drug interaction (F[3,47]= 5.18, p<0.05). A one-way ANOVA (F[7,40] = 7.75, p,< 0.0001) with Dunnett’s post-hoc test confirmed that the highest concentration of CPCCOEt (3 μM) moderately spared the decreases in NeuN immunofluorescence produced by CIE exposure by 22% while the lower concentrations (0.5 and 1.0 μM) failed to attenuate these changes (Figure 1). Table 1 shows that both 0.5 and 1.0 μM CPCCOEt produced significant reductions of NeuN immunofluorescence in the CA3 in ethanol-naïve tissue.
In the dentate gyrus, ANOVA revealed a significant main effects of medium (F[3,47] = 22.14, p<0.001) and drug (F[3,47] = 11.85, p<0.001). A one-way ANOVA (F[7,40] = 8.72, p,< 0.0001) with Dunnett’s post-hoc test confirmed that loss of NeuN immunoreactivity was not spared in hippocampi co-exposed to CIE and CPCCOEt in the granule cell layer of the dentate gyrus (Figure 1). Table 1 shows that a 1.0 μM CPCCOEt application produced significant reductions of NeuN immunofluorescence in this cell layer in ethanol-naïve tissue. Representative images of NeuN immunofluorescence of control hippocampi, hippocampi exposed to ethanol, or hippocampi co-exposed to ethanol and CPCCOEt (3 μM) are also depicted in Figure 1.
3.2. The Selective mGlu5 Antagonist SIB-1893 Reduces CIE-induced Cytotoxicity
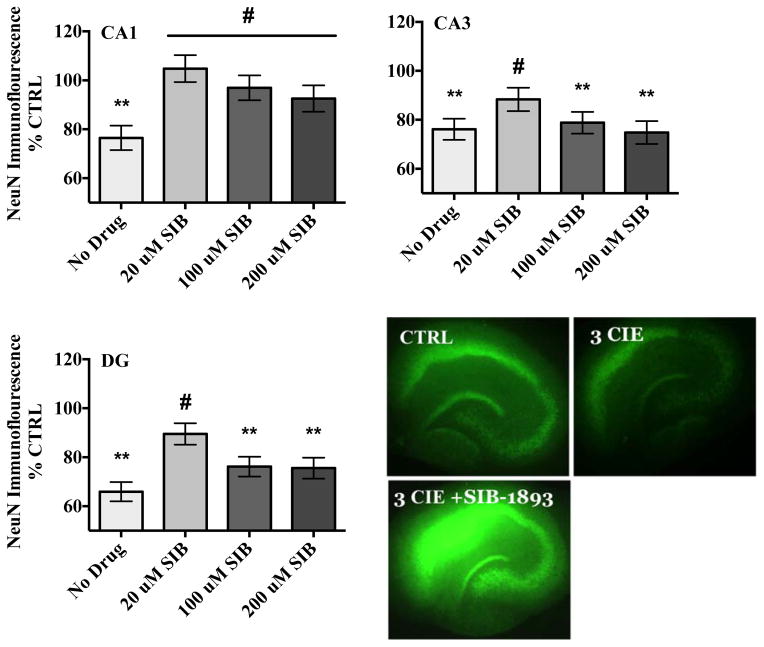
In the pyramidal cell layer of the CA1, ANOVA analyses revealed a significant medium-by-drug interaction (F[3,201] = 5.49, p<0.001). A one-way ANOVA (F[7,194] = 3.17, p= 0.003) with Dunnett’s post-hoc test confirmed that co-exposure to ethanol and 20 μM SIB-1893 prevented the loss of NeuN immunofluorescence produced by CIE whereas co-exposure to ethanol and SIB-1893 (100 μM) moderately attenuated these decreases. Table 2 shows that this compound did not alter levels of NeuN immunofluorescence in ethanol-naïve hippocampi.
Table 2.
Effects of noncompetitive mGlu5 antagonist SIB-1893 (20–200 μM) alone in ethanol-naïve medium. Details are the same as for Table 1. N = 24 for control hippocampi; N= 25 for hippocampi exposed to 20 μM SIB-1893; N = 27 for hippocampi exposed to 100 μM SIB-1893; N = 23 for hippocampi exposed to 200 μM SIB-1893 for each primary cell layer of the hippocampal formation.
| NeuN Immunofluorescence | CA1 ± SEM | CA3 ± SEM | DG ± SEM |
|---|---|---|---|
| Control hippocampi | 100.00 ± 5.27 | 100.00 ± 4.58 | 100.00 ± 4.19 |
| SIB-1893 (20 μM) | 92.31 ± 5.27 | 88.00 ± 4.89 | 91.67 ± 4.19 |
| SIB-1893 (100 μM) | 87.80 ± 5.07 | 79.52 ± 4.41 | 80.71 ± 4.03 |
| SIB-1893 (200 μM) | 82.13 ± 5.84 | 80.04 ± 4.77 | 80.33 ± 4.37 |
In the pyramidal cell layer of the CA3, ANOVA revealed significant medium-by-drug interaction (F[3,201] = 3.15, p<0.05). A one-way ANOVA (F[7,194] = 3.23, p= 0.003) with Dunnett’s post-hoc test confirmed that co-exposure to ethanol and the lowest concentration of SIB-1893 (20 μM) attenuated the decreases of NeuN immunofluorescence compared to ethanol-treated hippocampi whereas higher concentrations (100 and 200 μM) were toxic in ethanol-treated hippocampi as compared to control-treated hippocampi (Figure 2). Table 2 shows that higher concentrations (100 and 200 μM) of this compound were toxic alone in ethanol-naïve hippocampi.
Figure 2.
Effects of mGlu5 antagonist SIB-1893 on NeuN immunofluorescence in the primary cell layers of the hippocampal formation. Data are presented as percent control of the mean +/− the SEM. ** = statistical significance (p <0.05) compared to control hippocampi; # = statistical significance (p <0.05) compared to ethanol hippocampi. N = 25 for control hippocampi; N = 28 for hippocampi exposed to ethanol; N = 23 for hippocampi co-exposed to ethanol and 20 μM SIB-1893; N = 27 for hippocampi co-exposed to ethanol and 100 μM SIB-1893; N = 24 for hippocampi co-exposed to ethanol and 200 μM SIB-1893 for each hippocampal subregion. Representative images depict hippocampi exposed to ethanol-naïve media (control) or ethanol media (50 mM), or hippocampi co-exposed to 20 μM SIB-1893 and ethanol (50 mM).
Within the granule cell layer of the dentate gyrus, ANOVA revealed a significant medium-by-drug interaction (F[3,201] = 3.81, p<0.5) in this hippocampal cell layer. A one-way ANOVA (F[7,194] = 6.83, p= 0.001) with Dunnett’s post-hoc test confirmed that co-exposure to ethanol and the lowest concentration of SIB-1893 (20 μM) significantly attenuated levels of NeuN immunofluorescence compared to ethanol-treated hippocampi. Higher concentrations (100 and 200 μM) of SIB-1893 did not spare the decreases of NeuN immunofluorescence produced by three CIE in ethanol-treated hippocampi as compared to control-treated tissue (Figure 2). Table 2 shows that the higher concentrations (100 and 200 μM) of this compound were toxic in ethanol-naïve hippocampi.
3.3. Effects of Binge-like Ethanol Administration on Body Weight In Vivo
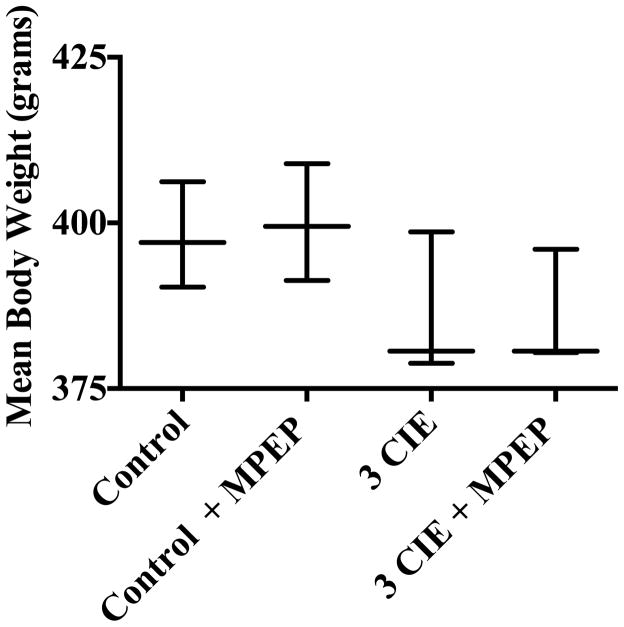
ANOVA revealed a significant interaction of day and diet (F[3,50] = 12.69, p<0.001) in subjects exposed to three cycles of CIE or an isocaloric diet with or without the addition of mGlu5 antagonist MPEP. A one-way ANOVA (F[3,25] = 6.06, p= 0.003) with Tukey’s post-hoc test confirmed that significant decreases in body weight (grams) were detected in subjects that were administered ethanol with or without the addition of MPEP compared to subjects administered an isocaloric diet during Week 3 (Figure 3). This figure also shows that MPEP administration did not have an effect on body weight in subjects exposed to CIE or an isocaloric diet.
Figure 3.
Body weight data were obtained daily on Week 1, 2, and 3. Data points show the range and means for subjects exposed to experimental groups. ** = statistical significance (p <0.05) compared to subjects administered an isocaloric control diet. N=7 for control subjects; N=8 for control with MPEP; N=6 for ethanol subjects; N=8 for subjects administered ethanol and MPEP
3.4. Influence of Group 1-Family Proteins in the Development of Ethanol Dependence
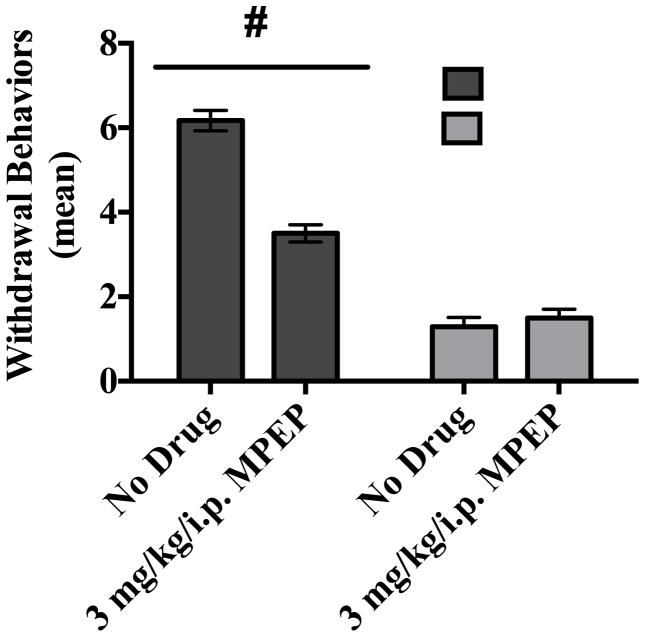
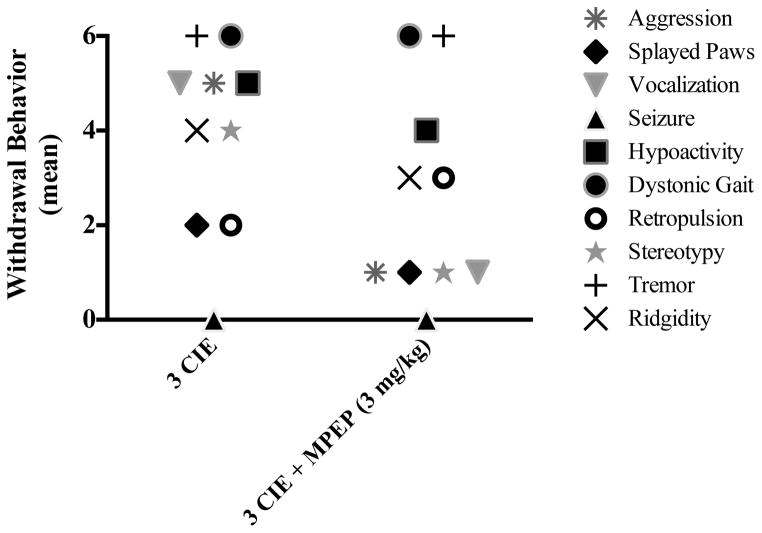
Subjects were administered CIE (or an isocaloric control diet) for three consecutive weeks with or without the addition of an oral and selective mGlu5 antagonist MPEP (3 mg/kg) to evaluate the influence of this particular receptor subtype in the development of ethanol dependence. ANOVA revealed a significant diet-by-drug interaction (F[1,28] = 44.87, p<0.001). A one-way ANOVA (F[3,25] = 101.6, p< 0.0001) with Tukey’s post-hoc test confirmed that subjects administered ethanol had significant increases in behavioral effects of withdrawal compared to subjects that received an isocaloric diet (Figure 4). The most prominent ethanol withdrawal-induced behaviors were tremor, other motor abnormalities (e.g., dystonic gait and stereotypy), aggression, and vocalization. This figure also shows that oral administration of mGlu5 antagonist MPEP significantly attenuated the physical manifestations of withdrawal in ethanol-dependent rats. Interestingly, MPEP administration did not uniformly reduce the severity of all withdrawal symptoms. Those symptoms primarily reduced by MPEP treatment were vocalization, aggression, and stereotypy. The prevalence of tremors and abnormal gait were not altered by MPEP administration (Figure 5).
Figure 4.
Withdrawal behavior was assessed during withdrawal on Day 6 of Week 3. Data points show mean scores in withdrawal behavior observed during the third consecutive withdrawal from CIE. # = statistical drug by diet interaction (p <0.001). N=7 for control subjects; N=8 for control with MPEP; N=6 for ethanol subjects; N=8 for subjects administered ethanol and MPEP.
Figure 5.
Frequency of ethanol withdrawal behaviors observed in the present study in ethanol-dependent subjects that received oral administration of ethanol with or without the addition of MPEP (3 mg/kg).
3.5. Effects of MPEP on Blood Ethanol Levels
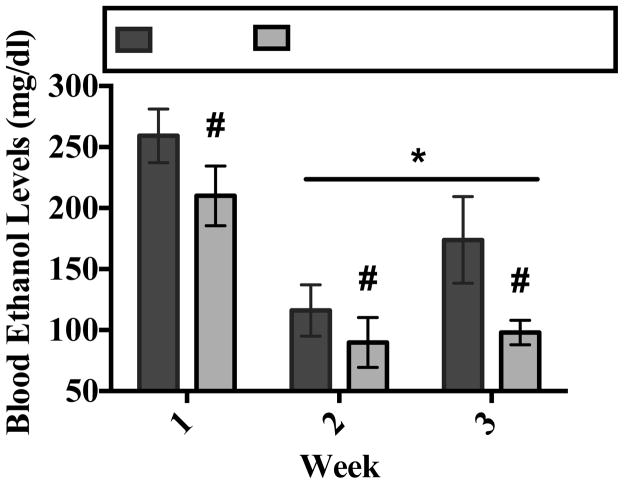
ANOVA revealed a significant main effect of drug (F[1,7] = 11.77, p<0.05) on BELs in ethanol-dependent subjects (Bonferroni adjustment for multiple comparisons). Figure 6 shows that MPEP administration modestly reduced BELs during Weeks 1, 2, and 3 of the CIE treatment regimen. ANOVA also revealed a significant main effect of week (F[1,14] = 35.00, p<0.001) on BELs in ethanol-dependent subjects (Bonferroni adjustment for multiple comparisons). Figure 6 also shows that average BELs on Weeks 2 and 3 were significantly lower as compared to Week 1.
Figure 6.
Peak BELs were assessed 90 minutes post-ethanol administration on Day 2 of Week 1, 2, and 3. Data points show mean score BELs for subjects exposed to ethanol with or without MPEP. * = statistical main effect of week (p <0.05). # = statistical main effect of MPEP on BELs in ethanol-dependent rats. N=4 for ethanol subjects; N=5 for ethanol with MPEP.
4. DISCUSSION
4.1. Role of Group-1 Family Proteins in Promoting Hippocampal Cytotoxicity of CIE
The present studies demonstrate that CIE exposure produces significant hippocampal cytotoxicity characterized by loss of NeuN immunofluorescence in the primary cell layers of the hippocampal formation. Exposure to higher concentrations of the mGlu1 antagonist CPCCOEt significantly attenuated the loss of NeuN immunoreactivity in the CA3 but not within the CA1 or and dentate subregions of the hippocampal formation. By contrast, exposure to the noncompetitive mGlu5 antagonist SIB-1893 prevented hippocampal cytotoxicity produced by CIE exposure in each primary cell layer of the hippocampal formation. The differential pattern of sparing of NeuN loss could reflect differential distribution of the mGlu1- and mGlu5-containing receptors in the hippocampus. For example, while the mGlu1 is differentially expressed in neurons throughout various areas of the brain and spinal cord (Shigemoto et al., 1993), levels of mGlu1 expression are highest in the granule cell layer of the dentate gyrus and pyramidal cell layer of the CA3 but are expressed at very low levels in the CA1 region (Davies et al., 1995; Petralia et al., 1997). By contrast, noncompetitive mGlu5 antagonists (e.g., SIB-1893) are thought to bind to the 7-transmembrane domain and alter conformational changes without affecting the extracellular ligand-binding site (for a review, see Spooren et al., 2001) and are localized throughout the cell layers of the hippocampal formation (Shigemoto et al., 1993). Thus, the present findings extend upon prior work suggesting a region-specific functional role for mGlu1- and mGlu5-containing receptors in the development of ethanol dependence and hippocampal cytotoxicity, independent of voluntary ethanol intake or alterations in ethanol pharmacokinetics.
It is interesting that in the current studies mGlu5 antagonist SIB-1893 exposure in the presence of ethanol prevented the hippocampal NeuN loss produced by CIE exposure. The reasons for the neuroprotective effects of SIB-1893 are likely associated with the effects of the mGlu5 on NMDA receptor activity in the modulation of glutamatergic tone. For example, NMDA receptor activity is influenced by activation of the mGlu5 in the hippocampus via second messenger effector protein kinase C in vitro (Chen et al., 2011). Others suggest that Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα) binds to the intracellular C terminus of the mGlu5 to produce a release of calcium from intracellular stores and that subsequent phosphorylation of adjacent GluN2Bs can potentiate NMDA receptor activity (Jin et al., 2013). Consistent with this notion, a prior report employing electrophysiological techniques demonstrated that mGlu5-containing receptors activates NMDA receptors to contribute to synaptic plasticity (Park et al., 2013). Therefore, it is likely that the neuroprotective effects of mGlu5 antagonist SIB-1893 include the modulation of glutamatergic tone via downstream effects at the NMDA receptor.
Worthwhile to note is that the loss of NeuN immunoreactivity could reflect a down-regulation of NeuN protein expression in the present report. However, we have previously demonstrated that decreases in NeuN immunoreactivity correlate with decreases in thionine, a cellular marker, using this model of CIE (Reynolds et al., 2015A). The exact cause of these effects that adversely affect “physiological status” (Weyer and Schilling, 2003) may reflect group 1 mGlu-family protein activation of downstream signaling cascades, as stimulation of these receptors can activate second messenger effectors, such as mitogen-activated protein kinase (Berkeley and Levey, 2003; Gallagher et al., 2004; for a review, see Wang et al., 2007). Gene expression can then be altered via CREB activation, producing increases in membrane-bound NMDA receptor subunit GluN2B (Rani et al., 2005). The exact cause of these effects probably reflects cellular toxicity rather than a decrease in NeuN protein expression. Given that cytotoxicity was observed in ethanol-naïve tissue within the hippocampus in the present report, there could be consequences of chronic exposure to mGlu5 antagonist SIB-1893 in vivo. For this reason, the present report used mGlu5 antagonist MPEP rather than SIB-1893 to assess the influence of group 1 mGlu-family proteins in the development of dependence in vivo.
These neuroadaptive changes to NMDA receptors likely confer sensitivity to neurotoxicity produced by the ethanol exposure in the current studies. This interpretation is supported by the work of Ticku and colleagues (Qiang et al., 2007) who demonstrated the upregulation of GluN1 and GluN2B subunits in cortical neurons exposed to binge-like ethanol in vitro (Qiang et al., 2007). Prior work suggests that this model of ethanol dependence reflects increases in NMDA receptor activation upon withdrawal, thus the activity of group 1 mGlu-family proteins may well contribute to this specific neuroadaptation to ethanol (Reynolds et al., 2015A).
4.2. Influence of Group 1 mGlu-Family Proteins in the Development of Ethanol Dependence
The present report examined the influence of the mGlu5 receptor in the development of ethanol dependence using an oral administration of MPEP in adult, ethanol-dependent and ethanol-naïve male rodents. MPEP is a selective mGlu5 antagonist that has been used previously as a neuroprotective compound in several models of drug and alcohol dependence, as well as in several models of neurodegenerative disorders (for a review, see Nickols and Conn, 2014). In the present report, subjects administered CIE demonstrated significant decreases in body weight when compared to subjects administered a control diet. These findings are not unexpected and are likely produced by the sedative effects of binge-like ethanol administration. This notion is supported by the work of others demonstrating that the administration of binge-like ethanol produces decreases in body weight (e.g., Broadwater et al., 2011A, 2010B). Alternatively, given that the CIE administration elicited physical dependence in the present report, these decreases in body weight could also reflect signs of illness although no overt signs of illness were observed. Notably, while chronic MPEP administration (3 mg/kg/day) did not produce significant changes in body weight in ethanol-dependent rats or ethanol-naïve rats, significant attenuation of BELs was observed in ethanol-dependent rats.
These studies demonstrate that CIE administration produces physical dependence manifested in physical symptoms of withdrawal, as has been previously reported (Reynolds et al. 2015B). In the prior report, it was found that periods of CIE shorter than the three cycles reported here were not able to produce robust ethanol withdrawal when similar BELs were achieved. In general, the most consistent features of withdrawal-like behavior in the current study were retropulsion (e.g., backing into a corner), dystonic gait, extreme tail rigidity, and severe head tremors. While no seizures were observed in the current report, withdrawal behaviors related to motor abnormalities were more consistent across subjects. For example, moderate to severe head tremors were observed in nearly all ethanol-dependent subjects. These findings are consistent with the work of others that unequivocally demonstrated hallmark characteristics of central nervous system hyper-excitability during periods of withdrawal from binge-like ethanol exposure (for a review, see Becker, 2013; Pérez and DeBiasi, 2015). Findings from a recent report demonstrated a significant increase in mGlu1 and NMDA GluN signaling within the central nucleus of the amygdala in mice following binge alcohol consumption for 30 days (Cozzoli et al., 2014). Thus, the findings in the present report of the ability of MPEP to blunt the behavioral effects of withdrawal could reflect attenuation of concomitant neuroadaptations in group 1 mGlu-family proteins and perhaps NMDA GluN proteins within areas of the limbic system, such as the central amygdala.
Notably, chronic and oral administration of mGlu5 antagonist MPEP significantly attenuated the behavioral effects of withdrawal in the present report, such as sparing the extreme stereotypy observed during the third consecutive period of withdrawal in ethanol-dependent subjects administered CIE. This may suggest that activation of mGlu5-family proteins, in particular, is a key underlying biochemical means of inducing ethanol dependence via alterations in glutamate receptor signaling, as noted above in the discussion of in vitro data. Cozzoli and colleagues (2009) found that binge-like ethanol administration produced a three-fold increase in mGlu1 polypeptide levels within the hippocampus, as well as significant increases in mGlu5 and GluN2 protein expression. This report also demonstrated ethanol-dependent increases in Homer2a/b expression as well as Homer2-phosphatidylinositol 3-kinase (PI3K) signaling in other limbic areas of the brain, such as the nucleus accumbens. These effects were significantly blocked with MPEP. This study also found that pretreatment with mGlu5 antagonist MPEP dose-dependently reduced binge ethanol consumption in rodents. Others have shown that MPEP administration (10 mg/kg) attenuated ethanol withdrawal anxiety-like behaviors (Kumar et al., 2013) or have shown a reduction in ethanol reinstatement following acute MPEP administration elicited by drug-associated cues (Bäckström et al., 2004), as well as attenuation of ethanol withdrawal-elicited behaviors in rodents (Blendov and Harris, 2008).
It is critical to note that in the present studies MPEP administration significantly reduced BELs during the CIE treatment regimen, a finding that has not previously been reported in the extant literature. This suggests that the reduced withdrawal behavior observed with chronic, oral MPEP treatment should alter the severity of withdrawal. Prior reports suggest that mGlu5 receptors are located in the liver (e.g., Storto et al., 2000; Wu et al., 2012), and although mGlu5 activity clearly contributes to hepatotoxicity under some circumstances, the role of these receptors in enzyme inhibition or induction has not been characterized. The present findings may demonstrate a role for mGluR5 inhibition via MPEP administration in promoting alcohol dehydrogenase activity and/or synthesis though this is not yet confirmed. However, we note that the in vitro data included in the present report demonstrate a role for hippocampal mGlu5-family proteins in the development of ethanol dependence that is entirely independent of effects on tissue ethanol levels.
In sum, these findings extend upon the current literature by characterizing the distinct roles that mGlu1- and mGlu5-containing receptors have in contributing to ethanol withdrawal-associated cytotoxicity in vitro and promoting the behavioral effects of withdrawal in vivo. Collectively, these findings suggest that the concomitant neuroadaptations in group 1 mGlu-family proteins are likely associated with both the behavioral and neurodegenerative effects observed following multiple bouts of heavy ethanol consumption (Duka et al., 2003, 2004; Sullivan et al., 1996), as well as in the development of dependence. Importantly, these effects are clearly independent of effects on voluntary ethanol intake. Group 1 mGlu-family proteins, in particular the mGlu5, may be therapeutic targets for treatment of alcohol use disorders.
The present studies examine the role of mGluR signaling in ethanol dependence.
Hippocampal explants are effective as a high-throughout screen of toxicity.
Group 1 metabotrophic receptor antagonism attenuates hippocampal cytotoxicity.
Blocking these receptors prior to withdrawal reduces development of dependence.
Acknowledgments
Role of Funding Source: T32 DA035200, AA013388
We would like to thank Lynda Sharrett, Jennifer Berry, and Tracy Butler for their assistance.
Footnotes
Conflict of interest: No conflict declared.
Contributors: The following individuals contributed substantially to the design, implementation, and/or interpretation of the data: Anna R. Reynolds, Luke A. Williams, Meredith A. Saunders, and Mark A. Prendergast. All authors have read and approve of the submission of this manuscript to Drug and Alcohol Dependence.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. doi:101038/sjnpp1300381. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacol. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJM, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley JL, Levey AI. Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol Pharmacol. 2003;63:128–135. doi: 10.1124/mol.63.1.128. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. doi:101017/s1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Different chronic ethanol exposure regimens in adolescent and adult male rats: effects on tolerance to ethanol-induced motor impairment. Behav Brain Res. 2011A;225:358–362. doi: 10.1016/j.bbr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 2011B;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Prendergast MA. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neurosci. 2010;165:525–534. doi: 10.1016/j.neuroscience.2009.10.018. doi:101016/jneuroscience200910018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S, Gahwiler BH, Do KQ, Knopfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347:765–767. doi: 10.1038/347765a0. doi:101038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chen H, Liao P, Chan M. mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J Biomed Sci. 2011;18:19. doi: 10.1186/1423-0127-18-19. doi:101186/1423-0127-18-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, et al. Binge drinking upregulates accumbens mGluR5- Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. doi:101523/jneurosci5900-082009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Maliniak D, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact group 1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39:435–444. doi: 10.1038/npp.2013.214. http://doi.org/10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Clarke VR, Jane DE, Collingridge GL. Pharmacology of postsynaptic metabotropic glutamate receptors in rat hippocampal CA1 pyramidal neurones. Br J Pharmacol. 1995;116:1859–1869. doi: 10.1111/j.1476-5381.1995.tb16674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27:1563–1572. doi: 10.1097/01.alc.0000090142.11260.d7. [DOI] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens D, Veatch LM, Becker HC, Crews FT. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28:233–246. doi: 10.1097/01.alc.0000113780.41701.81. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal- regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. doi:101523/jneurosci5407-032004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri L, Poggesi E, Angelico P, Farina P, Leonardi A, Clarke DE, Testa R. Effect of selective antagonists of group I metabotropic glutamate receptors on the micturition reflex in rats. BJU Int. 2008;102:890–898. doi: 10.1111/j.1464-410X.2008.07748.x. doi:101111/j1464-410X200807748x. [DOI] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27:1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. doi:101097/01alc000009360133119e3. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Mao LM, Wang JQ. Differential regulation of CaMKIIα interactions with mGluR5 and NMDA receptors by Ca2+ in neurons. J Neurochem. 2013;127:620–631. doi: 10.1111/jnc.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW, Sternebring B. Kinetics of ethanol and methanol in alcoholics during detoxification. Alcohol Alcohol. 1992;27:641–647. [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. doi:101074/jbcM109052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Hapidin H, Bee YTG, Ismail Z. Effects of the mGluR5 antagonist MPEP on ethanol withdrawal induced anxiety-like syndrome in rats. Behav Brain Funct. 2013;9:43. doi: 10.1186/1744-9081-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litschig S, Gasparini F, Rueegg D, Stoehr N, Flor PJ, Vranesic I, Prezeau L, Pin JP, Kuhn R. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol Pharmacol. 1999;55:453–461. [PubMed] [Google Scholar]
- Morin N, Grégoire L, Morissette M, Desrayaud S, Gomez-Mancilla B, Gasparini F, Di Paolo T. MPEP, an mGlu5 receptor antagonist, reduces the development of L-DOPA-induced motor complications in de novo Parkinsonian monkeys: biochemical correlates. Neuropharmacology. 2013;66:355–364. doi: 10.1016/j.neuropharm.2012.07.036. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis. 2014;61:55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Akinfiresoye LR, Allard JS, Lovinger DM. alcohol withdrawal-induced seizure susceptibility is associated with an upregulation of CaV1.3 channels in the rat inferior colliculus. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. http://doi.org/10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154:637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez EE, De Biasi M. Assessment of affective and somatic signs of ethanol withdrawal in C57BL/6J mice using a short-term ethanol treatment. Alcohol. 2015;49:237–243. doi: 10.1016/j.alcohol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Singh S, Wu C, Shi L, Wei J, Wenthold RJ. A monoclonal antibody shows discrete cellular and subcellular localizations of mGluR1 alpha metabotropic glutamate receptors. J Chem Neuroanat. 1997;13:77–93. doi: 10.1016/s0891-0618(97)00023-9. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. J Neurosci. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. doi:101016/jneuroscience200312013. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. doi:101124/mol106033043. [DOI] [PubMed] [Google Scholar]
- Rani CS, Qiang M, Ticku MK. Potential role of cAMP response element- binding protein in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene transcription in fetal mouse cortical cells. Mol Pharmacol. 2005;67:2126–2136. doi: 10.1124/mol.104.007872. doi:101124/mol104007872. [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Berry JN, Sharrett-Field L, Prendergast MA. Ethanol withdrawal is required to produce persisting N-methyl-D-aspartate receptor- dependent hippocampal cytotoxicity during chronic intermittent ethanol exposure. Alcohol. 2015A;49:219–227. doi: 10.1016/j.alcohol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AR, Saunders MA, Brewton HB, Winchester SR, Elgumati IS, Prendergast MA. Acute oral administration of the novel, competitive and selective glucocorticoid receptor antagonist ORG 34517 reduces the severity of ethanol withdrawal and related hypothalamic-pituitary-adrenal axis activation. Drug Alcohol Depend. 2015B doi: 10.1016/j.drugalcdep.2015.06.018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Tu JC, Petralia RS, Xiao B, Wenthold RJ, Worley PF. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274:25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Goldsworthy J, Johnson BG, Salhoff CR, Baker SR. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide- linked metabotropic glutamate receptors in the rat hippocampus. J Neurochem. 1994;63:769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. http://doi.org/10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self RL, Smith KJ, Butler TR, Pauly JR, Prendergast MA. Intra-cornu ammonis 1 administration of the human immunodeficiency virus-1 protein trans-activator of transcription exacerbates the ethanol withdrawal syndrome in rodents and activates N-methyl-D-aspartate glutamate receptors to produce persisting spatial. J Neurosci. 2009;163:868–876. doi: 10.1016/j.neuroscience.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Berry JN, Reynolds AR, Prendergast MA. Mifepristone pretreatment reduces ethanol withdrawal severity in vivo. Alcohol Clin Exp Res. 2013;37:1417–23. doi: 10.1111/acer.12093. http://doi.org/10.1111/acer.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- Storto M, de Grazia U, Knöpfel T, Canonico PL, Copani A, Richelmi P, Nicoletti F, Vairetti M. Selective blockade of mGlu5 metabotropic glutamate receptors protects rat hepatocytes against hypoxic damage. Hepatology. 2000;31:649–655. doi: 10.1002/hep.510310315. http://doi.org/10.1002/hep.510310315. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res. 1996;20:348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. doi:101111/j1471-4159200604208x. [DOI] [PubMed] [Google Scholar]
- Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- Wu Y, Le Wang NN, Gu L, Yang HM, Xia N, Zhang H. The suppressive effect of metabotropic glutamate receptor 5 (mGlu5) inhibition on hepatocarcinogenesis. Biochimie. 2012;94:2366–2375. doi: 10.1016/j.biochi.2012.06.006. [DOI] [PubMed] [Google Scholar]