Abstract
Objective
Resistance to obesity is observed in rodents and humans treated with Rapamycin (Rap) or Nebivolol (Neb). Since cardiac miR-208a promotes obesity, we tested whether the modes of actions of Rap and Neb involve inhibition of miR-208a.
Methods
Mouse cardiomyocyte HL-1 cells and Zucker obese (ZO) rats were used to investigate regulation of cardiac miR-208a.
Results
Angiotensin II (Ang II) increased miR-208a expression in HL-1 cells. Pre-treatment with an AT1 receptor (AT1R) antagonist, losartan (1µM), antagonized this effect, whereas a phospholipase C inhibitor, U73122 (10µM) and an NADPH oxidase inhibitor, apocynin (0.5mM) did not. Ang II-induced increase in miR-208a was suppressed by Rap (10nM), an inhibitor of nutrient sensor kinase mTORC1, and Neb (1µM), a 3rd generation β-blocker that suppressed bioavailable AT1R binding of 125I-Ang II. Thus, suppression of AT1R expression by Neb, inhibition of AT1R activation by losartan, and inhibition of AT1R-induced activation of mTORC1 by Rap attenuated the Ang II-induced increase in miR-208a. In ZO rats, Rap treatment (750µg/kg/day; 12 weeks) reduced obesity despite similar food intake, suppressed cardiac miR-208a, and increased cardiac MED13, a suppresser of obesity.
Conclusion
Rap and Neb suppress cardiac miR-208a. MiR-208a suppression and increase in MED13 correlated with attenuated weight gain despite leptin resistance.
Keywords: Anti-obesity, Nebivolol, Rapamycin, AT1 receptor, miR208a, MED13
INTRODUCTION
The worldwide epidemic of obesity now affects more than 2 billion people, costing the world’s economy two trillion dollars per year (1). Since obesity is a risk factor for cardiovascular disease and diabetes (2–4), it is critical to examine the causal mechanisms and interrelationships of these diseases. Elegant studies have established the significance of cardiac microRNA miR-208a in cardiac development and pathology (5–8). Encoded by intron 27 of the cardiac specific α-myosin heavy chain (MHC) gene Myh6, miR-208a is a member of the miR-208 family of miRNAs. Up-regulation of miR-208a in response to stress suppresses transcriptional repression of β-cardiac muscle myosin heavy chain gene (Myh7) and promotes switching of expression from αMHC to βMHC, resulting in myocardial contractile dysfunction. A unique trait of miR-208a is that pharmacological inhibition of miR-208a by anti-miR-208a confers resistance to obesity (9). This effect of miR-208a is attributed to its ability to inhibit Mediator Complex 13 (MED13) synthesis, since overexpression of cardiac specific MED13 confers resistance to obesity and controls systemic energy homeostasis (9). MED13 is also known as thyroid hormone–associated protein 1 (THRAP1) and is implicated in the anti-obesity effects of thyroid hormone signaling (9).
Although β-Adrenergic receptor blockers are effective in treating cardiovascular diseases (10–12) some cause weight gain (13). However, reports from our lab and others indicate that treatment with the 3rd generation β-blocker nebivolol (Neb) inhibits weight gain (14, 15). The exact mechanisms by which Neb inhibits weight gain are unclear. It is known that the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1) integrates inputs from nutritional stimuli with the cellular growth machinery, and activation of mTORC1 is central to the onset of obesity (4, 16, 17). We have reported that Neb attenuates activation of mTORC1 in heart tissues of Zucker obese (ZO) rats, an animal model for hyperinsulinemia, leptin resistance, and obesity (14). Therefore, inhibition of mTORC1 could be the mechanism by which Neb suppresses body weight gain in ZO rats. It is not known whether suppression of cardiac miR-208a, which can also induce resistance to weight gain, is involved in Neb’s mode of action.
Chronic Rapamycin (Rap) treatment also inhibits weight-gain in rodent models of obesity (18–20). Originally identified as an anti-fungal compound, Rap is now widely used as an immunosuppressant and anticancer therapeutic (21, 22). Rap is the canonical inhibitor of mTOR (mammalian target of rapamycin). Though the modes of action of Rap and Neb are different, both drugs confer resistance to obesity and attenuate mTORC1. We hypothesized that signaling by both Rap and Neb converges in a common mechanism that involves mTORC1 and miR-208a. To test this idea, we assessed the effects of angiotensin II (Ang II), Rap and Neb on mTORC1 activation and miR-208a expression using mouse cardiomyocyte HL-1 cells (23). We also tested whether chronic Rap treatment of leptin resistant and hyperphagic Zucker obese rats (ZO) causes resistance to weight gain that is correlated with suppression of cardiac miR-208a and increased expression of cardiac MED13. Data presented here shows for the first time that Rap and Neb inhibit AT1R-induced activation of mTORC1 and also inhibit upregulation of miR-208a expression. Moreover, we report that chronic Rap treatment conferred resistance to weight gain in leptin resistant ZO rats, attenuated cardiac miR-208a expression and increased the obesity suppressor cardiac MED13.
METHODS
Cell culture and treatments
Mouse atrial cardiomyocyte HL-1 cells were a gift from Dr. William Claycomb at Louisiana State University Medical Center and were cultured as described previously (18, 23). Nebivolol was a gift from Forest Laboratories Inc. (New York) and rapamycin was purchased from Cell Signaling Technology (Boston, MA). Human Ang II was purchased from Sigma-Aldrich. Losartan (AT1R inhibitor), PD123319 (AT2R inhibitor), apocynin (NADPH Oxidase inhibitor) and U73122 (Phospholipase C inhibitor) were purchased from TOCRIS Biosciences.
Rapamycin treatment of rats, body composition, food intake and tissue collection
All animal procedures used in this study were approved by the Harry S. Truman Veterans Memorial Hospital (HSTVMH) Subcommittee for Animal Safety and University of Missouri IACUC before commencing. All animals were cared for in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23). Zucker obese (fa/fa) (ZO) and lean (ZL) rats (Charles River Laboratories) were used in this study. Rats were maintained on ad libitum food and water and housed singly at the HSTVMH animal housing facility under standard laboratory conditions (room temperature: 21– 22°C; light and dark cycles: 12h). Food intake was monitored by placing a pre-weighed amount of food in the cage and determining the weight of leftover food after 24 hrs. At 8-weeks of age, rapamycin pellets designed to deliver Rap at a concentration of 750µg/kg/day for 21 days (from Innovative Research of America, Inc, Sarasota, FL) or placebo pellets were placed surgically under the skin behind the shoulder blades under brief isoflurane anesthesia and this procedure was repeated 3 times to achieve a 12-week treatment. Body composition was determined using the EchoMRI 4in1/1100 as described previously (18). Hearts were harvested at time of sacrifice as described before (18), flash frozen in liquid nitrogen, and stored at -80°C for future use.
RNA isolation, quantitative RT-PCR, and Immunoblotting
The mirVana miRNA isolation kit (Ambion) was used for isolation of miRNA and mRNA. Quantitative RT-PCR (qRT-PCR) was performed as described previously (18). Taqman microRNA assay primers for miR-208a and small nuclear RNA (snRNA) (Taqman microRNA Assays) and rat MED13 and 18S RNA primers (Gene Expression Assays) were from Applied Biosystems. Experiments were performed in triplicate for each biological sample. Relative quantification (RQ) values were obtained by determining ΔCt values followed by determining ΔΔCt values and then RQ values via the equation 2−ΔΔCt.
Cell lysates of HL-1 cells were prepared and Western blotting was performed as described previously (18). Experiments were performed in at least in triplicate for each biological sample. All antibodies except anti-β-MHC antibody were from Cell Signaling Technology Inc. (Boston, MA). The mouse monoclonal anti-β-MHC antibody which is highly specific for Myh7 product was from Sigma, (St. Louis, MO; Mouse Monoclonal Anti-myosin (skeletal, slow) antibody, M8421). The blots were blocked with 5% bovine serum albumin (BSA) in Trisbuffered saline-Tween 20 (TBST) for one hr. After blocking, PVDF membranes were probed with primary antibodies (1:1000 dilution of each antibody) for mTOR, phospho-mTOR (Ser2448), p70S6K, phospho-p70S6K (Thr389), RPS6, phospho-RPS6 (Ser235/236), Jak2, phospho-Jak2 (Tyr1007/1008), STAT1, phospho-STAT1(Tyr701), or β-MHC, in 5% BSA in TBST overnight and washed with TBST prior to the addition of horseradish peroxidase-conjugated secondary antibody (1:20,000). After 1 hr incubation at room temperature with secondary antibodies and washing with TBST, chemiluminescent substrate (Supersignal West Femto Maximum Sensitivity Substrate kit; Thermo Scientific) was used to visualize antibody binding. Images were captured using a Bio-Rad ChemiDoc XRS image-analysis system. Quantitation of phosphorylated protein band density normalized to the density of total protein or protein band density normalized to the density of β-actin band for each sample, was performed using Quantity One software (Bio-Rad Laboratories Inc. Berkeley, Ca). Data are reported as the normalized protein band density in arbitrary units.
Immunofluorescence
Immunofluorescence was used to determine the changes in the expression of β-MHC in HL-1 cells in response to different treatments. Briefly, HL-1 cells were grown on cover slips as described previously (18). All treatments were performed in triplicates. After treatments with Ang II (100nM:12hr) or Neb (1µM: 12hr) coverslips were washed with HEPES (Sigma), fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.5% Triton-X-100, washed with HEPES-T (1mL Tween-20/L), and blocked with 1% bovine serum albumin (BSA) (Jackson ImmunoResearch), 10% goat serum (Sigma) and 0.1% Tween 20 (Fisher Scientific). Cells were incubated with anti- β-MHC antibody (Sigma) (1:100 dilution) overnight at 4°C and repeatedly washed with HEPES. The coverslips were then incubated with Alexa Fluor 488 goat anti-rabbit (Invitrogen Inc.) (1:200 dilution) for 1 hr at room temperature. Coverslips were washed with HEPES and mounted with Fluoroshield with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) and visualized using a Leica DMI 4000B inverted confocal microscope using Leica Application Suite software. Imaging was done at 60x magnification using oil immersion.
Radioligand binding studies
HL-1 cardiomyocytes were incubated in serum-free Claycomb medium with or without Neb (1µM) or Losartan (1µM) or PD123319 (5µM) for 12 hrs. Each treatment was performed at least in triplicate. Cells were collected by trypsinization (0.05% Trypsin), and resuspended in ice-cold Claycomb medium. The number of cells per sample was determined using Millipore Scepter and cell numbers in all samples were adjusted to have 106 cells/mL. Next, 200µl of cell suspension for each treatment group (untreated, or Neb-, losartan-, and PD123319-treated) was transferred to microfuge tubes. Cells were collected by centrifugation, and re-suspended in 190 µl of ice-cold pre-incubation medium (24) with or without Ang II (1µM). Cells that received 12hr treatment with Neb or Losartan were re-suspended in pre-incubation medium containing Neb (1µM) or losartan (1µM). In addition, different aliquots of untreated cells were treated with pre-incubation medium containing either Neb (1µM) or losartan (1µM) or neither drugs to determine whether short-term pretreatment with Neb or losartan prior to the ligand-binding procedure had any effect on 125I-Ang II binding to HL-1 cells.
The 125I-Ang II was prepared using the chloramine T procedure (25). Monoradioiodinated Ang II was purified by HPLC as described previously (26). After a 30 min pre-incubation, 10µl of pre-incubation medium containing 125I-Ang II was added to achieve a final concentration of 1nM. After a2 hour incubation on ice, cells were collected by centrifugation, washed three times with ice-cold PBS and the radioactivity in the pellet was monitored using Perkin Elmer-Wallac Wizard 1480 gamma counter. Specific binding for the cells from each group was determined by subtracting the counts obtained from cell pellets that were incubated in the pre-incubation medium containing non-radioactive Ang II (1µM). The specific binding obtained for the untreated sample under these conditions was taken as 100%. The % specific binding of 125I-Ang II to HL-1 cells after each treatment in comparison with the untreated sample was calculated.
Statistics
The SPSS 20 software package was used for statistical analysis. Results were expressed as mean ± SEM (standard error of mean). Differences among groups were tested by using One-Way ANOVA followed by Tukey's test or Student’s t-test, as appropriate, and two-tailed p values are reported. A p-value of ≤ 0.05 was considered statistically significant.
RESULTS
Effects of Angiotensin II, Rapamycin and Nebivolol on miR-208a expression in HL-1 cardiomyocytes
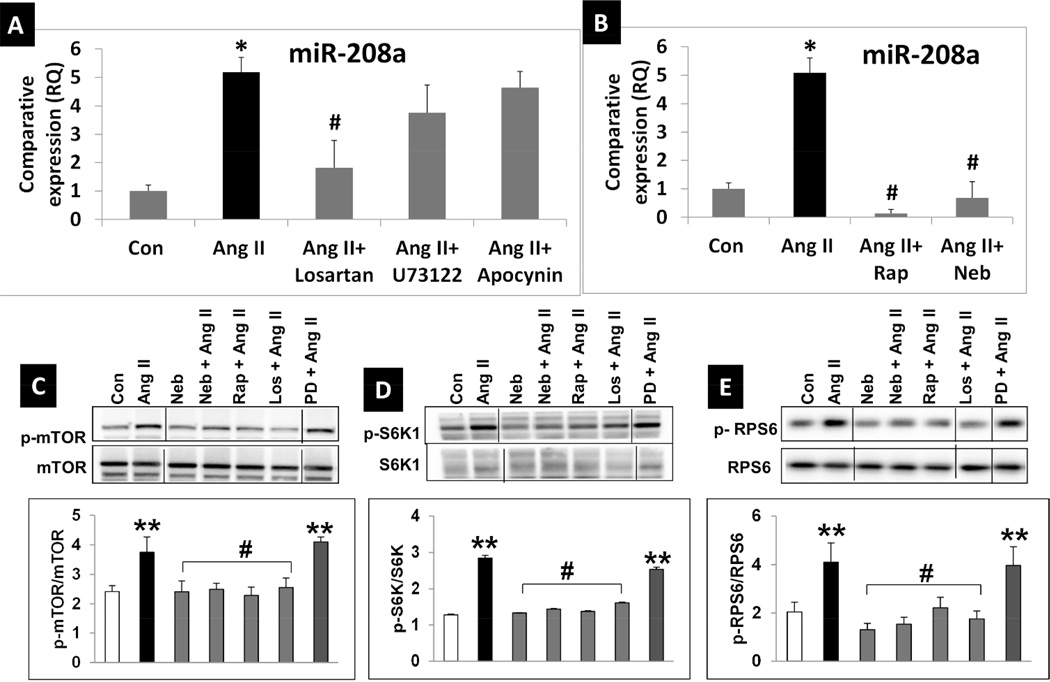
We investigated whether Ang II would increase, and Neb and Rap would suppress Ang II-induced miR-208a expression in HL-1 cardiomyocytes. Ang II treatment (100nM: 12hr) of serum starved HL-1 cardiomyocytes increased miR-208a expression by ~5 fold (Fig. 1A). Pretreatment with AT1R blocker losartan (1µM) suppressed this effect, however, pre-treatment with the NADPH oxidase inhibitor apocynin (0.5mM) and phospholipase C (PLC) inhibitor U73122 (10µM) did not significantly attenuate the Ang II-induced increase in miR-208a expression (Fig. 1A). Importantly, Rap (10nM) and Neb (1µM) suppressed the Ang II-induced increase in miR- 208a expression (Fig. 1B). These observations suggest that the Ang II induced increase in miR-208a expression is mediated via a mechanism regulated by Rap and Neb. Since Rap inhibits mTORC1, we tested whether Ang II activated mTORC1, and whether Rap and Neb suppressed this effect in HL-1 cells. We examined phosphorylation (p) status of Ser2448 of mTOR, Thr389 of p70 S6 kinase (S6K1), the substrate of mTORC1, and Ser235/236 of Ribosomal Protein S6 (RPS6), the substrate of S6K1. Ang II (100nM:12hr) increased stimulatory phosphorylation of mTOR (pSer2448), S6K1 (pThr389) and RPS6 (p Ser235/236), and this effect was suppressed by pretreatment with Rap (10nM), Neb (1µM) and losartan (1µM), but not by the AT2R inhibitor PD123319 (1µM) (Figs 1C-E). Thus, Ang II activates mTORC1 via AT1R, while both Rap and Neb suppress this effect.
Fig. 1. Ang II activates, while Rap and Neb suppress Ang II activated mTORC1 signaling and miR-208a expression in mouse cardiomyocyte HL-1 cells.
A: qRT-PCR data expressed as RQ values shows that Ang II (100nM: 12hrs) increases miR-208a while the AT1R blocker losartan suppressed Ang II-induced increases in miR-208a expression. Neither the Phospholipase C inhibitor U73122 (10µM) nor the NADPH oxidase inhibitor apocynin (0.5mM) could effectively suppress Ang II-induced miR-208a expression. B: qRT-PCR data expressed as RQ values show that addition of Neb (1µM) or Rap (10nM) to the incubation medium during the overnight treatment suppresses the Ang II-induced increase in miR-208a expression. n ≥3 treatments for each treatment group except for Ang II. Since Ang II treatment was repeated with each data set shown in Fig.1 A and B and in Fig. 3, biological replicates for Ang II was ≥10. qRT-PCR was performed in triplicate for RNA isolated from each biological sample. Values are means ± SEM. *p < 0.05 for Ang II treated vs. untreated (Con) cells. #p < 0.05 for Ang II treated vs. Neb, Rap, or losartan treated cells. C-E: Representative images of autoradiograms showing elevated levels of pSer2448 mTOR (C), pThr389 S6K1 (D), and pSer235/236RPS6 (E) in HL-1 cells incubated with Ang II (100nM: 12hrs) and suppression of this effect by co-treatment with Neb (10nM) Rap (1µM) or the AT1R blocker losartan (1µM), but not by AT2R blocker PD123319 (5µM). Images are from same gel with intervening lanes excluded for clarity. Graphs show results of densitometric analysis of the intensity of the phosphorylated protein bands after adjusting for the intensity of total protein bands (tmTOR, tS6K1, tRPS6). Values are means ± SEM. N ≥3 for each treatment group. **p < 0.05 for untreated (Con) vs. Ang II treated or PD+AngII treated. #p < 0.05 for Ang II treated vs. Neb, Rap, or losartan treated cells.
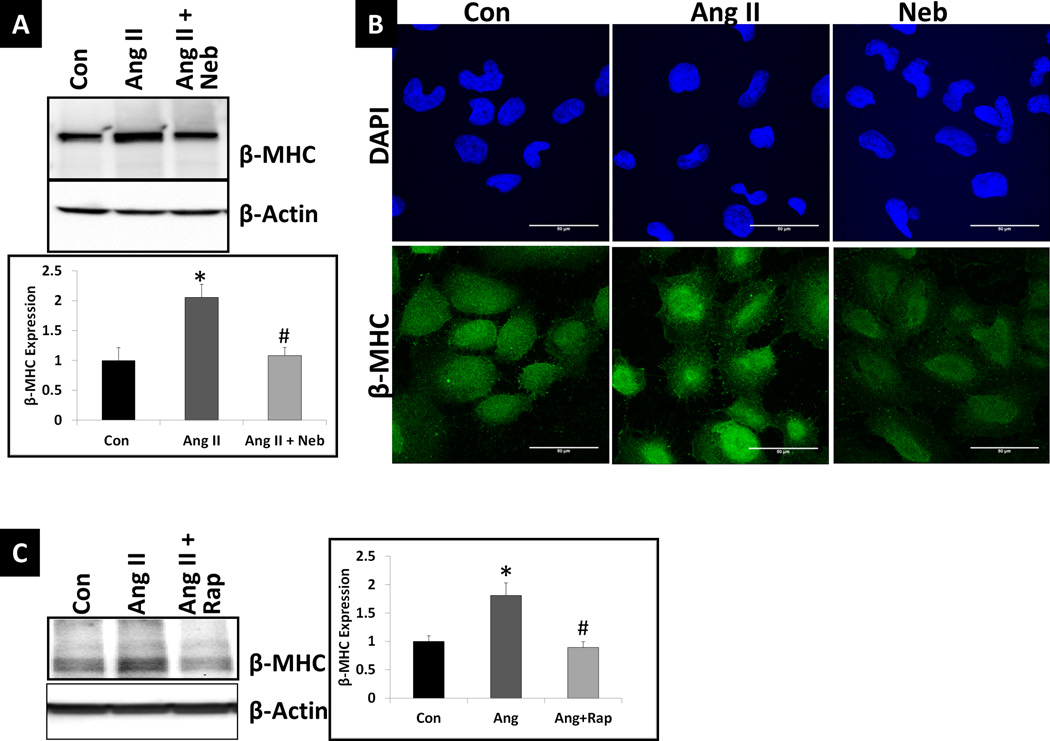
Increased miR-208a is reported to increase β-myosin heavy chain (βMHC) protein levels (5–8). To further confirm that Ang II and Neb treatments have opposing effects on βMHC expression, we treated HL-1 cardiomyocytes with Ang II (100nM:12hr), Neb (1µM:12hrs), and Ang II plus Neb (100 nM and 1 µM, respectively:12hr). Western blotting analysis showed that Ang II increased the expression of βMHC in HL-1 cells and Neb suppressed the Ang II–induced increase (Fig. 2A). Immunofluorescence analysis further confirmed Neb’s inhibitory effect on βMHC expression (Fig. 2B). Western blotting analysis also showed that Rap (10nM:12hrs) suppressed the Ang II-induced increase in βMHC expression (Fig. 2C)
Fig. 2. Ang II increases while Neb and Rap suppress the Ang II-induced increase in miR-208a effector β-MHC.
(A) Representative autoradiogram and densitometric analysis using Quantity One software (graph) after Western blotting of untreated and treated HL-1 cell lysates (treated with Ang II or Ang II+Neb) and probing with anti- β-MHC antibody. (B) Representative images from immunofluorescence analysis of untreated and treated HL-1 cells. (C) Representative autoradiogram and densitometric analysis using Quantity One software (graph) after Western blotting of HL-1 cell lysates (Untreated (Con) or treated with Ang II or Ang II+Neb) and probing with anti- β-MHC antibody. Treatments with Ang II (100nM:12hrs) Neb (1µM:12 hrs)) or Rap (10nM: 12hrs) were similar to those performed for the data shown in Fig. 1. Treatments were performed in triplicate. Neb- and Rap-treatment suppressed Ang II-induced increase in β-MHC (A, C). Immunofluorescence staining with anti- β-MHC antibody and nuclear stain DAPI indicated that in individual cells Ang II increased and Neb suppressed β-MHC (B). *p < 0.05 for untreated (Con) vs. Ang II treated. #p < 0.05 for Ang II treated vs. Ang II+Neb or Ang II+ Rap treated cells.
Mode of action of Neb in miR-208a regulation
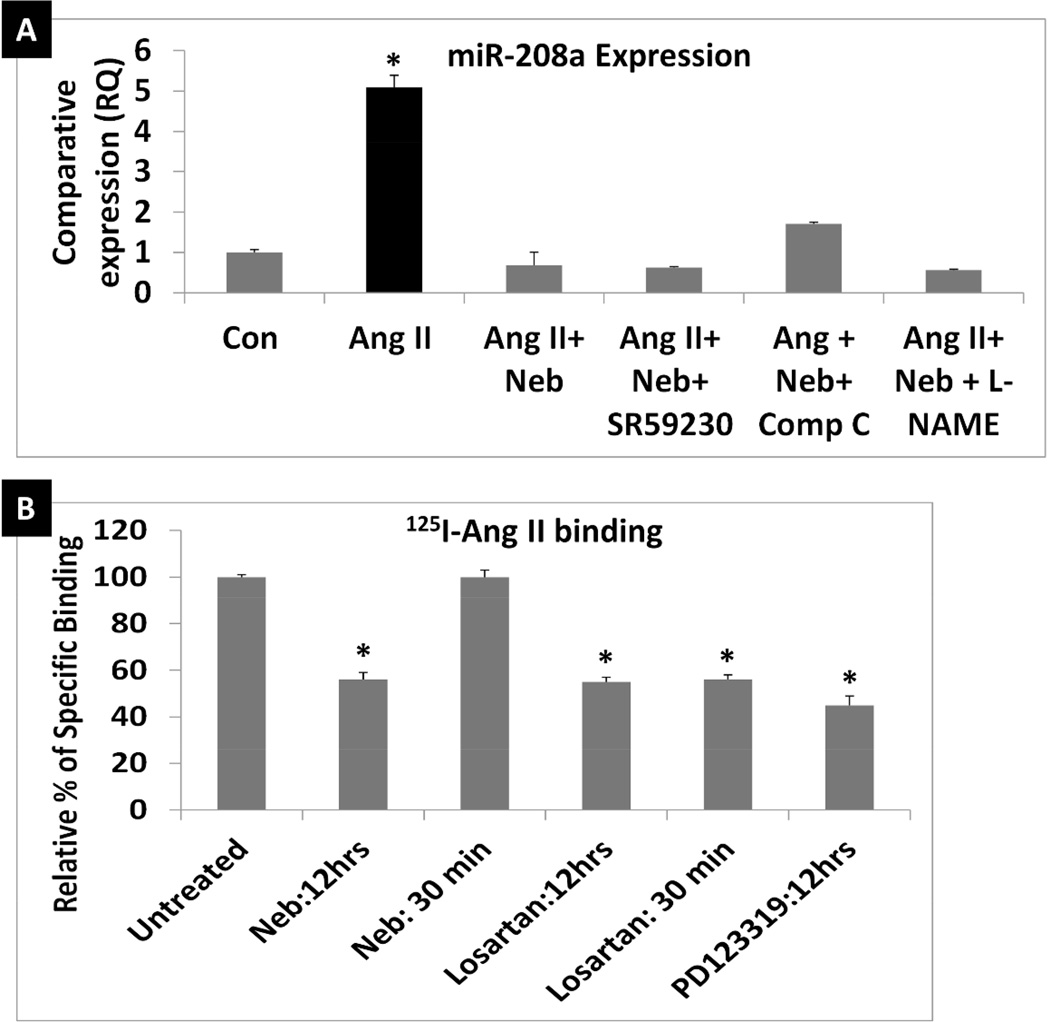
Since Neb suppressed Ang II-induced increases in miR-208a we investigated which Neb-mediated signaling mechanism(s) contributed to this effect. In addition to being a β1-AR blocker, Neb is known to act as an agonist of β3-AR and also to activate endothelial nitric oxide synthase (eNOS) and AMP kinase (27–31). Therefore, it is conceivable that Neb may inhibit miR-208a expression via mechanisms independent of Ang II-induced AT1R activation, such as activation of β3-AR, NOS, or AMP kinase. However, treatment with β3-AR blocker SR59230 (10µM), AMP kinase inhibitor Compound C (10µM) or NOS inhibitor L-NG-Nitroarginine methyl ester (L-NAME) (10µM) failed to reverse the Neb-mediated suppression of Ang II-induced increase in miR-208a expression in HL-1 cardiomyocytes (Fig. 3A). These observations suggest that Neb inhibits Ang II-induced increases in miR-208a by a mechanism that does not involve Neb induced activation of β3-AR-AMK-eNOS pathway.
Fig. 3. Suppression of Ang II mediated miR-208a expression by nebivolol may involve reduction of bioavailable AT1R.
A: qRT-PCR determination of miR-208a expression induced by Ang II that shows that Neb with or without the β3-AR blocker SR59230 (10µM), the AMP kinase inhibitor Compound C (10µM) or the NOS inhibitor L-NG-Nitroarginine methyl ester (L-NAME) (10µM) suppressed Ang II-induced increases in miR-208a expression. N ≥3 for each treatment. qRT-PCR was performed in triplicates for each biological sample. *p < 0.05 for untreated (Con) vs. Ang II treated, or cells treated with Ang II+Neb, Ang II+ Neb + SR59230, Ang II+ Neb+Compound C (Comp C), and Ang II+ Neb+ L-NAME. B: Comparison of 125I-Ang II binding to HL-1 cells subjected to pre-treatments with Neb or losartan or PD123319 for indicated times. Treatments were repeated at least 3 times. For each group of ligand binding, triplicates of each biological sample were used. *p < 0.05 for untreated (Con) vs. Neb treated (12hrs), losartan treated (12 hrs and 30 min samples) and PD123319 treated (12 hrs).
To further understand the mechanism of Neb-mediated suppression of Ang II-induced miR-208a expression, we investigated whether Neb suppressed bioavailable AT1R levels in HL-1 cardiomyocytes that bind 125I-Ang II. When HL-1 cells were pre-treated with either Neb (1µM: 12hrs) or losartan (1µM:12hrs) before a ligand binding experiment was performed, both treatments caused equivalent reductions in 125I-Ang II binding to HL-1 cells (Figure 3B). However, when cells were not pre-treated with Neb or losartan overnight and these drugs were added only 30 minutes prior to the addition 125I-Ang II for the radioligand binding assay, Neb did not suppress 125I-Ang II binding, whereas losartan did. These results suggest that losartan competitively inhibited 125I-Ang II binding because it inhibited 125I-Ang II binding after both preincubation time intervals, but that Neb did not compete for 125I-Ang II binding to HL-1 cells because it did not inhibit 125I-Ang II binding when added 30 minutes before the binding assay. Therefore, Neb reduced 125I-Ang II binding by decreasing bioavailable AT1 receptors.
Effect of Rapamycin treatment on weight gain, fat, lean muscle, and expression levels of cardiac miR-208a and MED13
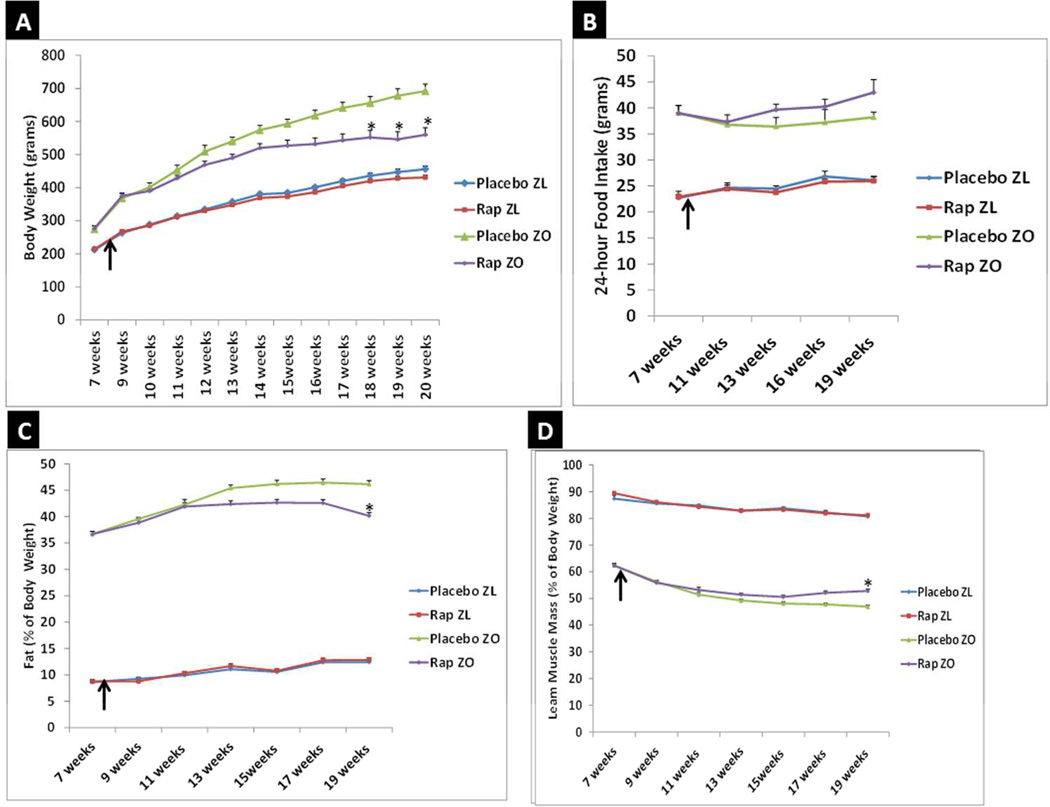
To determine whether Rap-mediated suppression of cardiac miR-208a occurs in an obese rodent model, we investigated the effect of chronic Rap treatment (750µg/kg/day delivered by subcutaneous implantation of Rap pellets) for 12 weeks on ZO rats. ZL rats were used as healthy lean controls and ZL and ZO rats receiving placebo pellets served as controls to distinguish the specific effects of Rap treatment on both ZL and ZO rats. Body weights of ZO rats were significantly different from that of ZL rats (Fig. 4A). ZO rats began to exhibit a reduction in body weight gain after two weeks of Rap treatment and their body weight was statistically significantly less than untreated ZO rats 10–12 weeks. (Fig. 4A). Rap treatment did not significantly affect body weight in ZL rats (Fig. 4A). Weight loss in Rap treated ZO rats was not due to a reduction in food intake (Fig. 4B). EchoMRI analysis showed that Rap treatment significantly lowered fat content in ZO rats (Fig. 4C) and increased their lean muscle mass (Fig. 4D). Rap treatment did not significantly change the fat content or lean muscle mass of ZL rats.
Fig. 4. Rap treatment reduces weight gain and fat content and increases lean muscle mass in leptin resistant ZO rat without reducing food intake.
Graphs show the time course of changes in body weight (A), food intake (B), fat content as percentage of body weight (C) and lean muscle mass as percentage of body weight (D). Arrow marks when Rap treatment was started (8 weeks of age). N=6 animals for all groups until 17th week and N=5 animals for Rap treated ZO for 19th and 20th week. One Rap treated ZO rat died of cardiac arrest in the 18th week. Body weight, food intake, fat content and lean muscle mass of ZO rat was significantly different from Rap treated or untreated ZL rats at all data points (p < 0.05). *p < 0.05 for untreated versus Rap treated ZO rats.
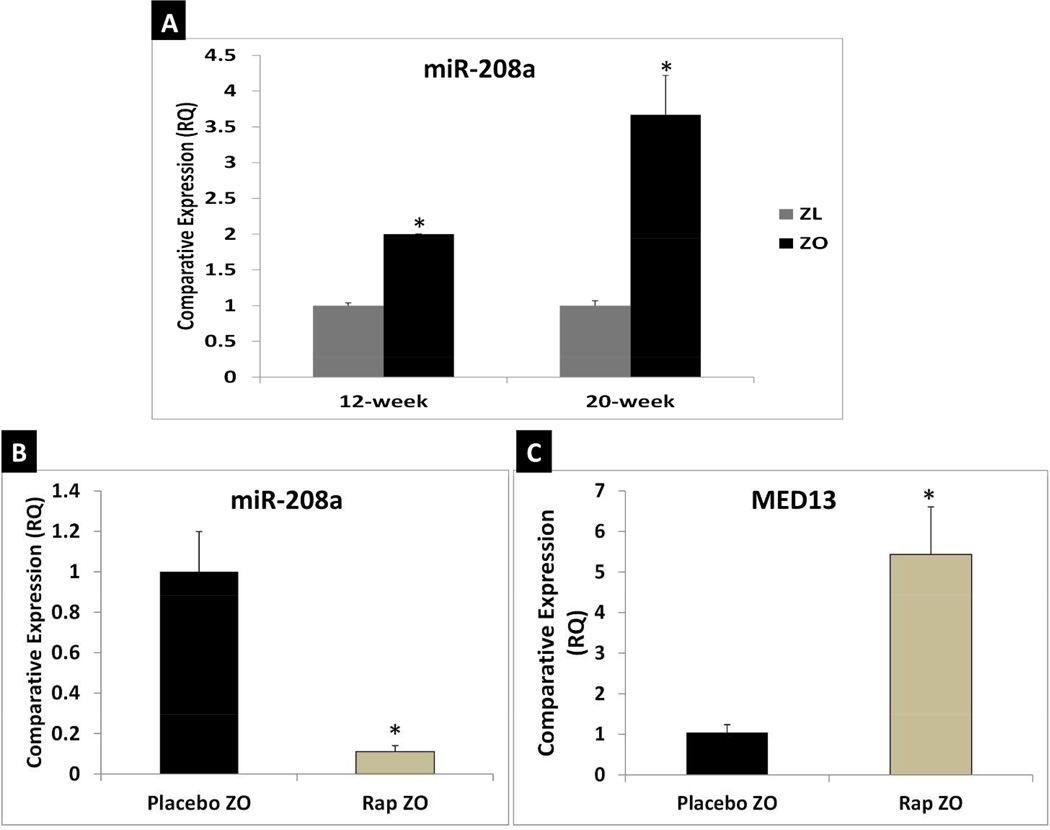
Since cardiac miR-208a is associated with obesity we determined expression levels of cardiac miR-208a in ZO and ZL rats at the age of 12 weeks and 20 weeks(Fig. 5A). Quantitative RT-PCR showed that ZO rats had significantly higher levels of cardiac miR-208a than ZL rats at the age of 12- and 20-weeks (Fig.5A). Next we tested whether Rap treatment could suppress miR-208a expression. As shown in Fig. 5B, Rap treatment suppressed cardiac miR-208a in ZO rats. Therefore, a possible mechanism by which Rap confers resistance to obesity is via suppression of cardiac miR-208a. Previous studies have shown that transgenic overexpression of cardiac MED13 confers resistance to obesity (9). Indeed, Quantitative RT-PCR analysis showed that Rap treatment increased cardiac MED13 mRNA expression levels in ZO rats (Fig. 5C). This is the first report showing concurrent Rap-induced reduction of cardiac miR-208a, increase of cardiac MED13 and reduction of weight gain in an animal model of obesity.
Fig. 5. Rap treatment attenuates cardiac miR-208a expression and increases cardiac MED13 which is known to confer resistance to obesity in ZO rats.
qRT-PCR data that shows ZO rat hearts expressed higher levels of miR-208a at the age of 12-weeks and 20-weeks compared to age-matched ZL rats (A); Rap treatment suppressed miR-208a expression in ZO rat heart (B); and Rap treatment increased MED13 expression in ZO rat heart (C). *p < 0.05 for ZL versus ZO and for untreated versus Rap treated ZO rats.
DISCUSSION
MicroRNA miR-208a serves as a link between cardiac pathology and obesity since mice treated with anti-miR- 208a do not gain weight in response to DIO (9). Given the role of miR-208a in the induction of obesity, it is reasonable to hypothesize that drugs that confer resistance to weight gain in rodent models that are hyperphagic due to deficient signaling of the satiety hormone leptin, may do so by suppressing cardiac miR-208a expression. The modes of action of Rap and Neb differ, Rap directly blocks mTORC1 whereas Neb binds β1-AR and does not directly interact with mTORC1 (15–;17, 28, 29). However, both Neb and Rap induce weight loss in rodent models and humans (14, 15, 19, 20). Rap-induced inhibition of weight gain is attributed to its ability to inhibit the nutrient sensor kinase mTORC1 signaling. Neb is an interesting 3rd generation β1-AR blocker that has pleotropic effects including activation of β3-AR and subsequent activation of AMP kinase that may lead to activation of eNOS (Fig. 6). Moreover, Neb may also function as a GRK/β-arrestin biased agonist of β1-AR (32). We previously observed that Neb suppresses mTORC1 in ZO rat heart (14) however the pathway by which this occurs is still unknown.
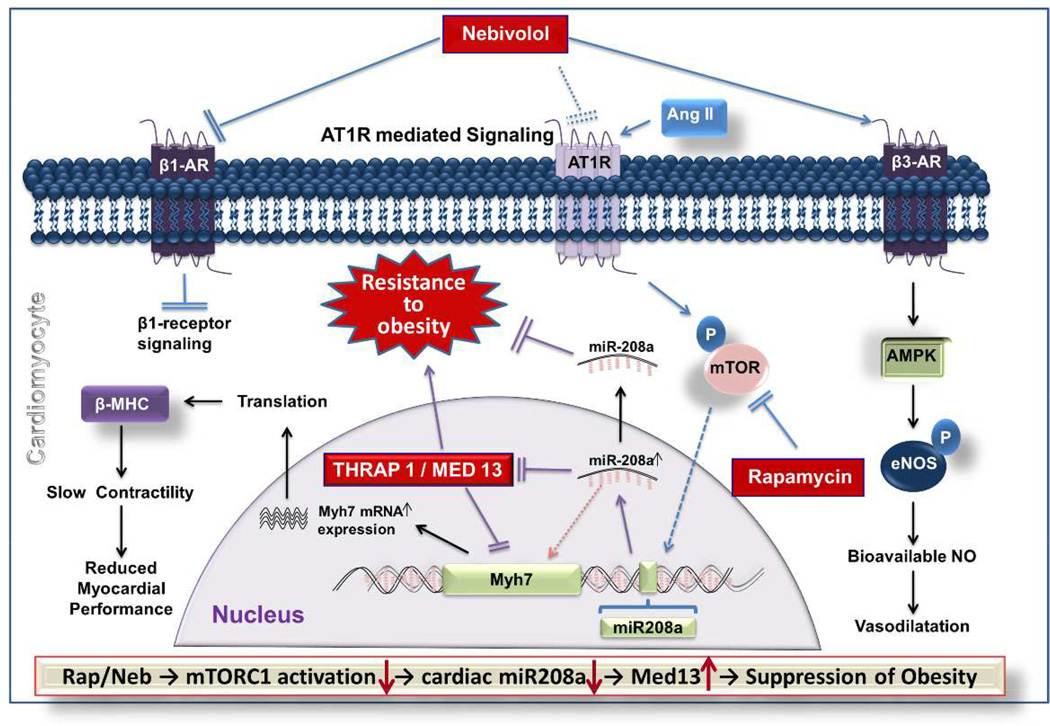
Fig. 6. Schematic diagram showing the regulation of mTORC1-miR-208a signaling axis by Ang II, Neb and Rap in h and its potential implications in the regulation of obesity.
Ang II acting through the AT1R activates the mTORC1-miR-208a signaling axis. Rap inhibits mTORC1 and thereby decreases miR-208a expression. According to the literature, Neb inhibits β1-AR and activates β3-AR. Neb also inhibits mTORC1 and suppresses miR-208a (this study and ref#14). Since inhibition of β3-AR or AMPK did not reverse Neb-induced suppression of Ang II-induced increase in miR-208a expression, we posit that Neb-mediated β3-AR activation is not involved in Neb-mediated suppression of Ang II-induced miR-208a expression. However, Neb suppressed bioavailable AT1R. Since AT1R is needed for Ang II-induced mTORC1 activation and miR-208a expression, this suggests that the Neb-mediated suppression of Ang II-induced miR-208a expression arises from down-regulation of the AT1R. Finally, suppression of miR-208a by Rap mediated inhibition of mTOR leads to up-regulation of MED-13 (also known as THRAP1) which, in turn, can improve energy homeostasis and reduce obesity.
Our observation that Neb and Rap suppress mTORC1 activation and miR-208a expression induced by Ang II in HL-1 cardiomyocytes suggests that both drugs use a common mechanism as part of their mode of action that involves mTORC1 and its ability to promote miR-208a formation. Rap is known to suppress stimulatory phosphorylation of Ser2448 of mTOR (33). Data presented here shows that Neb also suppresses stimulatory phosphorylation of Ser2448 of mTOR. Therefore, signaling activated by Rap and Neb in cardiomyocytes converge at inhibition of stimulatory phosphorylation of Ser2448 of mTOR and subsequent down-stream signaling (stimulatory phosphorylation of S6K1 at Thr389 and RPS6 at Ser235/236). We found that losartan, an AT1R blocker, could inhibit Ang II-induced mTOR phosphorylation and up-regulation of miR-208a expression whereas PD123319, an AT2R antagonist, did not inhibit mTOR phosphorylation and down-stream signaling. Interestingly, both Rap and Neb reduced miR-208a RNA expression more than losartan. This suggests that AT1 receptor signaling is not the sole activator of mTOR in the HL-1 cells. Since mTORC1 inhibition decreases miR-208a formation, it is likely that AT1R-mTORC1 signaling regulates miR-208a (Fig. 6). Antagonism of the β3-AR, inhibition of AMP kinase and NOS inhibition did not reverse Neb-mediated suppression of the Ang II-induced increase in miR-208a in HL-1 cells affirming the lack of β3-AR involvement in Neb’s effect on inhibition of Ang II-induced miR-208a expression. Interestingly, overnight treatment with Neb suppressed 125I-Ang II binding to HL-1 cells. However, a 30-min pre-treatment with Neb did not suppress 125I-Ang II binding to HL-1 cells whereas losartan did. These observations suggest that Neb does not directly compete with 125I-Ang II for binding sites; rather, the prolonged treatment with Neb substantially reduces bioavailable AT1R levels. Whether this involves inhibition of translation or transcription of the AT1R gene, or an impairment of trafficking of the AT1R to the membrane remains to be determined.
Importantly, results presented here show for the first time that in the ZO rat model for obesity, that Rap, an inhibitor of cardiac miR-208a increases cardiac MED13 levels, and confers resistance to obesity without reducing food intake. Previous studies have shown that inhibition of cardiac miR-208a by pharmacological intervention with anti-miR-208a oligonucleotide can confer resistance to obesity (9). Our observation that the cardiac mTORC1-miR-208a-MED13 signaling axis that modulates obesity can be regulated by the widely used drugs, Neb and Rap, expands the potential clinical utility of these drugs. However, given the role of mTORC1 and miR-208a in cardiomyocyte development and cardiac stress response, any approach to suppress this signaling axis must be taken with great caution (18, 34–37).
What is already known about this subject?
Treatment with Rapamycin and Nebivolol attenuates weight gain in humans and rodents.
Rapamycin and Nebivolol have different modes of action since Rapamycin inhibits mTOR Complex 1 formation and Nebivolol is a 3rd generation β1-Adrenergic receptor blocker.
Cardiac miR-208a-MED13 axis regulates systemic energy homeostasis and obesity.
What does this study add?
Modes of action of Rapamycin and Nebivolol converge in suppressing Angiotensin II-induced and AT1Rmediated increase in miR-208a expression in mouse atrial cardiomyocyte HL-1 cells.
In leptin resistant Zucker obese rats, a 12-week Rapamycin treatment suppresses weight gain without reducing food intake, attenuates the expression of the obesity inducer cardiac miR-208a, and increases the expression of cardiac MED-13, a promoter of systemic energy homeostasis.
Rapamycin-mediated regulation of the cardiac miR-208a-MED13 axis is a new mechanism that may explain Rapamycin-mediated suppression of weight gain which is a clinically important phenomenon.
ACKNOWLEDGMENTS
We greatly appreciate technical support from Ms. Lisa Watkinson and Mr. Terry Carmack in animal care and procedures.
Funding: This work was supported, in part, by the Life Science Mission Enhancement Fund from UM-Columbia (LP), NIH NHLBI 1R01HL118376-01 (LP), NHLBI Diversity Supplement awards 3 R01 HL118376-01S (CL), 5 R01 HL118376-02S (KL), Forest Research Institute grant (LP), Missouri foundation for Medical Research Funds (RG and LP), a Pilot Award from the Translational Technologies Component of the Georgetown, Howard Universities Center for Clinical and Translational Science, UL1TR000101 (RCS) and NIH HL-113905 (RCS). This work was also supported by Research Services facilities of HSTM Veterans Hospital.
Footnotes
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.How the world could better fight obesity. McKinsey Global Institute Web site. Available from: http://www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity#sthash.FLlIQ1a7.dpuf.
- 2.Raymond T, Raymond R, Lincoff AM. Management of the patient with diabetes and coronary artery disease: a contemporary review. Future Cardiol. 2013;9:387–403. doi: 10.2217/fca.13.22. [DOI] [PubMed] [Google Scholar]
- 3.Cleland SJ. Cardiovascular risk in double diabetes mellitus--when two worlds collide. Nat Rev Endocrinol. 2012;8:476–485. doi: 10.1038/nrendo.2012.47. [DOI] [PubMed] [Google Scholar]
- 4.Pulakat L, DeMarco VG, Ardhanari S, Chockalingam A, Gul R, Whaley-Connell A, et al. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011 Oct;301(4):R885–R895. doi: 10.1152/ajpregu.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009 Sep;119(9):2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009 Nov;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montgomery RL, Hullinger TG, Semus HM, Dickinson BA, Seto AG, Lynch JM, et al. Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure. Circulation. 2011 Oct 4;124(14):1537–1547. doi: 10.1161/CIRCULATIONAHA.111.030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Nakamura M. Expression of microRNA-208 is associated with adverse clinical outcomes in human dilated cardiomyopathy. J Card Fail. 2010 May;16(5):404–410. doi: 10.1016/j.cardfail.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Grueter CE, van Rooij E, Johnson BA, DeLeon SM, Sutherland LB, Qi X, et al. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012 Apr 27;149(3):671–678. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frishman WH, Saunders E. β-adrenergic blockers. J Clin Hypertens (Greenwich) 2011 Sep;13(9):649–653. doi: 10.1111/j.1751-7176.2011.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginelli P, Bella JN. Treatment of diastolic dysfunction in hypertension. Nutr Metab Cardiovasc Dis. 2012 Aug;22(8):613–618. doi: 10.1016/j.numecd.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 13.Boxall BW, Clark AL. Beta-blockers and weight change in patients with chronic heart failure. J Card Fail. 2012 Mar;18(3):233–237. doi: 10.1016/j.cardfail.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Gul R, Demarco VG, Sowers JR, Whaley-Connell A, Pulakat L. Regulation of overnutrition-induced cardiac inflammatory mechanisms. Cardiorenal Med. 2012 Aug;2(3):225–233. doi: 10.1159/000339565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladage D, Reidenbach C, Rieckeheer E, Graf C, Schwinger RH, Brixius K. Nebivolol lowers blood pressure and increases weight loss in patients with hypertension and diabetes in regard to age. J Cardiovasc Pharmacol. 2010 Sep;56(3):275–281. doi: 10.1097/FJC.0b013e3181eb4ff2. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010 Oct 22;40(2):310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011 Jan;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold N, Koppula PR, Gul R, Luck C, Pulakat L. Regulation of cardiac expression of the diabetic marker microRNA miR-29. PLoS One. 2014 Jul 25;9(7):e103284. doi: 10.1371/journal.pone.0103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012 Aug 9;75(3):425–436. doi: 10.1016/j.neuron.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012 Apr;165(7):2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiarini F, Evangelisti C, McCubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015 Feb;36(2):124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013 Mar;123(3):980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White SM, Constantin PE, Claycomb WC. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–H829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 24.Matute C, Pulakat L, Rio C, Valcarcel C, Miledi R. Properties of angiotensin II receptors in glial cells from the adult corpus callosum. Proc Natl Acad Sci USA. 1994;91(9):3774–3778. doi: 10.1073/pnas.91.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter WM, Greenwood FC. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- 26.Speth RC, Harding JW. Radiolabeling of angiotensin peptides. In: Wang DH, editor. Methods in Molecular Medicine: Angiotensin Protocols. Vol. 51. Totowa NJ: Humana Press; 2001. pp. 275–295. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro RF, Jr, Potratz FF, Pavan BM, Forechi L, Lima FL, Fiorim J, et al. Carvedilol prevents ovariectomy-induced myocardial contractile dysfunction in female rat. PLoS One. 2013;8(1):e53226. doi: 10.1371/journal.pone.0053226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Y, Vanhoutte PM. Nebivolol: an endothelium-friendly selective β1-adrenoceptor blocker. J Cardiovasc Pharmacol. 2012 Jan;59(1):16–21. doi: 10.1097/FJC.0b013e3182073e27. [DOI] [PubMed] [Google Scholar]
- 29.Maffei A, Di Pardo A, Carangi R, Carullo P, Poulet R, Gentile MT, et al. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodis J, Vaclavikova R, Farghali H. Beta-3 agonist-induced lipolysis and nitric oxide production: relationship to PPARgamma agonist/antagonist and AMP kinase modulation. Gen Physiol Biophys. 2011;30:90–99. doi: 10.4149/gpb_2011_01_90. [DOI] [PubMed] [Google Scholar]
- 32.Erickson CE, Gul R, Blessing CP, Nguyen J, Liu T, Pulakat L, et al. The β-blocker nebivolol is a GRK/β-arrestin biased agonist. PLoS One. 2013 Aug 20;8(8):e71980. doi: 10.1371/journal.pone.0071980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem. 2005 Jul 8;280(27):25485–25490. doi: 10.1074/jbc.M501707200. [DOI] [PubMed] [Google Scholar]
- 34.Fonarow GC. Diabetes medications and heart failure: recognizing the risk. Circulation. 2014 Oct 28;130(18):1565–1567. doi: 10.1161/CIRCULATIONAHA.114.012883. [DOI] [PubMed] [Google Scholar]
- 35.Yao H, Han X, Han X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am J Cardiovasc Drugs. 2014 Dec;14(6):433–442. doi: 10.1007/s40256-014-0089-9. [DOI] [PubMed] [Google Scholar]
- 36.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014 Jan 31;114(3):549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubli DA, Gustafsson AB. Cardiomyocyte health: adapting to metabolic changes through autophagy. Trends Endocrinol Metab. 2014 Mar;25(3):156–164. doi: 10.1016/j.tem.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]