Abstract
Background
Comorbid diabetes and substance use diagnoses (SUD) represent a hazardous combination, both in terms of healthcare cost and morbidity. To date, there is limited information about the association of SUD and related mental disorders with type 2 diabetes mellitus (T2DM).
Methods
We examined the associations between T2DM and multiple psychiatric diagnosis categories, with a focus on SUD and related psychiatric comorbidities among adults with T2DM. We analyzed electronic health record (EHR) data on 170,853 unique adults aged ≥18 years from the EHR warehouse of a large academic healthcare system. Logistic regression analyses were conducted to estimate the strength of an association for comorbidities.
Results
Overall, 9% of adults (n=16,243) had T2DM. Blacks, Hispanics, Asians, and Native Americans had greater odds of having T2DM than whites. All 10 psychiatric diagnosis categories were more prevalent among adults with T2DM than among those without T2DM. Prevalent diagnoses among adults with T2MD were mood (21.22%), SUD (17.02%: tobacco 13.25%, alcohol 4.00%, drugs 4.22%), and anxiety diagnoses (13.98%). Among adults with T2DM, SUD was positively associated with mood, anxiety, personality, somatic, and schizophrenia diagnoses.
Conclusions
We examined a large diverse sample of individuals and found clinical evidence of SUD and psychiatric comorbidities among adults with T2DM. These results highlight the need to identify feasible collaborative care models for adults with T2DM and SUD related psychiatric comorbidities, particularly in primary care settings, that will improve behavioral health and reduce health risk.
Keywords: anxiety disorder, comorbidity, diabetes mellitus, electronic health records, mood disorder, substance use disorder
1. INTRODUCTION
The prevalence of diagnosed diabetes among United States (U.S.) adults aged ≥18 years increased steadily from 3.5% in 1980 to 9.0% in 2011 (CDC, 2014a). Currently, an estimated 29.1 million Americans have diabetes (ADA, 2015). Type 2 diabetes mellitus (T2DM) accounts for 90–95% of individuals with diabetes (ADA, 2014). In the U.S., individuals with diagnosed diabetes have, on average, medical expenditures 2.3 times higher than those without diabetes (ADA, 2013). The total estimated cost of diagnosed diabetes (type 1 or 2) was $245 billion in 2012 (a 41% increase from a prior estimate in 2007), which included $176 billion in direct medical costs and $69 billion in increased absenteeism and reduced productivity (ADA, 2013). The economic burden is expected to escalate as the prevalence of T2DM continues to rise. Increasing costs will be particularly driven by adults with multiple chronic conditions, including substance use disorders (SUDs; Ashman et al., 2014; Jiang et al., 2014). Having diabetes plus SUD (and/or other psychiatric disorders) represents a hazardous combination, both in terms of healthcare cost and morbidity. SUD and mental health comorbidity can compromise patients’ adherence to treatment for T2DM, exacerbate existing medical conditions, and heighten the risk for premature mortality among individuals with diabetes (Ducat et al., 2014; Ghitza et al., 2013; Vinogradova et al., 2010). Notably, inpatient care constitutes the major part of medical expenditures for diabetes (ADA, 2013). In the nonelderly Medicaid population (<65 years), individuals with either a diabetes diagnosis with complications, SUD, or a mental disorder (mood, schizophrenia, psychotic) use more inpatient care than patients without such diagnoses (Jiang et al., 2014). Diabetes, SUD, and mental diagnoses also are among the leading medical conditions associated with elevated 30-day hospital readmission rates (Jiang and Wier, 2010).
The fact that some antipsychotics and antidepressants may induce metabolic side effects or significant weight gain is one reason for the co-comorbidity of mood/anxiety disorders and schizophrenia with T2DM (De Hert et al., 2009; Nielsen et al., 2010; Sharma et al., 2014). However, there are limited data about SUD and mental diagnoses other than mood/anxiety and schizophrenia diagnoses among adults with T2DM. Self-reported diabetes data from community sample surveys are limited by a non-specific question about diabetic history, which may not be equivalent to patients in real-world clinical settings who have medical documentation of a diabetic diagnosis. Specifically, cigarette smoking is associated with T2DM in a dose-dependent manner, and it constitutes a risk factor for diabetes-related complications and premature mortality for many diseases (cardiovascular, pulmonary conditions; Ghitza et al., 2013; Willi et al., 2007). Therefore, quantifying the prevalence of nicotine dependence is critical for people with diabetes (Willi et al., 2007). Survey data from the Behavioral Risk Factor Surveillance System estimated that 15.4% of adults aged ≥18 years with self-reported diabetes (“Have you ever been told by a doctor that you have diabetes?”) were current cigarette smokers (Fan et al., 2013); however, the extent of tobacco use disorder among adults with T2DM is unclear.
Similarly, data is lacking on the prevalence of alcohol and (illicit) drug use disorders in medical settings to inform SUD screening and treatment for adults with T2DM (Ghitza et al., 2013). While low-to-moderate alcohol use is associated with decreased odds of having T2DM, binge or heavy alcohol use increases the risk of T2DM (Baliunas et al., 2009; Pietraszek et al., 2010), demonstrating the need to identify and treat alcohol use diagnoses among individuals with diabetes. A prior study of 65,996 adults with diabetes who received care through Kaiser Permanente Northern California and responded to a survey of alcohol use indicated that 51% of adults with diabetes reported current alcohol use (Ahmed et al., 2006). A greater number of drinks per day was associated with a decreased probability of complying with diabetes care. Another study of male outpatients with diabetes (n=3,930) from 7 Veterans Affairs sites suggested that 13% of adults with diabetes had alcohol use problems (Alcohol Use Disorders Identification Test-Consumption [AUDIT-C] score ≥4) and that higher AUDIT-C scores were associated with poorer diabetes self-care (Thomas et al., 2006). Both studies of adults with diabetes in medical settings indicate that problematic or frequent alcohol use can impair diabetes self-care behaviors, yet alcohol use diagnosis data were not available (Ahmed et al., 2006; Thomas et al., 2006). Chronic misuse of illicit or nonmedical psychoactive drugs may also increase psychiatric problems, worsen medical sequelae of diabetes, or complicate diabetes self-care (Brick, 2004; Leung et al., 2011a,b; Volkow et al., 2014). Reliable estimates of drug use disorders by T2DM status are lacking, but are needed to inform targeted screening and interventions.
Taken together, the heavy economic burden associated with diabetes is disproportionally influenced by individuals with comorbid diabetes and SUD disorders, particularly those with both SUD and mental disorders (ADA, 2013; Ghitza et al., 2013; Jiang et al., 2014). Given the lack of information on patterns of comorbidity, we leveraged data from an electronic health record (EHR) warehouse to determine the extent of SUD and related psychiatric comorbidities by T2DM status. Since <1% of young people aged <20 years have diagnosed diabetes (CDC, 2014b), we focused on T2DM in adults. We examined the prevalence of T2DM and determined associations of psychiatric diagnoses (alcohol, tobacco, drug, schizophrenia/psychotic, mood, anxiety, personality, somatic, and disruptive behavioral disorder diagnoses) with T2DM status. Among adults with T2DM, we examined associations of SUD with mental diagnoses, in order to gauge multi-comorbidity. To control for age-related increases in medical problems, we stratified the analyses by age group.
2. METHODS
2.1. Data sources
We analyzed the EHR data of unique adults aged ≥18 years from the Duke Medicine Enterprise Data Warehouse (EDW) between January 1, 2007 and December 31, 2011 (i.e., patients were ≥18 years as of January 1, 2007). The primary group of interest was comprised of patients with a T2DM diagnosis (n=16,243); this group was compared with patients without T2DM (n=154,610). Patients aged ≥18 years with type 1 diabetes (ICD-9-CM diagnosis codes) were excluded from the analysis (n=2,650), resulting in a sample of 170,853 patients. Briefly, the EHR dataset for this analysis was identified and developed for the Durham Diabetes Coalition project, which leverages EHR data to inform community-based interventions that seek to improve population-level diabetes management, health outcomes, and quality of life for adults living with T2DM in Durham County (Spratt et al., 2015). A geographic health information system was employed to link the EHR and patients’ social and environmental data in order to provide a multidimensional understanding of environmental contexts and vulnerabilities for adults living with T2DM in Durham, North Carolina, and to develop tailored community-based interventions. An estimated 80% of Durham County residents received care from a Duke Medicine provider at some point during the interval of 2007–2011. Durham County is located in the Central Piedmont region of North Carolina. Compared with the overall U.S. population, Durham County has a higher proportion of the “Black/African American alone” population (13.2% vs. 38.7%) and lower proportions of the “White alone” (77.7% vs. 53.1%) and “Hispanic/Latino” populations (17.1% vs. 13.5%) (U.S. Census Bureau, 2015).
2.2. Study variables
Demographic variables included age (as of January 1, 2007), sex, patient-identified race (white or Caucasian, American Indian or Alaska Native, Asian, black or African American, multiracial, Native Hawaiian or Pacific Islander, other, declined, or unavailable), and patient-identified ethnicity (Hispanic or non-Hispanic). Diagnostic variables were based on ICD-9-CM codes (CMS, 2008). Common conditions that tend to be associated with diabetes, including chronic obstructive pulmonary disease (COPD), hypertensive disease, ischemic heart disease, and renal disease (nephritis, nephrotic syndrome, nephrosis), were included as control variables for the analysis of comorbidity (Heron, 2013). We also controlled for the overall number of healthcare encounters (outpatient, inpatient, emergency department) during 2007–2011 to mitigate the confounding effects of healthcare utilization, since those who use healthcare frequently may have an increased diagnostic probability (Jiang and Wier, 2010).
We used the published crosswalk of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) codes to ICD-9-CM codes from the American Psychological Association (APA) Practice Organization to define psychiatric diagnoses that are consistent with DSM-IV-TR categories (APA, 2002). The crosswalk lists each specific ICD-9-CM diagnosis code corresponding to a DSM-IV-TR diagnosis code. Psychiatric disorder diagnoses included alcohol use, tobacco use, drug use (cannabis, amphetamines, cocaine, hallucinogens, inhalants, opioids, phencyclidine, sedatives, hypnotics, or anxiolytics), schizophrenia or psychotic mood (major depressive, bipolar, manic, manic-depressive), anxiety (panic, generalized anxiety, phobia, obsessive-compulsive, posttraumatic stress), personality (paranoid, affective, chronic hypomanic, chronic depressive, schizoid, introverted, schizotypal, explosive, compulsive, histrionic, dependent, antisocial, narcissistic, avoidant, borderline, passive-aggressive), somatic (somatization, conversion, hypochondriasis, psychogenic pain-site unspecified), and disruptive behavioral disorder (conduct, impulse-control) diagnoses (APA, 2002). A patient was considered to have a given diagnosis if an ICD-9-CM code for that condition was found in the list of discharge or final diagnosis codes for any type of encounter (inpatient, outpatient) at least once during 2007–2011.
2.3. Statistical analysis
We examined frequencies for study variables by T2DM status and used logistic regression analyses to determine associations of demographics, medical variables, number of healthcare encounters, and inpatient treatment with T2DM status. We then examined the prevalence of psychiatric diagnoses by T2DM status. To control for age difference in health status, we stratified the analysis by age group. We conducted logistic regression analyses to determine associations of each psychiatric diagnosis with T2DM, adjusting for age, sex, race, ethnicity, and number of healthcare encounters. Finally, among patients with T2DM, we conducted logistic regression analyses to determine the association of SUD with psychiatric diagnoses, adjusting for age, sex, race, ethnicity, COPD, hypertensive disease, ischemic heart disease, renal disease, and number of healthcare encounters. We report 95% confidence intervals (CIs) for prevalence and adjusted odds ratio (OR) estimates. All analyses were performed using SAS® software, version 9.2 (SAS Institute, Inc., Cary, NC).
3. RESULTS
3.1. Demographics
Of 170,853 adults aged 18–90 years, 57.5% were female, 11.5% were older (aged 65–90 years), 34.9% were black, and 6.7% were Hispanic.
3.2. Factors associated with T2DM (Table 1)
Table 1.
Characteristics of adults aged 18 or older by T2DMa
| Variables | Overall n=170,853 | With T2DM n=16,243 | Without T2DM n=154,610 | Adjusted OR of T2DM (95% CI) |
|---|---|---|---|---|
| Age (overall)b | 1.22 (1.20, 1.23) | |||
| Mean (SD) | 41.6 (17.4) %c | 56.6 (15.6) %d | 40.0 (16.8) %d | |
| Age groups, years | ||||
| 18 to 35 | 76297 (44.7%) | 1586 (2.1%) | 74711 (97.9%) | 1.00 |
| 36 to 50 | 44514 (26.1%) | 4016 (9.0%) | 40498 (91.0%) | 2.24 (2.10, 2.39) |
| 51 to 64 | 30441 (17.8%) | 5638 (18.5%) | 24803 (81.5%) | 3.41 (3.19, 3.64) |
| 65 and older | 19601 (11.5%) | 5003 (25.5%) | 14598 (74.5%) | 3.23 (3.01, 3.47) |
| Sex | ||||
| Female | 98178 (57.5%) | 9130 (9.3%) | 89048 (90.7%) | 1.00 |
| Male | 72591 (42.5%) | 7113 (9.8%) | 65478 (90.2%) | 1.24 (1.20, 1.29) |
| Unknown | 83 (0.0%) | 0 (0.0%) | 83 (100.0%) | |
| Race | ||||
| Alaskan Native or American Indian | 587 (0.3%) | 51 (8.7%) | 536 (91.3%) | 2.43 (1.75, 3.37) |
| Asian | 6115 (3.6%) | 269 (4.4%) | 5846 (95.6%) | 1.50 (1.31, 1.73) |
| Black or African American | 59552 (34.9%) | 8705 (14.6%) | 50847 (85.4%) | 2.07 (1.98, 2.15) |
| Multiracial | 1179 (0.7%) | 75 (6.4%) | 1104 (93.6%) | 2.12 (1.63, 2.76) |
| Others/unknown | 21553 (12.6%) | 870 (4.0%) | 20683 (96.0%) | 1.56 (1.41, 1.73) |
| White or Caucasian | 81867 (47.9%) | 6273 (7.7%) | 75594 (92.3%) | 1.00 |
| Ethnicity | ||||
| Hispanic | 11503 (6.7%) | 545 (4.7%) | 10958 (95.3%) | 1.62 (1.43, 1.84) |
| Non-Hispanic | 159350 (93.3%) | 15698 (9.9%) | 143652 (90.1%) | 1.00 |
| Medical diagnosis, yes | ||||
| COPD | 16120 (9.4%) | 3472 (21.5%) | 12648 (78.5%) | 1.17 (1.11, 1.22) |
| Hypertensive disease | 46496 (27.2%) | 13365 (28.7%) | 33131 (71.3%) | 6.94 (6.59, 7.31) |
| Ischemic heart disease | 10108 (5.9%) | 3908 (38.7%) | 6200 (61.3%) | 1.51 (1.43, 1.59) |
| Renal diseasee | 8657 (5.1%) | 3612 (41.7%) | 5045 (58.3%) | 1.58 (1.50, 1.67) |
| Log base 10 of the number of encountersf | ||||
| Median (25th, 75th) | 8.0 (3.0, 24.0) | 28.0 (11.0, 56.0) | 7.0 (2.0, 20.0) | 1.86 (1.79, 1.93) |
Note: All ORs, with the exception of those for the age group variable, were derived from this logistic model: T2DM as predicted by continuous age, sex, race, ethnicity, individual comorbidities, and log base 10 of the number of patient encounters. A separate model used to calculate ORs for the age group variable was the same as above, with the exception that age group was substituted for continuous age.
Population excludes patients who have type 1 diabetes.
Reference for computation of OR is 10-year increase in age.
Proportions are within the column.
Proportions are across rows.
Renal disease: nephritis, nephrotic syndrome, or nephrosis.
Base 10 log transformation was applied to the number of healthcare encounters due to skewness.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Overall, 9% (n=16,243) of the sample had T2DM. Prevalence of T2DM increased with age strata (18.5%, ages 51–64; 25.5%, ages 65–90 years), was higher in men (9.8% vs. 9.3% in women) and black adults (14.6% vs. 7.7% in whites), and was elevated among adults with COPD (21.5%), hypertensive disease (28.7%), ischemic heart disease (38.7%), and renal disease (41.7%).
In the adjusted logistic regression model—which included age, sex, race, ethnicity, COPD, hypertensive disease, ischemic heart disease, renal disease, and number of encounters—all nonwhite groups had greater odds than whites of having T2DM; each medical diagnosis and number of encounters were associated with increased odds of having T2DM.
3.3. Prevalence of psychiatric diagnoses by T2DM (Table 2)
Table 2.
Prevalence of psychiatric conditions by T2DM (percent and 95% CI)
| Psychiatric diagnosis, prevalence | T2DM | Overall | Aged 18 to 35 | Aged 36 to 50 % (95% CI) | Aged 51 to 64 | Aged 65 and older |
|---|---|---|---|---|---|---|
| Alcohol | Yes | 4.00 (3.70–4.31) | 3.66 (2.79–4.70) | 5.85 (5.15–6.62) | 4.35 (3.83–4.91) | 2.22 (1.83–2.67) |
| No | 2.27 (2.20–2.35) | 1.57 (1.48–1.66) | 3.18 (3.01–3.36) | 3.18 (2.96–3.40) | 1.83 (1.62–2.06) | |
| Tobacco | Yes | 13.25 (12.74–13.79) | 11.35 (9.83–13.01) | 17.43 (16.27–18.64) | 15.36 (14.43–16.33) | 8.14 (7.39–8.93) |
| No | 5.58 (5.47–5.70) | 3.21 (3.08–3.34) | 7.67 (7.41–7.93) | 8.97 (8.62–9.33) | 6.19 (5.81–6.60) | |
| Drugs | Yes | 4.22 (3.91–4.54) | 5.93 (4.82–7.20) | 6.60 (5.85–7.41) | 3.57 (3.10–4.08) | 2.50 (2.08–2.97) |
| No | 2.07 (2.00–2.14) | 1.80 (1.71–1.90) | 2.98 (2.81–3.15) | 1.73 (1.57–1.90) | 1.47 (1.28–1.68) | |
| Any substance | Yes | 17.02 (16.44–17.60) | 16.14 (14.36–18.05) | 21.74 (20.47–23.05) | 18.84 (17.82–19.88) | 11.45 (10.58–12.37) |
| No | 7.96 (7.83–8.10) | 5.41 (5.25–5.58) | 10.53 (10.24–10.84) | 11.19 (10.80–11.59) | 8.38 (7.93–8.84) | |
| Schizophrenia or psychotic | Yes | 3.38 (3.11–3.67) | 3.97 (3.07–5.05) | 3.49 (2.94–4.10) | 2.66 (2.26–3.11) | 3.92 (3.40–4.49) |
| No | 0.98 (0.93–1.03) | 0.65 (0.59–0.71) | 0.97 (0.87–1.07) | 1.15 (1.02–1.29) | 2.44 (2.19–2.70) | |
| Mood | Yes | 21.22 (20.59–21.86) | 19.67 (17.74–21.72) | 22.61 (21.32–23.94) | 22.24 (21.16–23.35) | 19.45 (18.36–20.57) |
| No | 9.55 (9.40–9.69) | 6.41 (6.24–6.59) | 11.29 (10.98–11.60) | 12.93 (12.51–13.35) | 15.00 (14.43–15.59) | |
| Anxiety | Yes | 13.98 (13.45–14.52) | 11.79 (10.24–13.48) | 14.64 (13.56–15.77) | 14.37 (13.46–15.31) | 13.71 (12.77–14.70) |
| No | 7.53 (7.39–7.66) | 5.64 (5.47–5.81) | 8.44 (8.17–8.71) | 9.74 (9.38–10.12) | 10.88 (10.38–11.39) | |
| Personality | Yes | 0.91 (0.77–1.07) | 1.70 (1.12–2.47) | 1.54 (1.19–1.97) | 0.71 (0.51–0.96) | 0.38 (0.23–0.59) |
| No | 0.36 (0.33–0.39) | 0.34 (0.30–0.38) | 0.47 (0.41–0.55) | 0.30 (0.23–0.37) | 0.27 (0.19–0.37) | |
| Somatic | Yes | 0.44 (0.34–0.55) | 0.76 (0.39–1.32) | 0.72 (0.48–1.04) | 0.39 (0.24–0.59) | 0.16 (0.07–0.31) |
| No | 0.18 (0.16–0.20) | 0.11 (0.09–0.14) | 0.23 (0.19–0.29) | 0.24 (0.18–0.31) | 0.21 (0.14–0.30) | |
| Disruptive behavioral | Yes | 0.16 (0.10–0.23) | 0.50 (0.22–0.99) | 0.27 (0.14–0.49) | 0.11 (0.04–0.23) | 0.02 (0.00–0.11) |
| No | 0.06 (0.05–0.07) | 0.07 (0.05–0.09) | 0.07 (0.05–0.11) | 0.04 (0.02–0.07) | 0 | |
| None of the above | Yes | 62.64 (61.89–63.38) | 66.08 (63.69–68.41) | 60.13 (58.60–61.65) | 60.77 (59.48–62.04) | 65.66 (64.33–66.98) |
| No | 81.07 (80.88–81.27) | 86.57 (86.32–86.81) | 77.80 (77.39–78.20) | 74.75 (74.20–75.29) | 72.79 (72.06–73.51) |
Note: Due to small patient counts in several cells, all reported confidence intervals are exact.
All abbreviations can be found in Table 1.
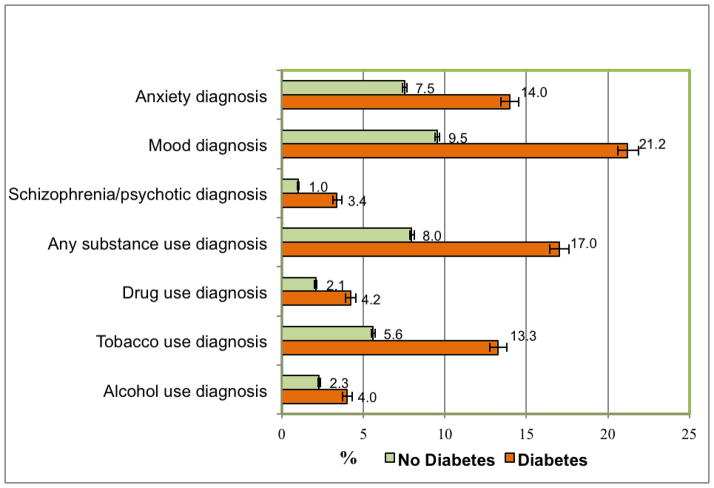
In the overall sample (n=170,853), all of the psychiatric diagnosis categories examined were more prevalent among adults with T2DM than among those without (Fig. 1). Overall, 37.36% of adults with T2DM had a psychiatric diagnosis compared with 18.93% of adults without T2DM. Prevalent diagnoses among adults with T2DM were mood (21.22%), SUD (any SUD 17.02%: tobacco 13.25%, alcohol 4.00%, illicit drugs 4.22%), and anxiety diagnoses (13.98%), followed by schizophrenia/psychotic (3.38%) and other psychiatric diagnoses (<1%).
Figure 1. Prevalence of psychiatric conditions.
This figure displays the prevalence of psychiatric conditions among adults aged 18 or older, by T2DM (percent and 95% CI)
Abbreviations: CI, confidence interval; T2DM, type 2 diabetes
3.4. Adjusted OR of psychiatric diagnoses by T2DM (Table 3)
Table 3.
Adjusted logistic regression analysis of psychiatric diagnosis in relation to T2DM
| Psychiatric diagnosis | Overall (n=170,853) | Aged 18 to 35 (n=76,297) | Aged 36 to 50 (n=44,514) | Aged 51 to 64 (n=30,441) | Aged 65 and older (n=19,601) |
|---|---|---|---|---|---|
|
| |||||
| Adjusted OR (95% CI) | |||||
| Alcohol | 1.041 (0.950–1.143) | 1.488 (1.124–1.970) | 1.110 (0.954–1.292) | 0.923 (0.791–1.078) | 0.906 (0.718–1.143) |
| Tobacco | 1.226 (1.160–1.296) | 1.425 (1.199–1.694) | 1.413 (1.283–1.555) | 1.170 (1.069–1.280) | 1.041 (0.916–1.182) |
| Drugs | 1.217 (1.109–1.335) | 1.375 (1.095–1.728) | 1.156 (0.999–1.338) | 1.272 (1.062–1.523) | 1.445 (1.147–1.820) |
| Any substance (alcohol, tobacco, or drugs) | 1.236 (1.176–1.299) | 1.488 (1.284–1.724) | 1.349 (1.236–1.472) | 1.195 (1.099–1.298) | 1.124 (1.007–1.254) |
| Schizophrenia or psychotic | 1.673 (1.500–1.866) | 2.731 (2.051–3.638) | 1.993 (1.619–2.454) | 1.603 (1.297–1.980) | 1.498 (1.246–1.802) |
| Mood | 1.364 (1.301–1.429) | 1.607 (1.397–1.848) | 1.465 (1.339–1.603) | 1.429 (1.317–1.549) | 1.238 (1.133–1.354) |
| Anxiety | 1.127 (1.068–1.190) | 1.094 (0.925–1.293) | 1.186 (1.069–1.316) | 1.087 (0.990–1.194) | 1.126 (1.016–1.248) |
| Personality | 1.780 (1.455–2.176) | 2.057 (1.345–3.145) | 1.838 (1.353–2.496) | 1.959 (1.303–2.943) | 1.439 (0.815–2.540) |
| Somatic | 1.070 (0.807–1.417) | 1.827 (0.961–3.476) | 1.280 (0.827–1.983) | 0.885 (0.529–1.478) | 0.607 (0.273–1.350) |
| Disruptive behavioral | 1.771 (1.094–2.869) | 2.615 (1.170–5.845) | 1.465 (0.713–3.010) | 1.449 (0.500–4.199) | ……. |
Note: For all age category columns, the following model was used to calculate ORs and CIs: psychiatric condition as predicted by T2DM, continuous age, sex, race, ethnicity, and log base 10 of the number of patient encounters.
The race variable in the model consists of these collapsed values: black, white, and other. For the overall column, the same model as above was used to calculate ORs and CIs, with the addition of stratification by age category.
All abbreviations can be found in Table 1.
We conducted an adjusted logistic regression analysis to determine the strength of the association between T2DM and each psychiatric diagnosis, while controlling for age, sex, race, ethnicity, and number of encounters. Except for alcohol use and somatic diagnoses, T2DM was associated with elevated odds of having each of the other psychiatric categories in the overall sample (n=170,853). We also stratified the analysis by age, and a similar pattern was observed across age groups. Additionally, T2DM was associated with elevated odds of having alcohol use disorder in the 18–35 age group.
3.5. Adjusted OR of comorbid SUD and psychiatric diagnoses among adults with T2DM (Table 4)
Table 4.
Adjusted logistic regression model of psychiatric disorder in relation to substance use disorder diagnosis among adults with a T2DMa diagnosis
| Psychiatric diagnosis | Overall n=16,243 | Aged 18 to 35 n=1,586 | Aged 36 to 50 n=4,016 | Aged 51 to 64 n=5,638 | Aged 65 and older n=5,003 |
|---|---|---|---|---|---|
|
| |||||
| Adjusted OR (95% CI) | |||||
| Schizophrenia or psychotic | 2.975 (2.454–3.607) | 3.774 (2.160–6.597) | 2.735 (1.904–3.928) | 4.496 (3.141–6.437) | 1.993 (1.345–2.953) |
| Mood | 2.229 (2.016–2.465) | 2.546 (1.814–3.573) | 3.052 (2.522–3.694) | 2.084 (1.767–2.458) | 1.638 (1.324–2.026) |
| Anxiety | 1.857 (1.656–2.081) | 1.977 (1.324–2.952) | 1.960 (1.583–2.428) | 1.638 (1.357–1.978) | 1.974 (1.565–2.490) |
| Personality | 3.649 (2.559–5.203) | 7.836 (3.046–20.158) | 3.726 (2.144–6.475) | 2.571 (1.291–5.118) | 2.031 (0.707–5.835) |
| Somatic | 2.831 (1.707–4.694) | 6.675 (1.666–26.750) | 2.474 (1.134–5.400) | 2.141 (0.864–5.306) | 2.542 (0.529–12.212) |
| Disruptive behavioral | 4.682 (2.033–10.782) | 3.915 (0.803–19.075) | 8.286 (2.097–32.739) | 2.888 (0.505–16.512) | ……. |
Analysis sample: patients had a T2DM diagnosis.
Note: For all age category columns, the following model was used to calculate ORs and CIs: psychiatric condition as predicted by any substance use diagnosis, continuous age, sex, race, ethnicity, COPD, hypertensive disease, ischemic heart disease, renal disease, and log base 10 of the number of patient encounters. The race variable in the model consists of these collapsed values: black, white, and other. For the overall column, the same model as above was used to calculate ORs and CIs, with the addition of stratification by age category. All reported ORs are for the variable “any substance use diagnosis” (alcohol, tobacco, or drug use).
All abbreviations can be found in Table 1.
Finally, we conducted adjusted logistic regression analyses of adults with T2DM (n=16,243) to determine the strength of the association between SUD and each of the mental diagnoses, respectively, while controlling for age, sex, race, ethnicity, COPD, hypertensive disease, ischemic heart disease, renal disease, and number of encounters. An SUD diagnosis was positively associated with having schizophrenia/psychotic, mood, anxiety, personality, somatic, and disruptive behavioral diagnoses, respectively. A similar pattern was found when the analysis was stratified by age group.
4. DISCUSSION
This analysis of EHR data from 170,853 adult patients provides important clinical evidence of the high prevalence of SUD and other psychiatric comorbidities in adults with T2DM. First, all non-white groups had higher odds of T2DM than whites. Second, all psychiatric diagnosis categories that we examined were found to be more prevalent among adults with T2DM than among those without T2DM; a similar pattern (except for somatic diagnosis) was identified by adjusted logistic regression analyses. Third, among adults with T2DM, SUD was positively associated with schizophrenia, mood, anxiety, personality, somatic, and disruptive behavioral disorders, respectively, indicating multi-comorbidity. These findings increase our understanding of the prevalence of SUD and related psychiatric comorbidities by T2DM status, and have implications for SUD, psychiatric screening, and the development of collaborative care models to improve health outcomes.
4.1. What this study adds to our knowledge
Prior research on mental health conditions in patients with diabetes has mainly focused on depression or anxiety, and used self-reported psychiatric and medical status derived from survey questions answered by the sampled participants (Roy and Lloyd, 2012; Smith et al., 2013). SUDs are among the leading conditions contributing to high rates of hospital readmissions (Jiang and Wier, 2010), but little is known about SUD prevalence and SUD-related comorbidity in adults with T2DM. Due to health care reform (e.g., the 2010 Affordable Care Act and the Mental Health Parity and Addiction Equity Act of 2008), SUD treatment services are considered an essential health benefit, and the development of integrated behavioral (especially SUDs) and physical care models to improve behavioral health care in primary care has become a priority (Tai and Volkow, 2013). The use of patients’ medical records data from EHRs to aide data collection and monitor clinical outcomes is recognized as a fundamental element in practical clinical research for developing learning healthcare systems (IOM, 2010). The Health Information Technology for Economic and Clinical Health (HITECH) Act also promotes national adoption of the EHR in clinical care. Consequently, the EHR is a pivotal tool for facilitating the implementation of integrated SUD care and tracking clinical outcomes for both clinical and research purposes (Tai et al., 2012). The EHR captures a wide range of psychiatric diagnoses from a large and broad patient population. Our study analyzed EHR data from a large sample, in hopes of providing much-needed SUD and related psychiatric comorbidity data on patients in real-life medical settings to inform EHR-enabled research and clinical efforts related to screening and integrated care. The results of our study add new and comprehensive psychiatric profiles (e.g., SUDs, schizophrenia, personality, somatic diagnoses) for adults with and without T2DM in medical settings, which may not be captured by community surveys.
One critical finding concerns the high prevalence of any SUD (17.02%) among adults with T2DM compared with adults without T2DM (7.96%); there is a pervasive pattern in comorbid SUD with mental diagnoses. National survey data estimate that 12.9–13.6% of adults aged ≥18 had current nicotine dependence, and that 7.0% and 2.5% of adults had an alcohol and drug use disorder in the past year, respectively (SAMHSA, 2014). We found that 13.25%, 4.00%, and 4.22%, had a documented tobacco, alcohol, and drug use disorder diagnosis, respectively. Moreover, among adults with T2DM, more than 1 in 3 (37.36%) had a documented psychiatric diagnosis in their EHRs, and SUD was positively associated with each of the 6 mental diagnosis categories examined, highlighting a need to increase SUD research in individuals with T2DM and to improve their SUD care. The co-occurrences of SUD with mental disorders may be influenced by multiple pathways (e.g., self-medication, common risk factors, diathesis-stress) (Conway et al., 2006; Green, 2005; Ingram and Luxton, 2005). Importantly, our study adds new estimates by revealing a particularly burdensome SUD and psychiatric multi-comorbidity among adults with T2DM in medical settings, which may also be related to treatment factors, including selection bias (severity increasing treatment use and diagnoses; De Hert et al., 2009; Vinogradova et al., 2010).
Integrated healthcare models aimed at improving SUD care among adults with T2DM should also consider other psychiatric conditions, especially mood and anxiety diagnoses. Consistent with EHR data from individuals seeking care in behavioral healthcare clinics (Wu et al., 2013a,b), we found that mood (21.22%), SUD (17.02%), and anxiety (13.98%) diagnoses are the most common disorders in the sample. National survey data estimate that 19.54% of adults aged ≥18 have a mood disorder and 16.16% have an anxiety disorder in their lifetime (Kessler et al., 2005). Additionally, depression was nearly twice as common among adults with T2DM (19.1%, range 6.5–33%) compared with those without T2DM (10.7%, range 3.8–19.4%) (Roy and Lloyd, 2012). Another review found that 14% of adults with diabetes had a current anxiety disorder (Grigsby et al., 2002). The association between T2DM and mood/anxiety have been suggested to be multifactorial in nature, including lifestyle and treatment factors that are associated with depression or obesity (e.g., physical inactivity, use of some antidepressants or antipsychotics; Faith et al., 2011; Grundy et al., 2014; Patten et al., 2011; Ramaswamy et al., 2006) as well as diabetes-related stress (De Hert et al., 2009; Ducat et al., 2014). This study adds newer clinical evidence that reveals a high prevalence of SUDs and a pervasive pattern of SUD-psychiatric comorbidity among adults with T2DM. These results demonstrate a critical need to address the potential impact of mood/anxiety diagnosis on severity and treatment compliance for adults living with both T2DM and SUD (Ducat et al., 2014; Najt et al., 2011).
4.2. Limitations and strengths
Our study results should be interpreted within the following limitations. This analysis focuses on understanding the prevalence of SUD and mental diagnoses among patient with T2DM, and results reflect associations, not causality. Results are based on patients who accessed healthcare at one of the clinics/practices of a large academic healthcare system. Although the EHR data that we studied included diverse racial/ethnic groups in the communities, results are not completely generalizable to patients in different regions. The EHRs may tend to include severe or frequent treatment-seeking people, so those with T2DM, but without manifested medical conditions, might not be diagnosed. Nonetheless, disorders with objective diagnostic features like diabetes have a high level of EHR coding accuracy (Jordan et al., 2004; Pringle et al., 1995).
Similar to other medical conditions, underdiagnoses or misclassification of SUD and psychiatric diagnoses are possible (Banta and Montgomery, 2007; Menchetti et al., 2009; Rockett et al., 2003). Diagnoses in EHRs are based on actual treatment as part of usual care settings, which are determined by using the available information from medical histories and evaluations, patient reports, and interactions among providers, patients, and family members. Since it is not feasible to assess all diagnoses systematically, people with mild forms of a disorder or those who did not disclose symptoms might not be recognized. Detection of SUD and psychiatric disorders may be influenced by patient demographics, presentation of symptoms, treatment-seeking frequency, and clinicians’ specialties (Docherty, 1997; Garland et al., 2005; Herran et al., 1999; Menchetti et al., 2009). Nevertheless, having a longstanding provider-patient relationship, using criteria for diagnoses, and using EHRs systematically (allowing reevaluation and monitoring of the problems) are important factors for improving diagnostic accuracy (Pringle et al., 1995; van Weel, 1995; van Weel-Baumgarten et al., 2000; van Weel-Baumgarten and Lucassen, 2009). Overall, our results should be considered conservative estimates, particularly for SUDs, which may be underestimated due to perceived stigma and a lack of systematic screening in general medical settings (Tai et al., 2012).
Our examination of EHR data also has important strengths. Results reflect clinical patterns among patients in real-world general medical settings in the Southeastern United States. To our knowledge, our study includes the largest sample of patients ever examined for SUD and related psychiatric comorbidity by T2DM status in the United States. This large sample size allowed stratified analyses by age group to inform reliability of estimates. The Duke University Health System is among the pioneers developing EHRs for its clinics/practices. In 1968, Duke investigators began developing a working prototype of a general purpose electronic medical record (EMR) that eventually evolved into one of the first EMRs in the United States (Duke University, 2010). The long-term use of an EMR to enhance healthcare and the longitudinally captured EHRs may improve completeness of diagnostic and treatment data (Horvath et al., 2014; Pringle et al., 1995; Silfen, 2006; Silow-Carroll et al., 2012; Weiner et al., 2007).
4.3. Conclusion and clinical implications
The prevalence of T2DM in this study is consistent with the national estimate (ADA, 2015). We found that more than 1 in 3 adults with T2DM had a documented psychiatric diagnosis. Individuals that have diabetes with complications, SUD, or chronic mental diagnoses use more costly inpatient care than those without such diagnoses (Jiang and Wier, 2010; Jiang et al., 2014); these coexistences further aggravate morbidity and increase healthcare expenditures (Ducat et al., 2014; Vinogradova et al., 2010). The most costly 10% of the patient population in the United States accounted for 66% of total healthcare expenditures (Cohen, 2014). Therefore, the identified SUD-psychiatric comorbidities among adults with T2DM highlight a critical need to apply preventive services (e.g., screening, intervention) in primary care in order to enhance early detection and intervention for SUD and associated psychiatric problems. There is a tremendous demand for coordinated care models aimed at improving behavioral health and reducing avoidable hospitalizations for patients with multi-comorbidities (Katon et al., 2012). Research efforts are needed to identify effective approaches for screening substance misuse/SUD, implementing interventions, and coordinating referrals to SUD treatment and follow-ups for individuals with diabetes (Ghitza et al., 2013). There are limited data available to inform smoking cessation in people with diabetes. Given that tobacco use disorder was the most prevalent SUD in our sample, this finding reaffirms the need for clinical research to test tailored smoking cessation interventions for people with diabetes (Nagrebetsky et al., 2014). Collaborative care models have been found to improve health outcomes for individuals with depression and diabetes, and this line of efforts should be expanded to also address SUD and related psychiatric comorbidity for people with diabetes (Huang et al., 2013).
HIGHLIGHTS.
Prevalence of type 2 diabetes mellitus (T2DM) has increased substantially.
Comorbid T2DM and substance use disorders (SUD) increase morbidity.
The extent of T2DM and SUD related comorbidities was examined.
Prevalence of all psychiatric categories was elevated in adults with T2DM.
Adults with T2DM had more SUD related comorbidities than those without.
Acknowledgments
Udi E. Ghitza is an employee of the Center for the Clinical Trials Network, National Institute on Drug Abuse, which is the funding agency for the National Drug Abuse Treatment Clinical Trials Net-work. Dr. Ghitza’s participation in this publication arises from his role as a project scientist on a cooperative agreement (CTN-0057), and Dr. Ghitza has not had and will not have any programmatic involvement with the grants cited. The authors thank Robert M. Califf, MD, former Director of Duke Translational Medicine Institute and Vice Chancellor for Clinical and Translational Research, Professor of Medicine in the Division of Cardiology at Duke University Medical Center, and Principal Investigator for the Southeastern Diabetes Initiative (SEDI) for his support of this project. The authors also thank Morgan Deblecourt and Erin Hanley for assisting with the manuscript preparation.
Role of the funding source:
This work was made possible by research support from the U.S. National Institutes of Health (U10DA013727; R01MD007658; UG1DA040317), the Centers for Medicare and Medicaid Services (1C1CMS331018), and the Bristol-Myers Squibb Foundation. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The opinions expressed in this paper are solely those of the authors and do not represent the official position of the U.S. government and the Bristol-Myers Squibb Foundation.
Footnotes
Contributors: Li-Tzy Wu originated research questions and wrote the drafts of the paper; Michael J. Pencina, Leoncio Flavio Rojas, Benjamin A. Goldstein, and Tony Schibler conducted data analyses; all authors contributed to designs, critical revisions, and interpretations of the findings to result in the final manuscript.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institutional review board approval:
This work has been approved by the Duke University Health System Institutional Review Board.
References
- Ahmed AT, Karter AJ, Liu J. Alcohol consumption is inversely associated with adherence to diabetes self-care behaviors. Diabet Med. 2006;23:795–802. doi: 10.1111/j.1464-5491.2006.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) Fast Facts: Data and Statistics about Diabetes. American Diabetes Association; 2015. [Accessed August 19, 2015]. web site http://professional.diabetes.org/admin/UserFiles/0%20-%20Sean/Documents/Fast_Facts_3-2015.pdf Updated March 2015. [Google Scholar]
- American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association (APA) Practice Organization. Covered Diagnoses & Crosswalk of DSM-IV Codes to ICD-9-CM Codes. American Psychological Association Practice Central; 2002. [Accessed August 19, 2015]. web site http://www.apapracticecentral.org/reimbursement/billing/dsmiv-to-icd9cm-codes-chart.pdf. [Google Scholar]
- Ashman JJ, Talwalkar A, Taylor SA. NCHS Data Brief, no 161. Centers for Disease Control and Prevention; 2010. [Accessed August 19, 2015]. Age differences in visits to office-based physicians by patients with diabetes: United States, 2010. web site http://www.cdc.gov/nchs/data/databriefs/db161.pdf Updated July 2014. [PubMed] [Google Scholar]
- Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;32:2123–2132. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta JE, Montgomery S. Substance abuse and dependence treatment in outpatient physician offices, 1997–2004. Am J Drug Alcohol Abuse. 2007;33:583–593. doi: 10.1080/00952990701407546. [DOI] [PubMed] [Google Scholar]
- Brick J, editor. Handbook of the Medical Consequences of Alcohol and Drug Abuse. The Haworth Press; New York: 2004. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Crude and age-adjusted rate per 100 of civilian, noninstitutionalized adults with diagnosed diabetes, United States, 1980–2011. Centers for Disease Control and Prevention; 2014a. [Accessed on August 19, 2015]. web site http://www.cdc.gov/diabetes/statistics/prev/national/figageadult.htm Updated September 5, 2014. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National diabetes statistics report, 2014: estimates of diabetes and its burden in the United States. Centers for Disease Control and Prevention; 2014b. [Accessed August 19, 2015]. web site http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- Centers for Medicare and Medicaid Services (CMS) ICD-9-CM official guidelines for coding and reporting. UCLA Health Office of Compliance Services; 2008. [Accessed August 19, 2015]. http://compliance.uclahealth.org/Workfiles/PDFs/ICD_9_CM_Official_Guidelines_for_Coding_and_Reporting_Effective_October_1_2008.pdf. [Google Scholar]
- Cohen SB. The Concentration Of Health Expenditures Across Population Subgroups In The U.S., 2012. Agency for Healthcare Research and Quality; 2014. [Accessed August 19, 2015]. Statistical Brief #448: Differentials. web site http://meps.ahrq.gov/mepsweb/data_files/publications/st448/stat448.shtml Updated September 2014. [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatr. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24:412–424. doi: 10.1016/j.eurpsy.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Docherty JP. Barriers to the diagnosis of depression in primary care. J Clin Psychiatry. 1997;58:5–10. [PubMed] [Google Scholar]
- Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312:691–692. doi: 10.1001/jama.2014.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke University. From research to practice. And back Duke Center for Health Informatics, Transforming healthcare through informatics Duke Center for Health Informatics; 2010. [Accessed August 19, 2015]. web site https://www.dchi.duke.edu/about-us/dchi-book/dchi_b_12oct10_full_final.pdf. [Google Scholar]
- Faith MS, Butryn M, Wadden TA, Fabricatore A, Nguyen AM, Heymsfield SB. Evidence for prospective associations among depression and obesity in population-based studies. Obes Rev. 2011;12:e438–453. doi: 10.1111/j.1467-789X.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Fan AZ, Rock V, Zhang X, Li Y, Elam-Evans L, Balluz L. Trends in cigarette smoking rates and quit attempts among adults with and without diagnosed diabetes, United States, 2001–2010. Prev Chronic Dis 2013. 2013;10:E160. doi: 10.5888/pcd10.120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland AF, Lau AS, Yeh M, McCabe KM, Hough RL, Landsverk JA. Racial and ethnic differences in utilization of mental health services among high-risk youths. Am J Psychiatry. 2005;162:1336–1343. doi: 10.1176/appi.ajp.162.7.1336. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Wu LT, Tai B. Integrating substance abuse care with community diabetes care: implications for research and clinical practice. Subst Abuse Rehabil. 2013;4:3–10. doi: 10.2147/SAR.S39982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AI. Schizophrenia and comorbid substance use disorder: effects of antipsychotics. J Clin Psychiatry. 2005;66:21–26. [PubMed] [Google Scholar]
- Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–1060. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- Grundy A, Cotterchio M, Kirsh VA, Kreiger N. Associations between anxiety, depression, antidepressant medication, obesity and weight gain among Canadian women. PLoS One. 2014;9:e99780. doi: 10.1371/journal.pone.0099780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2010. Centers for Disease Control and Prevention National Center for Health Statistics; 2013. [Accessed August 19, 2015]. National Vital Statistics Reports. web site http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_06.pdf Updated December 20, 2013. [PubMed] [Google Scholar]
- Herran A, Vázquez-Barquero JL, Dunn G. Recognition of depression and anxiety in primary care. Patients’ attributional style is important factor. BMJ. 1999;318:1558. [PMC free article] [PubMed] [Google Scholar]
- Horvath MM, Rusincovitch SA, Brinson S, Shang HC, Evans S, Ferranti JM. Modular design, application architecture, and usage of a self-service model for enterprise data delivery: the Duke Enterprise Data Unified Content Explorer (DEDUCE) J Biomed Inform. 2014;52:231–242. doi: 10.1016/j.jbi.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:260. doi: 10.1186/1471-244X-13-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, Luxton DD. Vulnerability-stress models. In: Hankin BL, Abela JRZ, editors. Development of Psychopathology: A Vulnerability-Stress Perspective. Sage Publications; London: 2005. pp. 32–46. [Google Scholar]
- Institute of Medicine (IOM) Initial National Priorities for Comparative Effectiveness Research. The National Academies Press; Washington, DC: 2009. [Google Scholar]
- Institute of Medicine (IOM) Clinical Data as the Basic Staple of Health Learning: Creating and Protecting a Public Good: Workshop Summary. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- Jiang HJ, Barrett ML, Sheng M. HCUP Statistical Brief #184: Characteristics of hospital stays for nonelderly Medicaid super-utilizers, 2012. Healthcare Cost and Utilization Project; 2014. [Accessed August 19, 2015]. web site http://www.hcup-us.ahrq.gov/reports/statbriefs/sb184-Hospital-Stays-Medicaid-Super-Utilizers-2012.jsp Updated November 2014. [PubMed] [Google Scholar]
- Jiang HJ, (AHRQ), Wier LM., (Thomson Reuters) HCUP Statistical Brief #89: All-cause hospital readmissions among non-elderly Medicaid patients, 2007. National Center for Biotechnology Information; 2010. [Accessed August 19, 2015]. web site http://www.ncbi.nlm.nih.gov/books/NBK53601/?report=printable Updated April 2010. [Google Scholar]
- Jordan K, Porcheret M, Croft P. Quality of morbidity coding in general practice computerized medical records: a systematic review. Fam Pract. 2004;21:396–412. doi: 10.1093/fampra/cmh409. [DOI] [PubMed] [Google Scholar]
- Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, Peterson D, Young B, Von Korff M. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506–14. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Leung GY, Zhang J, Lin W, Clark RE. Behavioral disorders and diabetes-related outcomes among Massachusetts Medicare and Medicaid beneficiaries. Psychiatr Serv. 2011a;62:659–665. doi: 10.1176/ps.62.6.pss6206_0659. [DOI] [PubMed] [Google Scholar]
- Leung GY, Zhang J, Lin W, Clark RE. Behavioral health disorders and adherence to measures of diabetes care quality. Am J Manag Care. 2011b;17:144–150. [PubMed] [Google Scholar]
- Menchetti M, Belvederi M, Bertakis K, Bortolotti B, Berardi D. Recognition and treatment of depression in primary care: effect of patients’ presentation and frequency of consultation. J Psychosom Res. 2009;66:335–341. doi: 10.1016/j.jpsychores.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Nagrebetsky A, Brettell R, Roberts N, Farmer A. Smoking cessation in adults with diabetes: a systematic review and meta-analysis of data from randomized controlled trials. BMJ Open. 2014;4:e004107. doi: 10.1136/bmjopen-2013-004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P, Fusar-Poly P, Brambilla P. Co-occurring mental and substance abuse disorders: a review on the potential predictors and clinical outcomes. Psychiatr Res. 2011;186:159–164. doi: 10.1016/j.psychres.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology. 2010;35:1997–2004. doi: 10.1038/npp.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Williams JV, Lavorato DH, Khaled S, Bulloch AG. Weight gain in relation to major depression and antidepressant medication use. J Affect Disord. 2011;134:288–293. doi: 10.1016/j.jad.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Pietraszek A, Gregersen S, Hermansen K. Alcohol and type 2 diabetes. A review. Nutr Metab Cardiovasc Dis. 2010;20:366–375. doi: 10.1016/j.numecd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Pringle M, Ward P, Chilvers C. Assessment of the completeness and accuracy of computer medical records in four practices committed to recording data on computer. Br J Gen Pract. 1995;45:537–541. [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry. 2006;18:183–194. doi: 10.1080/10401230600801234. [DOI] [PubMed] [Google Scholar]
- Rockett IR, Putnam SL, Jia H, Smith GS. Assessing substance abuse treatment need: a statewide hospital emergency department study. Ann Emerg Med. 2003;41:802–813. doi: 10.1067/mem.2003.189. [DOI] [PubMed] [Google Scholar]
- Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142:S8–21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- Sharma E, Rao NP, Venkatasubramanian G. Association between antipsychotic-induced metabolic side-effects and clinical improvement: a review on the evidence for metabolic threshold: author’s response. Asian J Psychiatr. 2014;11:76. doi: 10.1016/j.ajp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Silfen E. Documentation and coding of ED patient encounters: an evaluation of the accuracy of an electronic medical record. Am J Emerg Med. 2006;24:664–678. doi: 10.1016/j.ajem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Silow-Carroll S, Edwards JN, Rodin D. Using electronic health records to improve quality and efficiency: the experiences of leading hospitals. Issue Brief (Commonw Fund) 2012;17:1–40. [PubMed] [Google Scholar]
- Smith KJ, Béland M, Clyde M, Gariépy G, Pagé V, Badawi G, Rabasa-Lhoret R, Schmitz N. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Spratt SE, Batch BC, Davis LP, Dunham AA, Easterling M, Feinglos MN, Granger BB, Harris G, Lyn MJ, Maxson PJ, Shah BR, Strauss B, Thomas T, Califf RM, Miranda ML. Methods and initial findings from the Durham Diabetes Coalition: integrating geospatial health technology and community interventions to reduce death and disability. J Clin Trans Endocrin. 2015;2:26–36. doi: 10.1016/j.jcte.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Series H-48, HHS Publication No (SMA) 14-4863. Substance Abuse and Mental Health Services; 2014. [Accessed August 20, 2015]. Results from the 2013 national survey on drug use and health: summary of national findings. web site http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf Updated September 2014. [Google Scholar]
- Tai B, Wu LT, Clark HW. Electronic health records: essential tools in integrating substance abuse treatment with primary care. Subst Abuse Rehabil. 2012;3:1–8. doi: 10.2147/SAR.S22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai B, Volkow ND. Treatment for substance use disorder: opportunities and challenges under the affordable care act. Soc Work Public Health. 2013;28:165–174. doi: 10.1080/19371918.2013.758975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006:CD002968. doi: 10.1002/14651858.CD002968.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. State and county QuickFacts beta: Durham County, North Carolina. United States Census Bureau web site; [Accessed March 12, 2015. Accessed August 20, 2015]. http://www.census.gov/quickfacts/table/PST045214/37063. [Google Scholar]
- van Weel-Baumgarten E, Lucassen P. Clinical diagnosis of depression in primary care. Lancet. 2009;374:1817. doi: 10.1016/S0140-6736(09)62051-1. author reply 1817–1818. [DOI] [PubMed] [Google Scholar]
- van Weel-Baumgarten EM, van den Bosch WJ, van den Hoogen HJ, Zitman FG. The validity of the diagnosis of depression in general practice: is using criteria for diagnosis as a routine the answer? Br J Gen Pract. 2000;50:284–287. [PMC free article] [PubMed] [Google Scholar]
- Vinogradova Y, Coupland C, Hippisley-Cox J, Whyte S, Penny C. Effects of severe mental illness on survival of people with diabetes. Br J Psychiatry. 2010;197:272–277. doi: 10.1192/bjp.bp.109.074674. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MG, Lyman JA, Murphy S, Weiner M. Electronic health records: high-quality electronic data for higher-quality clinical research. Inform Prim Care. 2007;15:121–127. doi: 10.14236/jhi.v15i2.650. [DOI] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Wu LT, Blazer DG, Gersing KR, Burchett B, Swartz MS, Mannelli P NIDA AAPI Workgroup. Comorbid substance use disorders with other Axis I and II mental disorders among treatment-seeking Asian Americans, Native Hawaiians/Pacific Islanders, and mixed-race people. J Psychiatr Res. 2013a;47:1940–1948. doi: 10.1016/j.jpsychires.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Gersing KR, Swartz MS, Burchett B, Li TK, Blazer DG. Using electronic health records data to assess comorbidities of substance use and psychiatric diagnoses and treatment settings among adults. J Psychiatr Res. 2013b;47:555–563. doi: 10.1016/j.jpsychires.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]