Abstract
Background
Existing literature suggests that metformin, the most commonly used biguanide, may lower colorectal cancer (CRC) risk. Because most CRCs originate in pre-cancerous adenomas, we examined whether metformin use lowered colorectal adenoma risk after polypectomy in patients with type-2 diabetes.
Methods
Retrospective cohort study of 40-89-year-old Kaiser Permanente Northern California patients who had type-2 diabetes, and ≥1 adenoma detected at baseline colonoscopy during 2000-2009 and a repeat colonoscopy 1-10 years from baseline adenoma diagnosis through 2012. Cox models evaluated the association between metformin use during follow-up and subsequent adenoma diagnoses, controlling for age, race/ethnicity, sex, body mass index, and repeat examination indication.
Results
Study included 2,412 patients followed for a median of 4.5 years; cumulatively, 1,117 (46%) patients had ≥1 adenoma at repeat colonoscopy. Compared to patients not receiving diabetes medications (n=1,578), metformin-only use (n=457) was associated with lower adenoma recurrence risk (adjusted hazard ratio (HR)=0.76, 95% confidence interval (CI): 0.65-0.89), and the association was stronger with increasing total metformin dose (quartile (Q) 1: HR=0.90, CI 0.72–1.12; Q2: HR=0.89, CI 0.70-1.12; Q3: HR=0.80 CI 0.63–1.01; Q4: HR=0.50 CI 0.42–0.60, P-value for trend<0.001). Findings were unchanged in sensitivity analyses including evaluating only outcomes during the 3-10-year period from baseline.
Conclusion
Our study suggests a potential benefit of metformin use in lowering the risk of subsequent adenomas after polypectomy in patients with type-2 diabetes.
Impact
Metformin may lower CRC risk by reducing the formation of pre-cancerous lesions, reinforcing the potential additional benefits of its use.
Keywords: chemoprevention, colonoscopy, colorectal adenoma, colorectal cancer, metformin, pharmacoepidemiology, polypectomy, type 2 diabetes, screening, surveillance
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States,(1) with most originating as precancerous adenomas.(2) Many patients in whom colorectal adenomas have been removed develop subsequent lesions.(3-5) In the United States, based on Clinical Outcomes Research Initiative data, surveillance procedures aimed at detecting such new or recurrent lesions account for about 22% of all colonoscopies among persons 50 years and older and is the most common reason for colonoscopy among those 75 years and older.(6, 7) Thus, therapies that reduce adenoma recurrence may reduce both CRC risk and the need for surveillance colonoscopy.
Previous studies have found that commonly used medications, such as aspirin and celecoxib, may reduce adenoma and CRC risk,(8-11) but there is a paucity of published studies evaluating metformin’s effect and its potential chemopreventive role. Metformin is the most commonly prescribed drug for the prevention or treatment of type 2 diabetes mellitus and associated conditions.(12) Studies show that a diagnosis of diabetes is associated with an increased risk of diagnosis with colorectal adenomas and adenocarcinoma.(13, 14) A number of studies have suggested that metformin may reduce CRC risk,(15-18) and others have found no association.(19, 20) However, there is limited literature on metformin use and colorectal adenoma risk,(21) and no prior studies have examined whether metformin reduces the risk of adenoma formation after polypectomy. In this study, we examined the relationship between metformin use and detection of new or recurrent adenomas at follow-up examination after polypectomy in patients with type 2 diabetes.
Materials and Methods
Study Design and Setting
This is a retrospective cohort study of patients receiving care in Kaiser Permanente Northern California (KPNC), an integrated healthcare delivery organization serving approximately 3.3 million people in urban, suburban, and semirural regions within a large geographic area. The integrated structure allows access to stable enrolled populations for longitudinal studies. The study was approved by the Institutional Review Boards of Kaiser Permanente Northern California and the University of Pennsylvania.
Population
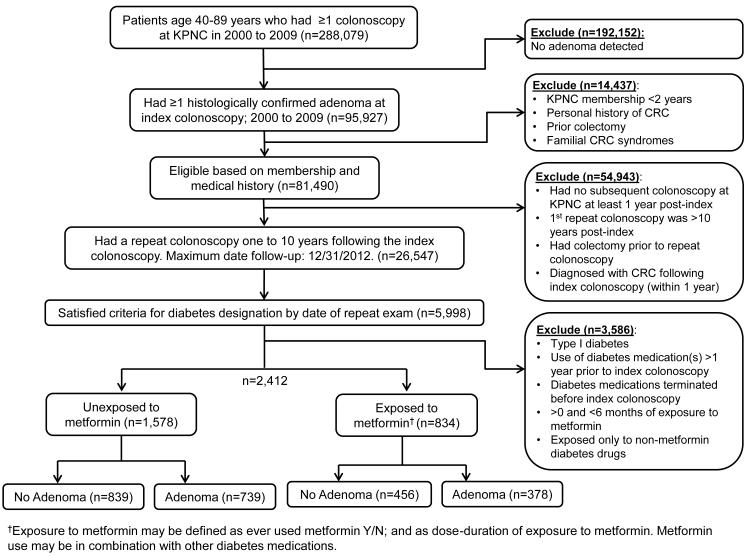
The study cohort was comprised of patients with type 2 diabetes who were 40-89 years old at the time they underwent a colonoscopy between January 1, 2000 and December 31, 2009, in which ≥1 histologically confirmed colorectal adenomas were found and removed. Patients were followed from the index examination date to the date of a follow-up colonoscopy on or before December 31, 2012, the last date of follow-up. We restricted the study to patients who had a repeat colonoscopy one to ten years after the index examination (Figure 1).
Figure 1.
Flow diagram of study participant selection
Representative flow chart of the study design and selection of patient data for inclusion in the study.
Patients with diabetes were identified using the Northern California Kaiser Permanente Diabetes Registry, which was started in 1993.(22) The updated criteria for inclusion in the registry are: 1) one or more prescriptions within the diabetes therapeutic class, one or more inpatient diabetes diagnosis, or two or more outpatient diabetes diagnoses in a prior five-calendar-year period; or 2) two or more pertinent abnormal labs (serum glycosylated hemoglobin (HbA1c) ≥6.5%, fasting glucose ≥126 mg/dL, or glucose ≥ 200 mg/dL) in a prior two-calendar-year period. Patients with gestational diabetes, type 1 diabetes, or who were prescribed diabetes medications for indications other than type 2 diabetes, such as lipodystrophy, metabolic syndrome, pre-diabetes, polycystic ovary syndrome, or amenorrhea, were excluded. Other exclusion criteria were: <2 years of health plan membership prior to cohort entry; history of CRC diagnosis or colectomy prior to or within one year after the index colonoscopy; and documented familial CRC syndromes such as Lynch syndrome or familial adenomatous polyposis.
To focus on new metformin users, we excluded those on diabetes medications more than one year prior to the index colonoscopy. This approach minimizes time-related biases (23) and also minimizes the potential to selectively include lesions that may have been resistant to the effect of metformin and thus are destined to recur. This approach also minimizes the inclusion of patients who may have had diabetes for longer duration prior to study baseline or used metformin for indications other than type 2 diabetes. We also excluded patients who discontinued diabetes medication before the index colonoscopy; used metformin for less than six months; or used other diabetes medications without metformin (Figure 1).
Data Sources
Information on clinical diagnoses was obtained from electronic databases using International Classification of Disease, Ninth Edition, Clinical Modification (ICD-9-CM) codes. Drug dispensing data were obtained from pharmacy files using National Drug Codes. Receipt of initial and follow-up colonoscopies were ascertained using Current Procedural Terminology and ICD-9-CM codes, as previously described.(24) Adenoma diagnosis, location, and histology were obtained from pathology reports using Systematized Nomenclature of Medicine Clinical Terms (SNOMED) codes.(24)
Outcome Measurements
The primary outcome was colorectal adenoma recurrence, defined as ≥1 histologically confirmed adenoma or adenocarcinoma at the repeat colonoscopy. Person-time was computed from the index colonoscopy date. Adenoma location was classified as right colon (proximal to and including the splenic flexure, irrespective of whether adenomas were detected in the distal colon), left colon/rectum, or unspecified using SNOMED codes.(24) The location was unspecified for approximately one-third of baseline adenomas.
Exposure Measurement
The primary exposure of interest was metformin-only use during the follow-up period based on dispensings. We also evaluated metformin used in combination with other diabetes medications such as sulfonylureas (any-metformin). We computed the metformin total dose (with or without other diabetes medications) dispensed during the follow-up period to assess dose-related effects; discontinuous dispensing periods were summed together. Total dose quartiles (Q) were calculated among all metformin users as: 50-399 (Q1); 400-799 (Q2); 800-1499 (Q3); and ≥1,500 (Q4) grams. We assumed that patients used all dispensed metformin.
Covariates
Information was available on patient age, sex, race/ethnicity, and baseline body mass index (BMI). BMI is related to the risk of type 2 diabetes, type of diabetes medication prescribed, disease severity, and likelihood of receiving colonoscopy.(25, 26) The performing physician and colonoscopy indications were derived using previously validated algorithms.(27, 28) We obtained serum HbA1c levels from laboratory databases. HbA1c levels correlate with serum glucose levels, which may be related to disease risk.(29, 30)
Statistical Analyses
We first used the Kaplan-Meier product limit estimator to evaluate the association between metformin use and adenoma recurrence risk. Multilevel Cox proportional hazard models with clustering on the performing provider were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between exposure to metformin in the one-to-ten-year period after the index colonoscopy and risk of adenoma recurrence. The reference group in all analyses was patients who did not receive any diabetes medications during the study period. Multivariable models were adjusted for the covariates noted above, except HbA1c, which was not statistically significantly associated with adenoma recurrence risk (p-value>0.05), and did not influence the main effects. We stratified on repeat colonoscopy indication (surveillance vs. diagnostic) on some of our analyses because of statistically significant interaction with metformin use. We also performed analyses according to baseline adenoma location (right vs. left colon) due to a priori interest and possible biological differences in colorectal lesions according to location.(31) In secondary analyses, we evaluated the association between any-metformin exposure (with or without other diabetes medications) and adenoma recurrence risk, as either a binary variable, or according to total dispensed dose quartiles.
We performed several sensitivity analyses including limiting the cohort to new metformin initiators (n=2,213), and to those with repeat examinations three to six, or six to ten years from baseline consistent with surveillance recommendations.(32) Type 2 diabetes occurs insidiously and patients may remain in a pre-diabetic state or have undetected diabetes for many years prior to clinical diagnosis.(33-35) We assumed this pre-clinical phase to be <5 years and performed analyses in which accrued person-time was computed from no further than five years prior to the diabetes diagnosis date. We also assessed the duration of therapy in our sensitivity analyses (Supplementary Table S1). Because of reports of potential gender differences in the association between having diabetes and colorectal neoplasia risk, we performed further analysis stratified on gender,(36) although, there was no statistically significant gender-metformin interaction observed. We examined and did not find a statistically significant association with level of glycemic control based on an HbA1C of ≤7% versus higher. All analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
Results
Baseline Characteristics
We identified 288,079 patients who were 40-89 years old and had undergone colonoscopy during 2000-2009, of whom 95,927 had ≥1 histologically confirmed adenoma. Of those, 2,412 eligible patients with type 2 diabetes were included in the study (Figure 1). The median time to repeat exam (4.5 years) did not differ significantly across exposures. On average, metformin users were on therapy for 878 days (range: 146-3,066), received 0.99 grams per day (range: 0.10-3.17), and had a total dose of 800 grams (range: 50-5,900) during the study period.
Association with Adenoma Recurrence Risk
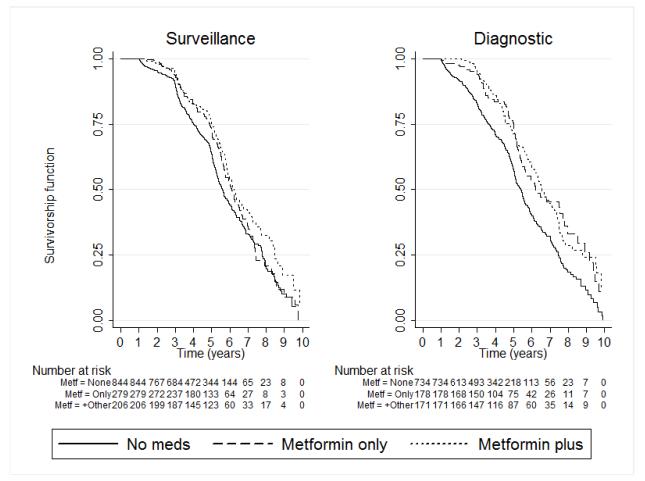
A total of 834 patients had used metformin, including 377 patients who received it in combination with other drugs, most commonly sulfonylurea, and 1,578 who did not receive diabetes therapy (Figure 1). Cumulatively, 196 (42.9%) of the 457 patients on metformin-only had adenoma recurrence compared to 739 (46.8%) patients who did not received diabetes therapy (untreated) (Table 1). In Kaplan-Meier analysis, untreated patients had a higher rate of adenoma recurrence than those on metformin (Figure 2, log-rank test p-values <0.001). Cox modeling showed a 24% lower risk of adenoma recurrence (adjusted HR=0.76, CI: 0.65-0.89) (Table 2) in those on metformin-only. This association was observed in analyses stratified by repeat colonoscopy indication (surveillance n=1,329, adjusted HR=0.89, CI: 0.73-1.09; diagnostic n=1,083, adjusted HR=0.62, CI: 0.50-0.79; p-value for test of interaction = 0.02), and by location of the index lesion (right colon n=1,117, adjusted HR=0.75 CI: 0.62-0.90; left colon/rectum n=421, adjusted HR=0.66 CI: 0.41-1.05; p-value for test of interaction = 0.95, Table 2). However, the association was not statistically significant for left colon lesions or surveillance examinations.
Table 1.
Characteristics of the cohort according to treatment type, KPNC 2000-2009 (n=2,412)
| Characteristics | No Diabetes Medication (n=1,578) |
Metformin-Only (n=457) |
Metformin Plus Other (n=377) |
|---|---|---|---|
| Age at Baseline, years | |||
| 40-49 | 54 (3.4) | 27 (5.9) | 33 (8.8) |
| 50-54 | 164 (10.4) | 71 (15.5) | 79 (21.0) |
| 55-59 | 199 (12.6) | 83 (18.2) | 80 (21.2) |
| 60-64 | 289 (18.3) | 94 (20.6) | 72 (19.1) |
| 65-69 | 322 (20.4) | 74 (16.2) | 64 (17.0) |
| 70-74 | 318 (20.2) | 73 (16.0) | 32 (8.5) |
| 75+ | 232 (14.7) | 35 (7.7) | 17 (4.5) |
| Sex | |||
| Female | 575 (36.4) | 178 (38.9) | 149 (39.5) |
| Male | 1,003 (63.6) | 279 (61.1) | 228 (60.5) |
| Race/Ethnicity | |||
| Non-Hispanic white | 943 (59.8) | 253 (55.4) | 222 (58.9) |
| Hispanic | 199 (12.6) | 64 (14.0) | 57 (15.1) |
| Black | 112 (7.1) | 33 (7.2) | 16 (4.2) |
| Asian/Pacific Islander | 216 (13.7) | 76 (16.6) | 48 (12.7) |
| Other* | 108 (6.8) | 31 ( 6.8) | 34 (9.0) |
| BMI Closest to Baseline Exam | |||
| <25.0 | 208 (13.2) | 42 (9.2) | 30 (8.0) |
| 25.0-29.9 | 484 (30.7) | 109 (23.9) | 76 (20.2) |
| 30+ | 801 (50.8) | 279 (61.1) | 252 (66.8) |
| Unknown | 85 (5.4) | 27 (5.9) | 19 (5.0) |
| Indication for Repeat Exam | |||
| Surveillance | 844 (53.5) | 279 (61.1) | 206 (54.6) |
| Diagnostic | 734 (46.5) | 178 (38.9) | 171 (45.4) |
| Recurrent Adenoma | |||
| No | 839 (53.2) | 261 (57.1) | 195 (51.7) |
| Any | 739 (46.8) | 196 (42.9) | 182 (48.3) |
| Mean HbA1c, Mean (SD) (n=2,293) | 6.18 (0.59) | 6.73 (0.72) | 7.35 (0.88) |
| Time to Repeat Exam, Median (IQR) | 4.1 (2.4) | 4.9 (2.5) | 5.2 (2.6) |
Other race/ethnicity includes Native American, Multiracial/other, and unknown
Figure 2.
Kaplan-Meier curves of relationship of metformin use and adenoma recurrence The curves are stratified by indication for the repeat examination. The log-rank test p-values were, for surveillance: 0.051 for metformin-only and 0.002 for metformin plus other; for diagnostic: <0.001 for both metformin-only and metformin plus other.
Table 2.
Associations between metformin use and risk of colorectal adenoma recurrence in patients with type 2 diabetes
| Exposure categories | Sample, n |
Adenoma detected, n |
Adjusted hazard ratio (95% confidence interval) |
||||
|---|---|---|---|---|---|---|---|
| Recurrent Adenoma (all indications and locations) |
Indication for Repeat Colonoscopy** |
Location at Baseline**¶ | |||||
|
| |||||||
| Diagnostic | Surveillance | Right colon | Left colon/rectum |
||||
|
Separate categories for metformin-
only and combination therapy |
|||||||
| No Diabetes Medication (Reference) | 1578 | 739 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Metformin-Only | 457 | 196 | 0.76 (0.65-0.89) | 0.62 (0.50-0.79) | 0.89 (0.73-1.09) | 0.75 (0.62-0.90) | 0.66 (0.41-1.05) |
| Metformin + Other | 377 | 182 | 0.72 (0.62-0.85) | 0.66 (0.52-0.83) | 0.80 (0.64-1.02) | 0.73 (0.58-0.92) | 0.96 (0.63-1.47) |
| Any metformin | |||||||
| No Diabetes Medication (Reference) | 1578 | 739 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Any Metformin | 834 | 378 | 0.74 (0.66-0.84) | 0.64 (0.53-0.77) | 0.85 (0.71-1.02) | 0.74 (0.64-0.86) | 0.79 (0.54-1.15) |
| Total Dose, Quartiles § | |||||||
| No Diabetes Medication (Reference) | 1578 | 739 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Quartile 1 | 205 | 88 | 0.90 (0.72-1.12) | 0.63 (0.46-0.86) | 1.32 (1.01-1.73) | 0.86 (0.66-1.13) | 1.17 (0.61-2.23) |
| Quartile 2 | 209 | 95 | 0.98 (0.79-1.21) | 0.97 (0.69-1.37) | 1.02 (0.79-1.31) | 0.91 (0.73-1.15) | 0.62 (0.32-1.21) |
| Quartile 3 | 205 | 95 | 0.80 (0.63-1.01) | 0.69 (0.48-0.97) | 0.91 (0.69-1.20) | 0.71 (0.51-0.98) | 1.14 (0.70-1.85) |
| Quartile 4 | 215 | 100 | 0.50 (0.42-0.60) | 0.47 (0.35-0.62) | 0.55 (0.41-0.72) | 0.58 (0.46-0.72) | 0.49 (0.28-0.88) |
Models were run separately for each set of exposure categories. All models are adjusted for age category, sex, race/ethnicity, BMI, and indication for repeat examination, as appropriate
P-value for interaction = 0.02 for indication, but was 0.95 for tumor location
Unspecified adenoma location at baseline (n=874)
Includes patients receiving metformin alone and metformin in combination with other medications Trend p<0.001
In secondary analyses, any-metformin use (alone and in combination with other diabetes medications) was similarly associated with a lower adenoma recurrence risk (adjusted HR=0.74, CI 0.66-0.84) (Table 2). The risk of adenoma recurrence was monotonically lower with increasing total dose. Compared to no therapy, and with increasing quartiles denoting increasing dose, the adjusted HR (95% CI) was 0.90 (CI 0.72–1.12) for Q1, 0.89 (CI 0.70-1.12) for Q2, 0.80 (CI 0.63–1.01) for Q3, and 0.50 (CI 0.42–0.60) for Q4 (p-value for trend <0.001). The findings were similar for analysis of average daily dose dispensed and the duration of therapy (Supplementary Table S1). The associations with metformin use were stable in sensitivity analyses excluding patients who had used anti-diabetes medication prior to the index colonoscopy, or person-time accrued >5 years prior to the diabetes diagnosis date (Supplementary Table S2), excluding repeat colonoscopies performed <3 years of index date (Supplementary Table S3), or according to gender (Supplementary Table S4).
Discussion
We found that, in patients with type 2 diabetes, metformin use was associated with a lower risk of colorectal adenoma recurrence when compared to no diabetes therapy. The observed risk was inversely related to metformin total dose and was stable in various sensitivity analyses, including models that were restricted to new therapy initiators. These findings suggest that, in addition to its established role in treating diabetes and related conditions, metformin use may also confer additional benefits in lowering the risk of adenoma.
The association of metformin with colorectal cancer or adenoma risk is controversial. Our findings are consistent with, and support previous reports that metformin use was associated with CRC risk. Our findings were stable in various secondary and sensitivity analyses. This study’s findings may also suggest a potential explanation for how metformin may lower CRC risk through the adenoma-carcinoma sequence. Metformin’s effect on adenoma risk could be mediated through several posited biological mechanisms such as mTOR (mammalian target of rapamycin) pathway inhibition and insulin-like growth factor signaling suppression.(37)
There are no previous studies to directly compare with ours, but Lee et al. reported that, in patients with type 2 diabetes who had undergone CRC resection, metformin use was associated with lower odds (odds ratio)=0.27, 95% CI: 0.10-0.76) of recurrent adenoma.(38) Kanadiya et al. also reported that metformin use was associated with lower odds (odds ratio=0.55, 95% CI: 0.34-0.87) of adenoma in a study of 405 patients with type 2 diabetes undergoing screening colonoscopy (n=148).(21) In contrast, our study examined adenoma risk during the surveillance phase of the cancer care continuum on a large cohort of 2,412 patients, with 834 exposed to metformin. Our design addressed several potential time-related biases of observational studies.(23)
Our study has some potential limitations. First, we restricted the follow-up time to first repeat colonoscopy, which limited the time interval for detecting recurrent lesions as well as our ability for direct causal inference. However, there was no substantive difference in the follow-up time according to exposure group. Also, we could not account for exposure to other potential adenoma chemopreventive strategies, such as cyclooxygenase-2 inhibitors, aspirin and other non-steroidal anti-inflammatory drugs, statins, lifestyle factors, smoking history, and dietary factors such as folic acid and calcium.(9-11, 39-41)
In conclusion, we found an inverse association between metformin use and risk of adenoma recurrence in patients with type 2 diabetes that was independent of other factors assessed. These findings suggest a possible role for metformin in the secondary chemoprevention of adenomas.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Sean Hennessy, PharmD, PhD and James D. Lewis, MD, MSCE for advice on study design, analysis and helpful comments and suggestions on the manuscript.
Funding: This work was funded in part from the Center for Pharmacoepidemiology Research and Training, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania to C. A. Doubeni. The work was also supported in part by grant K01CA127118 and U01CA151736 awarded to C. A. Doubeni and grant number U54CA163262 to C. A. Doubeni and D. A. Corley from the National Cancer Institute at the National Institutes of Health.
Footnotes
Conflict of interest: The authors report no actual, potential, or perceived conflict of interest with regard to this manuscript.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901–6. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 4.van Stolk RU, Beck GJ, Baron JA, Haile R, Summers R. Adenoma characteristics at first colonoscopy as predictors of adenoma recurrence and characteristics at follow-up. The Polyp Prevention Study Group. Gastroenterology. 1998;115:13–8. doi: 10.1016/s0016-5085(98)70359-2. [DOI] [PubMed] [Google Scholar]
- 5.Laiyemo AO, Doubeni C, Brim H, Ashktorab H, Schoen RE, Gupta S, et al. Short- and long-term risk of colorectal adenoma recurrence among whites and blacks. Gastrointest Endosc. 2013;77:447–54. doi: 10.1016/j.gie.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–83. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Williams JL, Holub JL, Morris CD, Logan JR, Eisen GM, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133–43. doi: 10.1016/j.gie.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 9.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 10.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 11.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 12.Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218–31. doi: 10.7326/0003-4819-156-3-201202070-00011. [DOI] [PubMed] [Google Scholar]
- 13.Luo S, Li JY, Zhao LN, Yu T, Zhong W, Xia ZS, et al. Diabetes mellitus increases the risk of colorectal neoplasia: An updated meta-analysis. Clin Res Hepatol Gastroenterol. 2015 doi: 10.1016/j.clinre.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol. 2015;21:6026–31. doi: 10.3748/wjg.v21.i19.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care. 2011;34:2323–8. doi: 10.2337/dc11-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smiechowski B, Azoulay L, Yin H, Pollak MN, Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiol Biomarkers Prev. 2013;22:1877–83. doi: 10.1158/1055-9965.EPI-13-0196. [DOI] [PubMed] [Google Scholar]
- 20.Kowall B, Stang A, Rathmann W, Kostev K. No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf. 2015;24:865–74. doi: 10.1002/pds.3823. [DOI] [PubMed] [Google Scholar]
- 21.Kanadiya MK, Gohel TD, Sanaka MR, Thota PN, Shubrook JH., Jr. Relationship between type-2 diabetes and use of metformin with risk of colorectal adenoma in an American population receiving colonoscopy. J Diabetes Complications. 2013;27:463–6. doi: 10.1016/j.jdiacomp.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 23.Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35:2665–73. doi: 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corley DA, Jensen CD, Marks AR, Zhao WK, de Boer J, Levin TR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11:172–80. doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis JD, Capra AM, Achacoso NS, Ferrara A, Levin TR, Quesenberry CP, Jr., et al. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf. 2007;16:1195–202. doi: 10.1002/pds.1441. [DOI] [PubMed] [Google Scholar]
- 26.Rosen AB, Schneider EC. Colorectal cancer screening disparities related to obesity and gender. J Gen Intern Med. 2004;19:332–8. doi: 10.1111/j.1525-1497.2004.30339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JK, Jensen CD, Lee A, Doubeni CA, Zauber AG, Levin TR, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointest Endosc. 2015;81:575–82. e4. doi: 10.1016/j.gie.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2004;13:915–9. [PubMed] [Google Scholar]
- 30.Yang YX, Habel LA, Capra AM, Achacoso NS, Quesenberry CP, Jr., Ferrara A, et al. Serial glycosylated hemoglobin levels and risk of colorectal neoplasia among patients with type 2 diabetes mellitus. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:3027–36. doi: 10.1158/1055-9965.EPI-10-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laiyemo AO, Doubeni C, Sanderson AK, 2nd, Pinsky PF, Badurdeen DS, Doria-Rose VP, et al. Likelihood of missed and recurrent adenomas in the proximal versus the distal colon. Gastrointestinal endoscopy. 2011;74:253–61. doi: 10.1016/j.gie.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Friedman SM, Vallipuram J, Baswick B. Incidental findings of elevated random plasma glucose in the ED as a prompt for outpatient diabetes screening: a retrospective study. BMJ Open. 2013;3:e003486. doi: 10.1136/bmjopen-2013-003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R, Baltimore Longitudinal Study of A The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52:1475–84. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 35.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 36.Campbell PT, Deka A, Jacobs EJ, Newton CC, Hildebrand JS, McCullough ML, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139:1138–46. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 37.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov. 2012;2:778–90. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 38.Lee JH, Jeon SM, Hong SP, Cheon JH, Kim TI, Kim WH. Metformin use is associated with a decreased incidence of colorectal adenomas in diabetic patients with previous colorectal cancer. Dig Liver Dis. 2012;44:1042–7. doi: 10.1016/j.dld.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Broughton T, Sington J, Beales IL. Statin use is associated with a reduced incidence of colorectal adenomatous polyps. Int J Colorectal Dis. 2013;28:469–76. doi: 10.1007/s00384-012-1601-9. [DOI] [PubMed] [Google Scholar]
- 40.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 41.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.