Abstract
Adolescent females are particularly vulnerable to mental illnesses with comorbidity of anxiety, such as anorexia nervosa (AN). We used an animal model of AN, called activity-based anorexia (ABA), to investigate the neurobiological basis of vulnerability to repeated, food restriction (FR) stress-evoked anxiety. Twenty-one of 23 adolescent female mice responded to the 1st FR with increased wheel running activity (WRA), even during the limited period of food access, thereby capturing AN's symptoms of voluntary FR and over-exercise. Baseline WRA was an excellent predictor of FR-elicited WRA (severity of ABA, SOA), with high baseline-runners responding to FR with minimal SOA (i.e., negative correlation). Nine gained resistance to ABA following the 1st FR. Even though allopregnanolone (3α-OH-5α-pregnan-20-one, THP), the metabolite of progesterone (P4), is a well-recognized anxiolytic agent, subcutaneous P4 to these ABA-resistant animals during the 2nd FR was exacerbative, evoking greater WRA than the counterpart resistant group that received oil vehicle, only. Moreover, P4 had no WRA-reducing effect on animals that remained ABA-vulnerable. To explain the sensitizing effect of P4 upon the resistant mice, we examined the relationship between P4 treatment and levels of the α4 subunit of GABAARs at spines of pyramidal cells of the hippocampal CA1, a parameter previously shown to correlate with resistance to ABA. α4 levels at spine membrane correlated strongly and negatively with SOA during the 1st ABA (prior to P4 injection), confirming previous findings. α4 expression levels were greater among P4-treated animals that had gained resistance than of vehicle-treated resistant animals or of the vulnerable animals with or without P4. We propose that α4-GABAARs play a protective role by counterbalancing the ABA-induced increase in excitability of CA1 pyramidal neurons, and although exogenous P4's metabolite, THP, enhances α4 expression, especially among those that can gain resistance, it also interferes with α4-GABAARs’ protective role by desensitizing α4-GABAARs.
Keywords: Activity-based anorexia, anxiety, anorexia nervosa, resilience, exercise, allopregnanolone
1.INTRODUCTION
Adolescence is a period of great vulnerability to mental illness (Spear, 2000). The stress response system in the brain and periphery exhibit strong and long-lasting (Andersen, 2003; Romeo, 2010) plasticity during adolescence, perhaps because it is still continuing to develop (McCormick & Mathews, 2007; Romeo, 2005; Romeo et al., 2006). As a consequence, a stressful event during adolescence affects the mental health outcome into adulthood (Andersen, 2003; Romeo, 2010). In puberty and adolescence, there is an interaction of the following factors: an increase of environmental stressors, gonadal hormone fluctuations, and a growth spurt in the hippocampus (Chowdhury et al., 2014; Mannan & O'Shaughnessy, 1991; Palumbo et al., 1995; Romeo et al., 2006; Spear, 2000). Estrogen, progesterone and testosterone are the major peri-pubertal hormones influencing plasticity and signaling in the hippocampus, along with reproductive function (Sheryl S Smith & Woolley, 2004). In adulthood, estrogen has excitatory effects in several brain regions, including the hippocampus, hypothalamus, and amygdala, with the impact of increasing or decreasing anxiety, increasing stress responses and decreasing depression (Harte-Hargrove, MacLusky, & Scharfman, 2013; Isgor, Cecchi, Kabbaj, Akil, & Watson, 2003; Nabekura, Oomura, Minami, Mizuno, & Fukuda, 1986; Walf & Frye, 2006). Among the questions that remain to be answered is the role of progesterone (P4) in altering the stress response of adolescents. We sought to address this gap in knowledge by imposing an animal model of stress called activity-based anorexia (ABA) upon adolescent mice and testing P4's cellular and behavioral effects upon ABA response.
The stressor in the ABA model is food restriction and the response to this stress is a marked increase in voluntary wheel running activity (WRA), causing exacerbated weight loss. WRA is a reliable measure of stress response, because it correlates positively with levels of corticosterone (cort) released after food restriction (Duclos, Bouchet, Vettier, & Richard, 2005; Duclos, Gatti, Bessiere, & Mormede, 2009). The WRA is also robustly correlated to anxiety levels measured during ABA (G. Wable, Min, Chen, & Aoki, 2015). Stress usually afflicts an individual more than once. The change in physiology evoked by repeated stress can also be analyzed in this animal model by imposing a second episode of ABA after recovery from the first. Such repeated stress exposures enable investigation of individual variability in habituation, adaptation or sensitization to the repeated stressor. Indeed, one of the strengths of the ABA model is that there is considerable individual variability in the WRA response to the first as well as the repeated episode of the food restriction stressor. The variability in behavior is not noise, because it is correlated in meaningful ways to variability in biological markers, such as the levels of cort (Duclos et al., 2005; Duclos et al., 2009). The extent of GABAergic innervation as well as expression levels of the α4 subunit of GABAARs by pyramidal neurons in the CA1 of dorsal hippocampus is also correlated strongly and negatively with the food restriction-evoked increase of WRA, suggesting that up-regulation of the hippocampal GABAergic system may contribute towards suppression of WRA and thereby resilience to ABA (Aoki et al., 2014; Chowdhury, Wable, Sabaliauskas, & Aoki, 2013).
P4 plays an important role in regulating anxiety. P4's metabolite, THP (3αOH-5α[β]-pregnan-20-one), reduces anxiety (C. A. Frye & Paris, 2011) (Bitran, Shiekh, & McLeod, 1995) in late adolescence, adulthood (Canonaco, Tavolaro, & Maggi, 1993; Engin & Treit, 2007; C. A. Frye & A. A. Walf, 2004; Koonce & Frye, 2013; Reddy, O'Malley, & Rogawski, 2005) and pre-pubertally (Hui Shen et al., 2007) through positive modulation of GABAA receptors (GABAARs) (Belelli & Lambert, 2005). These findings, together with our recent observation of the strong correlation between WRA response and anxiety (G. Wable et al., 2015), led us to test the following prediction: P4 treatment could decrease an animal's excessive WRA response to food restriction when imposed at late adolescence, which in turn would minimize weight loss and serve to protect animals from ABA. We chose a dosage of subcutaneous P4 that has been demonstrated to be anxiolytic in female mice of similar age (Cheryl A Frye, Walf, Rhodes, & Harney, 2004).
To identify a possible molecular mechanism underlying the behavioral effect of P4, we measured plasmalemmal and cytoplasmic levels of the α4 subunit of GABAARs in pyramidal neurons of the dorsal hippocampal CA1 of these mice. Our reason for measuring the α4 subunit level in the CA1 was two-fold: 1), the administration as well as withdrawal of P4 and THP increase the expression of α4βδ-GABAAR in the hippocampus over 2 to 3 days (H. Shen et al., 2007); and 2) ABA was previously shown to increase the expression of α4βδ-GABAARs in the hippocampal CA1 (Aoki et al., 2012; Aoki et al., 2014). We surmised that, if heightened levels of α4βδ-GABAAR in the hippocampal CA1 contributed to the suppression of food restriction-evoked increase in WRA, thereby reducing ABA vulnerability (Aoki et al., 2014), then P4 could induce the expression of α4βδ-GABAAR, which would also be protective against ABA. However, while P4's metabolite, THP, is, in general, anxiolytic, THP can also be anxiogenic. This is because THP has an additional property of desensitizing α4βδ-GABAAR, when expressed in pyramidal cells of the CA1 (H. Shen et al., 2007). This additional desensitizing property of THP upon α4βδ-GABAARs expressed by CA1 pyramidal cells (as opposed to the consistently positive modulating effect of THP upon α4βδ-GABAARs expressed by granule cells of the dentate gyrus, involving the difference in the direction of Cl− flux across the two regions) has the potential consequence of increasing excitability of the CA1 pyramidal neurons and thus of anxiety (H. Shen et al., 2007). Because of this dual potential action of THP, knowledge about the changing level of α4 subunits in the hippocampal CA1 was needed for assessing the action of P4 that would include the interaction of α4βδ-GABAAR in the animal's response to ABA. Here, we report that, indeed, P4 led to exacerbated WRA during the second ABA, specifically among individuals that had attained resilience during recovery from the 1st ABA and a rise of α4βδ-GABAARs. These observations are consistent with the idea that up-regulation of α4βδ-GABAAR in the dorsal hippocampal CA1 during recovery from the first ABA contributed towards attainment of resilience to the second ABA, while desensitization of α4βδ-GABAARs by exogenous P4 contributed to anxiogenesis and exacerbated animals’ responses to the second ABA.
2. EXPERIMENTAL PROCEDURES
2.1 General aspects about the animals
All procedures relating to the use of animals were according to the NIH Guide for the Care and Use of Laboratory Animals and also approved by the Institutional Animal Care and Use Committee of New York University.
Altogether, 23 animals were used for this study. All animals were female C57BL6 mice, bred at New York University's animal facility in a 12 hour: 12 hour light: dark cycle (lights on at 7 am). At postnatal day (PND) 25, they were weaned and group-housed with same sex littermates. If any litter contained only a single female mouse, she was not included in the experiment. Food was provided ad libitum when the animals were not undergoing the food restriction portion of the paradigm [dry chow (PMI Mouse Diet 5001; 336 kcal per 100 g, 28.507% protein, 57.996% carbohydrates, 13.496% fat) and soft food (Clear H2O DietGel® 76A; 99.8 kcal per 100 g, 4.7% protein, 17.9% carbohydrates, 1.5% fat, 73.4% moisture)] and water was provided ad libitum throughout the experiment.
Non-ovariectomized mice were used for the study because ovariectomy has been shown to alter CNS neurosteroid metabolism following injection of exogenous P4 (Corpechot et al., 1993). Also, P4 is produced by the adrenal gland in stress so ovariectomy does not guarantee depletion of circulating P4 (Romeo, Lee, & McEwen, 2005).
2.2 ABA induction and P4 injection
ABA induction consisted of combining food restriction with access to a running wheel, as described in the ABA protocol established in our laboratory (Chowdhury et al., 2013).
Running wheels were purchased from Med Associates, called Low-Profile Wireless Running Wheel for Mouse (Product #: ENV-044). Wheel-running activity (WRA) was recorded continuously using Med Associates’ “Wheel Manager” software (Product #: SOF-860).
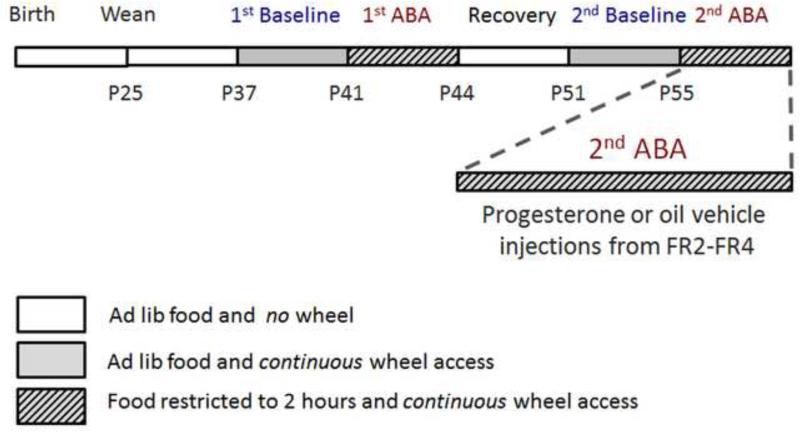
At noon on PND36, female mice were placed singly in standard cages with ad libitum access to laboratory chow while undergoing acclimation to the wheel (Fig 1). Beginning noon on PND41, mice were given access to food only for the first two hours of the dark cycle, from 7:00 pm to 9:00 pm, while having constant wheel access. At noon on PND44, ad libitum food access was restored and the mice were placed singly in a fresh cage with no running wheel. They were allowed to recover from ABA for a week, until PND51. From PND45 to 50, they were handled for five minutes every day, to acclimate them for vaginal lavage and injections. At noon on PND51, they were given access to a running wheel again for a re-acclimation period for four days. Vaginal lavages were collected starting PND53 until the end of the experiment. At noon on PND55, food access was again restricted to the first two hours of the dark cycle. Based on the running activity on this day called 2nd ABA FR1, we divided the mice into counter-balanced groups. One group received progesterone (P4) injections (1.0 mg progesterone in 0.1 cc of sesame oil, progesterone and sesame oil from Sigma-Aldrich, MO, USA, catalog number P3972 and S3547 respectively) and the other group received only oil. Injections were administered subcutaneously on the back of the mice, at 6 pm, an hour before feeding on 2nd ABA FR2, FR3, and FR4. Throughout the experiment, the mice and food were weighed at 6:40 pm, 20 minutes before the onset of the dark cycle. On the days that the mice were transferred to a fresh cage, i.e. PND36, PND44, PND51 and PND59 (day of perfusion), they were weighed at noon so as to not needlessly disturb them more than once a day. Wheel activity was monitored throughout the periods of wheel access. On PND59, all animals except one that was found dead were euthanized by transcardial perfusion with fixatives, so as to be able to analyze the membranous expression of α4-containing GABAARs in the hippocampus.
Figure 1. Experimental schedule.
Female mice were singly housed with access to a running wheel starting at noon at age P36. After 5 days of acclimation to the wheel (1st Baseline), their food access was restricted to two hours per day (from 7 - 9 PM) for 3 days (1st ABA). At the end of the 1st ABA, they were housed in fresh cages with ad libitum food access and no running wheel. Following 7 days of recovery, access to a running wheel was restored. Following 4 days of wheel access with ad libitum food access (2nd Baseline), the second induction of ABA (2nd ABA) started, on P55. Mice were divided into two groups on P56, i.e. the second day of food restriction or FR2. The two groups were counterbalanced in terms of running activity on FR1. One group received P4 in oil and the other received only oil. All mice were perfused at noon on P59, at the end of the 2nd ABA, after four days of food restriction.
2.3 Vaginal cytology to determine estrous stage
These were performed along with weighing of the animal beginning in the re-acclimation phase that preceded the 2nd ABA and continuing through the end of the experiment. A pipette containing 1-2 ml of PBS was used to perform vaginal lavage. The lavage was observed under a microscope at 100 times magnification and estrous stage was determined as described in (McLean, Valenzuela, Fai, & Bennett, 2012).
2.4 Brain preparation and immunocytochemistry
2.4.1 Materials
The primary antibody directed against the α4 subunit of GABAA receptor (GABAAR) was obtained from Santa Cruz Biotechnology (catalog #SC7355, lot J1912). Previous studies demonstrated specificity of this antibody for the α4-subunit of GABAAR using three EM-immunocytochemical procedures: reduction of immunoreactivity at the plasma membrane and in the cytoplasm of pyramidal neurons in the CA1 of hippocampus of α4-KO animals at the transition between prepuberty and puberty onset, which is when immunoreactivity for the α4 and δ subunits of GABAAR emerge at the spine plasma membrane of the hippocampal CA1 (Shen et al., 2007). Reduction of immunoreactivity was also verified in the CA1 when applying the antibody solution after preadsorption with the antigen corresponding to amino acids 1-14 of the α4-subunit and when the primary antibody was omitted from the incubation procedure (Sabaliauskas, Shen, Homanics, Smith, & Aoki, 2012). This antibody has also been shown to recognize a single band at 67kDa by Western blotting (Griffiths & Lovick, 2005; Sanna et al., 2003) and to disappear following preadsorption of the antibody with a peptide corresponding to the target sequence (Sanna et al., 2003).
The secondary antibody was obtained from Electron Microscopic Sciences [Rabbit anti goat IgG Gold ultrasmall gold (0.8 nm diameter of gold conjugated to secondary antibody) catalog #25220, lot 20126/1]. The silver intensification kit used was purchased from KPL (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD, USA). Electron microscopic supplies were purchased from Electron Microscopic Sciences, and all chemicals used for perfusion, tissue storage, and immunostaining were purchased from Sigma Chem (St. Louis, MO, USA).
2.4.2. Electron microscopic immunocytochemistry
Procedures for immunocytochemistry were as described before (Aoki et al., 2012; Aoki et al., 2014). Animals were anesthetized using urethane (i.p. 0.34 g/g body weight) during the hours of 12 noon to 4 pm, then euthanized by transcardial perfusion with fixatives, consisting of 4% paraformaldehyde buffered with 0.1M phosphate buffer (pH 7.4). Glutaraldehyde-fixation was withheld until after immunocytochemistry, so as to optimize antigen-retention. Brains were postfixed for a minimum of ten days and up to 4 months, then sectioned at a thickness of 50 μm in the coronal plane spanning the dorsal hippocampus, and stored at 4 °C, free-floating in phosphate buffered (0.01M PB, pH 7.6) saline (0.9% sodium chloride) (PBS), containing 0.05% sodium azide to prevent bacterial growth.
The immunocytochemical procedure commenced by incubating the free-floating sections in PBS azide containing 1% bovine serum albumin (Sigma Chem) and 1:100 dilution of the goat primary antibody directed against the α4 subunit of GABAARs. The free-floating sections were agitated continuously at room temperature for 3 days, after which time, excess unbound primary antibodies were removed by rinsing in PBS. Sections were then incubated overnight at room temperature in the rabbit secondary antibody, consisting of an anti-goat IgG conjugated to 0.8 nm colloidal gold particles. On the following day, excess unbound secondary antibodies were removed by rinsing in PBS, then postfixed by immersing the sections in PBS containing 2% glutaraldehyde (EMSciences EM grade) for 10 minutes. After rinsing in PBS, sections were stored overnight at 4°C , then processed for silver-intensification of the colloidal gold particles, so as to enlarge the gold particles to sizes detectable by EM. The silver-intensified colloidal gold particles (SIG) ranged in sizes from 10 to 100 nm, even though all procedures were run strictly in parallel. The silver-intensified sections were processed osmium-free (Phend, Rustioni, & Weinberg, 1995), so as to avoid oxidation of the SIG particles. The heavy metals used to generate contrast were uranyl acetate, which also served to improve ultrastructural preservation (Lozsa, 1974; Terzakis, 1968) and Reynold's lead citrate. Vibratome sections were then infiltrated with EMBED-812, flat-embedded between two sheets of Aclar plastic, then ultrathin sectioned at a thickness setting of 70 nm at a cutting plane tangential to the vibratome-cut surface, so as to maximize capture of the EPON-vibratome section surface, where penetration by immunoreagents were maximal.
2.4.3 Electron microscopic quantification of α4 subunits of GABAA receptors
200 spines receiving excitatory synapses in stratum radiatum (SR) of area CA1 in the dorsal hippocampus were observed at a direct magnification of 40,000× and images captured using a 1.2 megapixel Hamamatsu CCD camera from AMT (Boston, MA) from the JEOL 1200XL electron microscope were used for quantification. Spines were identified using criteria described before (Aoki et al., 2014), which consisted of a prominence of thick postsynaptic density (PSD), lack of mitochondria or microtubules, parallel alignment of the plasma membrane associated with the PSD with that of the axon terminal containing clusters of vesicles. All spines encountered at the surface-most regions of vibratome-cut surfaces were analyzed for the position and level of immunoreactivity to the α4 subunits, strictly in the order of encounter, so as to ensure randomness of sampling, until a minimum of 200 spines were encountered from tissue of each animal.
Dendritic shafts were also sampled per animal, also in SR of CA1 of the dorsal hippocampus. These were observed at a direct magnification of 20,000x using the same camera and microscope as stated above, and also counted in the order that they were encountered along the vibratome surface. Dendritic shafts were identified by the presence of mitochondria, and only those parallel to one another were sampled to ensure that they originated from the pyramidal cell layer.
For both, the spines and dendritic shafts, the location of the SIG in each dendritic shaft and spine was noted (at the plasma membrane or intracellular) (Fig 2) and the number of each type as well as the total number was recorded.
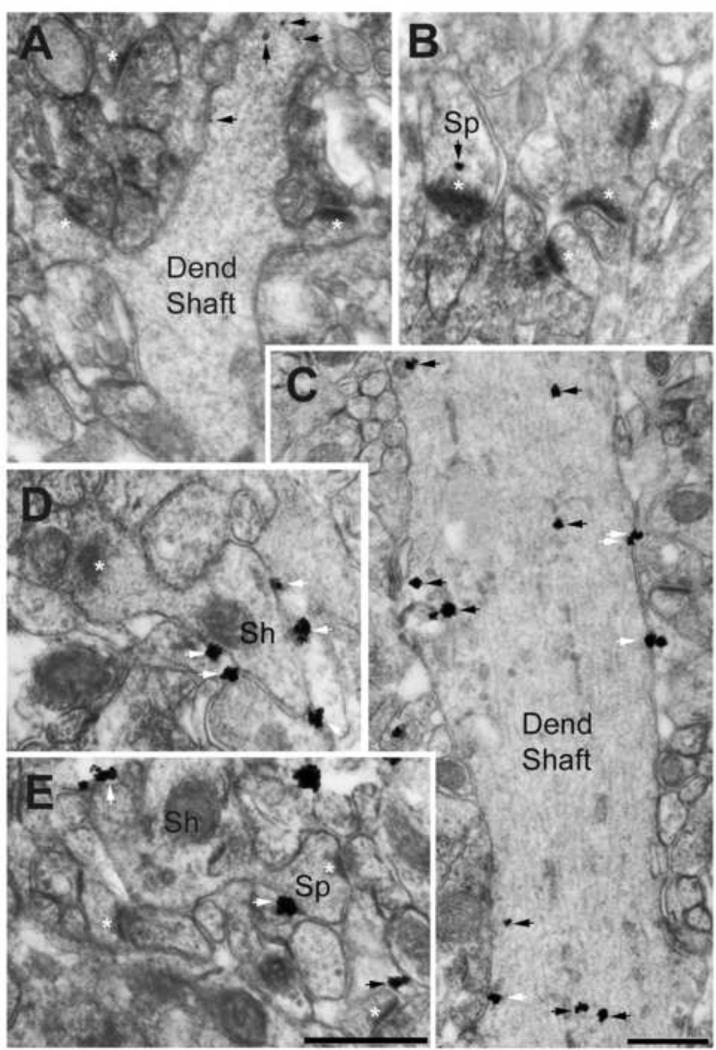
Figure 2. Electron micrographs showing α4-immunoreactivity in dendritic shafts and spines of the dorsal hippocampal CA1 of adolescent female mice that received P4 injection.
Panels A and B were taken from an animal categorized as vulnerable to ABA, while panels C, D and E were taken from an animal categorized as resistant to ABA. Animals had undergone two ABA inductions and were of age PND59. Black arrows point to cytoplasmic location of silver-intensified gold (SIG) particles reflecting α4-immunoreactivity, while white arrows point to SIG particles located at the extracellular surface of plasma membranes. Note the paucity of SIG particles in the dendritic shaft cytoplasm of the vulnerable animal (Panel A), compared to the dendritic shaft of the resistant animal (Panel C). Moreover, plasma membrane location of SIG particles is readily apparent in the dendritic shaft of the resistant animal (Panels C and ‘Sh’ in D), but not of the vulnerable animal. Similarly, SIG is sparse in spines of the vulnerable animal (Sp, white asterisks point to postsynaptic densities, Panel B) but more numerous and located more often at the plasma membrane of the resistant animal (panel E). The black arrow at the lower right corner of panel E points to cytoplasmic labeling in an astrocytic process immediately adjacent to an axo-spinous synapse with a PSD. Calibration bar in E = 500 nm and also applies to panels A, B, and D, all of which were captured at a magnification of 40,000x using AMT Camera system and Hamamatsu's CCD camera. Calibration bar in C = 500 nm, captured at a magnification of 40,000x.
The length of the plasma membrane and area of each dendritic shaft were measured using the segmented line tool and polygon tool, respectively, of NIH's software, Image J (version 1.46r). The entire plasma membrane of dendritic shafts was not always visible. Only the visible plasma membrane was counted in the length measure used for analysis. The number of plasma-membranous SIGs for each animal was divided by the total length of the plasma membrane for that animal to obtain a density value (# SIG/unit plasma membrane length). The total plasma membrane length sampled was equalized across all animals (~ 85 μm). A similar measure was calculated for intracellular SIG and total SIG where the total number of intracellular and total SIGs in dendritic shafts of an animal was divided by the total area of sampled dendritic shafts to obtain the number of SIG per unit area.
2.5 Statistics
All statistical analyses were performed using IBM's SPSS 21 and GraphPad Prism version 6. All data were tested using the D’Agostino-Pearson omnibus test for normality. Pearson's or Spearman R was computed to report correlation between measures that respectively were or were not distributed normally. Two-way ANOVA (analysis of variance) tests were conducted on measures of running wheel activity using the between-subject factors of drug and change in vulnerability to ABA as well as drug and change in severity of ABA defined in the next section. The SIG measures for total immunoreactivity and intracellular immunoreactivity in dendritic shafts were not distributed normally so they were square root transformed. Levene's test failed for plasma membranous immunoreactivity (spine and dendritic shaft, drug × change in vulnerability and drug × change in severity of ABA) and total spinous immunoreactivity (drug × change in severity of ABA). So a square root transform of the target variable was used in these instances. Mean and standard error of mean values reported in Table 6 pertain to the untransformed variable.
3. RESULTS
3.1 Quantification of wheel running activities
The goal of this study was to investigate the role of progesterone (P4) in adolescent females’ response to a repeated exposure of a stressor. We used food restriction as the stressor of the ABA induction paradigm and wheel running activity (WRA) as a measure of an animal's response to the stressor. All animals underwent two ABA inductions that were separated by 1 week of recovery: half of them received P4 during the 2nd ABA induction, while the other half received just the oil vehicle (Fig 1).
The baseline WRA to the 1st and 2nd ABA inductions were determined by taking an average of the 24-hour activity of the two days preceding the onset of food restriction. The WRA in the 1st ABA was quantified by taking an average of the 24-hour activity during the three days when food restriction was imposed. As for the 2nd ABA, either P4 or oil alone was systemically injected on the second, third and fourth day of food restriction but not on the first day. Therefore, WRA of the 2nd ABA was calculated by taking the average of the three days during which animals were food restricted and received either P4 or oil. WRA during the first food restriction day of the 2nd ABA provided another type of “baseline”, for comparing the effect of food restriction with and without P4 within individual animals (Fig 3A).
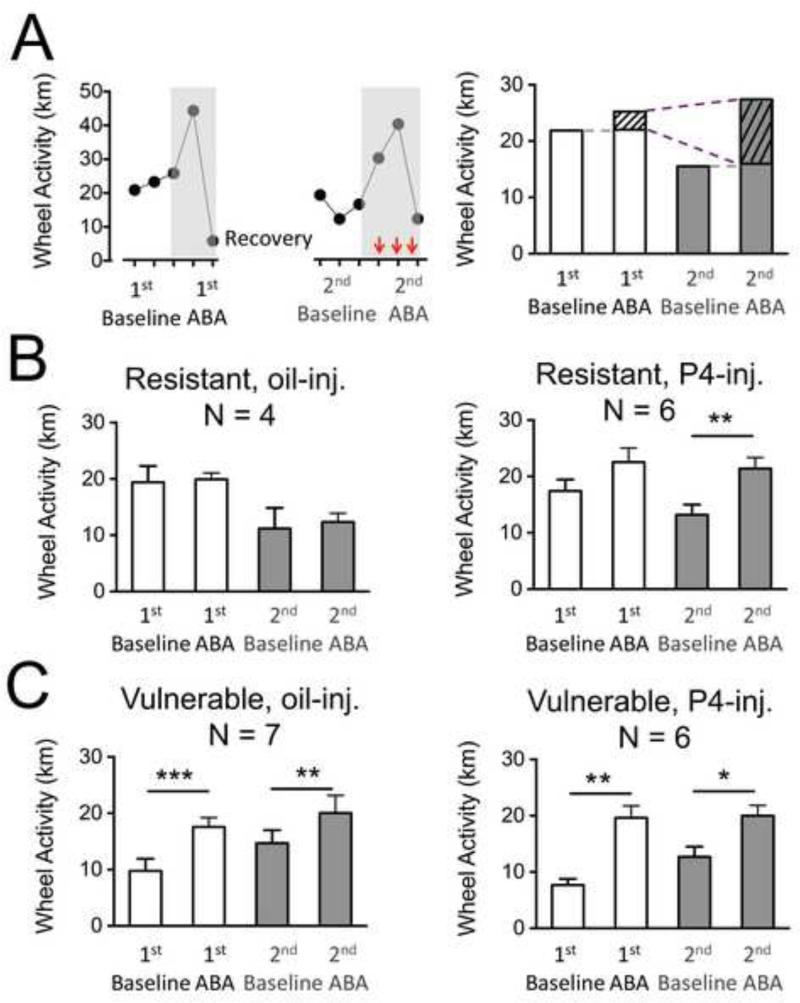
Figure 3. Averaged wheel activity per 24 hours during the four periods of a repeated ABA paradigm.
Panel A, left: Daily wheel running activity (WRA) of one animal in the relapse paradigm of ABA. The grey shaded boxes highlight the food restriction periods in the 1st and 2nd ABA. Red (dark gray) arrows indicate the last three days of the 2nd ABA when injections of P4 or oil were given.
Panel A, right: The 1st baseline and 2nd baseline WRA are averaged distances run on the wheel on the two days preceding food restriction in the 1st ABA and 2nd ABA respectively. In this example, the 2nd baseline is less than the 1st baseline, reflecting resistance that is acquired during recovery. Average distance run on the wheel during the three days of the 1st ABA and the last three days of the 2nd ABA (shown using the vertical red arrows in the left panel) are used as measure of response to ABA. The first day of food restriction (FR1) of the 2nd ABA was not used in the average because the mice did not receive an injection on that day. The dashed gray lines mark the level of baseline activity relative to the ABA activity. The portions of the average activity in the 1st and 2nd ABA marked with slanted lines refer to the measure called severity of ABA (SOA), reflecting the food restriction-evoked component of WRA. The dashed purple lines indicate the comparison of the SOAs of the 1st and 2nd ABAs, used to compute the change in SOA. In this example, the change in SOA is a positive value, indicating sensitization. This is a pattern observed for the P4-injected resistant animals (Fig. 3B-right).
Panels B and C: The wheel activities of all animals in a sub-group are averaged for each period of the experiment. For each animal, the values that are used for the group average are computed as shown in Panel A, right.
Panel B, left: Resistant animals (RWA 2nd baseline < 1st baseline) injected with oil show a non-significant increase in WRA in the ABA period compared to baseline in both episodes of ABA.
Panel B, right: Resistant animals (WRA 2nd baseline < 1st baseline) injected with P4 show a significant increase in WRA in the 2nd ABA period compared to baseline immediately prior to the 2nd ABA.
Panel C, left: Vulnerable animals (WRA 2nd baseline > 1st baseline) injected with oil show a significant increase in WRA in the ABA period compared to baseline in both episodes of ABA.
Panel C, right: Vulnerable animals (WRA 2nd baseline > 1st baseline) injected with P4 show a significant increase in WRA in the ABA period compared to baseline in both episodes of ABA.
* indicates p < 0.05 ; ** indicates p < 0.01 ; *** indicates p < 0.001.
3.2 The 1st baseline running is an indicator of vulnerability to ABA
Twenty-one out of the 23 animals that underwent two ABAs increased running in response to food restriction during the 1st ABA and 8 out of 9 of the oil vehicle-injected animals increased running in response to food restriction during the 2nd ABA. These animals also exhibited a wide range of WRA during the days preceding and during food restriction. During the 1st Baseline, preceding the 1st ABA, some ran greater than 20 km per day, while others ran less than 5 km per day. Similarly, in response to the food restriction during the 1st ABA, some ran 10 km per day, while others increased their running to 30 km per day. In spite of these great individual differences, WRA during the 1st Baseline correlated positively and strongly with WRA during the 1st ABA (R = 0.49, p = 0.016 for both groups combined) (Fig 4A-left). Similarly, both the oil-injected and P4-injected groups exhibited a strong positive correlation between the WRA of the 2nd Baseline and WRA of the 2nd ABA (R = 0.92, 0.69 and p < 0.0001, = 0.012 for the oil-injected, and P4-injected groups, respectively) (Fig 4B-left). Therefore, baseline WRA is an indicator of vulnerability to ABA, defined as the likeliness of an animal to develop hyperactivity in response to food restriction.
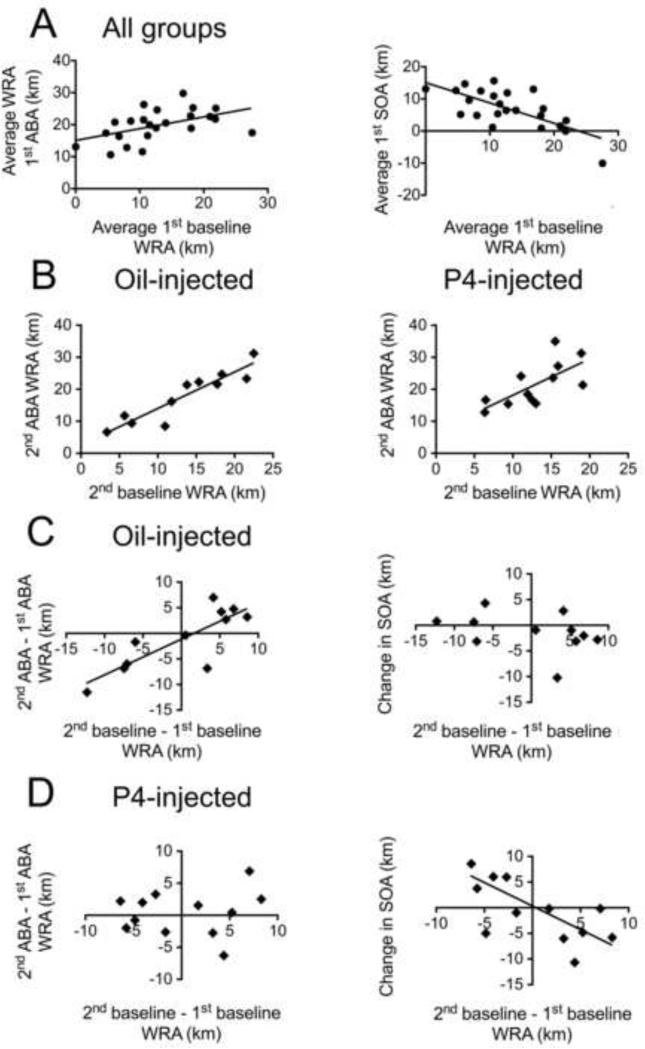
Figure 4. Pre-food restriction baseline WRA is a good predictor of vulnerability to ABA.
The measure of response to ABA induction is WRA during the food restriction period. Vulnerability to ABA is the likeliness that an animal will develop hyperactivity in response to ABA.
Panel A. Baseline WRA for both groups of animals, shown on the x-axis, is the average distance run per 24 hours on the wheel in the two days preceding food restriction in the 1st ABA. Y-axis of left graph shows average distance run per 24 hours on the wheel during the three days of the 1st ABA. The two measures show a positive correlation (R = 0.49, p = 0.01). Y-axis of right graph shows averaged WRA during the 1st ABA adjusted for the 1st baseline WRA, a measure that reflects food- restriction induced increase in activity (SOA). First SOA is negatively correlated with 1st baseline (R = − 0.66, p < 0.0001).
Panel B. 2nd baseline WRA, shown on the x-axis is a good predictor for WRA during the 2nd ABA for the oil- (R = 0.92, p < 0.0001) and P4-injected (R = 0.69, p = 0.012) groups. The first day of food restriction in the 2nd ABA was not included in the average because the mice did not receive an injection on that day.
Panels C and D. Graphs on the left show that the change in vulnerability (plotted on x-axis) is a good predictor for WRA of 2nd ABA compared to the 1st ABA (plotted on y-axis) for the oil-injected (R = 0.79, p = 0.0034) (Panel C) but not for the P4-injected animals (R = 0.12, p = 0.7) (Panel D).
Graphs on the right show that the change in vulnerability is a good predictor for the change in SOA (measurement of the worsening of sensitivity to food restriction) of the P4-injected animals (R = − 0.81, p = 0.0013) (Panel D), but not of the oil-injected animals (R = −0.53, p = 0. 091) (Panel C).
The animal's response to the 1st ABA is comprised of two portions: one is related to the preference of the animal for wheel running, measured as the 1st baseline. The other response is elicited by the stress of food restriction, which we will call severity of 1st ABA (1st SOA), calculated as the WRA during the 1st ABA minus WRA during the 1st baseline (explained verbally in Table 1 and pictorially in Fig. 3A, right). The 1st baseline WRA was negatively correlated with the 1st SOA. This negative correlation was statistically significant (R = −.703, p = 0.0002 for both groups combined, in Fig. 4A right). In other words, the more that mice ran during the pre-food restriction baseline phase (towards the right along the x-axis of Fig. 4A), the less that they increased running during the food restriction phase (smaller Y-axis value). This correlation between baseline running and food restriction-evoked running was not seen during the 2nd ABA (severity of 2nd ABA, 2nd SOA, calculated as the WRA during 2nd ABA minus WRA during the 2nd baseline, as defined in Table 1 and pictorially explained in Fig. 3A) for either the oil- (R = −0.04, p = 0.905) or P4-injected (R= −.317, p = 0.316) groups. This difference between the 1st and 2nd baseline may have arisen because the 2nd baseline was affected by the stressful experience of the 1st ABA, whereas the behavior in the 1st baseline and 1st ABA carried no history of stress exposure.
Table 1.
Measures and comparisons of wheel running activity
| Measure | Computation |
|---|---|
| Severity of ABA (SOA) in 1st ABA | Average of wheel running activity (WRA) during food restriction period (three days of 1st ABA) minus WRA in 1st baseline period |
| Severity of ABA (SOA) in 2nd ABA | Average of wheel running activity (WRA) during food restriction period (2nd, 3rd and 4th days of 2nd ABA) minus WRA in 2nd baseline period |
| Vulnerability to ABA | WRA during period preceding food restriction |
| Change in vulnerability | WRA in 2nd baseline minus WRA in 1st baseline |
| ➢ Resistant : | value of measure is negative |
| ➢ Vulnerable : | value of measure is positive |
| Change in SOA | WRA in (2nd ABA minus 2nd baseline) minus (1st ABA minus 1st baseline) |
| ➢ Non sensitized : | value of measure is negative |
| ➢ Sensitized : | value of measure is positive |
| Comparison of WRA in 2ndABA | Use |
| With SOA 1st ABA | Response to a repeated stressor |
| With WRA in 2nd baseline | Effect of food restriction in late adolescence |
| With WRA of first day of 2nd ABA | Effect of P4 injection as no injection is given on first day |
3.3 Change in baseline running (change in vulnerability) is a predictor of the change in severity to the 2nd ABA, a relationship altered by P4
Since baseline running in the absence of food restriction predicted vulnerability to ABA, we quantified the change in baseline running by comparing WRA during 2nd baseline, the period preceding the 2nd ABA, relative to the baseline WRA preceding the 1st ABA (Table 1). Henceforth, we refer to this measure as the “change in vulnerability”. We categorized the mice as ‘more vulnerable’ (henceforth referred simply as ‘vulnerable’), if the measure ‘2nd baseline minus 1st baseline’ running was positive and ‘more resistant’ (henceforth referred to as ‘resistant’ or ‘resilient’) if this value was negative (Fig. 3). High runners in the 1st baseline tended to reduce their baseline running after the 1st ABA, as shown by a negative correlation between 1st baseline WRA and the change in vulnerability (R = −.66, p = .001 for both groups combined).
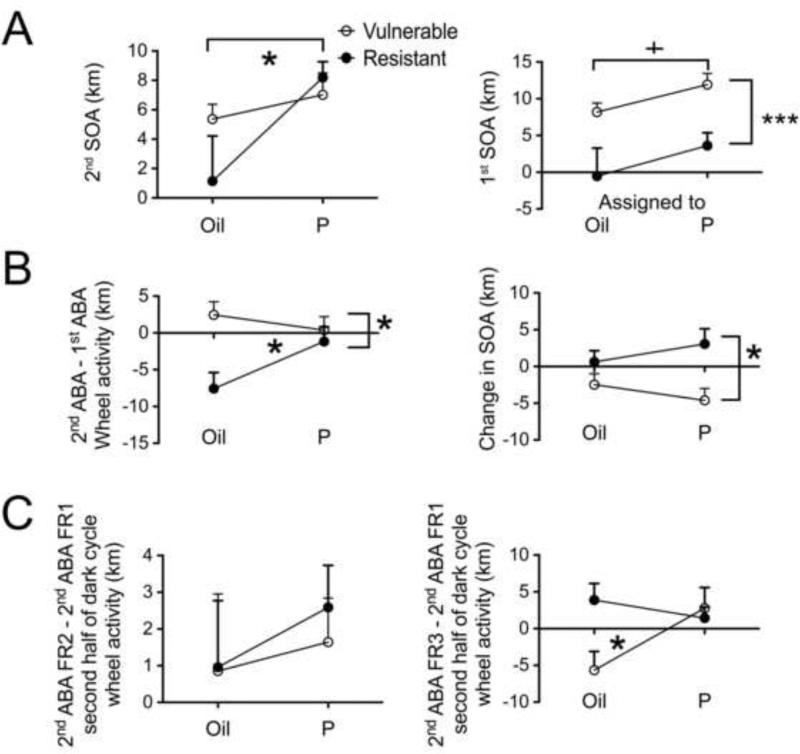
Mice classified as ‘vulnerable’ showed higher severity than the animals ‘resistant’ to the 1st ABA (1st SOA) (Fig 3-C, Fig 5A - right, Mvul = 9.707 ± 1.054, n=13; Mres = 3.282 ± 2.064, n=10). This result suggests that vulnerable mice were affected by the 1st ABA induction and this effect persisted through recovery and up to the 2nd baseline phase.
Figure 5. P4 exacerbates the response to the 2nd ABA.
Panel A, left graph shows that P4-injected mice responded with higher SOA to the 2nd ABA than did the oil-injected mice (* indicates p = 0.012 by two-way ANOVA using drug and change in vulnerability as fixed factors). The y-axis shows the averaged WRA during the 2nd ABA adjusted for the 2nd baseline WRA, a measure that reflects food- restriction induced increase in activity (SOA).
Panel A, right graph shows that animals categorized as vulnerable (P- or oil-injected) based on a positive value for 2nd baseline minus 1st baseline WRA showed a higher SOA to the 1st ABA than the animals categorized as resistant (P-or oil-injected) (*** indicates p = 0.0003 by two-way ANOVA using drug and change in vulnerability as fixed factors).
Despite a trend to a difference between the P4-injected (vulnerable plus resistant) and oil-injected (vulnerable plus resistant) mice in their SOA in the 1st ABA (+ indicates p = 0.058), post hoc tests showed no difference between the animals that would later receive P4 within the resistant or vulnerable groups or oil injections within the resistant or vulnerable groups. This indicates that there was no pre-existing difference between the animals assigned to the two drug groups within each category of resistant and vulnerable animals.
Panel B, left graph shows response severity in 2nd ABA compared to the 1st ABA. A two-way ANOVA using drug and change in vulnerability as fixed factors shows a main effect of vulnerability and a significant interaction between the factors of vulnerability and drug injection (* indicates p < 0.05). P4 injection removes the resistance of mice exhibited in the 2nd baseline period.
Panel B, right graph shows change in SOA. A two-way ANOVA using drug and change in vulnerability as fixed factors shows a main effect of vulnerability, no effect of P4 and no interaction between the factors of vulnerability and drug (* indicates p < 0.05). Post hoc tests reveal that the P4-injected resistant animals show a sensitization reflected in the change in SOA as compared to the P4-injected vulnerable animals.
Panel C, left graph shows the change in WRA during the second half of dark cycle (between 7 and 13 hours after P4 injection) from the first day of food restriction (FR1) to the second day of food restriction (FR2) in the 2nd ABA. There are no differences between either the vulnerable and resistant groups or the P4-injected and oil-injected groups.
Panel C, right graph shows the change in WRA during the second half of dark cycle (between 7 and 13 hours after P4 injection) from the first day of food restriction (FR1) to the third day of food restriction (FR3) in the 2nd ABA. There is a significant interaction between the vulnerability and drug groups indicated by * (p < 0.05), suggesting that P4 removes the resistance of animals causing them to responding severely, with a delay of 7 hours, specifically after the second injection.
The change in vulnerability (x-axis of Fig. 4-C) was a good predictor of RWA of 2nd ABA, compared to the 1st ABA (Y-axis of Fig. 4-C) in the oil-injected animals (R = 0.79, p = 0.0034) (Fig. 4C - left) but not for the P4-injected animals (R = 0.12, p = 0.7) (Fig 4D - left). We surmised that since P4 was injected during the food restriction phase of the 2nd ABA, P4 may have altered the animal's severity of response to food restriction during the 2nd ABA (2nd SOA), without altering the animal's response to other sources of stress, such as social isolation, that could have influenced the baseline WRA. In order to isolate the effect of food restriction upon WRA, we calculated the change in SOA, mathematically expressed as WRA in (2nd ABA minus 2nd baseline) minus (1st ABA minus 1st baseline) (Table 1). A positive value for this measure indicated that the animal had exacerbated its response to the 2nd ABA as compared to the 1st ABA. A negative or zero value indicated that the animal had not exacerbated its response to the 2nd ABA as compared to the 1st. The change in SOA correlated negatively with the change in vulnerability of the P4-injected animals (R = − 0.81, p = 0.0013, Fig. 4D - right), meaning that P4 exacerbated the ABA response especially more for those animals that had undergone a reduction in preference for running, following recovery from the 1st ABA. This relationship was absent for the oil-injected animals (p > 0.2) (Fig 4C - right), and the change in SOA observed among the resistant oil-injected individuals (those with negative values along the x-axis) were overall less than the values observed for the P4-injected group. Together, these results indicate that the exacerbated hyperactivity effect upon the resistant individuals was specific to the P4 treatment.
3.4 P4 worsens the severity of response in the 2nd ABA
The specificity of the effect of P4 upon ABA, relative to the pre-food restriction vulnerability, prompted us to search for additional behavioral effects of the drug that depended on vulnerability. WRA of the oil-injected group during the 2nd ABA (average of FR2, FR3 and FR4) was compared to two kinds of behavioral measurements (Table 1): 1) WRA during the 1st ABA, indicating change in response to a repeated stressor; 2) 2nd Baseline WRA, indicating the effect of food restriction in that repeated episode of ABA. WRA of the P4-injected group during the 2nd ABA was compared to three kinds of behavioral measurements: 1) WRA during the 1st ABA, indicating P4's modulation of a change in response to a repeated stressor; 2) 2nd Baseline WRA, indicating P4's modulation of the effect of food restriction in that repeated episode of ABA; and 3) WRA on FR1 of the 2nd ABA (a day before P4 injection began), which also indicated an effect of P4 upon the animal's response to a repeated stressor.
The oil- and P4-groups showed no differences in the WRA on 2nd ABA FR1 (two-way ANOVA, change in vulnerability X subsequent assignments to the drug groups). We measured the increase of WRA in response to food restriction with and without P4, averaged over FR2, FR3 and FR4 of 2nd ABA (2nd SOA, Table 1). P4-injected mice responded with higher 2nd SOA than did the oil-injected mice (MP = 7.75 ± 0.95, n=12, Moil = 3.82 ± 1.35, n=11, Mean difference = 3.93 ± 1.63) (Fig 5A - left). This P4-effect was not likely to be due to a pre-drug difference in stress response, as demonstrated by the two groups’ largely overlapping response to food restriction during the 1st ABA (i.e., 1st SOA, Table 1), which was without P4 injection (MPre-P = 8.52 ± 1.68, n=12, MPre-oil = 5.15 ± 1.78, n=11) (Fig. 5-A, graph on right).
Thus, P4 exacerbated the food restriction-evoked hyperactivity of animals to the 2nd ABA.
3.5 P4 removes the resistance acquired during recovery
To evaluate the effect of P4 upon response to repeated stress, we examined the measure of RWA to the 2nd ABA minus the 1st ABA WRA. To follow up a significant interaction between drug and change in vulnerability, we tested simple main effects which revealed response to repeated stress by the oil-injected resistant group (mean = −7.57 ± 2.33) to be significantly less than that of the oil-injected vulnerable group (mean = 2.46 ± 1.76, p = 0.003) (Fig 5-B, left graph). There were no differences between P4-injected resistant and vulnerable groups. These results indicate that P4 removed individual differences in the resistance to repeated stress that was acquired during recovery.
3.6 No acute effect of P4
Mice were not injected on FR1 of the 2nd ABA, so that FR1 WRA could serve as a baseline against which to compare the WRA of the same animals under the influence of P4 injections. On FR2, FR3 and FR4, P4 was injected at 6 pm, an hour before food access. To evaluate the acute (1 to 7 hours) effect of P4 injection on the mice, we computed the WRA during the first half of the dark cycle (7 pm to 1 am) after the first, second and third day's exposure to P4, on FR2, FR3, and FR4 respectively. These values will be referred to as “FR2 minus FR1 (first half of dark cycle)”, “FR3 minus FR1 (first half of dark cycle)” and “FR4 minus FR1 (first half of dark cycle)”. We found no acute effect of P4 (all p > 0.13). All sub-groups of mice, whether injected with P4 or with the oil vehicle alone, ran less in the first half of dark cycle on FR2, FR3 and FR4 than FR1 (data not shown). There were no group differences by two-way ANOVAs.
3.7 Delayed effect of P4:P4 removes the resistance 6 hours after the second injection
Having observed that P4 has no acute effect, we then set out to determine whether P4 has any delayed effect, i.e., during the second half of the dark cycle, from 1 am to 7 am. We computed the measures FR2 minus FR1, FR3 minus FR1 and FR4 minus FR1 (second half of dark cycle, i.e. 1 am to 7 am). The measures FR2 minus FR1 (second half of dark cycle) (Fig 5-C - left) and FR4 minus FR1 (second half of dark cycle) (data not shown) showed no group difference by a two- way ANOVA (all p > 0.47). However, the running activity on FR3 minus FR1 (second half of dark cycle) showed a significant interaction between the factors of drug and change in vulnerability (F (1,19) = 5.173, p = 0.034) (Fig 5C - right). Simple main effects showed no significant differences between any 2 sub-groups. These findings indicate that P4 removed the resistance that animals had acquired during the 7 days of recovery, with a delay of 7 to 13 hours after the second injection.
3.8 Sensitization to the 2nd ABA is observed in P4-injected resistant mice
The change in SOA measure compares the food restriction evoked hyperactivity in the 2nd ABA relative to the 1st ABA. It provides a measure of sensitization to repeated ABA. As described previously in the first section under Results and in Table 1, animals with positive values of change in SOA were categorized as ‘sensitized’ or worsened, while animals with negative or zero values were categorized as ‘non-sensitized’.
Resistant animals (P- and Oil-groups combined) exhibited a positive change in SOA (Mres = 2.097 ± 1.379), whereas the vulnerable animals exhibited a negative change in SOA (Mvul = −3.454 ± 1.097). Simple main effects revealed that the P4-injected resistant animals showed a sensitization reflected in the positive change in SOA (mean = 3.071 ± 2.075) as compared to the P4-injected vulnerable animals (mean = −2.463 ± 1.49) (Fig 5B - right). Oil-injected animals showed little change in SOA, whether vulnerable or resistant (Fig. 5B- right)
3.9 Weight loss
There were no group differences in the weight on baseline, or on any day of the 2nd ABA or at perfusion as a proportion of baseline weight (all p > 0.07). On average, the mice lost 18.8 ± 0.9 % of their pre-food restriction weight.
3.10 Open Field
P4 is converted to THP and exert sedating effects at certain doses (Korneyev & Costa, 1996). In order to ensure that P4 was not sedating, OF testing was conducted on FR4 at 6:30 pm, 30 minutes after the injections. Data were collected from 11 P4 – injected and 9 oil - injected mice. The distance travelled per minute in the OF was same between the two groups of mice by a t-test (t (18) = − 1.15, p = 0.276). A two- way ANOVA also did not show any effect of drug (F (1, 16) = 2.4, p = 0.14) or change in vulnerability (F (1,16) = 1.115, p =0.3) or any interaction (F (1, 16) = 0.007, p = 0.93). The time spent in the center of the OF was the same between the two groups of mice by a t-test (t (18) = 0.5, p = 0.62). A two- way ANOVA also did not show any effect of drug (F (1, 16) = 0.68, p = 0.42) or change in vulnerability (F (1,16) = 2.79, p =0.11) or any interaction (F (1, 16) = 0.35, p = 0.55). These findings demonstrate that P4 did not affect the level of anxiety of the mice 30 minutes after injection on FR4.
3.11 Estrous stage
Mice are known to run more on proestrus than on other days (Basterfield, Lumley, & Mathers, 2009) and have been shown to remain in diestrus following food restriction (Nelson, Gosden, & Felicio, 1985; Riddle et al., 2013). In order to determine whether the effects of P4 on WRA were also affected by the estrous stage, we assessed it starting 2 days before the onset of food restriction in the 2nd ABA to the end of the experiment. To examine whether the delayed effect of P4 during the second half of the dark cycle of FR3 in the 2nd ABA could be attributed to estrous stage, we performed Chi-Squared Test of Independence of Categorical Variables. Estrous stage had no impact on how much they ran on FR3 in the second half of the dark phase relative to FR1 (Pearson Chi-Square (2) = 2.79, p = 0.24, Likelihood Ratio (2) = 3.55, p = 0.169, Linear-by-Linear Association (1) = 0.754, p = 0.38). The estrous stage during 2nd baseline, FR1, FR2, FR3 or FR4 was not associated with changes in SOA (all p > 0.07).
3.12 α4-immunoreactivity in the stratum radiatum of the dorsal hippocampal CA1
To identify a possible cellular mechanism underlying the behavioral effect of P4, we examined the subcellular levels and localization of α4 subunits of GABAARs in pyramidal neurons of the dorsal hippocampal CA1. Our rationale for examining α4 subunits of GABAARs was two-fold: P4's metabolite, THP, is known to regulate the expression as well as the activity of α4-containing GABAARs in this brain (reviewed in (H. Shen et al., 2007)). Moreover, we had previously observed that adolescent female rats elevate levels of α4 and δ subunits of GABAARs at spines of the dorsal hippocampus within 4 days of ABA induction (Aoki et al., 2012).
The level of α4-immunoreactivity was measured in three ways, using silver intensified gold immunolabeling (SIG) as indications of the presence of α4 subunit proteins generating immunoreactivity: at the plasma membrane, in the cytoplasm and plasmalemmal+cytoplasmic (i.e., total) within two distinct subcellular domains of pyramidal neurons - in the spine or in the dendritic shaft (Fig. 2). Immunoreactivity at the plasma membrane is likely to reflect the presence of functional α4-containing GABAA receptors (α4-GABARs) and could directly impact the local membrane potential. Immunoreactivity in the cytoplasm is likely to reflect the presence of α4-GABAARs in the reserve pool that could be inserted into the plasma membrane upon appropriate signaling. The total α4-immunoreactivity reflects an increase or decrease in the protein level of the subunit, independent of the subcellular trafficking step. For α4-immunoreactivity at spines, the levels were compared across animals by normalizing to the total number of spines encountered, labeled or unlabeled. For α4 immunoreactivity at dendritic shafts, the levels were compared across animals by normalizing to the total lengths of the plasma membrane or total area of the cytoplasm analyzed, strictly in the order of encounter of dendritic profiles at the surface-most portions of vibratome sections. Tables 2, 3 and 4 and Fig. 6 summarize the correlations between measures of individual animals’ WRA and α4-immunoreactivity on spines and dendritic shafts.
Table 2.
Correlation between α4 levels at the plasma membrane of spines and dendritic shafts with measures of WRA
| Group | 1st Baseline | 2nd Baseline | Change in vulnerability | WRA 1st ABA | SDA in 1st ABA | WRA 2nd ABA | SOA in 2nd ABA | WRA in 2nd ABA minus 1st ABA | Change in SOA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil injected | Spinous plasma membrane SIG level |
Pearson Correlation Sig. (2-tailed) | 0.40 | 0.10 | −0.24 | 0.02 | −0.67 | −0.21 | −0.67 | −0.25 | −0.01 |
| 0.26 | 0.79 | 0.50 | 0.95 | 0.03 | 0.57 | 0.03 | 0.49 | 0.97 | |||
| P4 injected | Spinous plasma membrane SIG level |
Pearson Correlation Sig. (2-tailed) | 0.77 | 0.18 | −0.79 | 0.14 | −0.70 | 0.30 | 0.17 | 0.11 | 0.78 |
| 0.00 | 0.58 | 0.00 | 0.66 | 0.01 | 0.35 | 0.60 | 0.72 | 0.00 | |||
| Oil-injected | Shaft plasma membrane SIG density |
Pearson Correlation Sig. (2-tailed) | 0.36 | −0.03 | −0.33 | 0.45 | −0.10 | 0.06 | 0.21 | −0.18 | 0.28 |
| 0..30 | 0.92 | 0.36 | 0.19 | 0.78 | 0.87 | 0.55 | 0.61 | 0.46 | |||
| P4-injected | Shaft plasma membrane SIG dersity | Pearson Correlation Sig. (2-tailed) | 0.59 | −0.09 | −0.79 | 0.01 | −0.63 | −0.10 | −0.02 | −0.11 | 0.61 |
| 0.04 | 0.79 | 0.00 | 0.98 | 0.03 | 0.76 | 0.95 | 0.74 | 0.03 | |||
Pearson correlations between α4-immunoreactivity at the plasma membrane of spines and dendritic shafts of pyramidal neurons in the CA1 of the dorsal hippocampus in mice subjected to two episodes of ABA were computed with the following measures of running wheel activity in ABA: 1st baseline, 2nd baseline, change in vulnerability, WRA in 1st ABA, WRA in 2nd ABA, SOA in 1st ABA, SOA in 2nd ABA, WRA in 2nd ABA minus WRA in 1st ABA and change in SOA. For significant correlations with p < 0.05, the R and p values are bolded and in red (gray).
Table 3.
Correlation between cytoplasmic α4-immunoreactivity in spines and dendritic shafts with WRA
| Group | 1st Baseline | 2nd Baseline | Change in vulnerability | WRA 1st ABA | SOA in 1st ABA | WRA 2nd ABA | SOA in 2nd ABA | WRA in 2nd ABA minus 1st ABA | Change in SOA | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil-injected | Spinous cytoplasmic SIG level |
Pearson Correlation Sig. (2-tailed) | −0.52 | −0.34 | 0.13 | −0.68 | 0.12 | −0.52 | −0.63 | −0.22 | −0.70 |
| 0.27 | 0.34 | 0.77 | 0.03 | 0.75 | 0.13 | 0.05 | 0.55 | 0.02 | |||
| P4-injected | Spinous cytoplasmic SIG level |
Pearson Correlation Sig. (2-tailed) | 0.85 | 0.21 | −0.86 | 0.26 | −0.67 | 0.22 | 0.02 | −0.10 | 0.68 |
| 0.00 | 0.51 | 0.00 | 0.41 | 0.02 | 0.49 | 0.93 | 0.75 | 0.02 | |||
| Oil-injected | Shaft cytoplasmic SIG density |
Spearman Correlation Sig. (2-tailed) | 0.26 | 0.16 | 0.03 | 0.04 | 0.00 | 0.02 | 0.19 | 0.16 | 0.19 |
| 0.47 | 0.65 | 0.94 | 0.92 | 0.99 | 0.97 | 0.61 | 0.65 | 0.61 | |||
| P4 injected | Shaft cytoplasmic SIG level | Spearman Correlation Sig. (2-tailed) | 0.57 | −0.12 | −0.82 | 0.23 | −0.55 | 0.15 | 0.30 | 0.08 | 0.73 |
| 0.06 | 0.72 | 0.00 | 0.45 | 0.07 | 0.65 | 0.34 | 0.82 | 0.01 | |||
Correlations between cytoplasmic α4-immunoreactivity in spines and dendritic shafts of pyramidal neurons in the CA1 of the dorsal hippocampus in mice subjected to two episodes of ABA were computed with the following measures of running wheel activity in ABA: 1st baseline, 2nd baseline, change in vulnerability, WRA in 1st ABA, WRA in 2nd ABA, SOA in 1st ABA, SOA in 2nd ABA, WRA in 2nd ABA minus WRA in 1st ABA and change in SOA. For significant correlations with p < 0.05, the R and p values are bolded and in red (gray).
Table 4.
Summary of two-way ANOVAs performed on the measures of α4-immunoreactivity in spines and dendritic shafts of mice injected with P4 or oil
| Spine | Shaft | |||||||
|---|---|---|---|---|---|---|---|---|
| ANOVA | Effect | Membrane | Cytoplasm | Total | Membrane | Cytoplasm | Total | |
| Drug × change in vulnerability | Drug | F | 0.443 | 0.280 | 1.477 | 0.117 | 0.000 | 0.005 |
| p | 0.514 | 0.603 | 0.240 | 0.737 | 0.998 | 0.942 | ||
| Change in vulnerability | F | 11.139 | 6.511 | 4.862 | 12.211 | 9.515 | 10.209 | |
| p | 0.004 | 0.020 | 0.041 | 0.003 | 0.006 | 0.005 | ||
| Drug × change in | F | 3.579 | 8.271 | 2.735 | 5.287 | 10.897 | 11.263 | |
| p | 0.075 | 0.010 | 0.116 | 0.034 | 0.004 | 0.004 | ||
| Drug × change in SOA | Drug | F | 0.742 | 2.965 | 0.122 | 1.362 | 1.238 | 1.106 |
| p | 0.400 | 0.102 | 0.731 | 0.258 | 0.280 | 0.307 | ||
| Change in SOA | F | 9.853 | 4.165 | 3.855 | 9.582 | 17.830 | 17.852 | |
| p | 0.006 | 0.056 | 0.065 | 0.006 | 0.001 | 0.001 | ||
| Drug × change in | F | 14.180 | 13.137 | 11.379 | 4.136 | 12.640 | 12.668 | |
| p | 0.001 | 0.002 | 0.003 | 0.057 | 0.002 | 0.002 | ||
The two factors used in the two-way ANOVAs are drug and either change in vulnerability or change in SOA. The significant effects are bolded and in red (dark gray). The marginally significant effects are bolded and italicized. Note that Levene's test for homogeniety of variance was significant (p = 0.02) for the ANOVA on α4-immunoreactivity in the cytoplasm of dendritic shafts despite a square root transformation.
Figure 6. Relations of WRA with α4-immunoreactivity in spines of mice injected with P4 or oil.
Graphs on the left show correlation between α4-immunoreactivity (on the x-axis) and WRA (on the y-axis) for progesteroneP4-injected mice while graphs on the right show corresponding relationships for the oil-injected animals.
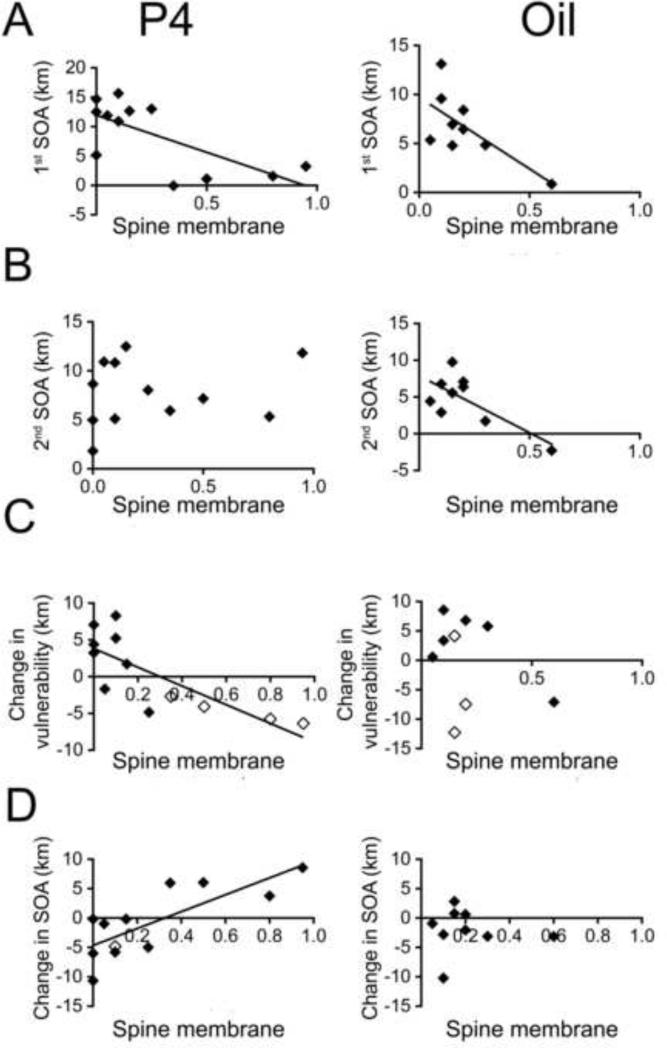
Panel A. α4-immunoreactivity at the spine plasma membrane (‘Spine membrane’ in the x-axes), seen as silver-intensified gold (SIG) particles, is significantly correlated with the severity of 1st ABA for the P4- injected (R = −0.69, p = 0.011, left graph) as well as oil-injected (R = −0.69, p = 0.037, right graph) animals.
Panel B. α4-immunoreactivity at the spine plasma membrane is significantly correlated with the severity of 2nd ABA for the oil- injected (R = −0.72, p = 0.027) but not the P4-injected animals.
Panel C. α4-immunoreactivity at the spine plasma membrane is significantly correlated with the change in vulnerability for the P4-injected animals (R = −0.785, p = 0.003) but not for the oil-injected animals. Open diamonds indicate animals that showed behavioral sensitization to the 2nd ABA and filled diamonds indicate animals that did not show sensitization. Panel D. α4-immunoreactivity at the spine membrane is significantly correlated with the change in SOA for the P4-injected animals (R = 0.781, p = 0.003) but not in the oil-injected animals.
3.12.1 α4-immunoreactivity at the plasma membrane of spines and dendritic shafts: α4-containing GABAARs may play different roles in the presence and absence of exogenous P4
In the absence of P4 (i.e., the oil-injected group), the level of α4-immunoreactivity at the plasma membrane of spines (but not dendritic shafts) was negatively correlated with SOA in the 1st ABA (= 1st ABA - 1st baseline) (Fig. 6A-right, Table 2) and with SOA in the 2nd ABA (= 2nd ABA - 2nd baseline), (Fig. 6-B, right; Table 2). This finding replicates an earlier study of adolescent female hippocampal spines following only one ABA (Aoki et al., 2014), indicating that α4-immunoreactivity contributes towards the animal's resistance to food restriction-evoked hyperactivity.
In the P4-injected group, α4-immunoreactivity in the plasma membrane of spines as well as at dendritic shafts was more than 100% greater than the values detected among the oil-injected group. This difference was significant (Table 5) and also evident by visual inspection of electron micrographs (Fig. 2). Moreover, this enhancement depended on the animal's pre-P4 behavioral trait: P4 enhanced α4 immunoreactivity preferentially among those with low 1st SOA (Fig. 6A-left) and among those that acquired resistance during recovery (negative y-axis values in Fig. 6C-left). In fact, Fig 6C-left indicates a strong correlation between a change in vulnerability during recovery (y-axis) and α4-immunoreactivity, indicating that the more that animals gained resistance during recovery (the more negative the y-axis value), the more that they could respond to the subsequent P4 injection with increased α4-immunoreactivity.
Table 5.
Mean and standard error of mean values for α4 – immunoreactivity for mice injected with P4 or oil
| Spine | Shaft | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Sub-group | N | Membrane | Cytoplasm | Total | Membrane | Cytoplasm | Total |
| P4 injected | Resistant | 6 | 0.48 ± 0.14 | 1.1 ± 0.2 | 0.97 ± 0.18 | 7.05 ± 1.75 | 9.8 ± 2.98 | 10.12 ± 3.02 |
| Vulnerable | 6 | 0.058 ± 0.027 | 0.17 ± 0.066 | 0.23 ± 0.17 | 0.76 ± 0.48 | 0.7 ± 0.21 | 0.68 ± 0.24 | |
| Sensitized | 4 | 0.65 ± 0.14 | 1.28 ± 0.23 | 1.21 ± 0.13 | 8.43 ± 2.03 | 12.97 ± 3.48 | 13.29 ± 3.5 | |
| Non-sensitized | 8 | 0.081 ± 0.031 | 0.31 ± 0.11 | 0.29 ± 0.072 | 1.65 ± 0.9 | 1.44 ± 0.58 | 1.46 ± 0.62 | |
| Oil injected | Resistant | 3 | 0.32 ± 0.14 | 0.52 ± 0.15 | 0.88 ± 0.32 | 2.74 ± 0.98 | 3.89 ± 1.73 | 3.99 ± 1.75 |
| Vulnerable | 7 | 0.18 ± 0.042 | 0.57 ± 0.16 | 0.78 ± 0.16 | 1.81 ± 0.61 | 3.75 ± 0.56 | 3.8 ± 0.57 | |
| Sensitized | 3 | 0.17 ± 0.017 | 0.37 ± 0.044 | 0.57 ± 0.073 | 2.73 ± 0.99 | 4.34 ± 1.33 | 4.43 ± 1.36 | |
| Non-sensitized | 7 | 0.24 ± 0.073 | 0.63 ± 0.16 | 0.91 ± 0.21 | 1.82 ± 0.61 | 3.56 ± 0.68 | 3.61 ± 0.68 | |
| Resistant | All | 9 | 0.43 ± 0.1 | 0.9 ± 0.17 | 0.94 ± 0.14 | 5.62 ± 1.37 | 7.87 ± 2.22 | 8.07 ± 2.26 |
| Vulnerable | All | 13 | 0.12 ± 0.03 | 0.39 ± 0.1 | 0.53 ± 0.13 | 1.33 ± 0.41 | 2.35 ± 0.53 | 2.36 ± 0.54 |
| Sensitized | All | 7 | 0.44 ± 0.12 | 0.89 ± 0.22 | 0.94 ± 0.15 | 5.99 ± 1.62 | 9.27 ± 2.6 | 9.49 ± 2.64 |
| Non-sensitized | All | 15 | 0.16 ± 0.042 | 0.46 ± 0.1 | 0.58 ± 0.13 | 1.73 ± 0.54 | 2.43 ± 0.51 | 2.46 ± 0.53 |
Measures for α4 –immunoreactivity on spines are computed as the number of SIGs found per 10 spines, whether on the plasma membrane, in the cytoplasm or overall in the spine (at the plasma membrane + cytoplasmic).
Measures for α4 –immunoreactivity on dendritic shafts were computed as follows. The number of membranous SIGs for each animal was divided by the total length of the plasma membrane sampled for that animal to obtain a density value (# SIG/100 μm of plasma membrane length). The total plasma membrane length sampled was equalized across all animals (~ 85 μm). A similar measure was calculated for cytoplasmic SIG and total (at the plasma membrane + cytoplasmic) SIG where the total number of cytoplasmic and total SIGs in the dendritic shafts of an animal was divided by the total area of sampled dendritic shafts to obtain the number of SIG per unit μm2.
Red italics indicate that among the P4-injected animals, values corresponding to the resistant and sensitized animals are higher than those corresponding to the vulnerable and non-sensitized animals, respectively.
Bolded and italicized values indicate a significant difference within the P4-injected animals as described for the red italicized values with an additional significant difference between the P4-injected and corresponding oil-injected group.
Bolded values corresponding to oil-injected animals indicate these are significantly higher than the corresponding P4-injected animals.
Bolded values corresponding to overall resistant and sensitized group indicates these are significantly higher than the vulnerable or non-sensitized group as applicable.
The strongly negative correlation of oil-injected brains’ α4-immunoreactivity with SOA of the 2nd ABA (Fig. 6B-right) was absent among the P4-injected animals (Fig. 6B-left). This indicates that the P4 injection during the 2nd ABA perturbed the relationship between α4-immunoreactivity and resistance to ABA. Instead, the levels of α4-immunoreactivity at the plasma membrane of spines as well as dendritic shafts of the P4-injected animals were correlated with the change in SOA (Fig. 6D-left). This correlation was positive, indicating that the higher the α4-immunoreactivity, the greater was the worsening of SOA.
3.12.2 Cytoplasmic (intracellular) α4-immunoreactivity in spines and dendritic shafts: P4 administration results in differential synthesis of the α4 subunit
In the P4-injected animals, the correlation of cytoplasmic α4-immunoreactivity in spines as well as dendritic shafts with WRA was similar to that seen for the α4-immunoreactivity at the plasma membrane: SOA in 1st ABA (negative correlation in spines, trend in dendritic shafts), and change in vulnerability (negative correlation for both spines and dendritic shafts) (Table 3). As was suggested for the plasma membrane localization of α4-immunoreactivity, these correlations indicate differential P4 injection induced synthesis of α4 subunits in animals, depending on their trait behavior reflected in the baseline WRA.
In the oil-injected group, there was a negative correlation between cytoplasmic α4-immunoreactivity in spines and change in SOA that was not seen at the plasma membrane of spines. This correlation was in a direction opposite to that of the P4-injected group (Table 3) and indicates that the higher the α4-immunoreactivity, the less that SOA worsened. This pattern is consistent with the relationship between behavior and α4-immunoreactivity at the plasma membrane (Table 2) and in the cytoplasm (Table 3) that were held constant throughout the 1st and 2nd ABA of oil-injected animals of this study (SOA in 1st ABA and SOA in 2nd ABA) and for previously studied cohorts that received no injection (Aoki et al., 2014): namely that α4 expression confers resistance against ABA through increased synthesis (in the cytoplasm) and trafficking of the α4-containing GABAARs to the plasma membrane. They further support the findings of the plasma membrane α4-immunoreactivity – namely, that α4-containing GABAARs influence behavior differently in the presence and absence of exogenous P4. Specifically, higher cytoplasmic α4-immunoreactivity was associated with more habituation in oil-injected animals but more sensitization in P4-injected animals.
3.12.3 Total (plasmalemmal + cytoplasmic) α4-immunoreactivity in spines and dendritic shafts
In the P4-injected animals, the total α4-immunoreactivity in spines and dendritic shafts showed the same relationships to measures of RWA as exhibited by the cytoplasmic α4-immunoreactivity (not shown). In the oil-injected animals, the total α4-immunoreactivity in spines (but not in dendritic shafts) showed a negative correlation with 2nd SOA and change in SOA (trend), parallel to those exhibited by the cytoplasmic α4-immunoreactivity (not shown).
3.13 Impact of change in vulnerability upon P4 effects
Given that the effect of P4 on ABA behavior depended upon whether they were resistant or vulnerable prior to P4 injection, we determined the mean value of α4-immunoreactivity for each group, categorized according to their behavior and drug treatment (Table 5). We then examined the effect of the change in vulnerability upon α4-immunoreactivity levels in the oil- and P4-injected mice (Table 4 and Fig. 7).
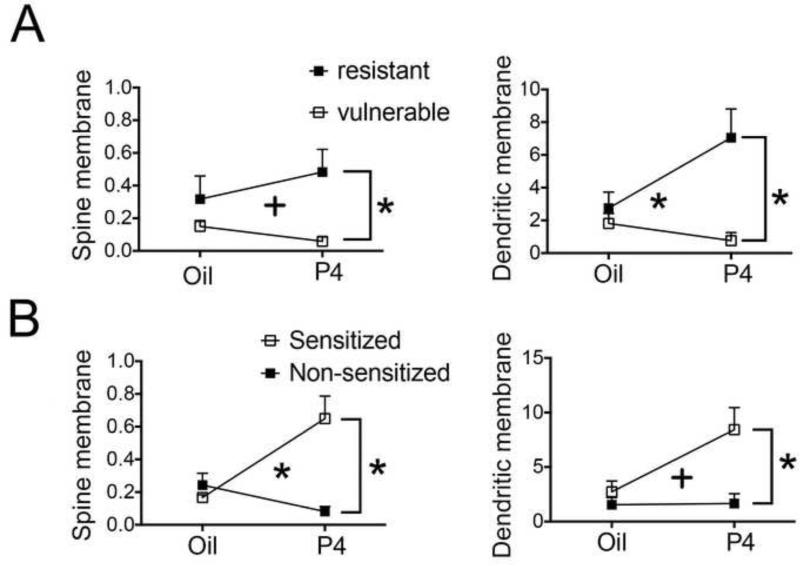
Figure 7. Effect of change in vulnerability and P4 on α4-immunoreactivity.
Panels A - left and A-right show mean values and standard errors of mean of α4-immunoreactivity along the plasma membrane in spines (left graphs) and dendritic shafts (right graphs) of vulnerable and resistant groups as well as P4- and oil-injected groups. Two-way ANOVAs using vulnerability and drug as fixed factors show a significant effect of vulnerability and interaction between the fixed factors for all measures.
Panels B left and right show mean values and standard errors of the mean of α4-immunoreactivity along the plasma membrane in spines (left graph) and dendritic shafts (right graph) of sensitized and non-sensitized groups as well as progesterone-injected and oil-injected groups. Two-way ANOVAs using change in SOA and drug as fixed factors show a significant effect of change in SOA and interaction between the fixed factors for all measures. ‘Spine membrane’ refers to immunoreactivity at the plasma membrane of spines.
* indicates p < 0.5 and + indicates p < 0.1.
3.13.1. Spinous α4-immunoreactivity is higher in resistant animals, especially those injected with P4
α4-immunoreactivity at spines was higher in the CA1 of resistant animals (oil- or P4-injected) than of the vulnerable animals (2.47 times higher at the plasma membrane, 1.32 times higher at the cytoplasm and 0.78 times higher for total SIG counts, Table 5). Simple main effects to follow up a trend to an interaction revealed that P4- injected resistant animals have higher α4-immunoreactivity at spines, compared to the values from the P4-injected vulnerable animals (7.27 times higher at the plasma membrane, p = 0.001 [Fig. 7A-left]; 5.24 times higher at the cytoplasm, p=0.001; 3.15 times higher for total counts, p=0.008 [not shown]) (Table 5). These observations indicate that P4 injection had opposite effects, depending on the vulnerability that the animals exhibited prior to P4 injections. As a consequence of the bidirectional action of P4, the oil-injected vulnerable animals also had 2.35 times higher level of total (plasmalemmal plus cytoplasmic) α4-immunoreactivity at spines than spines of P4-injected vulnerable animals (p = 0.032) ( Table 5).
There was no difference in α4-immunoreactivity between the oil-injected resistant and vulnerable animals at the plasma membrane and/or in the cytoplasm of spines.
3.13.2. Dendritic shaft α4-immunoreactivity is higher in resistant animals, especially those injected with P4
α4-immunoreactivity at dendritic shafts followed the same pattern observed for the spines. Specifically, α4-immunoreactivity was higher at dendritic shafts of resistant animals than of vulnerable animals (3.22 times higher at the membrane, 2.35 times higher in the cytoplasm and 2.42 times higher for total SIG counts, Table 5). Simple main effects to follow up a significant interaction revealed that P4-injected resistant animals showed 8.27 times higher α4-immunoreactivity than P4-injected vulnerable animals (8.27 times higher at the plasma membrane, p = 0.001 [Fig. 7A-right]; 13 times higher in the cytoplasm, p<0.001 [not shown]; 13.88 times higher for total SIG counts, p<0.001 [not shown]) (Table 4).
As a consequence of the bidirectional action of P4, the oil-injected vulnerable animals also had higher level of α4-immunoreactivity than P4-injected vulnerable animals (4.36 times higher at the cytoplasm, p = 0.016; 4.59 times higher for total counts, p=0.013). Levene's test for homogeneity of variance was significant for this ANOVA (p = 0.004).
These findings indicate that P4 injection increased α4-immunoreactivity at dendritic shafts of the resistant group and somewhat decreased α4-immunoreactivity for the vulnerable group.
3.14 Impact of P4 and change in SOA
To examine any possible association between the behavioral sensitization to repeated ABA and α4-immunoreactivity on the spine or dendritic shaft immunoreactivity, we conducted two-way ANOVA on the measures of immunoreactivity using the two fixed factors of drug and change in SOA.
3.14.1 Spinous α4-immunoreactivity is higher in P4-injected, behaviorally sensitized animals
A significant interaction between the two factors of drug and change in SOA in their effect on the plasmalemmal immunoreactivity on spines was found (Table 4, Fig 7B-left). The α4-immunoreactivity of P4-injected sensitized group was 1.67 times higher than that of the oil-injected sensitized group (p = 0.012) and 7 times higher than that of the P4-injected non-sensitized group (p < 0.001) (Table 5). These findings indicate that among the P4-injected animals, increased α4-immunoreactivity at the spine plasma membrane was associated with sensitization. Decreased α4-immunoreactivity among the P4-injected animals was associated with non-sensitization, as suggested by the twice higher α4-immunoreactivity in oil-injected non-sensitized animals compared to the P4-injected non-sensitized (p = 0.019).
Similarly to the plasma membrane component, there was a significant interaction between drug and change in SOA in their effect on cytoplasmic immunoreactivity in spines (Tables 4 and 5). α4-immunoreactivity of the P4-injected sensitized group was 2.47 times higher than that of the oil-injected sensitized group (p = 0.005) and 3.07 times higher than that of the P4-injected non-sensitized group (p < 0.001). These findings indicate that among the P4 injected animals, increased cytoplasmic α4-immunoreactivity in the spine was associated with sensitization. The interaction between drug and change in SOA in their effect was also significant for the total count of α4-immunoreactivity (at the plasma membrane plus cytoplasm). The P4-injected sensitized group had 3.12 times higher α4-immunoreactivity than the P4 injected non-sensitized group (p =0.001). Oil-injected non-sensitized group had 2.11 times higher α4-immunoreactivity than P-injected non-sensitized group (p = 0.004).
Together, these findings indicate that P4 has an effect on the relationship between α4-immunoreactivity in spines and behavior in ABA. Specifically, among the P4-injected animals, increased α4-immunoreactivity at spines was associated with sensitization and decreased α4-immunoreactivity with non-sensitization.
3.14.2. Dendritic shaft α4-immunoreactivity is higher in P4-injected, behaviorally sensitized animals
Analysis of dendritic shafts revealed a similar pattern as that seen for dendritic spines, in that, among the P4-injected animals, increased α4-immunoreactivity was associated with sensitization and decreased α4-immunoreactivity with non-sensitization. The one difference between the spine and dendritic shaft components was the subcellular component exhibiting the greatest interaction between the two factors of drug and change in SOA: it was the plasma membrane for spines and the cytoplasm for dendritic shafts. Specifically, sensitized animals exhibited 2.46 times higher α4-immunoreactivity at the dendritic shaft plasma membrane than of non-sensitized animals. Analysis of simple main effects to follow up a trend to an interaction revealed sensitized P4- injected group to have 4.11 times higher α4-immunoreactivity than P4 injected non-sensitized group (p = 0.001) (Fig. 7B-right). This is less than the 7-times higher α4-immunoreactivity (p<0.001) observed for the spine plasma membrane. There was a significant interaction between drug and change in SOA in their effect on cytoplasmic α4-immunoreactivity in dendritic shafts (Tables 4 and 5). The P4-injected sensitized group had thrice the α4-immunoreactivity of the oil-injected sensitized group (p = 0.001) and 8 times higher than that of the P4 injected non-sensitized group (p < 0.001). This latter difference between the sensitized versus non-sensitized group is greater than what was observed for the spine cytoplasm (3x, p=0.001).
There was a significant interaction between drug and change in SOA in their effect on total dendritic shaft α4-immunoreactivity (Table 4). The P4-injected sensitized group had thrice the α4-immunoreactivity than the oil-injected sensitized group (p = 0.012) and 8.1 times higher than that of the P4 injected non-sensitized group (p < 0.001). Oil-injected non-sensitized group also had 1.47 times greater α4-immunoreactivity than P4 injected non-sensitized (p = 0.038).
4. DISCUSSION
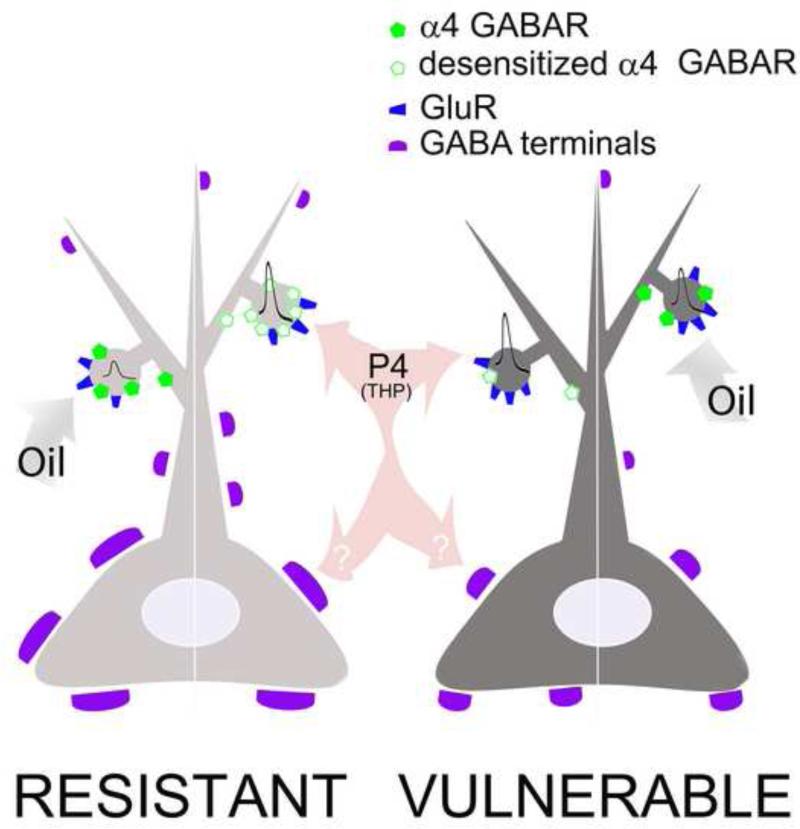
The main finding is that P4 reversed the effect of α4-immunoreactivity with respect to the animal's sensitivity to food restriction stress. In the absence of exogenous P4, elevation of α4-immunoreactivity was associated with the protective behavior, consisting of the suppression of high WRA to repeated ABA: suppression of high WRA is protective, because high WRA can be lethal to food-restricted adolescent female mice (Chowdhury et al., 2013). In the presence of P4, the higher the α4-immunoreactivity, the greater was the animal's sensitivity to repeated ABA. We provide a working hypothesis for the reversal of the role of α4-immunoreactivity evoked by P4.
4.1 Baseline voluntary WRA as predictor of vulnerability
Mice exhibit great variability in their behavioral response to ABA. We have systematically captured this variability by quantifying the change in vulnerability to ABA as well as the changes in severity of response to repeated ABA. The quantitative analysis revealed that WRA during the 1st baseline is a good predictor of an individual's vulnerability to ABA. From analysis of repeated ABA, we demonstrated that changes in the baseline WRA of an animal is also a good predictor of changes in the severity of the animal's response to ABA, i.e., the change in vulnerability. The stress response of high voluntary runners has not been studied directly by others, but we can compare our results with previous studies on the stress response of high aerobic capacity runners. High aerobic capacity runners are reported to exhibit lower cort responses and are proposed to have genetic similarities with animals selectively bred for high voluntary running (Waters et al., 2008). They also adopt a more active coping strategy to stress than low aerobic capacity runners in the forced swim test (Burghardt et al., 2010). Our data revealing a smaller increase in food restriction-evoked activity during the 1st ABA among animals with high baseline activity (Figure 4A-right) are consistent with Burghardt's result on high aerobic capacity runners, since suppression of hyperactivity during food restriction is a more adaptive coping strategy for animals reared in captivity.
4.2 Relationships between WRA and P4 administration
Prompted by previous findings that P4's metabolite, THP, is anxiolytic (Bitran et al., 1995; C. A. Frye & Paris, 2011; C. A. Frye & A. A. Walf, 2004; Koonce & Frye, 2013) and that the food restriction-evoked hyperactivity correlates positively with anxiety (G. Wable et al., 2015), we tested the hypothesis that P4 ameliorates responses to a second ABA induction. Contrary to our prediction, we found that P4 exacerbates the hyperactivity response (Figure 5A, left) as well as sensitize mice that had acquired resistance during recovery from the first ABA induction (Fig 5B, right). Sensitization to the 2nd ABA was quantified as the change in SOA. Among the oil-injected animals, the change in SOA was strongly and positively correlated to the 1st baseline WRA. In contrast, this correlation was lost by P4 injection. Moreover, for the P4-injected group, a stronger and negative correlation was seen between the change in SOA and change in vulnerability, indicating that acquisition of resistance during the recovery period (change in vulnerability is negative) rendered them to become more sensitized to the 2nd ABA (change in SOA is positive). That the resistant animals demonstrated low baseline WRA just prior to the 2nd ABA indicates that their P4 response during the 2nd ABA is specific to the combination of wheel and food restriction and that behavioral sensitization is a distinct effect of the repeated ABA.
Although the dose of P4 that we used has been shown to be anxiolytic in ad libitum fed female mice of similar ages (Cheryl A Frye & Alicia A Walf, 2004; Koonce & Frye, 2013), this was not effective for our food-restricted mice, as revealed by our open field test results. The mechanism of change in anxiety level due to food restriction may be via the rise in cort, leading to increased signaling of principal neurons in the amygdala and increasing its outflow (Joels, Sarabdjitsingh, & Karst, 2012). We have also observed a reduction of α4-GABAAR expression within inhibitory interneurons in the basolateral amygdala of ABA-induced rats, which could contribute to increased signaling of the principal neurons there (G. S. Wable et al., 2013). Possibly, the rise of cort prevents the anxiolytic action of P4, which is reported to be due to the interaction of THP and GABAARs (Canonaco et al., 1993; Engin & Treit, 2007; C. A. Frye & A. A. Walf, 2004; Koonce & Frye, 2013; Reddy et al., 2005). Pubertal stress is found to alter the hormone response to anxiety testing at 8 weeks of age (Olesen, Ismail, Merchasin, & Blaustein, 2011), supporting an idea that an interaction between the HPA axis and P4 could be at play during ABA induction.
4.3 Sensitization to ABA response in P4-injected resistant animals might be via α4-containing GABAARs
4.3.1. P4 affects α4-immunoreactivity differently, depending on the animal's behavioral phenotype that preceded P4 injection
P4 injection brought about an increase in α4-immunoreactivity in resistant animals, the same group that had shown high running activity in the first baseline period. A difference in motivation to run on the wheel is associated with considerable difference in neurobiology and other behaviors in rodents, such as nest building and stress response (reviewed in (Rhodes, Gammie, & Garland, 2005)). Mice that choose to run more show a higher increase in brain derived neurotrophic factor (BDNF) protein in the hippocampus (Johnson, Rhodes, Jeffrey, Garland Jr, & Mitchell, 2003). BDNF is an important regulator of the synthesis (Roberts, Hu, Lund, Brooks-Kayal, & Russek, 2006) and trafficking of α4βδ-GABAARs from the cytoplasm to the plasma membrane (Joshi & Kapur, 2009). P4 increases BDNF expression (Meyer et al., 2013; Singh & Su, 2013) and BDNF may possibly be the mediator of the trait behavior-dependent effect of neurosteroids such as THP on the expression of α4-containing GABAARs. The greater wheel running might result in a higher propensity for mature BDNF expression via enhanced post-translational modification (Sartori et al., 2011). P4 administration would lead to a greater increase in mature BDNF release, which, in turn, would lead to higher α4 subunit expression. The lack of correlation between α4-immunoreactivity and 1st baseline WRA in the oil-injected animals suggests that P4 contributed to the production of additional BDNF that led to an increase of α4 subunit.
The decrease in α4-immunoreactivity in P4-injected vulnerable animals could be due to an increase in proBDNF on P4 administration that did not ultimately convert to BDNF because they were low runners in baseline and possibly did not have any enhancement of enzymatic machinery for conversion of pro-BDNF to the mature form. proBDNF is known to have effects that are antagonistic to those of BDNF (Lu, Pang, & Woo, 2005). BDNF enhances and proBDNF represses synthesis and activity of synaptic GABAAR (Riffault et al., 2014). Thus, it is conceivable that an increase in proBDNF without increased BDNF to balance it might result in a decreased expression of α4 subunits as seen in the P4-injected vulnerable animals. The lack of a decrease of α4-immunoreactivity in oil-injected vulnerable animals as compared to the oil-injected resistant animals demonstrates that P4 administration contributed to the reduction of α4-immunoreactivity in the vulnerable animals.
It is known that estrogen influences the effect of exercise on BDNF induction (Berchtold, Kesslak, Pike, Adlard, & Cotman, 2001). Given that P4 interacts with estrogen in several ways to affect signaling, disease state and behavior, including wheel running (Baudry, Bi, & Aguirre, 2013; Rodier III & Segal, 1977), it is important that future studies investigate the role of P4 in BDNF induction by exercise.
Previous studies indicate that changes in the expression of α4-containing GABAARs are observed 48 hours following exposure to THP (allopregnanolone), the metabolite of P4 (H. Shen, Q. H. Gong, M. Yuan, & S. S. Smith, 2005; S. S. Smith, Shen, Gong, & Zhou, 2007), closely matching the timing of behavioral change in vulnerability that we observed (WRA on FR3, after the second P4 injection). Fluctuations in the level of P4 and THP due to the injection may have been superimposed with the release of these hormones from the adrenal glands due to the stress of food restriction, resulting in the increased expression of the α4-containing GABAAR in the P4-injected resistant animals. The sensitizing effect of P4 on behavior might then be mediated by the α4-containing GABAAR (as demonstrated by the correlation between α4-immunoreactivity and change in SOA) due to one or more of the following reasons.
4.3.2. α4-containing GABAAR may mediate sensitization of ABA response in P4-injected animals
Among the oil-injected animals, the negative correlation between plasma membrane α4-immunoreactivity and the 1st as well as the 2nd SOA (Table 2) is consistent with our past results in which we found α4-immunoreactivity to be associated with less severe response to ABA (Aoki et al., 2014). By comparison, the equally strong and negative correlation between plasma membrane α4-immunoreactivity and the 1st SOA among the animals that were subsequently assigned to receive P4-injection indicates that both the to-be-oil- and to-be-P4 groups were protected from severe ABA response, at least in part, by increased α4-GABARs, but that the subsequent P4-injection perturbed the α4-GABAAR-mediated protection. What might be the mechanism underlying the P4-perturbation of α4-GABAAR-mediated protection against ABA vulnerability?
Higher excitability of the pyramidal neurons of the CA1 dorsal hippocampus is associated with higher anxiety level (Huttunen & Myers, 1986). Although α4-containing GABAARs contribute towards anxiolysis through tonic inhibition of pyramidal neurons, the α4-containing GABAAR that are localized to the CA1 hippocampal pyramidal neurons are unusual in that they are desensitized by P4's metabolite, THP, thereby reversing these receptors’ and THP's joint role from being anxiolytic to being anxiogenic (Hui Shen et al., 2007). Although relatively less known, the anxiogenic role of α4-GABAARs and THP in the CA1 is solidly substantiated: the unusual property of THP is due to the Cl− flux direction-dependent desensitizing effect it has on the receptor, conferred by arginine 353 of the α4-subunit. THP desensitizes α4βδ-GABAARs only when expressed in cells with inward Cl− flux. The direction of Cl− flux is inward for α4βδ-GABAARs expressed by CA1 pyramidal neurons, but is outward for α4βδ-GABAARs expressed by the dentate gyrus granule cells. This difference in the Cl− flux across the two brain regions is likely to be the reason THP does not desensitize α4βδ-GABAARs in the dentate gyrus but operates consistently as a positive modulator there. The reason THP is uniquely anxiogenic at puberty (H. Shen et al., 2007) may be because α4βδ-GABAAR expression level is low prepubertally and in adulthood in the CA1 but rises at puberty. In contrast, α4βδ-GABAAR expression is consistently high throughout life in the dentate gyrus. This latter idea of CA1's α4βδ-GABAARs mediating anxiogenesis at puberty through the action of THP is supported by the finding that global knockout of the δ-subunit eliminates the THP-evoked anxiety at puberty (H. Shen et al., 2007). The current study extends upon this previous finding by demonstrating that CA1 pyramidal neurons can be evoked to continue express heightened levels of α4-containing GABAAR beyond puberty (ca PND 32), up to early adulthood (ca PND 59), rendering these neurons to be modulated throughout adolescence by THP, and generating anxiety (H. Shen et al., 2007; Hui Shen et al., 2007). Presumably, desensitization of α4-containing GABAAR increases excitability of the CA1 pyramidal neurons, because it removes the shunting inhibition that counteracts the glutamate receptor-mediated excitatory input to spines (Fig. 8). In support of this idea, we have observed that female adolescent rats express heightened levels of NR2A and NR2B subunits of NMDA receptors within dendritic spines of the hippocampal pyramidal cells in the CA1 following ABA induction (Aoki et al., unpublished observations).
Figure 8. Proposed mechanism for P4-induced behavioral sensitization of resistant animals.
P4 injection exacerbated WRA response to the 2nd ABA induction. α4-immunoreactivity was associated with resistance measured before the 2nd ABA, yet also with behavioral sensitization in P4-injected mice. We propose a mechanism to explain these dual associations of α4-GABAARs. Assumptions for the model are: 1) the injected P4 is readily converted to THP; 2) exposure to P4's metabolite, THP, causes insertion of α4-containing GABAARs into the membrane, along with up-regulation of its expression during the first 48 hours (Hui Shen et al., 2005); 3) A certain proportion of α4-GABAARs in the dorsal hippocampus of female adolescents are desensitized by the stress hormone THP and this desensitization is associated with increased anxiety (Shen et al., 2007) ; 4) anxiety is positively correlated with WRA (Wable et al., 2015). Our proposal is that α4-GABAARs play a protective role by counterbalancing the ABA-induced increased excitability of CA1 pyramidal neurons following food restriction, and although exogenous P4 enhances α4 expression, especially among those that can gain resistance, it also interferes with α4-GABAARs’ protective role by desensitizing α4-GABAARs.
The green polygons symbolize α4-GABAARs; while the unfilled green polygons symbolize desensitized α4-GABAARs. Blue symbols depict glutamatergic receptors. Animals that up-regulated α4-GABAARs in response to the 1st ABA became resistant (neuron to the left, with many α4-GABAARs on spines), while animals that failed to up-regulate α4-GABAARs remained vulnerable to ABA, as they were during the 1st ABA induction (neuron to the right, with fewer α4-GABAARs). P4 injection during the 2nd ABA increased α4-GABAARs expression more than by oil alone (compare the right versus left halves of the neuron belonging to the resistant animal). P4 injection upon vulnerable animals led to reduction of α4-GABAARs, relative to the expression level observed by neurons of vulnerable animal injected with oil vehicle, only (compare the left and right halves of the vulnerable animal's neuron). Exogenous P4 also desensitized α4-GABAARs. This desensitization is likely to have unmasked the glutamatergic receptors (GluR, blue symbols on spines) that would have been counterbalanced by α4-GABAARs.
Sensitization occurred for the P4-injected resistant animals (right half of the left neuron), because desensitization of α4-GABAARs by THP unmasked more of the glutamatergic receptors, thereby rendering the neurons hyper-excitable, as indicated by the amplitude of the unitary excitatory postsynaptic current (epsc) drawn within its spine. Oil-treated resistant animals were not sensitized, because α4-GABAARs continued to mask glutamatergic receptors (left half of the left neuron, note the small amplitude of the epsc). Neurons of the vulnerable animals were hyper-excitable, with or without P4 treatment, because the α4-GABARs were never sufficient in number to counterbalance the glutamatergic receptors (note the intermediate to high amplitudes of the unitary epscs). Since the extent of GABAergic innervation onto CA1 pyramidal neurons contributes to the animals’ resistance to ABA induction (magenta bars surrounding dendrites and somata) (Chowdhury et al., 2013), it would be worthwhile to test whether P4 and or its metabolite, THP, alters GABAergic innervation upon these neurons (question mark in the arrow). Elevation of α4-GABAARs is associated with a concomitant down-regulation of synaptic, i.e., α1-containing GABAARs (Hui Shen et al., 2005), another factor that may contribute towards P4-mediated sensitization.
α4βδ-GABAARs are exquisitely sensitive to THP, more so than are the α1βγ-GABAARs (Belelli & Lambert, 2005). Therefore, the developmental switch of THP action is due to a switch in the subtype of GABAARs that predominates the hippocampal CA1: THP's primary action during adulthood and pre-pubertally is upon the predominantly expressed α1-containing GABAARs, which THP potentiates, thereby inducing anxiolysis. In contrast, during puberty onset, THP's primary target becomes the α4βδ GABAARs, which it desensitizes, due to the developmentally regulated rise in the expression of these receptors, thereby inducing anxiogenesis (H. Shen et al., 2007; S. S. Smith, Aoki, & Shen, 2009). In addition to this developmental switch in the GABAARs, neurosteroid treatment in adulthood also induces an increase of α4 subunits, concomitant with a reduction of α1 subunit levels in the hippocampal CA1 (Hui Shen, Qi Hua Gong, Maoli Yuan, & Sheryl S Smith, 2005). There are a number of other reports indicating that heightened levels of α4-GABAARs are accompanied by reductions of α1-GABAARs (Brooks-Kayal & Russek, 2012; Kumar, Kralic, O'Buckley, Grobin, & Morrow, 2003; Liang, Cagetti, Olsen, & Spigelman, 2004; Roberts et al., 2006; H. Shen et al., 2005). The P4 treatment upon late adolescent ABA animals could have two combined effects: (1) an increase of α4-to-α1 ratio; and (2) desensitization of α4-GABAARs, which dominate over the potentiation of α1-GABAARs, due to a subunit switch. This study did not evaluate α1-immunoreactivity, so the contributions made by reductions of α1 subunits towards excitability of pyramidal cells following repeated exposures to food restriction stress remain undetermined. Also undetermined are the contributions made by other parts of the hippocampus, such as the dentate gyrus, or other brain regions to the behavioral phenotype.
One further limitation of our study is that anxiety testing was conducted only on FR4 and only in the acute phase, i.e. an hour after P4 administration. However, we have found that increased anxiety is correlated with higher RWA in the ABA model and that the change in WRA from baseline to the food-restricted phase is also correlated to the change in anxiety level (G. Wable et al., 2015). The correlation between WRA and anxiety level could be the final link between the expression of α4–containing GABAARs and WRA.
4.4 Implications for mental illness
The model of stress we used, ABA, is currently the most accepted animal model for anorexia nervosa (AN), an eating disorder characterized by self-imposed starvation and often by excessive exercise and anxiety, causing malnutrition and severe weight loss (American Psychiatric Association, 2013). AN has a very high relapse rate (greater than 30 %) and the highest mortality rate among all psychiatric disorders (10 -15 %);(Birmingham, Su, Hlynsky, Goldner, & Gao, 2005; Carter, Blackmore, Sutandar-Pinnock, & Woodside, 2004; Herzog et al., 1999; Strober, Freeman, & Morrell, 1997; Sullivan, 1995).
It has been suggested that P4 could ameliorate the symptoms of AN (Young, 1991). Our data indicate that Young's proposal should be considered cautiously, to the extent that findings from the animal model could be extrapolated to the human disease. This is because we have encountered at least one dose of P4 which, when combined with the individual's history of improved resilience, could exacerbate stress reactivity, while showing no efficacy for those individuals with vulnerability to stress. The lack of literature on the impact of administration of P4 in AN could suggest a lack of efficacy. A recent study about the administration of estrogen with P4 found no impact on eating attitudes or body shape perception in adolescents with AN (Misra et al., 2013). There was a decrease in trait anxiety (but not state anxiety) associated with estrogen replacement, but not with P4 replacement.
Since the level of anxiety and response to ABA in the form of wheel activity are correlated, the impact of P4 on the wheel running activity in late adolescence is important to consider in anxiety disorders as well. Future studies with altered doses of P4 and altered ages of ABA induction are needed to more fully understand the impact of P4 on anxiety, ABA and AN.
HIGHLIGHTS.
Stress reactivity is predicted by animals’ baseline voluntary wheel running.
Stress resilience is associated with α4-GABAAR levels in CA1 pyramidal cells.
Progesterone exacerbates stress reactivity in resilient mice.
Progesterone does not reduce hyperactivity in mice that fail to attain resilience.
Progesterone metabolite may cause the negative effect by desensitizing α4-GABAARs.
ACKNOWLEDGEMENTS
We are thankful to the members of the Office of Veterinary Services – Dr. Mark Klinger, Andrea Grappone, and Jennifer Goodwin - for their support in making the mouse ABA paradigm possible. We are grateful to Tara Chowdhury, Kei Tateyama, Alisa Liu, Clive Miranda and Jung-Yun Min for technical assistance and helpful discussions. This work was supported by The Klarman Foundation Grant Program in Eating Disorders Research to CA, R21MH091445-01 to CA, R01NS066019-01A1 to CA, R01NS047557-07A1 to CA, NEI Core grant EY13079 to CA, R25GM097634-01 to CA, NYU's Research Challenge Fund to CA, R21 MH105846 to CA, T32 MH019524 to GSW and Fullbright Scholarship to YWC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience & Biobehavioral Reviews. 2003;27(1):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Aoki C, Sabaliauskas N, Chowdhury T, Min JY, Colacino AR, Laurino K, Barbarich-Marsteller NC. Adolescent female rats exhibiting activity-based anorexia express elevated levels of GABA(A) receptor alpha4 and delta subunits at the plasma membrane of hippocampal CA1 spines. Synapse. 2012;66(5):391–407. doi: 10.1002/syn.21528. doi: 10.1002/syn.21528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Wable G, Chowdhury TG, Sabaliauskas NA, Laurino K, Barbarich-Marsteller NC. alpha4betadelta-GABAARs in the hippocampal CA1 as a biomarker for resilience to activity-based anorexia. Neuroscience. 2014;265:108–123. doi: 10.1016/j.neuroscience.2014.01.011. doi: 10.1016/j.neuroscience.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basterfield L, Lumley LK, Mathers JC. Wheel running in female C57BL/6J mice: impact of oestrus and dietary fat and effects on sleep and body mass. Int J Obes (Lond) 2009;33(2):212–218. doi: 10.1038/ijo.2008.253. doi: 10.1038/ijo.2008.253. [DOI] [PubMed] [Google Scholar]
- Baudry M, Bi X, Aguirre C. Progesterone–estrogen interactions in synaptic plasticity and neuroprotection. Neuroscience. 2013;239:280–294. doi: 10.1016/j.neuroscience.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain - derived neurotrophic factor mRNA and protein expression in the hippocampus. European Journal of Neuroscience. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. Int J Eat Disord. 2005;38(2):143–146. doi: 10.1002/eat.20164. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7(3):171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Russek SJ. Regulation of GABAA Receptor Gene Expression and Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- Burghardt PR, Flagel SB, Burghardt KJ, Britton SL, Gerard-Koch L, Watson SJ, Akil H. Risk-assessment and coping strategies segregate with divergent intrinsic aerobic capacity in rats. Neuropsychopharmacology. 2010;36(2):390–401. doi: 10.1038/npp.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonaco M, Tavolaro R, Maggi A. Steroid hormones and receptors of the GABAA supramolecular complex. Neuroendocrinology. 1993;57(5):965–973. doi: 10.1159/000126461. [DOI] [PubMed] [Google Scholar]
- Carter J, Blackmore E, Sutandar-Pinnock K, Woodside D. Relapse in anorexia nervosa: a survival analysis. Psychological medicine. 2004;34(04):671–679. doi: 10.1017/S0033291703001168. [DOI] [PubMed] [Google Scholar]
- Chowdhury TG, Ríos MB, Chan TE, Cassataro DS, Barbarich - Marsteller NC, Aoki C. Activity - based anorexia during adolescence disrupts normal development of the CA1 pyramidal cells in the ventral hippocampus of female rats. Hippocampus. 2014 doi: 10.1002/hipo.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TG, Wable GS, Sabaliauskas NA, Aoki C. Adolescent female C57BL/6 mice with vulnerability to activity-based anorexia exhibit weak inhibitory input onto hippocampal CA1 pyramidal cells. Neuroscience. 2013;241:250–267. doi: 10.1016/j.neuroscience.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz J, Touyer G, Sjövall J. Neurosteroids: 3 alpha-hydroxy-5 alpha-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133(3):1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Duclos M, Bouchet M, Vettier A, Richard D. Genetic differences in hypothalamic-pituitary-adrenal axis activity and food restriction-induced hyperactivity in three inbred strains of rats. J Neuroendocrinol. 2005;17(11):740–752. doi: 10.1111/j.1365-2826.2005.01367.x. doi: 10.1111/j.1365-2826.2005.01367.x. [DOI] [PubMed] [Google Scholar]
- Duclos M, Gatti C, Bessiere B, Mormede P. Tonic and phasic effects of corticosterone on food restriction-induced hyperactivity in rats. Psychoneuroendocrinology. 2009;34(3):436–445. doi: 10.1016/j.psyneuen.2008.10.008. doi: 10.1016/j.psyneuen.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The anxiolytic-like effects of allopregnanolone vary as a function of intracerebral microinfusion site: the amygdala, medial prefrontal cortex, or hippocampus. Behavioural pharmacology. 2007;18(5-6):461–470. doi: 10.1097/FBP.0b013e3282d28f6f. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ. Progesterone turnover to its 5alpha-reduced metabolites in the ventral tegmental area of the midbrain is essential for initiating social and affective behavior and progesterone metabolism in female rats. J Endocrinol Invest. 2011;34(7):e188–199. doi: 10.3275/7334. doi: 10.3275/7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci. 2004;118(2):306. doi: 10.1037/0735-7044.118.2.306. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Hippocampal 3alpha,5alpha-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78(3):531–540. doi: 10.1016/j.pbb.2004.03.024. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004(1):116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136(2):457–466. doi: 10.1016/j.neuroscience.2005.08.013. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Harte-Hargrove L, MacLusky NJ, Scharfman HE. Brain-derived neurotrophic factor–estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog DB, Dorer DJ, Keel PK, Selwyn SE, Ekeblad ER, Flores AT, Keller MB. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(7):829–837. doi: 10.1097/00004583-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Huttunen P, Myers RD. Tetrahydro-beta-carboline micro-injected into the hippocampus induces an anxiety-like state in the rat. Pharmacol Biochem Behav. 1986;24(6):1733–1738. doi: 10.1016/0091-3057(86)90513-7. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson S. Estrogen receptor β in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121(4):837–845. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64(4):901–938. doi: 10.1124/pr.112.005892. doi: 10.1124/pr.112.005892. [DOI] [PubMed] [Google Scholar]
- Johnson R, Rhodes J, Jeffrey S, Garland T, Jr, Mitchell G. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121(1):1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- Joshi S, Kapur J. Slow intracellular accumulation of GABA(A) receptor delta subunit is modulated by brain-derived neurotrophic factor. Neuroscience. 2009;164(2):507–519. doi: 10.1016/j.neuroscience.2009.08.008. doi: 10.1016/j.neuroscience.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce CJ, Frye CA. Progesterone facilitates exploration, affective and social behaviors among wildtype, but not 5α-reductase Type 1 mutant, mice. Behav Brain Res. 2013;253:232–239. doi: 10.1016/j.bbr.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Hormones and behavior. 1996;30(1):37–43. doi: 10.1006/hbeh.1996.0006. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic J, O'Buckley T, Grobin A, Morrow A. Chronic ethanol consumption enhances internalization of α1 subunit - containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86(3):700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. Journal of Pharmacology and Experimental Therapeutics. 2004;310(3):1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Lozsa A. Uranyl acetate as an excellent fixative for lipoproteins after electrophoresis on agarose gel. Clin Chim Acta. 1974;53(1):43–49. doi: 10.1016/0009-8981(74)90349-0. [DOI] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature Reviews Neuroscience. 2005;6(8):603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Mannan M, O'Shaughnessy P. Steroidogenesis during postnatal development in the mouse ovary. Journal of endocrinology. 1991;130(1):101–NP. doi: 10.1677/joe.0.1300101. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacology Biochemistry and Behavior. 2007;86(2):220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. Journal of visualized experiments: JoVE. 2012;(67) doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Deniselle G, Gargiulo - Monachelli G, Lima A, Roig P, Guennoun R, Nicola A. Progesterone attenuates several hippocampal abnormalities of the Wobbler mouse. J Neuroendocrinol. 2013;25(3):235–243. doi: 10.1111/jne.12004. [DOI] [PubMed] [Google Scholar]
- Misra M, Katzman DK, Estella NM, Eddy KT, Weigel T, Goldstein MA, Klibanski A. Impact of Physiologic Estrogen Replacement on Anxiety Symptoms, Body Shape Perception and Eating Attitudes in Adolescent Girls with Anorexia Nervosa: Data from a Randomized Controlled Trial. The Journal of clinical psychiatry. 2013;74(8):e765. doi: 10.4088/JCP.13m08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233(4760):226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32(3):515–522. doi: 10.1095/biolreprod32.3.515. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Hormones and behavior. 2011;60(4):318–326. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo M, Salvestroni C, Gallo R, Guo A-L, Genazzani A, Artini P, Genazzani A. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest. 1995;18(11):853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ. An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem. 1995;43(3):283–292. doi: 10.1177/43.3.7532656. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O'Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48(1):14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T. Neurobiology of mice selected for high voluntary wheel-running activity. Integrative and Comparative Biology. 2005;45(3):438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Riddle MC, McKenna MC, Yoon YJ, Pattwell SS, Santos PM, Casey BJ, Glatt CE. Caloric Restriction Enhances Fear Extinction Learning in Mice. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.268. doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffault B, Medina I, Dumon C, Thalman C, Ferrand N, Friedel P, Porcher C. Pro-Brain-Derived Neurotrophic Factor Inhibits GABAergic Neurotransmission by Activating Endocytosis and Repression of GABAA Receptors. The Journal of neuroscience. 2014;34(40):13516–13534. doi: 10.1523/JNEUROSCI.2069-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281(40):29431–29435. doi: 10.1074/jbc.C600167200. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Rodier WI, III, Segal S. The effect of progesterone on the activity-wheel running of ovariectomized rats. Hormones and behavior. 1977;9(3):214–221. doi: 10.1016/0018-506x(77)90057-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Neuroendocrine and behavioral development during puberty: a tale of two axes. Vitamins & Hormones. 2005;71:1–25. doi: 10.1016/S0083-6729(05)71001-3. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Developmental psychobiology. 2010;52(3):244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2005;80(6):387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the gamma-aminobutyric acid receptor subunit alpha4 reduces functional delta-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Busonero F, Talani G, Tranquilli S, Mameli M, Biggio G. Changes in GABA(A) receptor gene expression associated with selective alterations in receptor function and pharmacology after ethanol withdrawal. J Neurosci. 2003;23(37):11711–11724. doi: 10.1523/JNEUROSCI.23-37-11711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori C, Vieira A, Ferrari E, Langone F, Tongiorgi E, Parada C. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10(4):469–477. doi: 10.1038/nn1868. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nature neuroscience. 2007;10(4):469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49(5):573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABA< sub> A</sub> receptor subunit expression in rat CA1 hippocampus: Pharmacological and behavioral effects. Neuropharmacology. 2005;49(5):573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Su C. Progesterone, brain-derived neurotrophic factor and neuroprotection. Neuroscience. 2013;239:84–91. doi: 10.1016/j.neuroscience.2012.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Aoki C, Shen H. Puberty, steroids and GABA(A) receptor plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116(1):58–76. doi: 10.1016/j.pharmthera.2007.03.008. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Woolley CS. Cellular and molecular effects of steroid hormones on CNS excitability. Cleveland Clinic journal of medicine. 2004;71(Suppl 2):S4. doi: 10.3949/ccjm.71.suppl_2.s4. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Morrell W. The long - term course of severe anorexia nervosa in adolescents: Survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. International Journal of Eating Disorders. 1997;22(4):339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- Terzakis JA. Uranyl acetate, a stain and a fixative. J Ultrastruct Res. 1968;22(1):168–184. doi: 10.1016/s0022-5320(68)90055-5. [DOI] [PubMed] [Google Scholar]
- Wable G, Min J-Y, Chen Y-W, Aoki C. Anxiety is correlated with running in adolescent female mice undergoing activity-based anorexia. Behav Neurosci. 2015 doi: 10.1037/bne0000040. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wable GS, Barbarich-Marsteller NC, Chowdhury TG, Sabaliauskas NA, Farb CR, Aoki C. Excitatory synapses on dendritic shafts of the caudal basal amygdala exhibit elevated levels of GABAA receptor α4 subunits following the induction of activity-based anorexia. Synapse. 2013;68(1):1–15. doi: 10.1002/syn.21690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RP, Renner K, Pringle R, Summers CH, Britton S, Koch L, Swallow J. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol Behav. 2008;93(4):1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK. Estrogen and the etiology of anorexia nervosa. Neurosci Biobehav Rev. 1991;15(3):327–331. doi: 10.1016/s0149-7634(05)80025-9. [DOI] [PubMed] [Google Scholar]