Abstract
Objective
The effect of adolescent marijuana use on brain development remains unclear despite relaxing legal restrictions, decreased perceived harm, and increasing use rates among youth. The aim of this 3-year prospective study was to evaluate the long-term neurocognitive effects of adolescent marijuana use.
Method
Adolescent marijuana users with concomitant alcohol use (MJ+ALC, n=49) and control teens with limited substance use histories (CON, n=59) were given neuropsychological and substance use assessments at project baseline, when they were ages 16-19. They were then re-assessed 18 and 36 months later. Changes in neuropsychological measures were evaluated with repeated measures analysis of covariance (ANCOVA), controlling for lifetime alcohol use, and examined the effects of group, time, and group by time interactions on cognitive functioning.
Results
MJ+ALC users performed significantly worse than controls, across time points, in the domains of complex attention, memory, processing speed, and visuospatial functioning (ps<.05). Earlier age of marijuana use onset was associated with poorer processing speed and executive functioning by the 3-year follow-up (ps≤.02).
Conclusions
Frequent marijuana use throughout adolescence and into young adulthood appeared linked to worsened cognitive performance. Earlier age of onset appears to be associated with poorer neurocognitive outcomes that emerge by young adulthood, providing further support for the notion that the brain may be uniquely sensitive to frequent marijuana exposure during the adolescent phase of neurodevelopment. Continued follow-up of adolescent marijuana users will determine the extent of neural recovery that may occur if use abates.
Keywords: Adolescence, Cannabis, Alcohol, Cognition, Longitudinal
INTRODUCTION
Adolescence refers to the period from pubertal onset to the transition into adulthood (Spear & Swartzwelder, 2014) and is a dynamic period marked by changes in physical, psychological, and social development (Ernst, Pine, & Hardin, 2006). This coincides with substantial increases in marijuana and alcohol use (Brown et al., 2008). In the United States, alcohol remains the most commonly used substance among adolescents, despite a generalized downward trend in use over the past two decades (Johnston, O'Malley, Bachman, & Schulenberg, 2014). Marijuana is often used in combination with alcohol (Agosti, Nunes, & Levin, 2002), and use among teens continues to subtly rise as the perception of harm declines. Now, 36% of 12th graders report past year marijuana use, a gradual increase from approximately 23% two decades ago (Johnston, et al., 2014). As youth transition out of high school and into emerging adulthood, changes in substance use emerge with changing social roles and socioenvironmental demands (Arnett, 2000; Caldeira, O'Grady, Vincent, & Arria, 2012; Degenhardt & Hall, 2012). Prospective investigations of adolescent marijuana users will help clarify cognitive trajectories associated with adolescent use, and the longer-term neurocognitive implications.
The high rates of marijuana and alcohol use among adolescents is of great concern as ongoing neurodevelopment occurs throughout adolescence and emerging adulthood (Gogtay et al., 2004; Tamnes et al., 2010), and exposure to neurotoxic compounds during this period may alter healthy brain development (Squeglia, Jacobus, & Tapert, 2009). White matter tissue volume and density increases in a linear fashion through the second and third decades of human life, whereas region specific cortical gray matter volume increases and subsequently declines (Giedd et al., 1999; Jernigan, Trauner, Hesselink, & Tallal, 1991; Reiss, Abrams, Singer, Ross, & Denckla, 1996; Sowell et al., 2003). Over the past two decades, the advent of more sophisticated neuroimaging techniques has allowed researchers to identify functional and structural brain abnormalities in cerebral gray and white matter associated with heavy marijuana and alcohol use during adolescence (Batalla et al., 2013; Lorenzetti, Solowij, Fornito, Lubman, & Yucel, 2014). Included in these abnormalities are atypical morphometry and neurocognitive correlates in the hippocampus (Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007b), prefrontal cortex (Medina et al., 2009) and cerebellum (Medina, Nagel, & Tapert, 2010); disruptions in white matter integrity observed as increased diffusivity and decreased anisotropic diffusion, particularly in fronto-temporal and fronto-parietal pathways (Bava et al., 2009a; Bava, Jacobus, Thayer, & Tapert, 2013; Jacobus, et al., 2009; Jacobus, Squeglia, Bava, & Tapert, 2013b); and altered functional brain networks including increased frontal and parietal activation during verbal learning and spatial working memory tasks, suggesting potential compensatory neural recruitment to maintain task performance (Schweinsburg et al., 2005; Schweinsburg et al., 2010; Schweinsburg, Schweinsburg, Nagel, Eyler, & Tapert, 2011; Tapert et al., 2004).
Alcohol and marijuana use during adolescence have also been linked to cognitive deficits. Heavy drinking during adolescence has been consistently associated with neuropsychological impairment in many cognitive domains, including attention and information processing (Tapert & Brown, 2000; Tarter, Mezzich, Hsieh, & Parks, 1995), executive functioning (Giancola, Shoal, & Mezzich, 2001; Moss, Kirisci, Gordon, & Tarter, 1994); visuospatial functioning (Sher, Martin, Wood, & Rutledge, 1997; Squeglia, Spadoni, Infante, Myers, & Tapert, 2009), and verbal and nonverbal retention (Brown, Tapert, Granholm, & Delis, 2000). While the deleterious effects of heavy alcohol use during adolescence have been well documented, the cognitive sequelae of adolescent marijuana use are less well understood (Jacobus, Bava, Cohen-Zion, Mahmood, & Tapert, 2009). Within several hours to days of last use, heavy marijuana using adolescents and young adults have demonstrated deficits in attention, verbal learning and memory, psychomotor speed, and sequencing functioning (Fried, Watkinson, & Gray, 2005; Hanson et al., 2010; Harvey, Sellman, Porter, & Frampton, 2007; Solowij et al., 2011b). Cognitive deficits may also persist after longer periods of abstinence (Bosker et al., 2013; Hanson, et al., 2010). For example, adolescent marijuana users continue to show attention, verbal story memory, psychomotor speed and sequencing deficits after approximately one month of abstinence (Medina, et al., 2007). Evidence further suggests that adolescent onset (initiation prior to age 16, approximately) is an important risk factor and predicts poorer neural health and neurocognitive outcome over time (Gruber, Dahlgren, Sagar, Gonenc, & Lukas, 2014b; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012b; Pope et al., 2003; Solowij et al., 2011a; Zalesky et al., 2012). Meier and colleagues found that adolescent-onset cannabis users demonstrated IQ declines persisting years following cessation of use (Meier et al., 2012). While some longitudinal studies suggest deficits in processing speed and memory do not persist beyond several weeks of abstinence, most acknowledge that age of MJ use onset likely increases vulnerability to longer-term effects (Fried, et al., 2005; Pope, et al., 2003; Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001; Tait, Mackinnon, & Christensen, 2011).
Despite the prevalence of teenagers using marijuana, consistent evidence documenting cognitive sequelae are lacking. To date, few prospective studies have examined the longer-term neuropsychological consequences of marijuana use during adolescence with a comprehensive test battery (Fried, et al., 2005; Meier, et al., 2012; Tapert, Granholm, Leedy, & Brown, 2002). This study builds on previous work from our group (Medina et al., (2007), Bava et al., (2010), and Jacobus et al., (2013)). Our original cross sectional neuropsychological investigation found that adolescent marijuana users performed worse than non-users on psychomotor speed, attention, and memory performance at baseline (Medina, et al., 2007). Cross-sectional investigations with this sample have also focused on neuroimaging markers such as white matter integrity and the corresponding neurocognitive correlates (Bava, Jacobus, Mahmood, Yang, & Tapert, 2010). Subsequent longitudinal work added follow-up neuroimaging data and evaluated neurocognitive differences in smaller (e.g., N=54) subsamples (Bava, et al., 2013; Jacobus et al., 2013; Jacobus, Squeglia, Infante, Bava, & Tapert, 2013b). This study builds on this work by focusing exclusively on neurocognitive data obtained at three time points (N=108). The primary goal was to investigate the long-term impact of adolescent marijuana use on cognition. We hypothesized that adolescent marijuana users would demonstrate poorer neurocognitive performance across multiple domains than non-users at baseline, 1.5-year, and 3-year follow-up. We further suspected that early-onset marijuana users (initiation of regular use before 16 years of age) would demonstrate worse cognitive performance than late-onset users.
METHODS
Participants
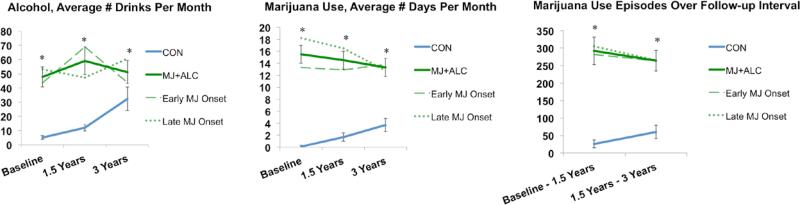
Adolescents (N=108; ages 16-19 at baseline) were recruited from San Diego area schools and followed for three years, which included a baseline and follow-up 1.5 and 3 years later, each with comprehensive substance use and neuropsychological assessments (Jacobus, et al., 2013; Medina, et al., 2007) administered by a trained Bachelor's or Master's level research assistant. All participants underwent written informed consent (or assent if under age 18 and consent from their guardians) in accordance with the University of California, San Diego Human Research Protections Program. Adolescents were classified at baseline as: marijuana users (with concomitant alcohol use) with ≥60 lifetime marijuana use episodes (MJ+ALC, n=49), or control teens with ≤9 lifetime marijuana use episodes and minimal alcohol use (CON) (see Table 1, Figure 1). At baseline, reported drinks per month ranged from 0-248 for MJ+ALC and 0-58 for CON, and cigarettes per day ranged from 0-10 for MJ+ALC and 0-1 for CON (see Table 1, Figure 1 for more detailed substance use characteristics). Marijuana users in our study are relatively consistent in their marijuana use patterns as over 80% continue to report >60 marijuana use episodes per year by 3-year follow-up. Similarly, marijuana users engage in consistent alcohol use, as 96% report > 150 lifetime alcohol use episodes by age 20. Only 22% report fewer than 10 drinks per month by 3-year follow-up and 16% deny heavy episodic drinking behaviors (≥ 4 drinks on one occasion for females and ≥ 5 drinks for males).
Table 1.
Demographic characteristics at baseline, unless otherwise noted.
| CON n=59 M(SD) | MJ+ALC n=49 M(SD) | |
|---|---|---|
| Age, Baseline | 17.6 (0.9) | 18.0 (0.8) |
| Age, Year 1.5 | 19.0 (0.9) | 19.5 (0.8) |
| Age, Year 3 | 20.5 (1.0) | 21.0 (0.8) |
| % Male | 68% | 62% |
| % Caucasian | 59% | 67% |
| Grade point average | 3.3 (0.6) | 3.2 (0.7) |
| Annual household income | 121K (72K) | 133K (104K) |
| % Family History positive for substance use disorder | 22% | 43% |
| Vocabulary T-score | 58.5 (9.1) | 57.2 (9.0) |
| Beck Depression Inventory total, Baseline | 2.1 (2.6) | 3.4 (4.6) |
| Beck Depression Inventory total, Year 1.5 | 2.3 (4.0) | 2.9 (3.5) |
| Beck Depression Inventory total, Year 3 | 2.1 (3.4) | 3.2 (5.5) |
| Spielberger State Anxiety T-score, Baseline | 37.8 (7.5) | 40.0 (8.4) |
| Spielberger State Anxiety T-score, Year 1.5 | 37.6 (6.0) | 37.6 (5.9) |
| Spielberger State Anxiety T-score, Year 3 | 36.7 (6.3) | 39.1 (8.1) |
| Lifetime MJ use episodes, Baseline to Year 3* | 87.2 (213.7) | 1086.4 (602.0) |
| Lifetime alcohol use episodes, Baseline to Year 3* | 180.2 (223.5) | 644.9 (406.1) |
| Lifetime other drug use episodes, Baseline to Year 3* | 7.5 (25.5) | 84.8 (120.0) |
| Average drinks per month, Baseline* | 5.1 (10.3) | 47.8 (49.2) |
| Average drinks per month, Year 1.5* | 12.0 (17.0) | 59.1 (68.7) |
| Average drinks per month, Year 3 | 32.3 (63.4) | 51.2 (57.7) |
| Alcohol use episodes from Baseline to Year 1.5* | 48.9 (84.4) | 207.6 (216.6) |
| Alcohol use episodes from Year 1.5 to Year 3* | 108.4 (136.0) | 201.4 (174.9) |
| Heavy episodic drinking episodes Year 1.5 to Year 3a* | 67.5 (111.2) | 151.4 (216.0) |
| Average # cigarettes per day, Baseline * | 0.1 (0.1) | 0.8 (2.2) |
| Average # cigarettes per day, Year 1. 5* | 0.1 (0.4) | 1.5 (3.6) |
| Average # cigarettes per day, Year 3 | 0.3 (1.2) | 0.8 (1.7) |
| Average MJ use days per month, Baseline* | 0.1 (0.4) | 15.5 (10.9) |
| Average MJ use days per month, Year 1.5* | 1.7 (5.4) | 14.5 (12.2) |
| Average MJ use days per month, Year 3* | 3.7 (8.7) | 13.3 (11.3) |
| Marijuana use episodes from Baseline to Year 3* | 26.0 (89.4) | 292.4 (276.6) |
| Marijuana use episodes from Year 1.5 to Year 3* | 60.2 (149.9) | 264.4 (208.0) |
| Days since last MJ use, Baseline | . | 52.8 (91.6) |
| Days since last MJ use, Year 1.5 | . | 81.1 (167.4) |
| Days since last MJ use, Year 3 | . | 68.9 (152.1) |
| Days since last alcohol use, Baseline | 128.6 (163.7) | 46.5 (79.9) |
| Days since last alcohol use, Year 1.5 | 115.1 (280.9) | 33.0 (103.7) |
| Days since last alcohol use, Year 3* | 41.1 (64.8) | 15.0 (15.5) |
| Age of onset, regular marijuana use b | . | 15.1 (1.9) |
| Age on onset, regular alcohol use b | . | 16.2 (1.9) |
p<.05;
≥ 4 drinks on one occasion for females and ≥ 5 drinks for males
Defined as >1x/week for 52+ weeks
Figure 1.
Substance use characteristics (N=108). Early and late refers to age of marijuana use initiation (early, < 16 years old, n=27; late, ≥16, n=22). *p<.05, MJ+ALC vs. CON. No significant differences observed between early and late MJ onset users (ps>.05)
Exclusionary criteria at study entry included: history of a lifetime DSM-IV Axis I disorder (other than cannabis or alcohol abuse/dependence), history of learning disability; history of neurological disorder or traumatic brain injury with loss of consciousness >2 minutes; history of a serious physical health problem; complicated or premature birth including prenatal substance use; uncorrectable sensory impairments; left handedness; and use of psychoactive medications.
Participants underwent biweekly urine toxicology for four weeks prior to cognitive assessments to confirm self-report of substance use at each time point and encourage abstinence from marijuana to avoid capturing the acute adverse effects of recent use. Compliance with the abstinence protocol is associated with decreasing 9-carboxy-tetrahydrocannabinol (THCCOOH)/creatinine excretion ratios. New cannabis use was determined by dividing each THCCOOH normalized collection by the previously collected specimen (urine 2/urine 1), per Huestis and Cone recommendations for determining new cannabis use as a function of time (Huestis & Cone, 1998; Smith, Barnes, & Huestis, 2009). Most participants reported at least three weeks of abstinence (self-report corroborated with decreasing excretion ratios) at each time point (89% at baseline and 70% at 1.5- and 3-year follow-up), and 13 reported <1 week of abstinence prior to assessment at 3-year follow-up (minimum was the day before testing).
Measures
Neuropsychological Battery
Standardized neuropsychological tests within the well-established domains of complex attention, processing speed, verbal memory, visuospatial functioning, and executive functioning (Jacobus, et al., 2013; Lezak, Howieson, & Loring, 2004; Medina, et al., 2007) included: (1) complex attention: California Verbal Learning Test-II (CVLT-II) Trial 1 total words and Trial 1-5 total words (Delis, Kramer, Kaplan, & Ober, 2001); Wechsler Adult Intelligence Scale Third Edition (WAIS-III) Arithmetic total and Digit Span forward, backwards, and total scores (Wechsler, 1997b); and the Paced Auditory Serial Addition Test (PASAT) 2-second trial total score (Gronwall, 1974); (2) processing speed: WAIS-III Digit Symbol Coding total score (Wechsler, 1997b); and the Delis-Kaplan Executive Functioning System (D-KEFS) Trail Making Test (TMT) Visual Scanning, Number Sequencing, Letter Sequencing, and Motor Speed total time to completion (Delis & Kaplan, 2000); (3) verbal memory: Wechsler Memory Scale-Third Edition (WMS-III) Logical Memory I, II, and Recognition total score (Wechsler, 1997a); and CVLT-II Short and Long Delay Free and Cued Recall total words and Recognition Discriminability z-score (Delis, et al., 2001); (4) visuospatial functioning: Wechsler Abbreviated Scale of Intelligence (WASI) Block Design total score (Wechsler, 1999); copy and delay accuracy conditions of the Rey Osterrieth Complex Figure (baseline), Taylor Complex Figure (1.5-year follow-up), and the Medical College of Georgia (MCG) Complex Figure (3-year follow-up) (Hubley & Tremblay, 2002; Loring & Meador, 2003; Rey & Osterrieth, 1993); and (5) executive functioning: D-KEFS Number-Letter Switching total completion time, Verbal Letter Fluency total score; and Tower Test total achievement score (Delis & Kaplan, 2000).
Both raw scores (to increase variability) and age-corrected scaled scores (e.g., CVLT-II, WAIS-III, WASI, D-KEFS, WMS-III) were examined and reported for each subtest if significant. Importantly, groups did not differ in age at any time point. Data were available for all participants except 4 cases (2 from each group) did not receive CVLT-II and D-KEFS tasks at 3-year follow-up (CON, n=57; MJ+ALC, n=47), 1 did not receive the WASI Block Design subtest at baseline (MJ+ALC, n=48), 2 did not receive the PASAT at 1.5-year follow-up (CON, n=57), and 6 (2 at 1.5-year follow-up and 4 at 3-year follow-up) did not receive the Complex Figure (MJ+ALC, n=43), therefore these individuals were excluded from the subtest analysis for which data were missing.
Substance Use and Mental Health Assessment
The Customary Drinking and Drug Use Record (CDDR) assessed quantity and frequency of lifetime alcohol, marijuana, cigarette, and other illicit drugs (Brown et al., 1998) including amphetamines, hallucinogens, cocaine, opiates, benzodiazepines, ecstasy, ketamine, and GHB. The Timeline Followback collected data on self-reported substance use in the past 28 days prior to cognitive assessment (Sobell & Sobell, 1992). The Beck Depression Inventory and the State Trait Anxiety Inventory (STAI) assessed depression symptoms and state anxiety at each visit (Beck, 1978; Spielberger, Gorsuch, & Lushene, 1970).
Data Analysis
Group Comparisons
Analysis of variance and chi-square tests were used to examine between group differences in demographic characteristics. Repeated-measures analyses of covariance (ANCOVA) were used to examine the trajectories of neuropsychological performance for each neuropsychological subtest identified within each domain above. The main effects of time, group status at baseline, and group by time interactions were specified for each ANCOVA, and run with lifetime alcohol use as a covariate given the high rate of alcohol use reported by the marijuana users. Multivariate results are reported for each ANCOVA unless homogeneity of variance assumptions were violated, in which case Greenhouse-Geisser corrections were applied. If an effect of group or group by time interaction was found to be significant (p<.05), the simple effects were explored with lifetime alcohol use as a covariate; the ANCOVA analysis was also re-run dividing the substance users (MJ+ALC) into early (< 16 years old, n=27) and late (≥ 16, n=22) onset of regular marijuana use (consistent with previous studies), defined as initiation of regular marijuana use > 1x/week for 52+ weeks (Ehrenreich et al., 1999; Gruber, Dahlgren, Sagar, Gonenc, & Lukas, 2014a; Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012a; Gruber, Silveri, Dahlgren, & Yurgelun-Todd, 2011).
Bivariate Correlations
Correlations were explored in the larger sample (N=108) between neuropsychological performance at 3-year follow-up, depression scores at 3-year follow-up, and state anxiety scores at 3-year follow-up. Within the substance use group (n=49), correlations were explored between neuropsychological performance at 3-year follow-up and age of onset of regular marijuana and alcohol use (defined as >1x/week for 52+ weeks), lifetime marijuana and alcohol use, and recency of marijuana and alcohol use at 3-year follow-up. For significant bivariate relationships identified, hierarchical linear regressions were run predicting neurocognitive performance at 3-year follow-up (dependent variable). To identify if emotional functioning, age of onset of use, or substance use severity was a significant predictor of neurocognitive performance at year 3 above and beyond baseline testing performance, the corresponding baseline neuropsychological performance was entered on step 1 and the appropriate independent predictors on step 2.
RESULTS
Demographics
Groups did not differ on demographic measures, but the MJ+ALC group had more substance use, as expected (see Table 1; Figure 1). The early-onset marijuana group reported initiating weekly marijuana use almost 3 years sooner than the late-onset heavy users, and regular alcohol use about 1 year earlier, but had no other significant demographic or substance use differences (see Table 2; Figure 1).
Table 2.
Demographic characteristics for MJ users (n=49) at baseline, unless otherwise noted.
| MJ+ALC Early MJ Onset n=27 M(SD) | MJ+ALC Late MJ Onset n= 22 M(SD) | |
|---|---|---|
| Age, Baseline | 17.7 (0.7) | 18.5 (0.6) |
| Age, Year 1.5 | 19.2 (0.7) | 19.9 (1.6) |
| Age, Year 3 | 20.7 (0.7) | 21.5 (0.6) |
| % Male | 59% | 64% |
| % Caucasian | 59% | 77% |
| Grade Point Average | 3.1 (0.8) | 3.2 (0.7) |
| Household Income | 102K (73K) | 172K (125K) |
| % Family History positive for substance use disorder | 46% | 41% |
| Vocabulary T-score | 55.3 (9.5) | 59.5 (8.0) |
| Beck Depression Inventory total, Baseline | 3.9 (5.5) | 2.7 (3.5) |
| Beck Depression Inventory total, Year 1.5 | 2.8 (3.4) | 3.0 (3.7) |
| Beck Depression Inventory total, Year 3 | 3.4 (6.4) | 3.0 (4.4) |
| Spielberger State Anxiety T-score, Baseline | 39.9 (8.2) | 40.1 (8.9) |
| Spielberger State Anxiety T-score, Year 1.5 | 37.5 (6.3) | 37.6 (6.3) |
| Spielberger State Anxiety T-score, Year 3 | 38.8 (8.8) | 39.6 (7.3) |
| Lifetime MJ use episodes, Baseline to Year 3 | 1222.9 (684.2) | 918.7 (442.2) |
| Lifetime alcohol use episodes, Baseline to Year 3 | 618.4 (424.2) | 677.5 (390.2) |
| Lifetime other drug use episodes, Baseline to Year 3 | 103.1 (142.8) | 62.5 (82.2) |
| Average drinks per month, Baseline | 43.6 (51.8) | 53.1 (46.5) |
| Average drinks per month, Year 1.5 | 68.8 (86.8) | 47.3 (34.7) |
| Average drinks per month, Year 3 | 43.7 (53.0) | 60.5 (62.9) |
| Alcohol use episodes from Baseline to Year 1.5 | 219.3 (260.2) | 193.3 (151.8) |
| Alcohol use episodes from Year 1.5 to Year 3 | 165.4 (138.0) | 245.5 (206.4) |
| Heavy episodic drinking episodes Year 1.5 to Year 3a | 138.9 (177.6) | 123.4 (173.8) |
| Average # cigarettes per day Baseline | 1.2 (2.9) | 0.2 (0.5) |
| Average # cigarettes per day, Year 1.5 | 2.1 (4.3) | 0.8 (2.3) |
| Average # cigarettes per day, Year 3 | 1.0 (1.9) | 0.5 (1.3) |
| Average MJ use days per month, Baseline | 13.3 (11.3) | 18.2 (10.0) |
| Average MJ use days per month, Year 1.5 | 12.9 (12.2) | 16.5 (12.2) |
| Average MJ use days per month, Year 3 | 13.8 (11.1) | 12.8 (11.8) |
| Marijuana use episodes from Baseline to Year 1.5 | 282.3 (326.8) | 304.8 (205.9) |
| Marijuana use episodes from Year 1.5 to Year 3 | 263.7 (206.7) | 265.3 (214.6) |
| Days since last MJ use, Year 3 | 83.3 (187.8) | 51.3 (92.9) |
| Days since last alcohol use, Year 3 | 18.6 (17.8) | 10.1 (11.3) |
| Age of onset, regular marijuana use*b | 13.9 (1.3) | 16.7 (0.8) |
| Age on onset, regular alcohol use*b | 15.6 (1.8) | 16.9 (1.7) |
p<.05;
≥ 4 drinks on one occasion for females and ≥ 5 drinks for males
Defined as >1x/week for 52+ weeks
Complex Attention/Working Memory Tasks
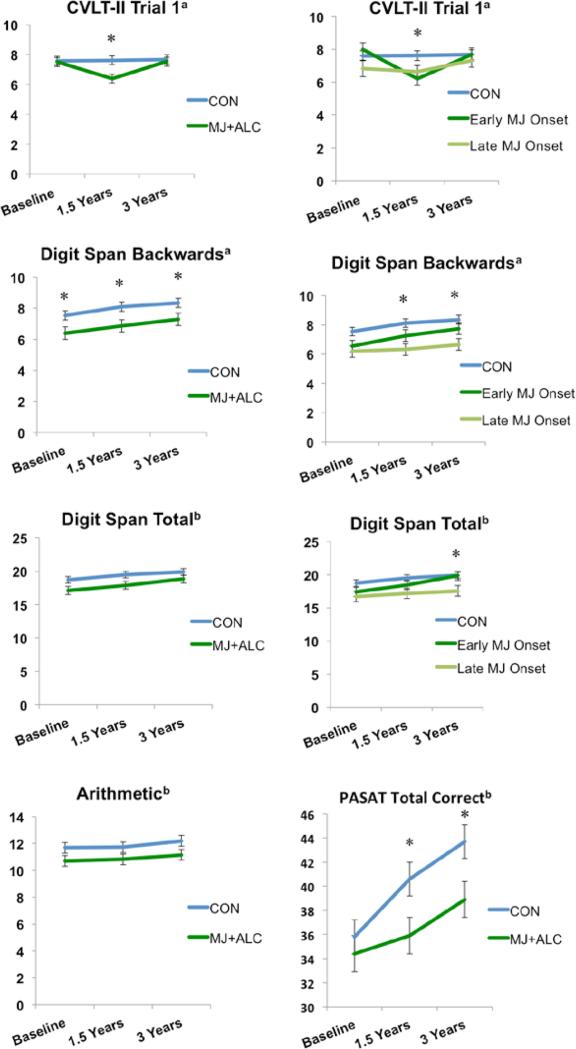
The MJ+ALC vs. CON group by time interaction significantly predicted CVLT-II Trial 1 performance (raw scores; F(2,100)=3.24 p=.04). MJ+ALC users remembered fewer words than controls at 1.5-year follow-up, F(1,101)=8.33, p<.01, but the difference was no longer present at 3-year follow-up (p>.05). Similarly, the early (n=26) vs. late MJ onset (n=21) group by time interaction predicted CVLT-II Trial 1 raw scores (F(3.7,188.6)=2.70, p=.03). Specifically, the early MJ onset group remembered fewer words than controls at 1.5-year follow-up (F(2,100)=4.51, p=.01), but this was no longer the case at 3-year follow-up (p>.05) (see Table 3; Figure 2,5).
Table 3.
Neuropsychological performance on subtests administered. Means below are unadjusted and presented as scaled scores. Cohen's d reflects between group differences (CON vs. MJ+ALC) adjusted for lifetime alcohol use.
| CON (n=59) M(SD) | MJ+ALC (n=49) M(SD) | Cohen's d | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 1.5 Years | 3 Years | Baseline | 1.5 Years | 3 Years | ||
| Complex Attention | |||||||
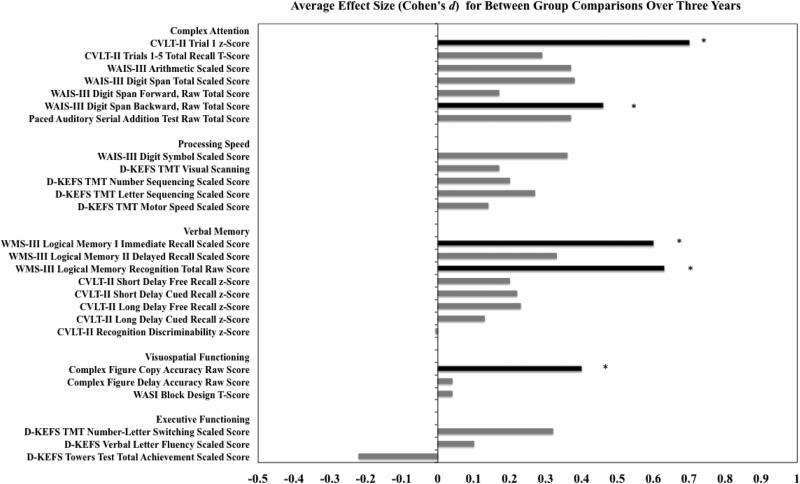
| CVLT-II Trial 1 z-Score | 0.1 (1.0) | −0.1 (0.8) | 0.2 (1.0) | 0.1 (1.1) | −0.4 (0.8) | 0.2 (1.1) | *0.7 |
| CVLT-II Trials 1-5 Total Recall T-Score | 54.1 (12.2) | 56.1 (9.5) | 59.1 (8.9) | 49.4 (19.3) | 53.6 (10.1) | 57.8 (9.1) | 0.3 |
| WAIS-III Arithmetic Scaled Score | 11.4 (2.5) | 11.5 (2.6) | 11.9 (2.8) | 11.0 (2.7) | 11.1 (2.4) | 11.5 (2.5) | 0.4 |
| WAIS-III Digit Span Total Scaled Score | 10.4 (2.8) | 11.3 (2.8) | 11.7 (2.5) | 10.4 (2.8) | 10.7 (2.5) | 11.1 (2.7) | 0.4 |
| WAIS-III Digit Span Forward, Raw Total Score | 10.9 (2.0) | 11.2 (2.2) | 11.4 (2.0) | 11.1 (2.1) | 11.2 (1.8) | 11.5 (1.9) | 0.2 |
| WAIS-III Digit Span Backward, Raw Total Score | 7.2 (2.2)* | 7.8 (2.3)* | 8.3 (2.0)* | 6.8 (2.2)* | 7.2 (2.3)* | 7.3 (2.5)* | *0.4-0.5 |
| Paced Auditory Serial Addition Test Raw Total Score | 34.9 (8.4) | 39.7 (8.7) | 42.6 (9.2) | 35.4 (10.4) | 37.0 (11.7) | 40.2 (10.4) | 0.4 |
| Processing Speed | |||||||
| WAIS-III Digit Symbol Scaled Score | 11.0 (2.0) | 11.5 (2.2) | 12.2 (2.4) | 10.4 (2.6) | 10.9 (2.7) | 11.6 (2.9) | 0.4 |
| D-KEFS TMT Visual Scanning | 11.5 (1.7) | 12.1 (1.5) | 12.3 (1.5) | 12.1 (1.4) | 12.1 (1.5) | 12.3 (1.4) | 0.2 |
| D-KEFS TMT Number Sequencing Scaled Score | 11.3 (2.6) | 12.2 (1.9) | 12.7 (1.7) | 11.3 (2.5) | 12.3 (1.9) | 12.6 (1.7) | 0.2 |
| D-KEFS TMT Letter Sequencing Scaled Score | 11.4 (2.4) | 12.7 (1.8) | 13.1 (1.4) | 11.6 (2.0) | 12.3 (2.1) | 12.7 (1.6) | 0.3 |
| D-KEFS TMT Motor Speed Scaled Score | 11.6 (2.2) | 12.5 (1.2) | 12.6 (1.0) | 12.2 (1.3) | 12.4 (1.0) | 12.4 (1.0) | 0.1 |
| Verbal Memory | |||||||
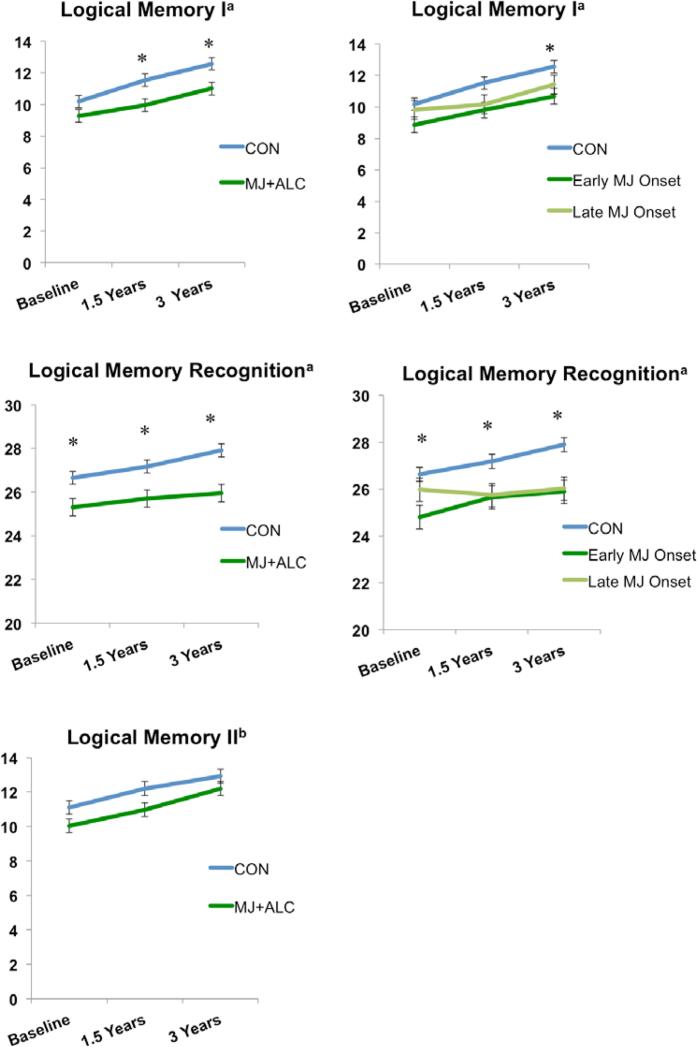
| WMS-III Logical Memory I Immediate Recall Scaled Score | 9.7 (2.9) | 11.1 (2.7)† | 12.1 (2.6)† | 9.9 (3.0) | 10.4 (2.8)† | 11.5 (3.0)† | †0.6 |
| WMS-III Logical Memory II Delayed Recall Scaled Score | 10.6 (2.9) | 11.9 (2.6) | 12.6 (2.8) | 10.6 (3.0) | 11.3 (2.9) | 12.6 (2.8) | 0.3 |
| WMS-III Logical Memory Recognition Total Raw Score | 26.3 (2.5)* | 26.7 (2.7)* | 27.5 (1.9)* | 25.7 (2.8)* | 26.2 (2.4)* | 26.4 (2.9)* | *0.5-0.8 |
| CVLT-II Short Delay Free Recall z-Score | 0.2 (0.9) | 0.4 (1.0) | 0.7 (0.8) | 0.2 (0.8) | 0.3 (1.0) | 0.6 (0.9) | 0.2 |
| CVLT-II Short Delay Cued Recall z-Score | 0.1 (1.0) | 0.5 (0.9) | 0.6 (0.7) | 0.0 (0.8) | 0.3 (1.1) | 0.6 (0.7) | 0.2 |
| CVLT-II Long Delay Free Recall z-Score | 0.1 (1.0) | 0.3 (1.0) | 0.6 (0.8) | 0.1 (0.9) | 0.1 (1.1) | 0.5 (0.8) | 0.2 |
| CVLT-II Long Delay Cued Recall z-Score | 0.0 (0.9) | 0.5 (0.8) | 0.5 (0.8) | −0.2 (1.1) | 0.3 (1.0) | 0.5 (0.7) | 0.1 |
| CVLT-II Recognition Discriminability z-Score | 0.1 (0.9) | 0.4 (0.8) | 0.5 (0.5) | 0.2 (0.8) | 0.1 (1.0) | 0.4 (0.7) | 0.0 |
| Visuospatial Functioning | |||||||
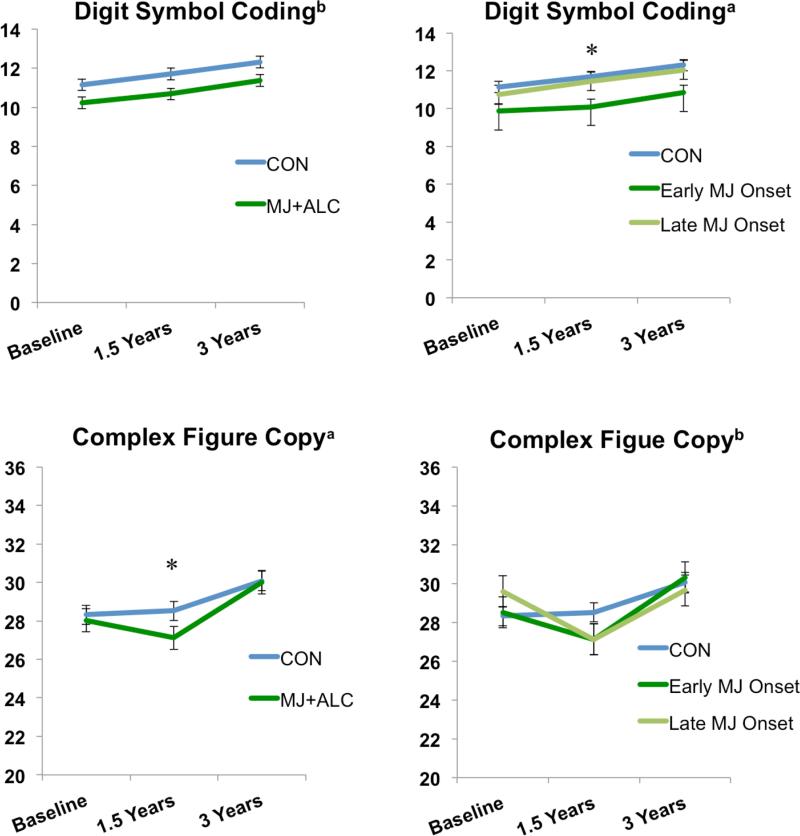
| Complex Figure Copy Accuracy Raw Score | 28.4 (3.3) | 28.4 (3.2)* | 30.1 (3.2) | 28.9 (3.9) | 27.2 (3.9)* | 30.0 (3.2) | *0.4 |
| Complex Figure Delay Accuracy Raw Score | 18.3 (4.5) | 21.0 (5.5) | 21.9 (6.3) | 17.8 (5.5) | 20.8 (4.6) | 21.8 (5.6) | 0.1 |
| WASI Block Design T-Score | 55.5 (7.9) | 59.7 (6.2) | 61.1 (6.4) | 57.2 (7.4) | 59.9 (5.4) | 60.7 (6.2) | 0.1 |
| Executive Functioning | |||||||
| D-KEFS TMT Number-Letter Switching Scaled Score | 10.6 (2.2) | 11.4 (1.8) | 11.7 (2.0) | 10.5 (2.1) | 11.2 (1.7) | 11.9 (1.4) | 0.3 |
| D-KEFS Verbal Letter Fluency Scaled Score | 12.3 (3.0) | 12.6 (3.2) | 13.0 (2.9) | 13.0 (3.2) | 12.6 (3.0) | 12.7 (2.9) | 0.1 |
| D-KEFS Towers Test Total Achievement Scaled Score | 9.6 (1.5) | 11.5 (2.5) | 12.7 (2.2) | 10.1 (2.5) | 11.7 (2.4) | 12.5 (2.3) | −0.2 |
p<.05, raw scores
p<05, scaled scores
Figure 2.
Complex attention subtest performance. Raw scores presented for California Verbal Learning Test-II Trial 1, Digit Span Backwards and Total, and Paced Auditory Serial Addition Test. Scaled scores presented for Arithmetic. a p<.05, group main effect or interaction; b p≤ .07, trend for group main effect or interaction; *p<.05, group effect at each time point. Means adjusted for lifetime alcohol use.
Figure 5.
Average effect sizes (Cohen's d, adjusted for lifetime alcohol use) for between-group comparisons (CON vs. MJ+ALC) over three years are presented for each subtest. Black (*) represents significant between group differences identified (see Table 3, p<.05); gray represents average effect sizes for non-significant findings (p>.05). Positive effect size represents CON>MJ+ALC.
The MJ+ALC vs. CON main effect for group predicted WAIS-III Digit Span backwards subtest performance (F(1,105)=6.47, p=.01). MJ+ALC performed poorer than CON at baseline (F(1,105)=5.01, p=.02), 1.5-year follow-up (F(1,105)=5.26, p=.02), and 3-year follow-up (F(1,105)=3.95, p=.04). A main effect of group was also observed between early/late MJ onset vs. CON (F(2,104)=4.36, p=.01), with the late onset group performing worse than CON at 1.5-year (F(2,104)=3.73, p=.02) and 3-year follow-ups (F(2,104)=3.50, p=.03) (see Table 3; Figure 2,5).
A MJ+ALC vs. CON trend for main effect for group was observed for Digit Span total raw score; MJ+ALC users showed poorer performance than CON (F(1,105)=3.28, p=.07) at baseline and 1.5-year follow-up (ps=.07). The late MJ onset users performed worse than early onset users and controls (F(2,104)=2.81, p=.06) at 3-year follow-up (ps=.02). Trends were also seen for a MJ+ALC vs. CON group main effect for Arithmetic scaled scores (F(1,105)=3.16, p=.07), and a group by time interaction for PASAT total score (F(2, 102)=2.6, p=.07). MJ+ALC performed poorer than CON at 1.5 and 3-year follow-up on PASAT total correct (ps<.05) (see Table 3; Figure 2,5).
Verbal Memory Tasks
MJ+ALC vs. CON (F(1,105)=5.67, p=.01) and early/late MJ onset vs. CON main effects of group (F(2,104)=3.31, p=.04) significantly predicted WMS-III Logical Memory I (immediate recall) scaled scores. Follow-up analyses revealed that MJ+ALC showed poorer performance than CON at 1.5 year (F(1,105)=5.75, p=.01) and 3-year follow-ups (F(1,105)=5.74, p=.01). Early MJ onset users showed poorer performance (p=.01) than CON at 3-year follow-up (see Table 3; Figure 3,5).
Figure 3.
Verbal memory subtest performance. Scaled scores presented for Logical Memory I and II. Raw scores presented for Logical Memory Recognition. a p<.05, group main effect; b p≤ .07, trend for group main effect; *p<.05, group effect at each time point Means adjusted for lifetime alcohol use.
MJ+ALC vs. CON (F(1,105)=11.44, p<.01) and early/late MJ onset vs. CON main effects of group (F(2,104)=6.04, p<.01) significantly predicted WMS-III Logical Memory Recognition scores. Follow-up analysis showed that MJ+ALC performed worse than CON at baseline (F(1,105)= 4.57, p=.03), 1.5 year (F(1,105)=6.36, p=.01), and 3-year follow-ups (F(1,105)=12.32, p<.01). Early MJ onset users correctly identified fewer words than CON at baseline (p=.01) and 1.5-year follow-up (p=.02); CON identified more words than both early and late MJ onset users at 3-year follow-up (ps<.01) (see Figure 2). A trend for a MJ+ALC vs. CON main effect of group was seen for WMS-III Logical Memory II scaled scores (delayed recall)(F(1,105)=3.12, p=.08), with CON showing better performance at 1.5-year follow-up (F(1,105)=3.37, p=.06) (see Table 3; Figure 3,5).
Processing Speed Tasks
A trend was observed for a MJ+ALC vs. CON main effect of group on WAIS-III Digit Symbol Coding scaled scores (F(1,105)=3.24, p=.07), however the early/late MJ onset vs. CON main effect of group was significant, F(2,104)=3.25, p=.04. CON performed better than early MJ onset users at 1.5-year follow-up (p=.01) (see Table 3; Figure 4,5).
Figure 4.
Processing speed and visuospatial subtest performance. Scaled scores presented for Digit Symbol Coding. Raw scores presented for Complex Figure data. a p<.05, group main effect or interaction; b p≤ .07, trend for group main effect or interaction; *p<.05, group effect at each time point. Means adjusted for lifetime alcohol use.
Visuospatial Functioning Tasks
The MJ+ALC vs. CON group by time interaction significantly predicted the Complex Figure copy condition, F(2,98)=3.45, p=.03. We saw improvement in CON performance from baseline to 3-year follow-up, but a significant decline and subsequent improvement in MJ+ALC performance from baseline to 1.5-year follow-up and 1.5-year follow-up to 3-year follow-up (ps<.01). A trend for the early/late MJ onset group vs. CON interaction effect was observed, F(4,194)=2.34, p=.05. CON improved in their performance from baseline to 3-year follow-up, whereas both the early/late onset groups decreased and then improved in their performance (ps<.01) (see Table 3; Figure 4,5).
Executive Functioning Tasks
We did not observe any main effects of group, or group by time interactions (or trends) in the domain of executive functioning (see Table 3; Figure 5).
Within Subjects Effects
In addition to the group main effects reported above, a main effect of time (increasing performance from baseline to follow-up) was found for the vast majority of measures as anticipated (20 of 26 tests), controlling for lifetime alcohol use, including CVLT-II Trials 1-5; WAIS-III Digit Span Total, Forward, and Backwards; WAIS-III Digit Symbol Coding; D-KEFS Trail Making Test Number, Letter, Switching, and Motor Speed subtests; WMS-III Logical Memory I and II; CVLT-II Short and Long Delay Free and Cued Recall conditions and Recognition Discriminability; Complex Figure Delay Accuracy condition; WASI Block Design; and D-KEFS Verbal Fluency and Tower Test total achievement scores, ps≤.04.
Bivariate Correlations
There are significant relationships in the larger group (N=108) between state anxiety T-score and WAIS-III Digit Symbol performance (raw and scaled scores), rs=-.21, ps=.02, Trail Making Test Number Sequencing condition raw scores, r=.20, p=.04, and CVLT-II Short Delay Cued Recall, r=-.20, p=.04. We did not see significant relationships between cognitive performance and depression, recency of substance use, or lifetime marijuana use and lifetime alcohol use.
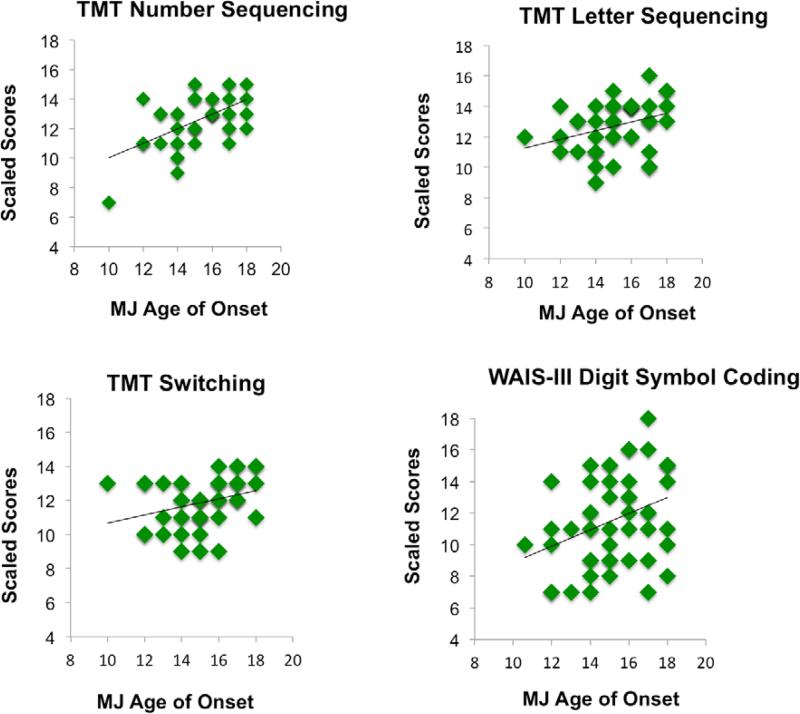
In the MJ+ALC group (n=49), earlier age of regular MJ use onset was associated with poorer performance on 9 measures: WAIS-III Digit Symbol raw and scaled scores (r=.35, p=.01; r=.34, p=.01), Trail Making Test Visual Scanning total time raw scores (r=-.29, p=.04), Trail Making Test Number Sequencing total time raw and scaled scores (r=-.44, p<.01; r=.40, p<.01), Trail Making Test Letter Sequencing total time raw and scaled scores (r=-.37, p=.01; r=.39, p<.01), and Trails Making Test Switching raw and scaled scores (r=-.29, p=.04; r=.32, p=.02) (see Figure 6).
Figure 6.
Bivariate correlations with age of marijuana use onset (defined as >1x/week for 52 weeks) and Digit Symbol Coding (n=49) and Trail Making Test (TMT) conditions (n=47).
Hierarchical linear regressions were run predicting WAIS-III Digit Symbol and Trail Making Test performance (scaled scores), with baseline performance entered on the first step (and anxiety T-score for prediction of Digit Symbol performance), and age of marijuana use onset on the second step. For Trail Making Test Number Sequencing and Switching, age of marijuana onset was found to be a significant predictor (β=-.49 p<.01; β=-.33 p=.01) and accounted for variance above and beyond baseline test performance, F(2,43)=12.77, p<.01, ΔR2=.23, p<.01; F(2,43)=7.2, p<.01, ΔR2=.12, p=.01 Age of onset (β=-.06, p=.57) was not found to be a significant predictor of performance beyond baseline performance and anxiety T-score(β=.24 p=.02) for Digit Symbol performance, or Trail Making Test Visual Scanning (β=.18 p=.19) and Letter Sequencing (β=.21 p=.18).
DISCUSSION
This study evaluated adolescents reporting heavy marijuana and alcohol use, and control teens with minimal substance over three-years. Group differences, controlling for lifetime alcohol use, were observed in the domains complex attention, memory, processing speed, and visuospatial functioning. While MJ+ALC performed worse than controls across all three time points, significant differences were more consistent at 19 years old (1.5-year follow-up) across domains, just prior to a narrowing substance use gap observed by age 20 (3-year follow-up) between our users and controls for both alcohol and marijuana use. Our sample use trajectories are consistent with epidemiological research that shows marijuana use tends to peak around 18-19 years old in the United States (spanning baseline to 1.5-year follow-up) for those who do not transition to chronic heavy use (Caldeira, et al., 2012; Johnston, et al., 2014). Poorer cognitive performance in our users (at 1.5-year follow-up) compared to those with more minimal use histories corresponds with the epidemiological literature on age of peak use, and differences are observed in domains important for optimal cognitive performance.
It is important to put these findings into the context of the broader marijuana literature by exploring 1) how these findings compare to other cross sectional and longitudinal work, and 2) the mechanism likely driving observed group differences in this study and others. Cross-sectional studies have found group differences (non-acute) between adolescent/young adult marijuana studies across neurocognitive domains. For example, studies that have required at least 24 hours of abstinence find that adolescent and young adult marijuana users do not perform as well as matched controls on processing speed, attention, and memory tasks (Bartholomew, Holroyd, & Heffernan, 2010; Gonzalez et al., 2012; Hanson, et al., 2010; Medina, Hanson, et al., 2007; Takagi et al., 2011), and executive functioning and inhibition tasks (Griffith-Lendering, Huijbregts, Vollebergh, & Swaab, 2012). Dose-dependent relationships have identified increased lifetime marijuana use associated with poorer processing speed, memory, and executive functioning in adolescents and young adults, which may be moderated by gender (Crane, Schuster, & Gonzalez, 2013; Lisdahl & Price, 2012). Our findings show performance discrepancies in domains overlapping with the existing literature and independently measured over the course of three years.
Longitudinal work, while still limited, also finds poorer neurocognitive outcomes associated with adolescent marijuana use. Meier and colleagues (2012) found that those with persistent cannabis use histories over a 20-year period showed widespread decline in areas such as memory, processing speed, and executive functioning from their premorbid childhood functioning (ages 7-13). However these individuals were not assessed throughout adolescence and likely represent the consequences of more severe and pervasive use patterns established by young adulthood (age 38), versus elucidating the cognitive sequelae of “subdiagnostic” use increasing in prevalence during adolescence (Johnston, et al., 2014; Meier, et al., 2012). While our observed group differences are subtler in nature, and widespread decrements in performance are not observed across all subtests, there remains evidence for adverse effects of use throughout the transition from adolescence to young adulthood on select aspects of neurocognitive functioning. As marijuana use differences gradually decrease between our groups, we see slightly fewer significant group differences by 3-year follow-up, which may speak to the possibility of “recovery” or resolving effects in some domains. Other longitudinal studies that have utilized a comprehensive test battery include research by Fried and colleagues (2005) who found that former heavy users by ages 17-21 (no regular use for at least three months) did not differ on a comprehensive neuropsychological battery compared to non-using controls. Current heavy users differed in memory and processing speed performance after accounting for preexisting differences.
Continued follow-up of longitudinal cohorts is greatly needed to assess the degree of enduring effects. Strong dose-dependent relationships with lifetime use and recency of use were not identified in this study. However abstinence was encouraged with four weeks of monitored toxicology (abstinence ranged 1 day to several years by 3-year follow-up) and most did not use within the week of testing (or longer), potentially washing out recency effects as reported in other studies (Solowij, et al., 2011b).
We saw associations between earlier age of onset and poorer processing speed and executive functioning performance as measured by several conditions on the D-KEFS Trail Making Test. Notably, age of onset was found to predict Number Sequencing and Switching 3-year follow-up performance above and beyond baseline performance. Several studies have identified poorer outcomes associated with earlier age of marijuana use onset (regular use before age 16), including poorer reaction time performance (Ehrenreich, et al., 1999), executive functioning (e.g., Stroop Color Word Test, Wisconsin Card Sorting Test) (Gruber, et al., 2012; Fontes et al., 2011), memory performance (Solowij, et al., 2011b; Solowij et al., 2012) and verbal abilities (Pope et al., 2003). Neuroimaging studies have also found that earlier age of marijuana use onset is associated with altered neural tissue health in gray and white matter, and functional brain activation patterns (Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, & Daumann, 2010; Gruber, Dahlgren, Sagar, Gonenc, & Killgore, 2012; Gruber, et al., 2014a; Wilson et al., 2000). Taken together, our study expands on these findings and provides more evidence that encouraging delayed initiation of cannabis is advantageous, as earlier initiation is likely to have negative neural consequences.
In our sample, our late MJ onset group reports more recent marijuana use, on average, at 3-year follow-up. While the late onset group reports slightly more marijuana use per month at baseline and 1.5-year follow-up, differences in monthly marijuana use are no longer evident a 3-year follow-up between our early- and late MJ onset groups. This is likely related to our late MJ initiation group (initiation after age 16) starting to use more regularly around the baseline time point (~age 18). In attention performance, we see the early- and late MJ onset groups performing more poorly compared to controls. While speculative, differences observed between the late MJ onset group and controls may represent recency of use to some degree, given that in some domains (i.e., processing speed) relationships with late onset are not observed, however in domains shown to be influenced by recency of use (i.e., attention; Bosker, et al., 2013; Hanson, et al., 2010; Solowij, et al., 2011b), the late MJ onset users perform poorly compared to controls. The early MJ onset group demonstrated poorer performance at 1.5-year follow-up, but not 3-year follow-up, on CVLT-II Trial 1 and Digit Symbol Coding subtests. Considering the early MJ onset group reports more drinks per month and less occasions at 1.5-year follow-up, it is possible that pattern of alcohol use at this time point may have contributed to this finding despite lifetime alcohol use included as a covariate in all analyses.
There are several outstanding questions that need to be addressed in the adolescent cannabis literature, including the degree to which pre-existing differences account for any between group differences observed (Cheetham et al., 2012; Fried, et al., 2005; Jacobus, et al., 2013; Meier, et al., 2012); how changing use trajectories over discrete adolescent and young adult periods of development impact neural and cognitive outcomes, particularly in the domains of attention, memory, and processing speed; and how unique relationships with co-occurring use (e.g., alcohol) impact neural outcomes (Jacobus et al., 2009; Mahmood, Jacobus, Bava, Scarlett, & Tapert, 2010). Despite the limited dose-dependent alcohol findings observed in the present study (i.e., bivariate relationships with lifetime alcohol use), we have seen divergent relationships with alcohol and marijuana use in other investigations in our laboratory (Jacobus, Squeglia, Sorg, Nguyen, & Tapert, in press).
The biological mechanisms that may underlie neurocognitive differences include a marijuana-related toxic impact (e.g., neuroinflammatory response) on the vulnerable developing brain, or altered development trajectories that result from reorganization of the endocannabinoid system, which is critical for development and modulation of several neurotransmitter systems and normal neuromaturation (Atakan, 2012; Cutando et al., 2013; Iversen, 2003; Rubino & Parolaro, 2008; Stella, 2013; Viveros et al., 2012). Interference with neurogenesis, plasticity, neural patterning, or any other neurobiological processes can lead to poorer behavioral outcomes, including increased risk taking behavior and poorer mental health functioning by young adulthood (Caldeira, et al., 2012; Jacobus, et al., 2013 ; Suarez-Pinilla, Lopez-Gil, & Crespo-Facorro, 2014; Terry-McElrath, O'Malley, & Johnston, 2014). Recent reviews of neural effects of cannabis use have corroborated many findings from our laboratory, including decreased hippocampal volume (Lorenzetti, et al., 2014; Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007a), decreased white matter integrity in fronto-thalamic connections (Batalla, et al., 2013; Bava et al., 2009b; Jacobus, Squeglia, Bava, & Tapert, 2013a; Jacobus, Squeglia, Infante, Bava, & Tapert, 2013a), and different activation patterns in frontal cortices (Batalla, et al., 2013; Schweinsburg, et al., 2011). Alterations, even subtle, in in these critical brain systems may result in marijuana users at a neurocognitive disadvantage resulting in decreased performance in several neurocognitive domains (e.g., attention, memory) reported in this study, despite less consistent evidence for associations between neurocognitive functioning and neuroimaging markers of structural brain integrity (Lorenzetti, et al., 2014)
Limitations include the lack of pre-existing neuropsychological data for this cohort. Unfortunately the suggestion that the observed differences existed prior the initiation of substance use in our sample cannot be ruled out, despite prospective (pre/post initiation) evidence of a marijuana-related effect on neurodevelopment (Jacobus, Squeglia, Infante, et al., 2013a). There may be biological and environmental factors present prior to marijuana initiation and contributing to marijuana use and cognitive vulnerabilities. These findings need to be expanded upon and replicated, as our effect sizes and Type I error control was modest. We took measures to decrease the practice effects on our repeated neuropsychological measures, particularly for tasks in which salience of stimuli were most likely to carry over from one assessment to another. While we recognize using different stimuli for the complex figure could systematically affect participants’ performance, several studies have supported the equivalence of these measures (Delaney, Prevey, Cramer, & Mattson, 1992; Strauss & Spreen, 1990; Tombaugh & Hubley, 1991).
Our groups were matched on gender; however, sex differences in the relationships between cannabis use and neural functioning is becoming a growing area of research and future work in our lab will focus on modeling these unique and complex relationships with larger sample sizes to identify if substance use and neurocognitive associations exist similarly for both sexes (Crane, Schuster, Fusar-Poli, & Gonzalez, 2012; Crane, et al., 2013). Self-reported alcohol and marijuana use continues to be a limitation of substance use research in general, and better objective measures of cannabis use may reduce inconsistencies in the research literature. Collecting additional information in future studies such as cannabis potency and content (e.g., THC/CBD ratios) will also help disentangle existing discrepancies. Further, co-occurring alcohol and marijuana use is common and consistent with epidemiological data (Agosti, et al., 2002; Johnston, et al., 2014). Marijuana users observed in this study tend to remain consistently heavy users of alcohol and marijuana use (Figure 1). In fact, 96% of our marijuana users reported more than 150 lifetime alcohol use episodes by age 20. Only 22% report less than 10 drinks per month by 3-year follow-up, and few (16%) deny heavy episodic drinking behaviors. Nevertheless, it is possible that subtle changes in alcohol use patterns (versus cumulative lifetime use) not captured in our data impact neurocognitive outcomes. Future work will examine interactions between differing use patterns of these substances.
Research suggests that adolescent cannabis use impacts cognitive and neural functioning. While there is potential and some evidence for neural recovery after prolonged use, the short-term implications suggest deleterious effects on neural and psychosocial functioning. Future prospective research studies will work to better delineate the impact of cannabis use on different stages of development with better objective markers of use; efforts to understand the timeline for both impairment and recovery will guide prevention, safety, and intervention development.
Acknowledgments
This research was supported by National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism Grants R01 DA021182, F32 DA032188, R01 AA013419, T32 AA013525, and U01 AA021692
References
- Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. The American Journal of Drug and Alcohol Abuse. 2002;28(4):643–652. doi: 10.1081/ada-120015873. doi: 10.1081/ADA-120015873. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. The American Psychologist. 2000;55(5):469–480. doi: 10.1037/0003-066X.55.5.469. [PubMed] [Google Scholar]
- Atakan Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Therapeutic Advances in Psychopharmacology. 2012;2(6):241–254. doi: 10.1177/2045125312457586. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? Journal of Psychopharmacology. 2010;24(2):241–246. doi: 10.1177/0269881109106909. doi: 10.1177/0269881109106909. [DOI] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research. 2009a;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Research. 2009b;173(3):228–237. doi: 10.1016/j.pscychresns.2009.04.005. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain and Cognition. 2010;72(3):347–354. doi: 10.1016/j.bandc.2009.10.012. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcoholism, Clinical and Experimental Research. 2013;37(Suppl 1):E181–189. doi: 10.1111/j.1530-0277.2012.01920.x. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A. Beck Depression Inventory (BDI) Psychological Corporation; San Antonio, TX: 1978. [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010;34(6):837–845. doi: 10.1016/j.pnpbp.2010.03.032. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bosker WM, Karschner EL, Lee D, Goodwin RS, Hirvonen J, Innis RB, Ramaekers JG. Psychomotor function in chronic daily cannabis smokers during sustained abstinence. Public Library of Science One. 2013;8(1):e53127. doi: 10.1371/journal.pone.0053127. doi: 10.1371/journal.pone.0053127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59(4):427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism, Clinical and Experimental Research. 2000;24(2):164–171. doi: 10.1111/j.1530-0277.2000.tb04586.x. [PubMed] [Google Scholar]
- Caldeira KM, O'Grady KE, Vincent KB, Arria AM. Marijuana use trajectories during the post-college transition: Health outcomes in young adulthood. Drug and Alcohol Dependence. 2012:267–275. doi: 10.1016/j.drugalcdep.2012.02.022. doi: 10.1016/j.drugalcdep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biological Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: Recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review. 2012 doi: 10.1007/s11065-012-9222-1. doi: 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Gonzalez R. Preliminary evidence for a sex-specific relationship between amount of cannabis use and neurocognitive performance in young adult cannabis users. Journal of the International Neuropsychological Society. 2013:1–7. doi: 10.1017/S135561771300088X. doi: 10.1017/S135561771300088X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutando L, Busquets-Garcia A, Puighermanal E, Gomis-Gonzalez M, Delgado-Garcia JM, Gruart A, Ozaita A. Microglial activation underlies cerebellar deficits produced by repeated cannabis exposure. The Journal of Clinical Investigation. 2013;123(7):2816–2831. doi: 10.1172/JCI67569. doi: 10.1172/JCI67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Delaney RC, Prevey ML, Cramer J, Mattson RH. Test-retest comparability and control subject data for the Rey-Auditory Verbal Learning Test and Rey-Osterrieth/Taylor Complex Figures. Archives of Clinical Neuropsychology. 1992;7(6):523–528. doi: 10.1016/0887-6177(92)90142-A. [PubMed] [Google Scholar]
- Delis DC, Kaplan E. Delis-Kaplan Executive Functioning Scale Manual. Psychological Corporation; San Antonio, Texas: 2000. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test- Second Edition. The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142(3):295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, Lacerda AL. Cannabis use before age 15 and subsequent executive functioning. The British Journal of Psychiatry : The Journal of Mental Science. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27(2):231–239. doi: 10.1016/j.ntt.2004.11.003. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Shoal GD, Mezzich AC. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Experimental and Clinical Psychopharmacology. 2001;9(2):215–227. doi: 10.1037//1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012 doi: 10.1080/13803395.2012.703642. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith-Lendering MF, Huijbregts SC, Vollebergh WA, Swaab H. Motivational and cognitive inhibitory control in recreational cannabis users. Journal of Clinical and Experimental Neuropsychology. 2012;34(7):688–697. doi: 10.1080/13803395.2012.668874. doi: 10.1080/13803395.2012.668874. [DOI] [PubMed] [Google Scholar]
- Gronwall DMA. Paced Auditory Serial-Addition Task: A measure of recovery from concussion. Perceptual and Motor Skills. 1974;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neuroscience Letters. 2012;511(2):89–94. doi: 10.1016/j.neulet.2012.01.039. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014a;231(8):1455–1465. doi: 10.1007/s00213-013-3326-z. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology (Berl) 2014b;231(8):1455–1465. doi: 10.1007/s00213-013-3326-z. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors. 2012a;26(3):496–506. doi: 10.1037/a0026269. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012b;26(3):496–506. doi: 10.1037/a0026269. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Dahlgren MK, Yurgelun-Todd D. Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology. 2011;19(3):231–242. doi: 10.1037/a0023034. doi: 10.1037/a0023034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26(3):309–319. doi: 10.1080/09595230701247772. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hubley AM, Tremblay D. Comparability of total score performance on the Rey-Osterrieth Complex Figure and a modified Taylor Complex Figure. Journal of Clinical and Experimental Neuropsychology. 2002;24(3):370–382. doi: 10.1076/jcen.24.3.370.984. doi: 10.1076/jcen.24.3.370.984. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. Journal of Analytical Toxicology. 1998;22(6):445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126(Pt 6):1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacology, Biochemistry, and Behavior. 2009;92(4):559–565. doi: 10.1016/j.pbb.2009.04.001. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31(6):349–355. doi: 10.1016/j.ntt.2009.07.006. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Research. 2013a;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: A 3-year investigation. Psychiatry Research. 2013b;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White Matter Integrity Pre-and Post Marijuana and Alcohol Initiation in Adolescence. Brain Sci. 2013a;3(1):396–414. doi: 10.3390/brainsci3010396. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre-and post marijuana and alcohol initiation in adolescence. Brain Sciences. 2013b;3(1):396–414. doi: 10.3390/brainsci3010396. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen NT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of Studies on Alcohol and Drugs. doi: 10.15288/jsad.2014.75.729. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors. 2013;27(2):431–442. doi: 10.1037/a0028235. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national results on adolescent drug use: Overview of key findings, 2013. Institute for Social Research, The University of Michigan; Ann Arbor: 2014. [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press; New York: 2004. [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Current Pharmaceutical Design. 2014;20(13):2138–2167. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ. The Medical College of Georgia (MCG) Complex Figures: Four forms for follow-up. In: Knight J, Kaplan E, editors. Handbook of Rey- Osterrieth Complex Figure Usage: Clinical and Research Applications. Psychological Assessment Resources; Odessa, FL: 2003. [Google Scholar]
- Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: Interactive effects. Journal of Studies on Alcohol and Drugs. 2010;71(6):885–894. doi: 10.15288/jsad.2010.71.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13(5):807–820. doi: 10.1017/S1355617707071032. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addiction Biology. 2009;14(4):457–468. doi: 10.1111/j.1369-1600.2009.00166.x. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007a;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007b;29(1):141–152. doi: 10.1016/j.ntt.2006.10.010. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1206820109. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism, Clinical and Experimental Research. 1994;18(1):159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. doi: 10.1016/S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rey A, Osterrieth PA. Corwin J, Bylsma FW, editors. Translations of excerpts from Andre Rey's “Psychological examiniation of traumatic encephalopathy,” and P.A. Osterrieth's “The complex figure copy test”. The Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Molecular and Cellular Endocrinology. 2008;286(1-2 Suppl 1):S108–113. doi: 10.1016/j.mce.2008.02.003. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug and Alcohol Dependence. 2005;79(2):201–210. doi: 10.1016/j.drugalcdep.2005.01.009. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs. 2010;42(3):401–412. doi: 10.1080/02791072.2010.10400703. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106(3):564–573. doi: 10.1111/j.1360-0443.2010.03197.x. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Martin ED, Wood PK, Rutledge PC. Alcohol use disorders and neuropsychological functioning in first-year undergraduates. Experimental and Clinical Psychopharmacology. 1997;5(3):304–315. doi: 10.1037//1064-1297.5.3.304. doi: 10.1037/1064-1297.5.3.304. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytical Toxicology. 2009;33(4):185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow back. A technique for assessing self- reported alcohol consumption. Humana Press; New York, NY: 1992. [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Yucel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 2011a;216(1):131–144. doi: 10.1007/s00213-011-2203-x. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Yucel M. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011b;216(1):131–144. doi: 10.1007/s00213-011-2203-x. doi: 10.1007/s00213-011-2203-x. [DOI] [PubMed] [Google Scholar]
- Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, Yucel M. Reflection impulsivity in adolescent cannabis users: A comparison with alcohol-using and non-substance-using adolescents. Psychopharmacology. 2012;219(2):575–586. doi: 10.1007/s00213-011-2486-y. doi: 10.1007/s00213-011-2486-y. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1970. [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience : Official Journal of the EEG and Clinical Neuroscience Society. 2009;40(1):31–38. doi: 10.1177/155005940904000110. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors. 2009;23(4):715–722. doi: 10.1037/a0016516. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N. Chronic THC intake modifies fundamental cerebellar functions. The Journal of Clinical Investigation. 2013 doi: 10.1172/JCI70226. doi: 10.1172/JCI70226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Spreen O. A comparison of the Rey and Taylor figures. Archives of Clinical Neuropsychology. 1990;5(4):417–420. [PubMed] [Google Scholar]
- Suarez-Pinilla P, Lopez-Gil J, Crespo-Facorro B. Immune system: A possible nexus between cannabinoids and psychosis. Brain, Behavior, and Immunity. 2014 doi: 10.1016/j.bbi.2014.01.018. doi: 10.1016/j.bbi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106(12):2195–2203. doi: 10.1111/j.1360-0443.2011.03574.x. doi: 10.1111/j.1360-0443.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- Takagi M, Yucel M, Cotton SM, Baliz Y, Tucker A, Elkins K, Lubman DI. Verbal memory, learning, and executive functioning among adolescent inhalant and cannabis users. Journal of Studies on Alcohol and Drugs. 2011;72(1):96–105. doi: 10.15288/jsad.2011.72.96. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown SA. Substance dependence, family history of alcohol dependence and neuropsychological functioning in adolescence. Addiction. 2000;95(7):1043–1053. doi: 10.1046/j.1360-0443.2000.95710436.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8(7):873–883. doi: 10.1017/s1355617702870011. doi: 10.1017/S1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. doi: 10.1097/01.ALC.0000141812.81234.A6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug and Alcohol Dependence. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. doi: 10.1016/0376-8716(95)01129-M. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, O'Malley PM, Johnston LD. Alcohol and marijuana use patterns associated with unsafe driving among u.s. high school seniors: High use frequency, concurrent use, and simultaneous use. Journal of Studies on Alcohol and Drugs. 2014;75(3):378–389. doi: 10.15288/jsad.2014.75.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley AM. Four studies comparing the Rey-Osterrieth and Taylor complex figures. Journal of Clinical and Experimental Neuropsychology. 1991;13(4):587–599. doi: 10.1080/01688639108401073. doi: 10.1080/01688639108401073. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, Lopez-Gallardo M, de Fonseca FR. The endocannabinoid system in critical neurodevelopmental periods: Sex differences and neuropsychiatric implications. Journal of Psychopharmacology. 2012;26(1):164–176. doi: 10.1177/0269881111408956. doi: 10.1177/0269881111408956. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-3rd Edition. Psychological Corporation; New York: 1997a. [Google Scholar]
- Wechsler D. WAIS-III Manual. New York: 1997b. [Google Scholar]
- Wechsler D, editor. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: A magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Solowij N, Yucel M, Lubman DI, Takagi M, Harding IH, Seal M. Effect of long-term cannabis use on axonal fibre connectivity. Brain. 2012;135:2245–2255. doi: 10.1093/brain/aws136. doi: 10.1093/brain/aws136. [DOI] [PubMed] [Google Scholar]