Abstract
We hypothesized that soy phytochemicals may have immunomodulatory properties that may impact prostate carcinogenesis and progression. A randomized, phase II trial was conducted in 32 prostate cancer patients with asymptomatic biochemical recurrence but no measurable disease on standard staging studies. Patients were randomized to 2 slices of soy bread (34 mg isoflavones/slice) or soy bread containing almond powder daily as a source of β-glucosidase. Flow cytometry and bioplex assays were used to measure cytokines or immune cell phenotype in blood at baseline (day 0) and following intervention (day 56). Adequate blood samples were available at enrollment and day 56 and evaluated. Multiple plasma cytokines and chemokines were significantly decreased on Day 56 versus baseline. Subgroup analysis indicated reduced Th1 (p=0.028) and MDSC-associated cytokines (p=0.035). Th2 and Th17 cytokines were not significantly altered. Phenotypic analysis revealed no change in CD8+ or CD4+ T cells, but showed increased CD56+ NK cells (p=0.038). The percentage of cells with a T regulatory cell phenotype (CD4+CD25+FoxP3+) were significantly decreased after 56 days of soy bread (p=0.0136). Significantly decreased monocytic (CD33+HLADRnegCD14+) MDSC were observed in patients consuming soy bread (p=0.0056). These data suggest that soy bread modulates systemic soluble and cellular biomarkers consistent with limiting inflammation and suppression of MDSCs. Additional studies to elucidate impact on the carcinogenic process or as a complement to immune-based therapy are required.
Keywords: Soy, isoflavones, inflammation, MDSC, T regulatory cells
Introduction
Soybeans and the foods derived from them are a rich source of bioactive phytochemicals. Among the phytochemicals, the isoflavones (primarily genistein and daidzein) have received the greatest attention regarding putative health promoting properties, ranging from bone health to cancer prevention. The concept of soy as an intervention for prostate cancer was initially founded upon prior epidemiologic studies demonstrating a lower risk of prostate cancer in populations consuming considerable dietary soy (1, 2). Soy enriched diets and pure phytochemicals from soy have subsequently shown anticancer activity in experimental models of prostate carcinogenesis (3–6).
Based on these data, human clinical trials testing multiple types, schedules and doses of dietary soy, or soy components have been conducted in humans with prostate cancer in both the pre- and post-prostatectomy setting. These studies have produced very interesting, yet sometimes seemingly divergent results given the heterogeneity in study population and design, as well as concerns for study size and statistical power (7–11). Our group and others are actively investigating the potential of diets rich in soy components to prevent or slow human prostate cancer progression, particularly in hormone sensitive disease (4, 5, 12–14). Soy isoflavones are hypothesized to act through various pathways to impact PCa progression, such as inhibition of tumor growth factor signaling (15), anti-angiogenesis (12, 16), cell cycle inhibition (4) and metastasis (17).
One emerging area in prostate carcinogenesis, progression, and therapy concerns the role of the host immune response. Indeed, activation of inflammatory pathways have been linked to early carcinogenesis and progression (18–20). More recently novel strategies to harness the host immune response for therapeutic benefit are the focus of extensive preclinical and clinical research. For example, multiple Phase III studies of novel strategies (e.g. PSA-TRICOM vaccine, antibodies targeting CTLA4 and PD1/PDL1) are building upon the 2011 approval of sipuleucel-T/Provenge immune therapy for PCa (18–21). Yet, how dietary components, such as soy, may impact the host to potentiate or possibly antagonize immune therapy remains unknown.
Complex networks of white blood cells and soluble factors control the type, magnitude, and duration of immune responses to pathogens, various environmental insults, or emerging cancer cells. Following an acute inflammatory response, the host orchestrates negative regulatory cells and soluble factors to limit residual tissue injury and promote healing. In contrast, chronic inflammation is associated with repeated tissue damage, elevated levels of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and aberrant expansion of immunoregulatory cells in a futile attempt to control smoldering immune responses (19). Myeloid-derived suppressor cells (MDSC) and T regulatory cells (T regs) have emerged as key cellular subsets that regulate inflammation, cancer development and progression.
Although heterogeneity in the phenotype of human MDSC and T regs is appreciated, both cell populations can promote the ability of cancers to evade CD8+ T and NK-cell mediated immune responses (22). These cells therefore represent a potential barrier to maximizing benefit from immunotherapy regimens for PCa. MDSC, present systemically and in the prostate microenvironment, act through a variety of mechanisms to suppress survival, signaling and cytotoxic function by immune effector cells (22). Several reports have shown that MDSC and T regs are relevant biomarkers that are elevated, and in some cases, associated with reduced overall survival in patients with advanced prostate cancer (23–29). Despite our increasing understanding as to the role of immune suppression in cancer, no studies to date have explored dietary intervention with soy phytochemicals as a means by which to modulate immunosuppressive cell populations in patients.
We hypothesized that dietary soy may benefit PCa patients by modulating the balance of pro-inflammatory cytokines and immunosuppressive cells. To address this hypothesis, peripheral blood was obtained from PCa patients with asymptomatic biochemical recurrence who were enrolled in a phase II clinical trial evaluating the safety, compliance and pharmacokinetic profile of a novel bread enriched with soy phytochemicals. In the present study, we demonstrate that the peripheral blood of PCa patients had significantly reduced pro-inflammatory cytokines, MDSC and T regulatory cells following soy bread intervention as compared to baseline. These data represent the first report suggesting dietary soy may modulate immune parameters in favor of anti-cancer immune responses in humans.
Materials and Methods
Clinical Trial Design
All studies were conducted according to guidelines under Institutional Review Board Approval at The Ohio State University. A phase II clinical trial of soy phytochemical enriched bread was conducted in men (n=32) with prostate cancer who were experiencing asymptomatic biochemical recurrence (rising PSA) with no evidence of measurable disease on staging studies. All patients had a good performance status (ECOG 0-1). The majority of patients (24 of 32 total patients) had undergone radical prostatectomy prior to entry into this study. Detailed eligibility criteria, response criteria, dietary constraints and other clinical parameters are described in our accompanying publication (30). The study protocol was approved by The Ohio State University Comprehensive Cancer Center’s Clinical Scientific Review Committee and the Biomedical Sciences Cancer Institutional Review Board (NCT:01682941). Briefly, a cross-over design was used in which men were randomized to receive 2 slices per day of either a soy bread or soy almond bread. Men received one bread formulation for 8 weeks (56 days) following an initial two week legume-free washout. After a second two week washout, men received an additional 8 weeks of the alternate bread formulation. The primary objective of this trial was to characterize the safety and compliance, pharmacokinetics of the soy phytochemicals (30). This study describes results from a series of immunologic correlative studies conducted in a retrospective manner, as a secondary objective using only data from the first 56 days of the study, prior to cross-over.
Soy Bread Formulation
A novel soy bread formulation was developed that delivers 34 mg total soy isoflavones per 50g slice. Two slices of this bread provided a dose of soy phytochemicals similar to the daily intake consumed by Asian populations in which lower cancer incidence has been noted in epidemiologic studies (1, 2). A second bread was formulated to accelerate the conversion of glucosides to aglycones and possibly alter pharmacokinetic properties by incorporating ground almonds (which are naturally high in β-glucosidase) and steaming the soy ingredients (to aid in conversion of the malonyls) (31). These soy isoflavone delivery vehicles are described in further detail as previously reported by our group (31).
Procurement of Peripheral Blood
Approximately 8–10 mL of blood was drawn from prostate cancer patients into sodium heparin tubes following a two-week, legume-free washout (day 0) and following eight weeks of daily soy bread intervention (day 56). PBMCs were separated using Ficoll-Paque and density gradient centrifugation and cryopreserved prior to batch analysis as previously described (32). Plasma was snap frozen and stored at -80°C prior to analysis.
Analysis of Plasma Cytokines
A panel of 54 cytokines and chemokines were analyzed in plasma from each patient at day 0 and day 56 using the commercially available, high-throughput Luminex Mulitplex Cytokine Kits (Procarta Cytokine Assay Kit, Affymetrix) as described previously (33). All samples were batch run in duplicate and quantified based on a unique standard curve for each analyte.
Flow Cytometric Analysis
Cryopreserved PBMCs from each patient obtained at day 0 and day 56 were assayed for phenotypic markers consistent with NK cells, T lymphocytes, myeloid derived suppressor cells (MDSC) and T regulatory cells as previously described (26, 32). Briefly, PBMC from each patient were suspended at a concentration of 1x107/mL in flow staining buffer (PBS containing 1% FBS). Cells were incubated with fluorochrome-labeled antibodies for one hour at 4°C. Specific antibodies include CD4-APC (Beckman Coulter), CD8-APC (Beckman Coulter), NKRD1 (Beckman Coulter), CD33-PE (BD Biosciences), HLA-DR PERCP-Cy5.5 (eBioscience), and CD14-Pacific Blue (BD Biosciences). PBMC were also labeled with the appropriate isotype control antibodies for each fluorochrome to use as negative controls. Cells were then washed with flow buffer, fixed with 1% formalin, and stored at 4°C until analysis. All samples were run on a BD LSR II flow cytometer, and were subsequently analyzed with FlowJo software (Tree Star, Inc.). Monocytic MDSC were defined as cells with a CD33+HLA-DRnegCD14+ phenotype. T regulatory cells were defined as CD4+CD25+FoxP3+ and assessed using the commercially available Human T regulatory cell staining kit per manufacturer’s recommendations (eBioscience).
CD33+ Cell Depletion Studies
Patient peripheral blood mononuclear cells (PBMC) from n=5 representative patients were depleted of MDSC by using anti-CD33/66b magnetic microbeads (STEMCELL Technologies, Vancouver, Canada) and negatively selected using an Easy Sep magnet. Isolated cells were washed twice prior to further studies. Patient PBMC (+/− CD33-positive cells) were labeled with 1 μM CFSE (Invitrogen, Grand Island, NY) and cultured with CD3/CD28 beads (Invitrogen) for 3 days. Cells were collected, stained for CD4+ or CD8+ T cell markers (Beckman Coulter, Brea, CA) and fixed for flow cytometric analysis on a FACS Calibur. Cells were gated on CD4+ or CD8+ T cells and the percentage of T cell proliferation was determined based on CFSE dilution.
Statistical Analysis
A global testing approach (34) was used to compare change from baseline to day 56 for groups of cytokines defined a priori according to putative function. Within patient differences in individual cytokine and chemokine levels and cellular data were compared using paired t-tests. Using the approach of Gordon et al. (35), the mean number of false discoveries in the cytokine/chemokine panel was set to two in order to address the issue of multiplicity. Comparisons between treatment groups were made using two-sample t-tests. Where necessary, data were log-transformed in order to meet test assumptions of homoscedasticity and normality.
Results
Compliance with soy bread and soy metabolism
The details of the clinical study and extensive examination of soy isoflavone pharmacokinetics and metabolism are presented in a parallel publication (Ahn-Jarvis, J. et al., In Press). In brief, prostate cancer patients (n=32) with asymptomatic biochemical recurrence were recruited and detailed patient characteristics and dietary composition, are presented in an accompanying publication (30). Both soy and soy-almond breads were without grade 2 or higher toxicity, and self-reported compliance was >92% of the targeted dose. Of the 32 patients enrolled, 25 remained on the clinical protocol for at least 56 days, provided adequate blood samples, and were therefore eligible for assessment of immunologic biomarkers, the primary endpoint of this report. Four of the ineligible patients were excluded due to cancer progression requiring intervention that arose during the course of the study.
Soy bread intervention is associated with decreased pro-inflammatory cytokines and chemokines in peripheral blood of prostate cancer patients
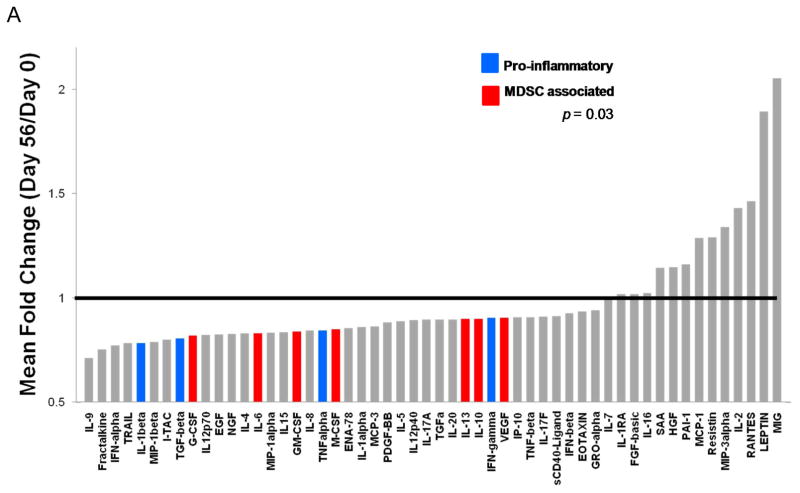
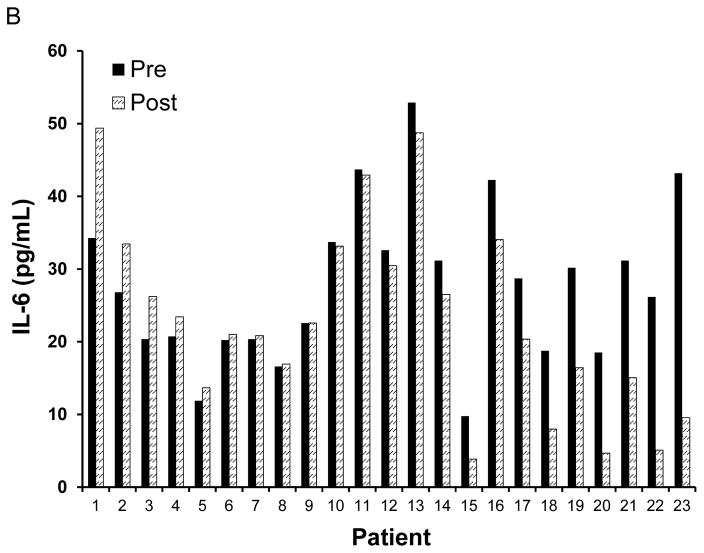
The concentrations of 54 cytokines and chemokines were profiled in plasma from patients obtained at baseline (following the 2 week washout) and after 56 days of soy bread intervention. Significant changes were evident when comparing the plasma concentrations of soluble immunologic mediators following soy bread consumption as compared to the baseline values (Figure 1A). Global tests of hypotheses were performed in which individual cytokines were grouped into well-characterized subclasses based on putative function. For example, cytokines associated with Th1 (IL-1β, IL-2, IL-12, IFN-γ, TNF-α), Th2 (IL-4, IL-5, IL-10), or Th17 (IL-17A, IL-17F) immune responses were grouped together. Similar groupings were devised for cytokines associated with differentiation and function of MDSC (IL-6, GM-CSF, G-CSF, M-CSF) or T regulatory cells (TGF-β, IL-10). There were significant reductions in the level of both Th1 type cytokines (p=0.028) and MDSC-associated cytokines (p=0.035) when comparing day 56 to baseline values across all patients (Table I). Although modest alterations were evident in the level of Th2 associated cytokines, Th17 associated cytokines and T regulatory cells, these data did not reach statistical significance (Table I). In addition to these biologically grouped analyses, paired t-tests were used to analyze differences of individual cytokines across all patient samples. These data revealed significant reductions in individual factors implicated in a variety of immunologic functions including: IL-6, IL-12, MIP-1β, IL-4, TRAIL, NGF-38, and IFN-α (Figure 1B and Supplemental Table 1). Setting the mean number of false discoveries at two for the 54 factor panel, significant differences at the 0.037 level were detected in 11 factors. Finally, the mean fold change was elevated across patients for a subset of individual soluble factors including MIG, LEPTIN, RANTES, IL-2, MIP-3alpha and Resistin at the day 56 time point, although significant variability across individual patients for these biomarkers precluded statistical significance. Importantly, the observed cytokine changes were consistent regardless of whether men consumed soy bread or soy-almond bread (all between group p-values > 0.5 for cytokine subsets; Supplemental Table 2A).
Figure 1. Soy bread consumption is associated with modulation of cytokines in plasma from patients with prostate cancer.
Plasma from patients obtained on Day 0 and Day 56 were analyzed via bioplex analysis for the expression of 54 soluble cytokine and chemokine mediators. (A) Data are presented as the mean fold change in expression for each factor across the 23 patients who were evaluable on study with adequate samples for analysis. Significantly reduced levels of both canonical pro-inflammatory cytokines (IL-1β, TGF-β, TNF-α, IFN-γ; shown in blue) and MDSC-associated cytokines (G-CSF, IL-6, GM-CSF, M-CSF, IL-10, IL-13, VEGF); shown in red) were observed following dietary soy intervention. (B) Representative raw data for plasma IL-6 (in pg/mL) from patients prior to (Day 0) and following soy intervention (Day 56).
Table I.
Changes in cytokines by groups in plasma from patients with prostate cancer following eight weeks of soy bread consumption. A global testing approach was used and differences were in terms of z-scores. CL = confidence limits.
| Cytokine Group | Mean Difference (z-score) | Lower 95% CL | Upper 95% CL | Pr > |t| |
|---|---|---|---|---|
| MDSC | −1.7442 | −3.3527 | −0.1357 | 0.035 |
| T reg | −0.7412 | −1.5748 | 0.0924 | 0.079 |
| Th1 | −2.0844 | −3.9275 | −0.2414 | 0.028 |
| Th17 | −0.6364 | −1.4361 | 0.1632 | 0.113 |
| Th2 | −1.0983 | −2.362 | 0.1654 | 0.085 |
Soy bread intervention is associated with alterations in the percentage of NK and T cells in patient peripheral blood
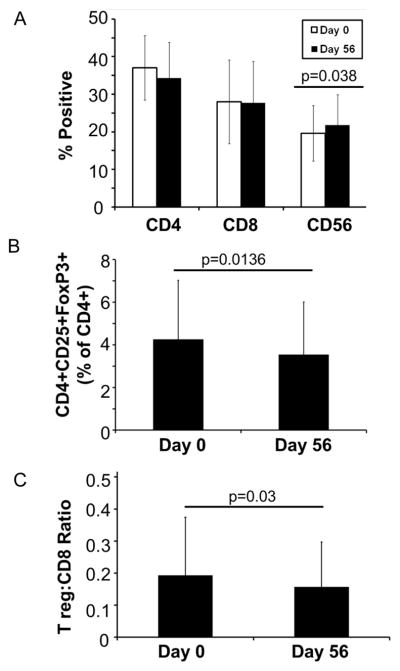
Based on the significant reduction in numerous plasma cytokines following the soy bread intervention, we postulated that this intervention may also alter immune cell phenotype within the peripheral blood of these patients. Therefore, phenotypic analysis of cryopreserved PBMCs obtained at baseline and day 56 (following soy intervention) was conducted by flow cytometry. As compared to baseline, there was a modest, but statistically significant increase in the percentage of CD56+ NK cells (p=0.038), but no significant change in the percentage of CD8+ or CD4+ T cells (p=0.75 and p=0.065, respectively; Figure 2A).
Figure 2. Soy bread consumption and phenotypic changes in circulating NK and T cell populations in patients with prostate cancer.
(A) Percentages of circulating CD4+, CD8+ and CD56+ lymphocytes or (B) CD4+CD25+FoxP3+ T regulatory cells were evaluated at Day 0 and Day 56 by flow cytometry. Data are presented as the mean % positive cells for each phenotypic subset across the 25 patients who were evaluable on study. (C) The ratio of CD4+CD25+FoxP3+ T regulatory cells to CD8+ cells was significantly reduced at Day 56 after soy bread intervention as compared to Day 0. Data are presented as the mean ratio of T reg:CD8+ cells across the 25 evaluable patients.
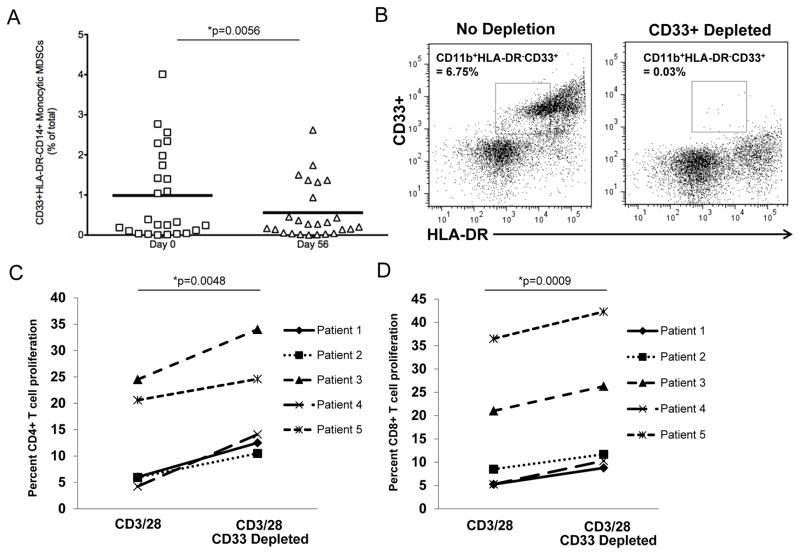
Soy bread reduced the ratio of T regulatory cells to CD8+ cells and MDSC in peripheral blood
Consistent with the cytokine data, the total percentage of CD4+CD25+FoxP3+ T regulatory cells (Tregs) and the ratio of Treg:CD8+ T cells were significantly reduced at day 56 as compared to baseline (p=0.0136 and p=0.03 respectively; Figure 2B-C). Compared to other cell subsets within the peripheral blood, we observed noticeable heterogeneity in the percentage of cells with phenotypic properties consistent with monocytic (CD33+HLA-DRnegCD14+) MDSC (Figure 3A). As expected for a population with excellent performance status (ECOG 0-1) (30), and low tumor burden the circulating percentage of these myeloid cell populations was modest. However, the percentages of the monocytic MDSC were also significantly lower in patients at day 56 as compared to baseline (p=0.0056; Figure 3A). No significant differences were detected between patients consuming soy bread vs. soy almond bread for any of these outcomes (all p-values > 0.2; Supplemental Table 2B). Importantly, ex vivo depletion of CD33+ cells from the PBMCs of representative patients significantly improved CD4+ (p=0.0048) and CD8+ (p=0.0009) T cell proliferation following stimulation with CD3/CD28-targeted beads (Figure 3B-D). These data support the concept that cells with an MDSC phenotype were in fact, functionally suppressive.
Figure 3. Soy bread consumption and phenotypic changes in circulating MDSC populations in patients with prostate cancer.
Peripheral blood mononuclear cells (PBMCs) were obtained from the 25 evaluable cancer patients on Day 0 and Day 56, and stained for MDSC using flurochrome-labeled antibodies targeting CD33, HLADR and CD14 or the appropriate isotype controls. MDSC levels were evaluated by flow cytometry based on a minimum of 20,000 live events and presented as the percentage of total cells. Data were analyzed and presented as the total percentage of (A) CD33+HLA-DRnegCD14+ monocytic MDSC. Each symbol represents data from an individual patient while the horizontal lines indicate the mean within each group. (B) Representative flow cytometric dot plots validating effective depletion of MDSC from patient peripheral blood samples. Depletion of CD33+ cells results in enhanced proliferation following a 72 hour stimulation with CD3/CD28 beads in CFSE-labeled (C) CD4+ and (D) CD8+ T cells from peripheral blood of patients. Data are presented as the percentage of T cell proliferation from blood taken on Day 0 from five representative patients on study. Cryopreserved cells were thawed, CFSE-labeled, stimulated, stained for CD4 or CD8 markers and analyzed by flow cytometry.
Discussion
Our study demonstrates significant reductions in circulating pro-inflammatory cytokines, T regs and MDSC after a soy bread intervention. These data represent the first evidence that dietary soy can modulate human immune markers relevant to inflammation and cancer progression. Collectively, these findings and preclinical studies (3–5), support the hypothesis that dietary soy may influence the human immune system in a manner that reduces inflammatory processes associated with early carcinogenesis and promote immune surveillance during cancer progression.
A key consideration in conducting informative human studies of foods and immune function is a fully characterized intervention agent. Studies of dietary patterns or individual foods provide layers of complexity that go far beyond pure drugs or even pure nutrients. First, we controlled the participant’s background diet to limit exposure to soy phytochemicals (30). In parallel, we standardized the vitamin and mineral supplement employed in order to further control potential confounding exposures. Second, we developed and fully characterized the soy bread products that are easily incorporated into the daily diet with high compliance while providing quantifiable exposure to the array of potentially bioactive phytochemicals found in a food product (31). In this study we also compared two variations of the soy-bread that modestly alters the pharmacokinetics of isoflavones (30). However, with regards to the immunologic markers examined, we saw no significant difference in men consuming the soy or soy-almond bread (enriched in β-glucosidase to increase aglycones). Overall, this whole-food based approach offers the added advantage of safety, and in our ability to deliver a defined dose of bioactives in the context of a food matrix which may positively impact absorption and downstream biologic effects (36). This bread provided soy isoflavones to our study participants in amounts similar to intake consumed by Asian populations and earlier linked to a lower risk of prostate cancer in epidemiologic studies (1, 2). Importantly, the sensory properties (taste, texture etc.) of this soy bread formulation were such that remarkably high self-reported compliance (>92%) was observed among patients.
The significant reduction in specific cytokines observed after soy bread intervention was consistent with alterations in the percentage of circulating MDSC and T regs:CD8+ ratios observed in these patients. Our finding is just the beginning of an effort to understand the mechanisms whereby soy phytochemicals act on the immune system and impact health. For example, most in vitro and rodent studies investigating immune readouts have been largely restricted to pure genestein, although a small number of reports have been published with equol or daidzein (37, 38). In these provocative reports, genistein has been shown to possess anti-inflammatory effects by virtue of its ability to suppress delayed-type hypersensitivity (DTH) responses to various stimuli, decrease thymocyte maturation, and decrease specific Ab titers in keyhole limpet hemocyanin (KLH) or ovalbumin-immunized mice (39–43). Interestingly, reports suggest that genistein increases the activity levels of cytotoxic T cells and natural killer cells, conferring resistance to tumor challenge (39, 44, 45). However, these data should be interpreted with the following considerations in mind. First, many studies showing an immune altering effect of genistein have used pharmacologic concentrations, much higher than what would be obtained in vivo through chronic dietary exposure (46). Second, the effects of genistein or other pure isoflavones on immunity may not necessarily correspond to the effects produced by exposure to the more complex phytochemical profiles at lower dosages within a food-matrix that people actually consume (46). Third, most available data are derived from in vitro studies and some rodent models rather than human cells or subjects. Finally, the mechanisms by which dietary soy modulates immune function deserves further investigation. It is possible that the immunologic changes observed may be a direct consequence of soy components acting upon immune cells. Alternatively, immune effects may be secondarily influenced by soy-mediated alterations in host metabolism. This is an interesting possibility given our observations that a significant reduction in body mass index (BMI) was observed in the study, while a subset of men with hypercholesterolemia had decreased LDL and cholesterol (30). The potential interactions between these physiologic events and immune modulation deserve future attention in subsequent studies.
Numerous studies have suggested a potential benefit of dietary soy for patients with prostate cancer, but these efforts have been compromised by their limited power and scope. Soy isoflavones are reported to augment the direct effect of radiation therapy on prostate cancer cells while limiting damage to normal tissues (47). However, the clinical results across all human studies have been heterogeneous, and are truly difficult to compare due to variation in study design, populations examined, compliance, and the soy products provided. For example, a recent study was completed in patients post-prostatectomy, comparing a milk protein to a soy protein drink showed no impact on risk of biochemical progression (7). Together these data highlight the complexity of dietary intervention studies, and demonstrate the need for further definitive research using highly-defined food delivery systems, such as those described in this study (8, 11). The present study does provide unique insight into the potential for dietary soy as a novel strategy to alter immunologic processes that may be relevant to prostate cancer at multiple stages of disease progression. For example, we can hypothesize that reduced inflammatory processes may inhibit the early progression of prostatic intraepithelial neoplasia to cancer. Optimizing anti-cancer immunity by dietary means may potentiate the impact of immunotherapy, including vaccines, for this disease.
Although our results highlight the potential for dietary soy as a novel immune modulatory intervention, there are a number of caveats to this phase II study that deserve mention. First, there was no placebo group per se as all men served as their own controls, and our team is now supported to pursue such studies in the near future. Second, we have not yet determined if the systemic immune changes are reflected in the tumor microenvironment, and is the focus of a future trial. This study was also not designed to determine whether the effects of a soy diet are lasting or are reversible upon returning to a usual diet. Based on these initial data, we are in the process of conducting a clinical trial of soy bread intervention to assess local and systemic immunologic biomarkers in the pre-prostatectomy setting. This study design will use a placebo group and allow for acquisition of tissue upon surgery to address this limitation. Third, due to limitations in sample quantity and the unavoidable use of cryopreserved cells in this retrospective study, our phenotypic analysis of PBMCs was limited to CD4+CD25+FoxP3+ T regulatory cells, and the monocytic subset of MDSC. Certainly we are interested in understanding the impact of dietary soy on more precisely defined, freshly isolated, immune cell populations such as inducible T regulatory cells or other MDSC subsets (i.e. CD15+ granulocytic MDSC) that may be altered via cryopreservation. However, in representative patients with sufficient quantities of cells, we did demonstrate that CD33+ cell depletion led to significantly improved T cell proliferation upon in vitro stimulation. These data confirm the functional properties of cells with an MDSC phenotype in the present study. Finally, it was also interesting to note the strong concordance between reduced cytokines in the PBMC compartment and circulating levels of these cells. Although not previously reported for soy isoflavones, other dietary agents with putative chemopreventative properties including 25-hydroxyvitamin D3, curcumin and EGCG have been shown to modulate aberrant myeloid cell expansion (48–50).
Our initial observations documenting an impact of diet on biomarkers of human immune function have important implications. First, this finding supports the concept that immune-based mechanisms may underlie dietary associations with human cancer risk. As the role of immunologic processes in carcinogenesis is further elucidated for specific cancers, informative studies can be undertaken. Perhaps more critically, our human study implicates diet as one of the variables that may impact the efficacy of our rapidly expanding portfolio of immunologic therapies for cancer. Ongoing and future clinical trials of novel immune therapies may benefit from considering diet and nutrition as potential modulators of outcome that contribute to heterogeneous responses. Indeed the value of immunotherapeutic studies may be enhanced by controlling for the multitude of supplements and dietary strategies employed by cancer patients to thwart disease progression. Many of these are unsubstantiated, yet may impact relevant processes such as immune function.
In summary, these data indicate that dietary soy can influence the composition of specific types of immune cell populations and the orchestration of the cytokine network impacting immune responses. Together these research findings suggest that dietary interventions represent a novel means to tune the immune system in a manner that benefits cancer therapy and prevention.
Supplementary Material
Acknowledgments
Financial support: Supported by NIH grants 1R01CA169363-01, 5T32CA009338-34, R21CA125909, a grant from the Ohio Soybean Council, the Ohio Agricultural Research and Development Center (OARDC), The Center for Advanced Functional Foods Research and Entrepreneurship (CAFFRE), The Molecular Carcinogenesis and Chemoprevention Program of The Ohio State University Comprehensive Cancer Center. This work was also supported by the Pelotonia Fellowship Program of The Arthur G. James and Richard Solove Research Institute. Any opinions, findings and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program.
We thank the OSUCCC Analytical Cytometry, Biostatistics and Nutrient and Phytochemical Shared Resources. We are especially grateful to all patients who participated in this study.
Footnotes
Conflict of Interest: There are no conflicts of interest related to this work.
References
- 1.Messina M. Western soy intake is too low to produce health effects. The American journal of clinical nutrition. 2004;80:528–9. doi: 10.1093/ajcn/80.2.528. author reply 9–30. [DOI] [PubMed] [Google Scholar]
- 2.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 3.Trottier G, Bostrom PJ, Lawrentschuk N, Fleshner NE. Nutraceuticals and prostate cancer prevention: a current review. Nat Rev Urol. 2010;7:21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–35. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 5.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–82. [PubMed] [Google Scholar]
- 6.Zuniga KE, Clinton SK, Erdman JW., Jr The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev Res (Phila) 2013;6:548–57. doi: 10.1158/1940-6207.CAPR-12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E, Melamed J, et al. Effect of soy protein isolate supplementation on biochemical recurrence of prostate cancer after radical prostatectomy: a randomized trial. Jama. 2013;310:170–8. doi: 10.1001/jama.2013.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein MA, Nahin RL, Messina MJ, Rader JI, Thompson LU, Badger TM, et al. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J Nutr. 2010;140:1192S–204S. doi: 10.3945/jn.110.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar NB, Kang L, Pow-Sang J, Xu P, Allen K, Riccardi D, et al. Results of a randomized phase I dose-finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. Journal of the Society for Integrative Oncology. 2010;8:3–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar NB, Krischer JP, Allen K, Riccardi D, Besterman-Dahan K, Salup R, et al. A Phase II randomized, placebo-controlled clinical trial of purified isoflavones in modulating steroid hormones in men diagnosed with localized prostate cancer. Nutr Cancer. 2007;59:163–8. doi: 10.1080/01635580701432678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Die MD, Bone KM, Williams SG, Pirotta MV. Soy and soy isoflavones in prostate cancer: a systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113:E119–30. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
- 12.Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993;90:2690–4. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranse R, Dagnelie PC, van Kemenade MC, de Jong FH, Blom JH, Tijburg LB, et al. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. International journal of cancer Journal international du cancer. 2005;113:835–40. doi: 10.1002/ijc.20653. [DOI] [PubMed] [Google Scholar]
- 14.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61:117–31. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–76. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18:408–17. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Pavese JM, Krishna SN, Bergan RC. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. The American journal of clinical nutrition. 2014;100:431S–6S. doi: 10.3945/ajcn.113.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–93. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurel B, Lucia MS, Thompson IM, Jr, Goodman PJ, Tangen CM, Kristal AR, et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:847–56. doi: 10.1158/1055-9965.EPI-13-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madan RA, Gulley JL, Kantoff PW. Demystifying immunotherapy in prostate cancer: understanding current and future treatment strategies. Cancer J. 2013;19:50–8. doi: 10.1097/PPO.0b013e31828160a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. The New England journal of medicine. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 22.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox SB, Launchbury R, Bates GJ, Han C, Shaida N, Malone PR, et al. The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2alpha but not HIF-1alpha. Prostate. 2007;67:623–9. doi: 10.1002/pros.20538. [DOI] [PubMed] [Google Scholar]
- 24.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 25.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–55. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 27.Vergati M, Cereda V, Madan RA, Gulley JL, Huen NY, Rogers CJ, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother. 2011;60:197–206. doi: 10.1007/s00262-010-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012;1:152–61. doi: 10.4161/onci.1.2.18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. 2014;63:1177–87. doi: 10.1007/s00262-014-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn-Jarvis JH, Clinton SK, Grainger EM, Riedl KM, Schwartz SJ, Lee M-L, et al. Isoflavone pharmacokinetics and metabolism after consumption of a standardized soy and soy-almond bread in men with asymptomatic prostate cancer. 2014 doi: 10.1158/1940-6207.CAPR-14-0465. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn-Jarvis JH, Riedl KM, Schwartz SJ, Vodovotz Y. Design and selection of soy breads used for evaluating isoflavone bioavailability in clinical trials. Journal of agricultural and food chemistry. 2013;61:3111–20. doi: 10.1021/jf304699k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, et al. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-alpha signaling in CD4(+) T cells from patients with GI malignancy. Cancer Immunol Immunother. 2011;60:1269–79. doi: 10.1007/s00262-011-1029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace TA, Ameen Z, Collins A, Wojcik SE, Mair M, Young GS, et al. Pancreatic cancer associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–87. [PubMed] [Google Scholar]
- 35.Gordon A, Glazko G, Qui X, Yakovlev A. Control of the mean number of false discoveries, Bonferroni and stability of multiple testing. Annals of Applied Statistics. 2007;1:179–90. [Google Scholar]
- 36.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 37.Sakai T, Kogiso M. Soy isoflavones and immunity. J Med Invest. 2008;55:167–73. doi: 10.2152/jmi.55.167. [DOI] [PubMed] [Google Scholar]
- 38.Yum MK, Jung MY, Cho D, Kim TS. Suppression of dendritic cells' maturation and functions by daidzein, a phytoestrogen. Toxicol Appl Pharmacol. 2011;257:174–81. doi: 10.1016/j.taap.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Guo TL, McCay JA, Zhang LX, Brown RD, You L, Karrow NA, et al. Genistein modulates immune responses and increases host resistance to B16F10 tumor in adult female B6C3F1 mice. J Nutr. 2001;131:3251–8. doi: 10.1093/jn/131.12.3251. [DOI] [PubMed] [Google Scholar]
- 40.Kogiso M, Sakai T, Mitsuya K, Komatsu T, Yamamoto S. Genistein suppresses antigen-specific immune responses through competition with 17beta-estradiol for estrogen receptors in ovalbumin-immunized BALB/c mice. Nutrition. 2006;22:802–9. doi: 10.1016/j.nut.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Santell RC, Kieu N, Helferich WG. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J Nutr. 2000;130:1665–9. doi: 10.1093/jn/130.7.1665. [DOI] [PubMed] [Google Scholar]
- 42.Yellayi S, Naaz A, Szewczykowski MA, Sato T, Woods JA, Chang J, et al. The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci U S A. 2002;99:7616–21. doi: 10.1073/pnas.102650199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yellayi S, Zakroczymski MA, Selvaraj V, Valli VE, Ghanta V, Helferich WG, et al. The phytoestrogen genistein suppresses cell-mediated immunity in mice. J Endocrinol. 2003;176:267–74. doi: 10.1677/joe.0.1760267. [DOI] [PubMed] [Google Scholar]
- 44.Guo TL, White KL, Jr, Brown RD, Delclos KB, Newbold RR, Weis C, et al. Genistein modulates splenic natural killer cell activity, antibody-forming cell response, and phenotypic marker expression in F(0) and F(1) generations of Sprague-Dawley rats. Toxicol Appl Pharmacol. 2002;181:219–27. doi: 10.1006/taap.2002.9418. [DOI] [PubMed] [Google Scholar]
- 45.Guo TL, Zhang XL, Bartolucci E, McCay JA, White KL, Jr, You L. Genistein and methoxychlor modulate the activity of natural killer cells and the expression of phenotypic markers by thymocytes and splenocytes in F0 and F1 generations of Sprague-Dawley rats. Toxicology. 2002;172:205–15. doi: 10.1016/s0300-483x(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 46.Cooke PS, Selvaraj V, Yellayi S. Genistein, estrogen receptors, and the acquired immune response. J Nutr. 2006;136:704–8. doi: 10.1093/jn/136.3.704. [DOI] [PubMed] [Google Scholar]
- 47.Hillman GG, Singh-Gupta V, Runyan L, Yunker CK, Rakowski JT, Sarkar FH, et al. Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2011;101:329–36. doi: 10.1016/j.radonc.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santilli G, Piotrowska I, Cantilena S, Chayka O, D'Alicarnasso M, Morgenstern DA, et al. Polyphenol E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res. 2013;19:1116–25. doi: 10.1158/1078-0432.CCR-12-2528. [DOI] [PubMed] [Google Scholar]
- 50.Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G, et al. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev Res (Phila) 2012;5:205–15. doi: 10.1158/1940-6207.CAPR-11-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.