Abstract
The neuropeptides vasopressin (VP) and oxytocin (OT) and their receptors in the brain are involved in the regulation of various social behaviors and have emerged as drug targets for the treatment of social dysfunction in several sex-biased neuropsychiatric disorders. Sex differences in the VP and OT systems may therefore be implicated in sex-specific regulation of healthy as well as impaired social behaviors. We begin this review by highlighting the sex differences, or lack of sex differences, in VP and OT synthesis in the brain. We then discuss the evidence showing the presence or absence of sex differences in VP and OT receptors in rodents and humans, as well as showing new data of sexually dimorphic V1a receptor binding in the rat brain. Importantly, we find that there is lack of comprehensive analysis of sex differences in these systems in common laboratory species, and we find that, when sex differences are present, they are highly brain region- and species- specific. Interestingly, VP system parameters (VP and V1aR) are typically higher in males, while sex differences in the OT system are not always in the same direction, often showing higher OT expression in females, but higher OT receptor expression in males. Furthermore, VP and OT receptor systems show distinct and largely non-overlapping expression in the rodent brain, which may cause these receptors to have either complementary or opposing functional roles in the sex-specific regulation of social behavior. Though still in need of further research, we close by discussing how manipulations of the VP and OT systems have given important insights into the involvement of these neuropeptide systems in the sex-specific regulation of social behavior in rodents and humans.
1. Introduction
The neuropeptides vasopressin (VP) and oxytocin (OT) are involved in the regulation of diverse social behaviors such as social recognition, pair-bonding, and social cognition in mammals, including humans (Veenema & Neumann, 2008; Ross & Young, 2009; Meyer-Lindenberg et al., 2011; Albers, 2014). VP and OT are evolutionarily conserved, differing from each other by only two amino acids. Importantly, VP and OT often regulate social behavior in sex-specific ways. This may be due to sex differences in the brain VP and OT systems, which will be the overarching topic of this review. Importantly, VP and OT have been implicated in the etiology of psychiatric disorders, such as schizophrenia (Jobst et al., 2014), autism (Yang et al., 2010; Xu et al., 2013; LoParo and Waldman, 2014), depression (Yuen et al., 2014), and borderline personality disorder (Bertsch et al., 2013), disorders which show sex biases in prevalence, symptom severity, and treatment responses. Knowledge of sex differences in these systems, as well as how OT and VP may mediate sex-specific social behavior, may therefore provide useful insight into sex-specific treatment strategies for men and women diagnosed with psychiatric disorders characterized by social dysfunction.
We will start with a discussion on sex differences in VP and OT in the brains of rodents and humans (Section 2,). We briefly summarize the well-known sex differences in VP synthesis and fiber distribution in the brain of rodents and other species (for a more extensive review, see De Vries & Panzica, 2006). Interestingly, compared to VP, there is much less research regarding sex differences in OT synthesis in the brain. We generally find that, while there are robust sex differences in VP synthesis in conserved brain regions across species, there are fewer sex differences in OT synthesis in the brain and such sex differences are specific to particular brain regions and species.
Compared to sex differences in VP and OT peptide synthesis, even less is known about sex differences in OT and VP receptors in the brain. We therefore discuss the current knowledge of sex differences in these receptor systems in rodent and human brains (section 3), as well as show new data of sex differences in the VP V1a receptor (V1aR) in the rat brain (section 3, Figs 1 and 2). Interestingly, of the relatively few studies across various species, males seem to have higher V1aR and OT receptor (OTR) expression compared to females.
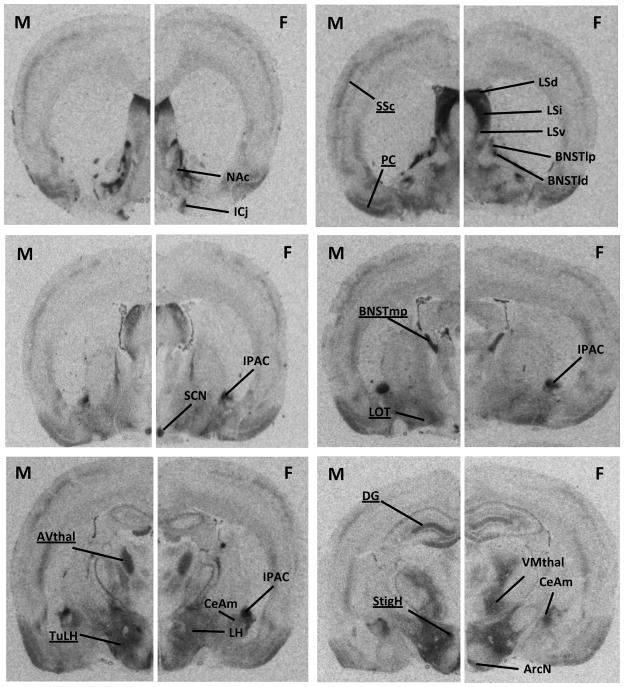
Fig. 1.
Representative coronal sections showing V1aR binding densities in forebrain areas of male (M) and female (F) Wistar rats. Receptor autoradiography was performed on 16 μm cryocut coronal brain sections according to Dumais et al. (2013) using the 125I linear VP antagonist [125I]-d(CH2)5(Tyr[Me])-AVP (Perkin Elmer, Shelton, CT) as tracer. Brain sections were exposed to film for 7 days. The optical density of V1aR binding was measured using Image J (NIH, http://rsb.info.nih.gov/ij/). Each measurement was subtracted by tissue background and V1aR binding densities were calculated by taking the mean of 4–10 (depending on the region being analyzed) bilateral measurements of the region of interest per rat. The data was converted to dpm/mg (disintegrations per minute/milligram tissue) using a [125I] standard microscale (American Radiolabeled Chemicals Inc., St Louis, MO). Compared to females, males have higher V1aR binding densities in the somatosensory cortex (SSC), piriform cortex (PC), nucleus of the lateral olfactory tract (LOT), medial posterior BNST (BNSTmp), anteroventral thalamus (AVthal), tuberal lateral hypothalamus (TuLH), stigmoid hypothalamus (StigH), and dentate gyrus (DG). No sex differences were found in the Islands of Calleja (ICj), nucleus accumbens (NAc), dorsal lateral septum (LSd), intermediate LS (LSi), ventral LS (LSv), lateral dorsal BNST (BNSTld), lateral posterior BNST (BNSTlp), lateral hypothalamus (LH), arcuate nucleus of the hypothalamus (ArcN), suprachiasmatic nucleus of the hypothalamus (SCN), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), ventromedial thalamus (VMthal), and medial part of the CeA (CeAm). Regions which show sex differences in V1aR binding density are underlined.
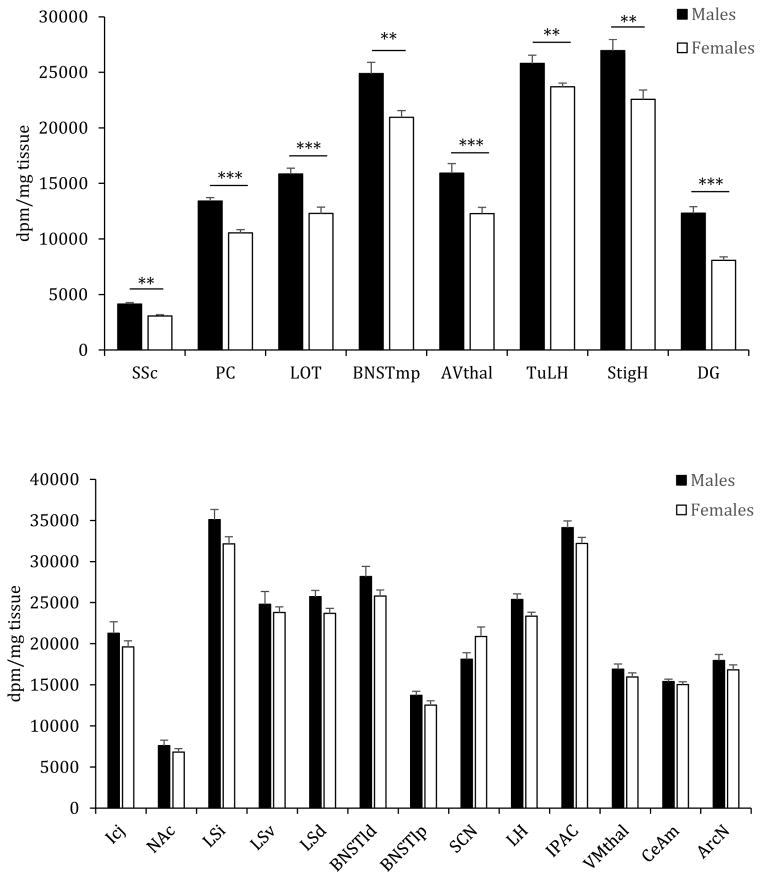
Fig. 2.
V1aR binding densities in forebrain regions of male and female Wistar rats. Males have higher V1aR binding densities than females in 8 out of 21 forebrain regions analyzed (top graph). These regions include the somatosensory cortex (SSC), piriform cortex (PC), nucleus of the lateral olfactory tract (LOT), medial posterior BNST (BNSTmp), anteroventral thalamus (AVthal), tuberal LH (TuLH), stigmoid hypothalamus (StigH), and dentate gyrus (DG). There are no sex differences in 13 forebrain regions analyzed (bottom graph). These regions include the Islands of Calleja (ICj), nucleus accumbens (NAc), dorsal lateral septum (LSd), intermediate LS (LSi), ventral LS (LSv), lateral dorsal BNST (BNSTld), lateral posterior BNST (BNSTlp), lateral hypothalamus (LH), arcuate nucleus of the hypothalamus (ArcN), suprachiasmatic nucleus of the hypothalamus (SCN), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), ventromedial thalamus (VMthal), and medial part of the CeA (CeAm). Bars indicate means + SEM. **p<0.01, ***p<0.001, one-way ANOVA, correcting for multiple comparisons (FDR α≤0.0214)
Despite the reported sex differences in VP and OT systems, surprisingly few comparative studies have investigated the behavioral functions of OT and VP systems in males and females using the same design. Importantly, those studies that do investigate the role of VP and OT in social behavior in both sexes often demonstrate a robust sex-specific modulation of social behavior by VP and OT systems in a variety of rodent species and humans (Section 4). Finally, we provide a short general discussion on the main findings and propose future directions (Section 5). Interestingly, the expression of VP and OT receptors in the rodent brain are non-overlapping (Section 5, Fig 3), suggesting possible implications for either complementary or distinct behavioral functions of VP and OT, a rather unexplored but important area for future research.
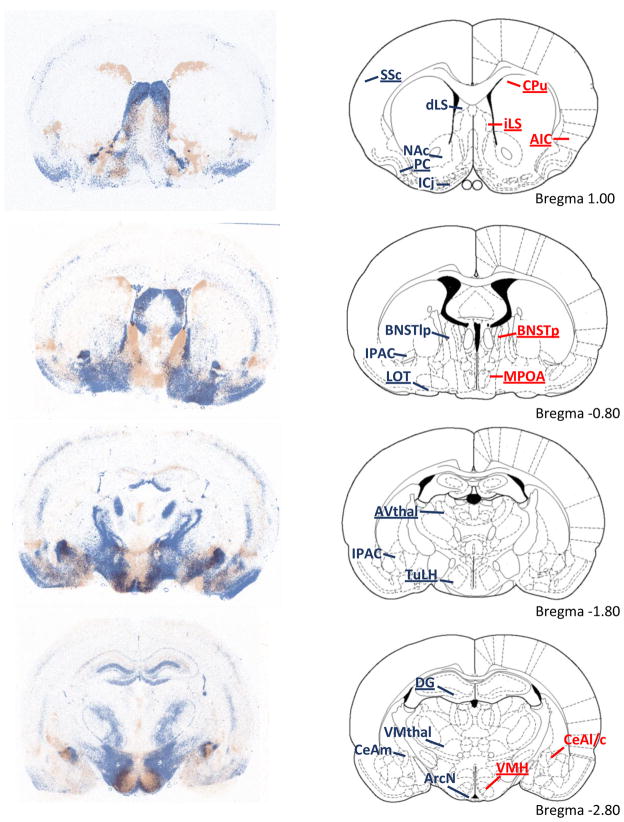
Fig. 3.
Overlay of V1aR binding densities (blue) and OTR binding densities (red) in forebrain areas of male Wistar rats from adjacent coronal sections. The right column depicts representative rat brain images adapted from The Rat Brain Atlas (Paxinos & Watson, 1998). Overall, there is little overlap between V1aR binding and OTR binding profiles. Dense V1aR binding is found in the somatosensory cortex (SSc), piriform cortex (PC), Islands of Calleja (ICj), nucleus accumbens (NAc), lateral septum (LS), lateral dorsal BNST (not shown), lateral posterior BNST (BNSTlp), medial posterior BNST (not shown), nucleus of the lateral olfactory tract (LOT), dentate gyrus (DG), tuberal lateral hypothalamus (TuLH), anteroventral thalamus (AVthal), suprachiasmatic nucleus of the hypothalamus (not shown), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), arcuate nucleus of the hypothalamus (ArcN), ventromedial thalamus (VMthal), and medial central amygdala (CeAm). Dense staining of OTR binding is found in the dorsal caudate putamen (CPu), agranular insular cortex (AIP), posterior BNST (BNSTp), medial preoptic area (MPOA), ventral medial hypothalamus (VMH), and lateral and capsular central amygdala (CeAl/c). Regions which show sex differences in V1aR or OTR binding are underlined.
2. Sex differences in vasopressin and oxytocin synthesis and projections in the brain
2.a. Vasopressin
VP acts as a hormone in the periphery and as a neuromodulator in the brain. VP-producing magnocellular neurons of the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus project to the posterior pituitary, where VP is released into the general circulation as a hormone (Brownstein et al., 1980; Young and Gainer, 2003). As a hormone, it is involved in the regulation of blood pressure and water retention in the body (Silva et al., 1969; Ishikawa, 1993). VP is also produced in parvocellular neurons of the PVN that project to the anterior pituitary (Armstrong, 2004). From here, VP stimulates the release of adrenocorticotropic hormone, which, in turn, stimulates glucocorticoid release from the adrenal cortex (Gillies et al., 1982). As such, VP is part of the hypothalamic-pituitary-adrenal (HPA) axis, a system involved in the neuroendocrine stress response (Herman, 1995; Volpi et al., 2004). In addition, VP-synthesizing cells in the PVN, SON, suprachiasmatic nucleus of the hypothalamus (SCN), bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA) project centrally to multiple areas in the brain (Buijs, 1978; Sofroniew & Weindl, 1978; Buijs & Swaab, 1979; Buijs, 1980; Sofroniew, 1980; Sofroniew, 1983; Rood & De Vries, 2011). It should be noted that a recent study in mice showed that the VP-synthesizing cells within the amygdala are located in the intra-amygdaloid BNST rather than the MeA (Otero-Garcia et al., 2014). However, it is not clear whether this is specific to mice or a more general feature. Therefore, we will refer to these VP-synthesizing cells as being located in the MeA across species. Upon its central release, VP can modulate the activation of many brain regions via binding to vasopressin receptors (discussed further in section 3.a.), thereby regulating social, emotional, and cognitive behaviors (Veenema & Neumann, 2008; Koshimizu et al., 2012; Albers, 2014).
Importantly, VP synthesis and VP fiber projections are sexually dimorphic in specific areas of the brain (Tables 1 and 3). De Vries et al. (1981) were the first to discover the sexually dimorphic nature of VP in the rat brain, and reported that there were more VP-immunoreactive fibers in the lateral septum (LS) and lateral habenular nucleus in males compared to females (De Vries et al., 1981). De Vries and colleagues further discovered that this sex difference was androgen-dependent. Testosterone injections in females or in neonatally castrated males resulted in VP fiber density levels similar to that in control males when given in the first, second, or third week of life (De Vries et al., 1983). The sex difference in LS-VP fiber density originates primarily from VP neurons in the BNST, as lesions to the BNST, but not the PVN, decreased VP-immunoreactive fiber density in the LS (De Vries & Buijs, 1983). Additional studies found that LS-VP fibers also originate in the MeA (Caffe et al., 1987). Not surprisingly, VP synthesis in these areas is also sexually dimorphic, with males having a higher number of VP-immunoreactive neurons and higher VP mRNA expression in the BNST and MeA compared to females (Van Leeuwen et al., 1985; Miller et al., 1989; Wang & De Vries, 1995). VP synthesis in the BNST and MeA is also dependent on gonadal hormones, as castration in males decreased the number of VP-immunoreactive neurons and VP mRNA expression in the BNST and MeA (Van Leeuwen et al., 1985; Miller et al., 1992).
Table 1.
Summary of studies in which VP system parameters are higher in males than in females
| VP measure | Species | Region | Reference |
|---|---|---|---|
| VP-ir neurons | Wistar rats | BNST | Van Leeuwen et al., 1985 |
| Prairie and meadow voles | BNST, MeA | Wang, 1995 | |
| Marmosets | BNST | Wang et al., 1997a | |
| Mandarin voles | PVN, SON (dominant voles) LH, AH (dominant and subordinate voles) | Qiao et al., 2014 | |
| Golden hamsters | SON | Delville et al., 1994 | |
| Humans | PVN, SON | Ishunina & Swaab, 1999 | |
| VP-ir fibers | Wistar rats | LS, LHn | De Vries et al., 1981 |
| MF1 mice | LS | De Vries et al., 2002 | |
| CD1 mice | LS, MeA | Bakker et al., 2006 | |
| C57BL/6 mice | LS, LHn, BNST, MeA, MPOA, LH, thalamus, midbrain, hindbrain | Gatewood, et al., 2006; Rood et al., 2013 | |
| Prairie, pine, and meadow voles | ventral pallidum (prairie voles), LS, LHn | Wang, 1995; Wang et al., 1996; Lonstein & De Vries, 1999; Lim et al., 2004a | |
| Gerbils | LS, medial SDA | Crenshaw et al., 1992 | |
| European hamsters | LS, LHn, MeA (only in spring [high testosterone] season) | Buijs et al., 1986 | |
| Garden dormice | LS, LHn, diagonal band, MeA ventral hippocampus, VTA, LC, central gray (only in spring [high testosterone] season) | Hermes et al., 1990 | |
| VP mRNA | Long Evans rats | MeA | Wang & De Vries, 1995 |
| Wistar rats | BNST | Miller et al., 1989 | |
| Wistar rats (juveniles) | BNST, MeA, SON | Taylor et al., 2012 | |
| V1aR binding | Wistar rats | SSc, PC, medial anterior-BNST, AVthal, tuberal LH, LOT, StigH, DG | Figs. 1 & 2 |
| Siberian hamsters (V1R) | VMH, medial tuberal-, ventral premammilary- hypothalamus | Dubois-Dauphin et al., 1991 | |
| Golden hamsters (V1R) | ventrolateral hypothalamus | Delville & Ferris, 1995 | |
| Prairie and montane voles | medial PFC | Smeltzer et al., 2006 | |
| Plasma VP | Sprague Dawley rats | n/a | Share et al., 1988 |
| Humans | n/a | Share et al., 1988; Asplund & Aberg, 1991; van Londen et al., 1997; Miller et al., 2013; Graugaard-Jensen et al., 2014 (at night only) | |
| Urinary VP | Sprague Dawley rats | n/a | Share et al., 1988 |
| Humans | n/a | Share et al., 1988 |
AH, anterior hypothalamus; AVthal, anteroventral thalamus; BNST, bed nucleus of the stria terminalis; DG, dentate gyrus; LC, locus coeruleus; LH, lateral hypothalamus; LHn, lateral habenular nucleus; LOT, nucleus of the lateral olfactory tract; LS, lateral septum; MeA, medial amygdala; MPOA, medial preoptic area; PC, piriform cortex; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; SDA, sexually dimorphic area found at the border between the medial preoptic area and the anterior hypothalamus in gerbils; SON, supraoptic nucleus of the hypothalamus; SSc, somatosensory cortex; StigH, stigmoid hypothalamus; VMH, ventral medial hypothalamus; VTA, ventral tegmental area; n/a, not applicable.
Table 3.
Summary of studies in which VP system parameters are higher in females than in males
| VP measure | Species | Region | Reference |
|---|---|---|---|
| V1aR binding | C57B6 mice | MPOA, mammillary nuclei | Dubois-Dauphin et al., 1996 |
| P. maniculatus & p. californicus mice | centromedial thalamic nucleus | Insel et al., 1991 |
MPOA, medial preoptic area
Although the aforementioned studies on sex differences were all performed in rats, similar sex differences in BNST and MeA VP have been found across a wide variety of rodent species (summarized in De Vries & Panzica, 2006), including mice (De Vries et al., 2002; Bakker et al., 2006; Gatewood et al., 2006; Rood et al., 2013), voles (Wang, 1995; Wang et al., 1996; Lonstein & De Vries, 1999), gerbils (Crenshaw et al., 1992), European hamsters (but season-dependent; Buijs et al., 1986), and garden dormice (but season-dependent; Hermes et al., 1990). Male prairie voles additionally show more VP-immunoreactive fibers in the ventral pallidum compared to females (Lim et al., 2004a). Non-mammalian vertebrates also show similar sex differences in vasotocin (homologous of VP) which is expressed in areas homologous to the BNST and MeA and in vasotocin projections from these areas (summarized in De Vries & Panzica, 2006). However, there are some exceptions, such as the Syrian hamster, which seems to lack VP cells in the BNST and MeA (Albers et al., 1991; Ferris et al., 1995; Miller et al., 1999). The presence of sex differences in BNST/MeA VP is less clear in primates, which could be due to limited number of studies. Of those studies that included both males and females, no sex differences in BNST-VP were found in macaques (Caffé et al., 1989) and humans (Fliers et al., 1986), whereas male marmosets have more VP-immunoreactive cells in the BNST than female marmosets (Wang et al., 1997a). None of these studies found VP-immunoreactive fibers in the LS, while VP-immunoreactive fibers were found in other brain areas (Caffé et al., 1989; Fliers et al., 1986; Wang et al., 1997b).
In contrast to the BNST and MeA, no sex differences in VP mRNA expression in the PVN have been found in adult rats (Table 4), nor in weanling (Paul et al., 2014) or juvenile (Taylor et al., 2014) rats. Less consistent are findings for VP synthesized in the SON. Although we did not find a sex difference in VP mRNA expression in the PVN of adult Wistar rats (Table 4), higher VP mRNA expression in the SON was found in juvenile male compared to juvenile female Wistar rats (Taylor et al., 2012) and larger VP neurons in the SON were found in adult male compared to adult female Sprague-Dawley rats (Madeira et al., 1993). The latter finding seems in line with higher plasma VP and urinary VP concentrations in male versus female Sprague-Dawley rats, a sex difference that was abolished by gonadectomy and restored by testosterone and ovarian hormone replacement in males and females, respectively (Share et al., 1988).
Table 4.
VP mRNA expression in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus of adult Wistar rats. Cryocut coronal brain sections (16 μm) were processed and hybridized according to Dumais et al (2013) and exposed to film. The optical density of VP mRNA was measured as arbitrary units using Image J (NIH, http://rsb.info.nih.gov/ij/). Values indicate means ± SEM.
| Region | Males (n=11) | Females (n=28) |
|---|---|---|
| PVN | 80.9 ± 4.0 | 81.1 ± 2.1 |
| SON | 95.6 ± 3.1 | 98.6 ± 1.8 |
VP synthesis in hypothalamic regions of most other rodent species studied is not different between males and females, except for two species that do show sex differences (Tables 1–3). In detail, VP synthesis in hypothalamic regions is similar between male and female mice (PVN and SON: Joca et al., 2013; Steinman et al., 2015), voles (PVN, SON, SCN in prairie, pine, meadow, and montane voles: Wang, 1995; Wang et al., 1996), Mongolian gerbils (PVN, medial preoptic area [MPOA], lateral hypothalamus [LH] and anterior hypothalamus [AH]: Wang et al., 2013), and Chinese striped hamsters (PVN, medial preoptic area, lateral hypothalamus and anterior hypothalamus: Wang et al., 2013). However, other rodent species show region- and social context-specific sex differences in hypothalamic VP-immunoreactivity. For example, dominant male mandarin voles had more VP-immunoreactive neurons in the PVN and SON compared to dominant females, and dominant and subordinate male mandarin voles had more VP-immunoreactive neurons in the AH and LH compared to dominant and subordinate females (Qiao et al., 2014). Also, male golden hamsters showed more VP-immunoreactive neurons in the SON, but not the PVN, compared to female golden hamsters (Delville et al., 1994). Males of several fish species show more vasotocin neurons in the preoptic area (homologous to PVN and SON in mammals; Moore and Lowry, 1998) than females (reviewed in Goodson and Bass, 2001). These species-specific sex differences in hypothalamic VP suggest a role for hypothalamic VP in mediating species-specific sex differences in the regulation and/or expression of behavior.
Data discussed above indicate that sex differences in BNST/MeA VP synthesis are found in most rodent species analyzed, while sex differences in hypothalamic VP are limited to a few rodent species (mandarin voles and hamsters). To what extent these sex differences in VP synthesis contribute to sex differences in VP peripheral release or VP release in the brain is still unknown. Local VP release in the brain has been measured in male (Veenema et al., 2010; Lukas et al., 2011a) and female (Bosch & Neumann, 2010; Bosch et al., 2010) rats during the expression of social behavior; however, to our knowledge, there are no studies which have compared brain VP release in males and females. We can hypothesize that, because males show higher VP synthesis in the BNST and MeA, males may have higher VP release in BNST- and MeA- projecting areas, such as the LS. This is an important area of research, as it would provide insights into the extent to which sex differences in static VP synthesis and fiber projections in the brain relate to sex differences in dynamic VP release.
The first study investigating VP neurons in the PVN and SON in humans reported no sex difference (age of subjects ranges from 10–93 years; Fliers et al., 1985). However, a more recent study found a sex difference depending on age, with larger VP neurons in the PVN and SON of men compared to women under 50 years of age, but no sex differences in subjects older than 50 years of age, which was likely due to an increase in VP neuron size in women (Ishunina and Swaab, 1999). This finding seems consistent with studies showing sex differences in plasma VP concentrations with higher plasma VP concentrations in preadolescent and adolescent boys (Miller et al., 2013), adult men (Share et al., 1988; Van Londen et al., 1997) but also in elderly men (Asplund & Aberg, 1991). A more recent study, however, found that plasma VP was higher in men compared to women only at night (Graugaard-Jensen et al., 2014), and another found no sex differences in plasma VP concentrations in adults (Gouin et al., 2012).
Taken together, across vertebrate species, VP synthesis and projections in the brain are often sexually dimorphic, VP within the BNST/MeA is modulated by gonadal hormones, and sex differences in VP may be sensitive to changes in status (dominant, subordinate), season, and photoperiod. Despite some important exceptions in rodent species (De Vries & Panzica, 2006) and some inconsistencies across human studies, VP synthesis, VP innervation in the brain, and VP plasma concentrations generally seem to be higher in males than in females. This could have important implications for the sex-specific regulation and/or expression of behaviors regulated by VP (see section 4).
Remarkably, the functional significance of sexual dimorphisms in VP is still largely unclear. It has been proposed that these consistent sex differences in VP could have a dual function, namely contributing to sex differences in some behaviors while preventing sex differences in other behaviors (De Vries & Boyle, 1998; De Vries, 2004). The latter may be less intuitive, but sex differences in some parameters, like VP, may serve to compensate for necessary sex differences in other parameters. This may allow males and females to display similar behaviors, despite major differences in their physiological and hormonal conditions.
Importantly, VP function in the brain is intrinsically dependent on its receptors in the brain. Therefore, we propose that in order to understand the function of sexual dimorphism in VP synthesis and innervation in the brain we should also have a closer look at VP receptors in the brain and how manipulations of the VP system (both VP and VP receptors) can give insight into the functional significance of the sexually dimorphic VP system (discussed further in sections 3.a. and 4.a.). For a more comprehensive read on sex differences in brain VP and vasotocin, the reader is referred to several excellent reviews (Goodson & Bass, 2001; De Vries & Panzica, 2006; Albers, 2014).
2.b. Oxytocin
Across species, most OT is synthesized in the PVN and SON, but some OT synthesizing cells are found in the accessory magnocellular nucleus of the hypothalamus (rats, humans: Fliers et al., 1985; Weirda et al., 1991; Meynen et al., 2007) or LH, AH, and MPOA (hamsters, gerbils, voles, naked mole rats: Brownstein et al., 1980; Dierickx, 1980; Rhodes et al., 1981; Swanson and Sawchenko, 1983; Wang et al., 1996; Rosen et al., 2008; Xu et al., 2010; Wang et al., 2013). OT-producing magnocellular neurons of the PVN and SON project to the posterior pituitary where OT is released into the general circulation as a hormone, in which it is involved in maternal responses such as milk ejection and uterine contractions (Fuchs and Poblete, 1970; Belin et al., 1984), in male reproductive functions such as ejaculation, in cardiovascular homeostasis, and other peripheral activities (Gimpl and Fahrenholz, 2001). Moreover, OT synthesized in parvocellular neurons of the PVN (Buijs, 1978; Buijs and Swaab, 1979; Sofroniew, 1980; Sofroniew, 1983) and magnocellular neurons in the PVN, SON, and accessory nucleus of the hypothalamus (Ross et al., 2009, Knobloch et al., 2012) project centrally, where OT can modulate the activity of many brain regions via binding to the widely distributed OT receptor (OTR; Jard et al., 1987; Gimpl and Fahrenholz, 2001).
Sex differences in OT-immunoreactive neurons have been found in hypothalamic nuclei in a few rodent species, in which OT is typically higher in females compared to males (Table 7). For example, females had more OT-immunoreactive neurons in the PVN, SON, and anterior hypothalamic periventricular nucleus of CD mice (Häussler et al., 1990), the PVN and SON of mandarin voles (Qiao et al., 2014), the PVN of Brandt’s voles (Xu et al., 2010), and the MPOA of Mongolian gerbils and Chinese-striped hamsters (Wang et al., 2013).
Table 7.
Summary of studies in which OT system parameters are higher in females than in males
| OT measure | Species | Region | Reference |
|---|---|---|---|
| OT-ir neurons | Brandt’s voles | PVN | Xu et al., 2010 |
| Mandarin voles | PVN, LH (dominant and subordinate voles) SON (dominant voles) | Qiao et al., 2014 | |
| Chinese striped hamsters | Intermediate MPOA | Wang et al., 2013 | |
| Mongolian gerbils | Intermediate MPOA | Wang et al., 2013 | |
| CD mice | PVN, SON, anterior hypothalamic periventricular nucleus | Häussler et al., 1990 | |
| OT-ir fibers | CD mice | LS, BNST | Häussler et al., 1990 |
| Mandarin voles | LH | Qiao et al., 2014 | |
| OTR binding | P. maniculatus & p. californicus mice | hippocampal CA1 | Insel et al., 2001 |
| ICR mice | VMH | Tribollet et al., 2002 | |
| Prairie voles | medial PFC | Smeltzer et al., 2006 | |
| Montane voles | medial PFC | Smeltzer et al., 2006 | |
| CSF OT | Humans | n/a | Altemus et al., 1999 |
| Plasma OT | Sprague Dawley rats | n/a | Kramer et al., 2004 |
| Prairie voles | n/a | Kramer et al., 2004 | |
| Mandarin voles | n/a | Cao et al., 2013 | |
| Humans (adolescents) | n/a | Miller et al., 2013 |
BNST, bed nucleus of the stria terminalis; LH, lateral hypothalamus; LS, lateral septum; MPOA, medial preoptic area; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; SON, supraoptic nucleus of the hypothalamus; VMH, ventral medial hypothalamus; n/a, not applicable.
OT mRNA expression or immunoreactivity in the brain of several other rodent species is similar between males and females (Table 6). For example, no sex difference was found for OT mRNA expression in Wistar rats (PVN, SON: Dumais et al., 2013) or for OT-immunoreactive neurons in prairie, pine, meadow, and montane voles (PVN, SON, MPOA, BNST: Wang et al., 1996), naked mole rats (PVN, SON: Rosen et al., 2008), and long tailed hamsters (PVN, MPOA, LH, AH: Xu et al., 2010). Studies in non-human primates have also reported an absence of sex differences in OT-immunoreactive neurons in macaques (PVN, SON: Caffé et al., 1989) and marmosets (PVN, SON, BNST, MeA: Wang et al., 1997). Similarly, there were no sex differences in the number of OT neurons in the PVN (Wierda et al., 1991) and in the size of OT neurons in the PVN and SON (Fliers et al., 1985; Ishunina and Swaab, 1999) in humans.
Table 6.
Summary of studies in which OT system parameters are not different between males and females
| OT measure | Species | Region | Reference |
|---|---|---|---|
| OT-ir neurons | Naked mole rats | PVN, SON, AH, preoptic area Posterior BNST, MeA | Rosen et al., 2008 |
| Prairie voles | PVN, SON, MPOA, BNST, LH preoptic periventricular nucleus, median preoptic nucleus | Wang et al., 1996 | |
| Pine voles | PVN, SON, MPOA, BNST, LH preoptic periventricular nucleus, median preoptic nucleus | Wang et al., 1996 | |
| Meadow voles | PVN, SON, MPOA, BNST, LH preoptic periventricular nucleus, median preoptic nucleus | Wang et al., 1996 | |
| Montane voles | PVN, SON, MPOA, BNST, LH preoptic periventricular nucleus, median preoptic nucleus | Wang et al., 1996 | |
| Brandt’s voles | MPOA, AH, LH, MeA, reticular thalamic nucleus | Xu et al., 2010 | |
| Long-tailed hamsters | MPOA, AH, LH, MeA, reticular thalamic nucleus | Xu et al., 2010 | |
| Mandarin voles | AH | Qiao et al., 2014 | |
| Chinese striped hamsters | PVN, AH, LH, anterior MPOA posterior MPOA | Wang et al., 2013 | |
| Mongolian gerbils | PVN, AH, LH, anterior MPOA posterior MPOA | Wang et al., 2013 | |
| Macaques | PVN, SON | Caffé et al., 1989 | |
| Common marmosets | PVN, SON, BNST, MeA | Wang et al., 1997 | |
| Humans | PVN, SON | Fliers, et al., 1985; Wierda et al., 1991; Ishunina & Swaab, 1999 | |
| OT-ir fibers | Prairie voles | nucleus accumbens | Lim et al., 2004 |
| Macaques | amygdala, NTS, marginal layer of cervical spinal cord | Caffé et al., 1989 | |
| OT mRNA | Wistar rats | PVN, SON | Dumais et al., 2013 |
| OTR mRNA | Mandarin voles | NAc | Smeltzer et al., 2006 |
| OTR binding | Sprague Dawley rats | CeA | Uhl-Bronner et al., 2005 |
| Wistar rats | anterior NAc, ventral LS, PVN, dorsal lateral BNST, CeA | Dumais et al., 2013 | |
| Prairie voles | VP, MeA, CeA, BNST, LS, cingulate ctx | Bales et al., 2007a | |
| C57BL/6J mice | hippocampus, LS, SSc | Hammock & Levitt, 2013 | |
| Golden hamster | endopiriform nucleus, cingulate ctx, islands of Calleja, LS, dorsal hippocampus, amygdala | Dubois-Dauphin et al., 1992 | |
| Humans | basal nucleus of Meynert, diagonal band, globus pallidus, VP, LS, hypothalamus, | Loup et al., 1991 | |
| Plasma OT | Humans | n/a | Zhong et al., 2012; Taylor et al., 2010; Grewen et al., 2005; Gordon et al., 2008; Gordon et al., 2010; Graugaard-Jensen et al., 2014 |
AH, anterior hypothalamus; AVthal, anteroventral thalamus; BNST, bed nucleus of the stria terminalis; CeA, central amygdala, Ctx, cortex; DG, dentate gyrus; IPAC, nucleus of the posterior limb of the anterior commissure; LC, locus coeruleus; LH, lateral hypothalamus; LS, lateral septum; MeA, medial amygdala; MPOA, medial preoptic area; NAc, nucleus accumbens, NTS, nucleus of the solitary tract; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus of the hypothalamus; SON, supraoptic nucleus of the hypothalamus; SSc, somatosensory cortex; VMH, ventral medial hypothalamus; VP, ventral pallidum; n/a, not applicable.
Remarkably, while sex differences in VP fiber projections have been extensively investigated, a quantitative comparison of OT fiber projections between males and females and across rodent species is limited. To our knowledge, the only comparisons between sexes thus far have been in CD mice, prairie voles, mandarin voles and macaques. It was found that females had more OT-immunoreactive fibers compared to males in the LS and BNST of CD mice (Häussler et al., 1990) and LH of mandarin voles (Qiao et al., 2014). In prairie voles, OT-immunoreactive fibers were analyzed only in the nucleus accumbens, where no sex difference was found (Lim et al., 2004a). In macaques, no sex differences were found in OT-immunoreactive fibers in the amygdala, solitary tract nuclei, and marginal layer of the cervical spinal cord (Caffé et al., 1989).
Importantly, it is still unclear as to whether sex differences in brain OT immuoreactivity or mRNA expression reflect sex differences in OT release in the brain. Similar to VP, there is little research on potential sex differences in brain OT release, because investigation into brain region-specific OT release patterns in rats have been performed in one sex only (males: Engelmann et al., 1999; Ebner et al., 2000; Waldherr and Neumann, 2007; or females: Nyuyki et al., 2011; Neumann et al., 1993; Bosch et al., 2010; Bosch et al., 2004). In humans, however, it was found that women had higher concentrations of OT in cerebral spinal fluid compared to men irrespective of health condition (i.e., in healthy controls and in patients with obsessive compulsive disorder; Altemus et al., 1999), suggesting that brain OT release may be higher in females, regardless of the lack of sex differences in OT synthesis in the brain (Fliers et al., 1985; Wierda et al., 1991; Ishunina and Swaab, 1999).
Furthermore, it is unclear as to whether sex differences in brain OT synthesis reflect sex differences in OT release in the periphery. For example, female mandarin voles (Cao et al., 2013) prairie voles (Kramer et al., 2004) and Sprague Dawley rats (Kramer et al., 2004) had higher plasma OT concentrations compared to males, yet only female mandarin voles had higher OT-immunoreactivity in the PVN and SON (Qiao et al., 2014). In humans, although there is a lack of sex differences in OT synthesis in the brain (Fliers et al., 1985; Wierda et al., 1991; Ishunina and Swaab, 1999), studies regarding the presence and direction of sex differences in plasma OT concentrations have been inconsistent. While preadolescent and adolescent girls had higher plasma OT concentrations than boys (Miller et al., 2013), adult men had higher plasma OT compared to adult women (van Londen et al., 1997; Weisman et al., 2013). Still, others reported no sex differences in plasma OT (Zhong et al., 2012; Taylor et al., 2010; Grewen et al., 2005; Gordon et al., 2008; Gordon et al., 2010; Graugaard-Jensen et al., 2014). Thus, in contrast to consistent reports of higher plasma VP in men compared to women, the presence and direction of sex differences in plasma OT concentrations are inconclusive. These inconsistencies may be due to methodological differences across studies, as commercially available methods to quantify plasma OT concentrations are in need of improvement (Szeto et al., 2011).
Overall, we can conclude that there is a lack of sex differences in OT synthesis in the brain in most species analyzed. Interestingly, in those rodent species that show a sex difference, OT-immunoreactivity is consistently higher in females compared to males (Table 7). This is in contrast with VP synthesis in the brain, where VP-immunoreactivity is consistently higher in males compared to females (Table 1). Importantly, the functional significance of the presence (and absence) of these sex differences is still to be determined, though sex differences in synthesis may suggest a greater role of OT in females, and a greater role of VP in males. This suggestion, however, is overly simplified, and OT and VP play a significant role in both male and female social behavior, which is discussed further in section 4.
3. Sex differences in vasopressin and oxytocin receptors in the brain
3.a. VP receptors
VP receptors are G protein coupled receptors consisting of two major subtypes, the V1 receptor (V1R) and the V2 receptor (V2R; Jard, 1983). The V1R is further divided into V1aR and V1bR receptor subtypes. V1aR and V1bR are both expressed in the brain and are the most well-known mediators of the effects of VP in the brain on social behavior. For example, V1aR has been implicated in pair bonding, maternal care, aggression, social recognition, and social play (Bielsky et al., 2004; Egashira et al., 2004; Lim et al., 2004b; Bosch and Neumann, 2008; Veenema and Neumann, 2008; Veenema et al., 2013; Albers, 2014). The V1bR has been more recently getting recognized as a modulator of various social behaviors, such as social recognition, aggression, and maternal care (Stevenson & Caldwell, 2012). Although the V2R has been reported to be found in the rat brain (Hirasawa et al., 1994; Kato et al., 1995), this has not recently been confirmed, and evidence of the presence of V2R in the brain is largely lacking.
Detecting specific V1a and V1b receptors in the brain has been hindered for a long time by the availability of only nonselective radioligands, such as tritium labelled (3H) VP ligands, which bind to both V1 and V2 receptors (Phillips et al., 1990). Using this 3H labeled VP ligand, VP receptor binding in the rat brain was found in areas such as the LS, BNST, nucleus accumbens (NAc), amygdala, diagonal band of Broca, cingulate gyrus, dorsal hippocampus, and caudate nucleus (Baskin et al., 1983; Dorsa et al., 1983; Pearlmutter et al., 1983; Dorsa et al., 1984; Petrecca et al., 1986; Freund-Mercier et al., 1988; Tribollet et al., 1991). However, because this ligand lacked specificity to VP receptor subtypes, specific subtype distributions were still unknown. Further, in all the aforementioned studies, only males were used, or sex was not specified.
The development of more specific and sensitive radioligands led to the visualization of V1R specific binding using iodine labeled (125I) V1R antagonist. For example, Gerstberger and Fahrenholz (1989) used (Mca1,125I-Tyr2,Sar7)AVP to localize V1R in the brain. While only male Wistar rats were used, this was the first study to show V1R-specific binding in the hypothalamus, NAc, LS, BNST, central amygdala (CeA), nucleus of the solitary tract, islands of Calleja, lateral olfactory tract, choroid plexi, and stigmoid hypothalamic nucleus (StigH; Gerstberger & Fahrenholz, 1989). Schmidt et al. (1991) further developed a linear antagonist specific to the V1aR. They found V1aR binding in several brain areas of Wistar rats, such as the LS, hippocampus, superior colliculus, substantia nigra, and central gray, however only female rats were used in this study. Using the same 125I linear V1aR antagonist, additional V1aR binding sites were found in the frontal cortex, dorsal hypothalamus, AH, hippocampal dentate gyrus (DG), substantia nigra, and ventral tegmental area (VTA; Johnson et al., 1993)of male rats.
The first comparative study in rats did not find sex differences in VP receptor binding in areas such as the BNST, DG, LS, and CeA (Tribollet et al., 1990). However, the authors used the nonselective radioligand [3H]VP and the number of males (n=3) and females (n=3) was too low to reliably run statistics. Yet, the VP receptor binding overall was higher in males compared to females.
Importantly, using the 125I linear VP antagonist we find sex differences in V1aR binding densities in several areas in the brains of Wistar rats (Figs. 1 and 2; Table 1; method details are described in the legend of Fig. 1). In detail, males show higher V1aR binding densities in 8 out of 21 forebrain regions, namely the somatosensory cortex (SSc; p<0.01), piriform cortex (PC; p<0.001), medial posterior BNST (BNSTmp; p<0.01), nucleus of the lateral olfactory tract (LOT; p<0.001), anterior ventral thalamic nucleus (AVthal; p<0.001), tuberal LH (tubLH; p=0.001), stigmoid hypothalamus (StigH; p<0.01), and DG (p<0.001; Figs. 1 and 2; Table 1). No sex differences were found in the remaining forebrain regions, i.e., Islands of Calleja, NAc shell, intermediate-, ventral- and dorsal- LS, lateral dorsal BNST, lateral posterior BNST, LH, arcuate nucleus of the hypothalamus, SCN, the nucleus of the posterior limb of the anterior commissure, ventral thalamic nuclei, and the medial central amygdala; Figs. 1 and 2; Table 2).
Table 2.
Summary of studies in which VP system parameters are not different between males and females
| VP measure | Species | Region | Reference |
|---|---|---|---|
| VP-ir neurons | C57BL/6J mice | PVN | Joca et al., 2013 |
| California mice | PVN, SON | Steinman et al., 2015 | |
| Prairie, pine, and meadow voles | PVN, SON, SCN | Wang, 1995; Wang et al., 1996 | |
| Mongolian gerbil | PVN, MPOA, LH, AH | Wang et al., 2013 | |
| Chinese hamster | PVN, MPOA, LH, AH | Wang et al., 2013 | |
| Golden hamsters | PVN | Delville et al., 1994 | |
| Macaques | BNSN, MeA, PVN, SCN, SON, dorsomedial hypothalamus, LC, NTS, diagonal band | Caffé et al., 1989 | |
| Humans | PVN, SON, BNST | Fliers et al., 1985; 1986 | |
| VP-ir fibers | Macaques | medial septum, BNST, amygdala, hippocampus, VTA, peraquaductal gray, raphe, LC, NTS, parabrachial nuclei, diagonal band | Caffé et al., 1989 |
| VP mRNA | Wistar rats (weanling) | PVN | Paul et al., 2014 |
| Wistar rats (juveniles) | PVN, SCN | Taylor et al., 2012 | |
| Wistar rats (adults) | PVN, SON | current article: Table 4 | |
| V1aR binding | Wistar rats | ICj, NAc, LS, BNSTld, BNSTlp, LH, ArcN, SCN, ventromedial thalamus, IPAC, CeA | Figs. 1 & 2 |
| C57B6 mice | CA1, SSc, LS, VTA | Hammock & Levitt, 2012 | |
| ICR mice | subfornical organ, LS, PVN, paraventricular thalamus, mammillary complex, parabrachial nucleus, area postrema- nucleus of the solitary tract, and hypoglossal nucleus | Tribollet et al., 2002 | |
| P. maniculatus & p. californicus mice | OB, LS, septohippocampal nucleus, VP, neocortex, lateral olfactory tract, PVN, reunion thalamic nuclei | Insel et al., 1991 | |
| Singing mice | OB, VP, LS, BNST, globus pallidus, indusium griseum, MeA, CeA, AH, LH, PVN, SON, supramammillary nucleus, thalamus, dorsal lateral geniculate, periaqueductal gray | Campbell et al., 2009 | |
| Prairie voles | VP, MeA, MPOA, BNST, LS, cingulate Ctx, mediodorsal thalamus | Bales et al., 2007a | |
| Tuco-tuco | OB, PFC, indusium griseum, NAc, VP, LS, ArcN, DG | Beery et al., 2008 | |
| Rhesus monkeys | PFC, cingulate Ctx, PC, insular Ctx, entorhinal Ctx, presubiculum, LS, BNST, diagonal band, MPOA, globus pallidus, SCN, SON, CeA, MeA, VMH, infundibulum, mammillary bodies, parabrachial nucleus, LC, inferior olive | Young et al., 1999 | |
| Humans | dorsal LS, BNST, midline and rostral thalamic nuclei, DG, basal amygdala, brainstem | Loup et al., 1991 | |
| Plasma VP | Humans | n/a | Gouin et al., 2012 |
AH, anterior hypothalamus; ArcN, arcuate nucleus; AVthal, anteroventral thalamus; BNST, bed nucleus of the stria; BNSTld, lateral dorsal BNST; BNSTlp, lateral posterior BNST; terminalis; CA1, CA1 region of the hippocampus; CeA, central amygdala, Ctx, cortex; DG, dentate gyrus; ICj, Islands of Calleja; IPAC, nucleus of the posterior limb of the anterior commissure; LC, locus coeruleus; LH, lateral hypothalamus; LS, lateral septum; MeA, medial amygdala; MPOA, medial preoptic area; NAc, nucleus accumbens, NTS, nucleus of the solitary tract; OB, olfactory bulb; PFC, prefrontal cortex; PVN, paraventricular nucleus of the hypothalamus; SCN, suprachiasmatic nucleus of the hypothalamus; SON, supraoptic nucleus of the hypothalamus; SSc, somatosensory cortex; StigH, stigmoid hypothalamus; VMH, ventral medial hypothalamus; VP, ventral pallidum; VTA, ventral tegmental area; n/a, not applicable.
Comparative analysis of V1aR binding between males and females of other rodent species is very limited, and of those that have included both sexes, only a few used specific radioactive ligands and a reasonable number of male and female subjects. An overview of the brain region- and species-specific sex differences in V1aR is provided in Tables 1 and 3 and details per species are discussed below.
Using the 125I linear V1aR antagonist, it was found that female C57B6 mice (n=5) had higher V1aR binding in the MPOA and mammillary nuclei compared to males (n=5; Dubois Dauphin et al., 1996). Although the authors reported V1aR in several other areas throughout the mouse brain, it is unclear whether these areas were tested for sex differences in V1aR binding. Likewise, although no sex differences were found in the hippocampal CA1 region, SSc, LS, and VTA of C57B6 mice (Hammock and Levitt, 2012), other brain regions were not tested for sex differences and the number of mice per sex (n=3) is too low to draw any definitive conclusions. In ICR mice, using the 125I linear V1aR antagonist, males (n=6) and females (n=6) showed a lack of sex differences in all regions analyzed (subfornical organ, LS, PVN, paraventricular thalamus, mammillary complex, parabrachial nucleus, area postrema-nucleus of the solitary tract, and hypoglossal nucleus; Tribollet et al., 2002). This suggests potential strain specific sex differences in V1aR in mice, i.e. a sex differences in the mammillary nucleus was found in C57B6 mice (Dubois Dauphin et al., 1996), but not in ICR mice (Tribollet et al., 2002). Although a thorough comparative analysis of the V1aR throughout the brain of commonly used laboratory mice is lacking, findings thus far suggests that mice, in general, may show few sex differences in V1aR binding density in the brain.
In Peromyscus (P.) maniculatus and P. californicus mice, no sex differences were found for VP receptor binding in any of the brain regions analyzed, except for higher binding in the centromedial thalamic nucleus in females (Insel et al., 1991). However, there are several limitations to this study, including the use of 3H VP and 125I-sarc-AVP, which may not be specific for the V1aR. Additionally, females were run separately from the males, and the number of females was very low (n=3).
Using 3H VP, it was found that male Siberian hamsters had higher V1R binding in the ventromedial, medial tuberal, and ventral premammillary hypothalamic nuclei when on a long photoperiod light cycle, and in the ventral premammillary hypothalamus when on a short photoperiod light cycle, compared to females (Dubois-Dauphlin et al., 1991). Displacement of 3H VP binding by co-incubation with a V1 agonist suggests that binding was of the V1 subtype. In addition, using 125I d(CH2)5,Sar7]AVP, a specific V1 antagonist, V1R binding was also higher in male golden hamsters in the ventrolateral hypothalamus compared to females (Delville & Ferris, 1995). Although VP receptor binding in these species show clear sexual dimorphisms, specificity to the V1aR is still lacking, and neither study included a comprehensive analysis of sex differences in other brain regions of the hamster.
Using the 125I linear V1aR antagonist, no sex differences were found in prairie voles in any region analyzed in Bales et al., (2007a). However, a sex difference for V1aR was found in the medial prefrontal cortex with higher V1aR binding in male prairie and montane voles compared to females in Smeltzer et al., (2006). It should be noted that this was the only region analyzed (Smeltzer et al., 2006) and that this region was not included in the analysis in Bales et al., (2007a). Thus, across several common laboratory rodent species (laboratory mice, hamsters, voles), a comprehensive analysis of sex differences in brain V1aR using a specific V1aR antagonist is still lacking.
Using the 125I linear V1aR antagonist, no sex differences in V1aR binding were found in several brain regions analyzed in singing mice (Campbell et al., 2009), tuco-tuco (Beery et al., 2008), and rhesus monkeys (Young et al., 1999), albeit the number of male (n=2) and female (n=3; 1 gonadally intact, 1 overiectomized, 1 lactating) rhesus monkeys was too low to be conclusive. Likewise, there were no sex differences in V1R binding in several brain regions analyzed in humans (Loup et al., 1991), but this also remains inconclusive because 3H VP was used and the number of women was very low (n=4).. V1aR binding was also found throughout the brain of male and female marmosets (Wang et al., 1997) and the coppery titi monkey (Freeman et al., 2014), but there was no mention of an analysis of sex. The lack of comparative studies in non-human and human primates with specific ligands and proper number of subjects highlights the need for further investigation into potential sex differences in brain V1aR expression in these species. Recently, a radiolabeled human V1aR antagonist has been developed for the use in PET and SPECT imaging, which may lead to a further understanding of sex differences in the brain V1aR system in humans, including the potential relation of V1aR binding with brain function and vasopressin-related disorders (Fabio et al., 2012).
Like VP neurons and fiber projections, V1R binding has also been found to be dependent on gonadal hormones in various species. Gonadectomy abolished V1aR binding in the BNST and in several hypothalamic areas (namely the ventrolateral hypothalamus, ventromedial MPOA, lateral MPOA, posterior lateral preoptic area, anterior LH, and ventromedial hypothalamus[VMH]) of male hamsters (Delville & Ferris, 1995; Johnson et al., 1995; Young et al., 2000). Gonadectomy also reduced V1aR binding in the ventrolateral hypothalamus in female hamsters (Delville & Ferris, 1995), but this was the only region measured. However, short photoperiod-induced anestrus female Syrian hamsters have lower V1aR binding in the medial preoptic nucleus, MPOA, LH, CeA, and BNST compared to females on a long photoperiod (Caldwell and Albers, 2004b), suggesting gonadal hormonal control of V1aR in various brain regions of both male and female hamsters. Furthermore, testosterone treatment increased vasotocin V1aR binding in nuclei of the song system in female canaries (Voorhuis et al., 1988) and male white-throated sparrows (Grozhik et al., 2014).
Given the hormonal modulation of the VP receptor system in these species, we determined whether the estrus phase of female Wistar rats alters V1aR binding densities (see Table 8). Surprisingly, no significant differences were found in V1aR binding densities between non-estrus and estrus females in any of the 21 forebrain regions analyzed (Table 8). This may suggest that changes in V1aR are not required for estrus-induced changes in behaviors associated with mating, at least in rats. This is in contrast with estrus-induced changes in OTR binding in the VMH, MPOA, posterior BNST, and NAcc (Dumais et al., 2013). It would be important to study the influence of estrus on V1aR in the brain in other species as this may help to better understand the behavioral significance of sex differences in V1aR. To our knowledge, none of the above-mentioned studies on V1aR comparisons between males and females indicated the estrus phase of females.
Table 8.
V1aR binding in forebrain regions of non-estrus and estrus, and nulliparous and primiparous Wistar rats. V1aR binding densities were analyzed in non-estrus and estrus Wistar rats. Estrus phase was determined immediately after death, and was based on cell characteristics defined by Goldman et al. (2007). Rats were categorized as being in proestrus/estrus (n=13; cells characteristic of proestrus and estrus phases in which females show higher levels of estradiol and progesterone) or in non-estrus (n=15; cells characteristic of diestrus and metestrus in which females show lower levels of estradiol and progesterone). V1aR binding densities were also analyzed in virgin (nulliparous, n=12) females and females who have gone through one experience of pregnancy and parturition (primiparous, n=16). Brains of primiparous females were processed three weeks post-weaning. Receptor autoradiography was performed as described in the legend of Figure 1. Values indicate means (dpm/mg tissue) ± SEM.
| Region | Non-estrus | Estrus | Nulliparous | Primiparous |
|---|---|---|---|---|
| Nucleus accumbens | 6095 ± 520 | 7683 ± 545 | 6984 ± 711 | 6718 ± 472 |
| Islands of Calleja | 20200 ± 1086 | 20079 ± 991 | 20859 ± 882 | 19656 ± 1047 |
| Somatosensory cortex | 2855 ± 158 | 3265 ± 172 | 2686 ± 127 | 3377 ± 152** |
| Piriform cortex | 9861 ± 397 | 11125 ± 393 | 10022 ± 414 | 10859 ± 406 |
| Nucleus of the lateral olfactory tract | 11444 ± 838 | 13151 ± 691 | 11416 ± 875 | 12885 ± 715 |
| Lateral Septum dorsal | 23587 ± 907 | 23788 ± 883 | 23867 ± 943 | 23558 ± 849 |
| Lateral septum intermediate | 32886 ± 797 | 31686 ± 1310 | 29704 ± 1206 | 33644 ± 988 |
| Lateral septum ventral | 23503 ± 1022 | 24107 ± 963 | 23925 ± 1064 | 23719 ± 938 |
| BNST lateral dorsal | 25469 ± 1183 | 26138 ± 947 | 26119 ± 1043 | 25579 ± 1102 |
| BNST lateral posterior | 12200 ± 789 | 12848 ± 716 | 11451 ± 547 | 13261 ± 751 |
| BNST medial posterior | 20733 ± 1096 | 20625 ± 838 | 19091 ± 784 | 21670 ± 817* |
| Suprachiasmatic nucleus | 18039 ± 1290 | 22722 ± 1507 | 18773 ± 665 | 22027 ± 1714 |
| Interstitial nucleus of the posterior limb of the anterior commissure | 31650 ± 1100 | 32758 ± 981 | 31083 ± 1084 | 33138 ± 940 |
| Anteroventral thalamus | 12454 ± 809 | 12125 ± 815 | 11667 ± 833 | 12746 ± 764 |
| Ventromedial thalamic nucleus | 16580 ± 814 | 15310 ± 593 | 15364 ± 988 | 16309 ± 560 |
| Lateral hypothalamus | 23341 ± 704 | 23378 ± 666 | 22917 ± 871 | 23689 ± 536 |
| Lateral hypothalamus tuberal | 23642 ± 413 | 23951 ± 552 | 23342 ± 483 | 23928 ± 449 |
| Stigmoid hypothalamus | 22036 ± 1251 | 22722 ± 1507 | 21696 ± 1302 | 23515 ± 1056 |
| Arcuate nucleus | 17441 ± 1043 | 16135 ± 644 | 17008 ± 1175 | 16656 ± 658 |
| Dentate gyrus | 7910 ± 360 | 8234 ± 547 | 8082± 524 | 8064 ± 418 |
| Medial central amygdala | 15362 ± 568 | 14242 ± 464 | 15015 ± 583 | 15029 ± 447 |
p=0.0073,
p=0.0035 versus nulliparous, one-way ANOVA correcting for multiple comparisons (FDR α≤0.0085).
Moreover, V1aR binding density may change depending on the reproduction status and experience of males and females. Indeed, V1aR binding was higher in the BNST and MPOA in lactating rats compared to virgin rats (Bosch and Neumann, 2008; Bosch et al., 2010). Furthermore, V1aR binding was lower in the PVN and CeA during lactation as compared with parturition in the rat (Caughey et al., 2011). However, the authors did not include virgin female rats, making it unclear in what direction the change in V1aR across the peripartum period is occurring. Given the role of VP in modulating maternal behavior in rodents (Bosch & Neumann, 2008; Nephew & Bridges, 2008), it is likely that such changes in V1aR may mediate optimal maternal care. In this respect, it is interesting that V1aR binding in the LS positively correlated with maternal aggression in rats (Caughey et al., 2011). Interestingly, pregnancy and parturition induce long-lasting enhancements in maternal behaviors during subsequent maternal experiences (Bridges, 1975, 1977; Nephew et al., 2009; Macbeth & Luine, 2010). This suggests that long term changes in neural systems which regulate these behaviors, such as the VP system, may also occur. We therefore compared V1aR binding densities in virgin (nulliparous) female Wistar rats and Wistar rats who have gone through one experience of pregnancy and parturition (primiparous) at three weeks post-weaning. We found that, among 21 forebrain regions analyzed primiparous females had higher V1aR binding in the SSc (p<0.01) and medial posterior BNST (p<0.01) compared to nulliparous females (Table 8). Our current data therefore suggests that maternal experience induces long-term changes in V1aR binding in restricted brain regions. Notably, the BNST shows both short-term (Bosch et al., 2010) and long-term (Table 8) changes in V1aR binding, suggesting a permanent increase in V1aR in this area which may contribute to long-lasting enhancements in maternal responsiveness. Additionally, paternal experience enhanced V1aR immunolabeling in the prefrontal cortex of marmosets (Kozorovitskei et al., 2006). Clearly, more research is needed to determine the functional significance of such long-lasting changes in V1aR in response to maternal and paternal experiences. These findings further illustrate the importance of studying sex differences in V1aR, OTR, or any other parameter, in the context of sexual and parental experiences in order to better understand their function in behaviors related to reproduction and parental care.
Because of the absence of selective and high-affinity V1b ligands, characterization of the V1bR remains poorly understood. Using techniques such as immunohistochemistry and RT-PCR, the V1bR has been detected in several areas throughout the brains of rats, including V1bR mRNA expression in the olfactory bulb, hippocampus, caudate putamen, septum, amygdala, hypothalamus, cortex, and cerebellum in male Sprague Dawley rats (Lolait et al., 1995; Vaccari et al., 1998) and V1bR-immunoreactivity in the olfactory system, cortex, BNST, diagonal band of Broca, amygdala, NAc, hippocampus, thalamus, hypothalamus, and cerebellum in male Wistar rats (Hernando et al., 2001). In C57BL/6mice, V1bR mRNA expression was predominantly found in the CA2 field of the hippocampus, with lower expression levels in the PC, caudate putamen, septum, midbrain, pons, cerebellum and medulla (Young et al., 2006). Finally, V1bR mRNA expression was found in the CA2 and CA3 of the hippocampus in humans (Young et al., 2006), although other brain regions were not analyzed. Moreover, V1bR mRNA expression in humans was not analyzed according to sex, most likely because of the low number of subjects (1 male and 1 female; Young et al., 2006). To our knowledge, there are no studies which have investigated sex differences in V1bR mRNA expression or V1bR binding.
In summary, sex differences in V1aR binding in various species remain inconclusive. While sex differences in V1aR binding have been found in multiple brain regions of rats (Figs. 1 and 2), sex differences in V1aR binding have only been found in a few brain regions in mice, hamsters, and voles. However, these data might be far from conclusive because most of these studies only analyzed a few brain regions expressing V1aR, included too few animals of each sex, and did not use specific ligands for the V1aR. Furthermore, comprehensive analysis of sex differences in VP receptor binding in humans and non-human primates are still needed. Given our extensive knowledge of sex differences in VP synthesis and VP fiber densities in various species (see section 2), it is surprising that the investigation into sex differences in VP receptors is still so limited. Despite these limitations, several studies have shown a sex-specific role of the VP system in the regulation of social behavior in a variety of species, which is discussed in section 4.a.
3.b. OT receptor
Thus far only one type of OT receptor (OTR) has been identified and, similar to VP receptors, the OTR is a G protein-coupled receptor (Gimpl and Fahrenholz, 2001). We discussed above that few rodent species show sex differences in OT-immunoreactivity. However, sex differences in OTR rather than OT could underlie the observed sex-specific regulation of OT-modulated social behaviors (see section 4b). Therefore, knowledge of sex differences in brain OTR is important for a better understanding of how the OT system often regulates social behavior in sex-specific ways.
Similar to the VP receptor, sex differences in OTR binding are both brain region- and species-specific (Tables 5–7). The first comparative study was carried out in Sprague Dawley rats and no sex differences in OTR binding density was found in any of the brain regions analyzed including the LS, BNST, VMH, and CeA (Tribollet et al., 1990). However, this study used [3H] OT and, like [3H] VP, [3H] OT may not exclusively bind OTR (Mouillac et al., 1995; Manning et al., 2012), and thus possibly may mask potential sex differences in OTR binding. Indeed, later studies consistently found higher OTR mRNA expression (Bale and Dorsa, 1995) and higher OTR binding density ([using the specific OTR antagonist d(CH2)5[Tyr(Me)2,Thr4,Orn8,[125I]Tyr9-NH2]-OVTA; Uhl-Bronner et al., 2005) in males compared to females in the VMH of Long-Evans and Sprague-Dawley rats, respectively. We recently confirmed and expanded findings of sex differences in OTR binding in the brains of Wistar rats using d(CH2)5[Tyr(Me)2,Thr4,Orn8,[125I]Tyr9-NH2]-OVTA. Specifically, we found that males had higher OTR binding densities than females in 9 out of 15 forebrain regions analyzed. These brain regions included the NAc, dorsal caudate putamen, intermediate LS, posterior BNST, MPOA, agranular insular cortex, hippocampal CA1 region, MeA, and VMH (Dumais et al., 2013). The largest sex difference in OTR binding was found in the posterior BNST, with males showing almost three times higher density than females. We have confirmed a similar sex difference in OTR binding density in the posterior BNST of Sprague-Dawley rats (data not shown).
Table 5.
Summary of studies in which OT system parameters are higher in males than in females
| OT measure | Species | Region | Reference |
|---|---|---|---|
| OTR mRNA | Long Evans rats | VMH | Bale & Dorsa, 1995 |
| Mandarin voles | MeA | Smeltzer et al., 2006 | |
| OTR binding | P. maniculatus & P. californicus mice | mitral cell layer, granule cell layer, cingulate ctx, dorsal LS, lateral-, medial anterior-, and medial posterior- BNST | Insel et al., 2001 |
| Singing mice | MeA, hippocampal CA1 | Campbell et al., 2009 | |
| Sprague Dawley rats | VMH, spinal cord | Uhl-Bronner et al., 2005 | |
| Wistar rats | posterior NAc, CPu, LS, BNST, MPOA, agranular insular ctx, hippocampal CA1, MeA, VMH | Dumais et al., 2013 | |
| Colonial tuco-tuco | MPOA, VMH | Beery et al., 2008 | |
| Plasma OT | Humans | n/a | van Londen et al., 1997; Weisman et al., 2013 |
BNST, bed nucleus of the stria terminalis; CPu, caudate putamen; Ctx, cortex; LS, lateral septum; MeA, medial amygdala; MPOA, medial preoptic area; NAc, nucleus accumbens; VMH, ventromedial hypothalamus; n/a, not applicable.
Furthermore, sex differences in OTR binding in multiple brain regions have been found in two deer mouse species, P. maniculatus and P. californicus. Sex differences in OTR binding were found in 8 out of 20 brain regions analyzed, including the olfactory bulb, cingulate cortex, dorsal LS, BNST (lateral, medial anterior, and medial posterior parts), and the hippocampal CA1 region (Insel et al., 1991). Males had higher OTR binding compared to females in all regions except for the CA1 region. The fact that the sex difference in OTR binding densities in rats and deer mice are so consistently found in one direction (higher in males) and in so many different brain regions suggest an important sex-specific role of the OTR in OT-mediated social behaviors (see section 4.b. for further details).
In contrast, limited or no sex differences in brain OTR have been found in other rodent species and in humans. For example, female prairie and montane voles showed higher OTR binding densities in the medial prefrontal cortex compared to males (Smeltzer et al., 2006), while male mandarin voles showed higher OTR mRNA expression in the MeA (Cao et al, 2013). It was also found that male ICR mice had lower OTR binding in the VMH than female ICR mice (Tribollet et al., 2002). Male S. xerampelinus singing mice had higher OTR binding in the MeA and hippocampal CA1 compared to females (Campbell et al., 2009). Tuco-tuco (Ctenomys sociabilis) males had higher OTR binding than did females in the MPOA and VMH (Beery et al., 2008). No sex differences in OTR binding were found in C57Bl/6J mice (Hammock and Levitt, 2013), the solitary golden hamster (Dubois-Dauphin et al., 1992), prairie voles (Bales et al., 2007a; except for the medial prefrontal cortex in Smeltzer et al., 2006), and humans (Loup et al., 1991). However, Loup et al. (1991) had a very low number of women (n=4). Moreover, most rodent studies only analyzed for sex differences in OTR in a few brain regions (Dubois-Dauphin et al., 1992; Smeltzer et al., 2006; Bales et al., 2007a; Cao et al., 2013; Hammock and Levitt; 2013). A recent study investigated OTR binding in the coppery titi monkey in males and females, however an analysis of sex was not performed (Freeman et al., 2014). Together, this highlights the need for more extensive analysis in more forebrain regions in these species, as well as the need for more species to be investigated for potential sex differences in OTR.
Unlike the V1R, the OTR is modulated by gonadal hormones, which may help to explain the mechanisms underlying the sex differences in OTR. Interestingly, estrogens may directly regulate OTR gene expression through binding to the estrogen receptor alpha, which is a transcription factor that modulates gene transcription via interaction with estrogen response elements located on the promotor region of the OTR gene (Carson-Jurica et al., 1990; Young et al., 1998; Ivell & Walther, 1999). Female rats treated neonatally with testosterone (which is likely converted into estrogen) show higher levels of OTR binding densities in the VMH, BNST, and MeA compared to control female Sprague-Dawley rats (Uhl-Bronner et al., 2005). However, it is unclear whether these changes reach levels of OTR binding densities as seen in males. Furthermore, gonadectomy of adult Sprague-Dawley rats decreased OTR binding in areas such as the VMH, dorsolateral BNST, and dorsal caudate putamen in both males and females (Tribollet et al., 1990). In Wistar rats, OTR binding was higher in estrus females (characteristic of higher levels of estradiol and progesterone) compared to non-estrus females in the NAc, posterior BNST, MPOA, and VMH (Dumais et al., 2013). Importantly however, estrus females still showed significantly lower OTR binding compared to males in the posterior BNST, MPOA, and VMH (Dumais et al., 2013). Adult hormonal influences on OTR binding densities are therefore unlikely to explain all sex differences in OTR binding. In further support, we found sex differences in areas that may not be sensitive to gonadal steroids (Tribollet et al., 1990), such as the cortex, caudate putamen and hippocampal CA1 (Dumais et al., 2013).
Overall, these findings clearly indicate that, similar to OT-immunoreactivity, there are robust brain region- and species-specific sex differences in OTR expression in the brain. These findings further suggest that when sex differences are found in OT and in OTR, it not necessarily is in the same direction or exists in both parameters (Tables 5 and 7). For example, female mandarin voles, hamsters, and gerbils show higher OT-immunoreactivity (Wang et al., 2013; Qiao et al., 2014), while male rats and deer mice show higher OTR binding (Insel et al., 1991; Uhl-Bronner et al., 2005; Dumais et al., 2013). Importantly, both the brain region- and species- specificity of sex differences in OTR suggests that the OT system in these brain regions and species may be implicated in sex-specific regulation of social behavior. This is an understudied area of research. Particularly, research specifically linking these sex differences in OTR (and OT-immunoreactivity; section 2.b.) with sex-specific regulation of OT system-modulated behaviors is lacking. However, in section 4.b., we discuss the few findings showing sex-specific regulation of social behavior by OT..
4. Implications for sex-specific regulation of social behavior by the VP and OT systems
4.a. VP system
Although sex differences in VP have been known for several decades and have since been found in various rodent species, there is a surprising lack of comparative studies investigating sex-specific function of VP in the regulation of social behavior. Here we discuss the rodent studies that found sex-specific roles of the VP system in the regulation of partner preference in voles, social recognition in rats, social play in rats, and aggression in hamsters. We also discuss the studies that reported sex-specific effects of VP and of V1aR gene variants on social functioning in humans.
Early studies of the effects of VP on partner preference formation in prairie voles suggested that VP played a larger role in males than in females. In detail, intracerebroventricular (ICV) V1aR antagonist impaired partner preference formation in males (Winslow et al., 1993), but there was no effect in females (Insel & Hulihan, 1995). Furthermore, ICV VP (0.5ng) enhanced partner preference in males (Winslow et al., 1993), but had no effect in females (Insel & Hulihan, 1995). However, a higher dose of ICV VP (100ng) enhanced partner preference in both males and females (Cho et al., 1999). This suggests that males may be more sensitive to the enhancing effects of VP on partner preference formation. Additional studies implicated a role for the LS (Liu et al., 2001) and ventral pallidum (Pitkow et al., 2001) in the facilitating effects of VP on partner preference formation. However, these studies were done in males only. Therefore, potential brain region-specific effects of VP on partner preference formation in female prairie voles are still unclear.
In addition, early studies suggested a potential sex-specific role of the VP system in the regulation of social recognition in rats. For example, while subcutaneous injections of VP enhanced social recognition in male (Dantzer et al., 1987) and female (Bluthe & Dantzer, 1990) rats, a subcutaneous injection of the VP antagonist, dPTyr(Me)AVP, blocked social recognition in male rats only (Dantzer et al., 1987; Bluthe & Dantzer, 1990). Likewise, ICV VP antagonist had no effect on social recognition in females (Engelmann et al., 1998), although males were not tested in this study. Together, these findings suggest that endogenous VP may be more important for the regulation of social recognition in males than in females. Additional studies implicated a role of the VP system in the LS and olfactory bulb in the regulation of social recognition (Le Moal et al., 1987; Dantzer, 1998; Engelmann & Landgraf, 1994; Everts & Koolhaas, 1999; Landgraf et al., 2003; Tobin et al., 2010), but these studies were conducted in males only. Importantly, we recently showed that V1aR blockade, using the specific V1aR antagonist d(CH2)5[Tyr(Me)2]AVP), in the LS impaired social recognition in both male and female adult rats (Veenema et al., 2012). We further found that V1aR blockade in the LS of juvenile rats did not impair social recognition in male and female juvenile rats, but rather increased social investigation of the familiar over the novel stimulus rat (Veenema et al., 2012). This effect was stronger in male juveniles than in female juveniles (Veenema et al., 2012). Together, these findings indicate that despite initial sex-specific effects, it rather seems that the brain VP system in rats is important for the modulation of social recognition in both sexes. Findings in juvenile rats reveal age differences in the regulation of social recognition by the VP system, which underscores the importance of studying sex-specific regulation of social behavior by the VP system across the lifespan.
Similar to rats, the V1aR is important for social recognition in mice. For example, V1aR knockout male mice show deficits in social recognition (Bielsky et al., 2004; however, see Wersinger et al., 2007). Re-expression of V1aR in the LS of V1aR KO mice restored social recognition (Bielsky et al., 2005a), thereby confirming studies in rats showing the importance of the LS-VP system in social recognition. Unfortunately, these studies have been performed in males only. Given that V1aR KO male, but not female, mice show a decrease in anxiety-related behavior (Bielsky et al., 2004, 2005b), it would be of interest to study potential alterations in social recognition in female V1aR KO mice as well as in other social behaviors in both sexes.
We recently showed a sex-specific role of the VP system in the regulation of social play behavior in juvenile rats. ICV administration of the specific V1aR antagonist d(CH2)5[Tyr(Me)2]AVP) reduced social play behavior in male juveniles, while increasing social play behavior in female juveniles (Veenema et al., 2013). Interestingly, this sex-specific regulation of social play behavior by the VP system was also found when blocking V1aR in the LS, but in the opposite direction. V1aR blockade in the LS increased social play behavior in males, while decreasing social play behavior in females (Veenema et al., 2013; Bredewold et al., 2014). These brain region-specific effects might be explained by the involvement of distinct brain VP systems. In support, in male juvenile rats, social play levels correlated positively with VP mRNA expression in the PVN, but negatively with VP mRNA expression in the BNST (Paul et al., 2014). Although speculative, these findings may suggest that PVN-VP promotes social play, while a BNST-LS VP circuit reduces social play in juvenile male rats. Furthermore, the sex-specific regulation of social play by the VP system was found to be context specific. Here, VP injected into the LS did not have an effect on home cage social play behavior in either sex, but decreased novel cage social play in females only (Bredewold et al., 2014). These studies highlight the importance of both brain region- and context-specific effects in the sexually dimorphic involvement of the VP system in social play behavior.
VP has also been shown to play a sex-specific role in aggression in Syrian hamsters. Specifically, V1aR antagonist injection into the AH decreased intermale aggression, while VP increased intermale aggression (Ferris et al., 1997; Caldwell and Albers, 2004a). In contrast, V1aR antagonist increased interfemale aggression, while VP decreased interfemale aggression (Gutzler et al., 2010). These data suggest a strong sex difference in the functionality of the V1aR in the AH in the regulation of aggressive behaviors in Syrian hamsters. Since a sex difference in V1aR binding has not been reported in the AH in Syrian hamsters, these studies suggest that the activation of the V1aR in the AH may stimulate different neural circuits which may facilitate or inhibit aggressive behavior in males and females, respectively.
The role of VP in human social behavior is mostly investigated using men only, leaving little knowledge of potential sex-specific modulation of VP in human behavior and cognition. There are also far fewer studies examining the role of the VP system on human social behavior and social cognition than those investigating the OT system (see section 4.b.), leaving the effects of VP in humans largely understudied.
Our current knowledge of sex-specific actions of both VP and OT in humans relies on the use of intranasal administration. Although VP and OT do not pass the blood brain barrier, it has been suggested that intranasal VP and OT may reach the brain given the fast behavioral and cognitive effects of intranasal administration (Guastella & MacLeod, 2012). Furthermore, intranasal VP administration in humans was associated with an increase in VP concentration in cerebrospinal fluid (Born et al., 2002). Likewise, intranasal OT administration in rhesus macaques was associated with an increase in cerebrospinal fluid (Modi et al., 2014). However, this still does not provide evidence of direct access to the brain, as intranasal administration could indirectly, via peripheral actions, increase VP and OT release in the brain. Indeed, intranasal OT administration also leads to increases in OT in plasma and saliva in humans (Gossen et al., 2012; van Ijzendoorn et al., 2012) and rhesus macaques (Modi et al., 2014). Yet, irrespective of the mechanism of action, investigating the effects of intranasal VP and OT can still be informative on how these neuropeptides may be modulating social behavior and doing so differently in men and women. However, behavioral and neuronal effects of exogenous VP may say less about the involvement of the endogenous VP system. Therefore, we will end this section with a discussion of recent evidence for the sex-specific involvement of the human V1aR gene (AVPR1A) in various social behaviors, as variations in this gene have given some insight into the role of the V1aR in human social behavior (for reviews see Meyer-Lindenberg et al., 2011; Ebstein et al., 2012).
Most studies that administered VP intranasally have been carried out in men and have revealed a facilitating role of VP in the encoding of happy and angry faces (Guastella et al., 2010b; however, see Uzefovsky et al., 2012), the recognition of sexual cues (Guastella et al., 2011), the perception of neutral facial expressions as being angry (Thompson et al., 2004), and in musical working memory (Granot et al., 2013). When looking at the effects of VP on brain activation, again, women are largely left out of the subject pool. For example, in men, intranasal VP altered activation and connectivity in a medial prefrontal cortex-amygdala circuit during processing of facial emotions (Zink et al., 2010) and when viewing pictures illustrating socially threatening scenes (Brunnlieb et al., 2013b). In addition, intranasal VP altered social recognition-processing regions, such as the temporoparietal junction, caused by social unfamiliarity (Zink et al., 2011), and activation in the right superior temporal sulcus during reactive aggression (Brunnlieb et al., 2013a) in men.
The few studies that included men and women reveal sex-specific effects of VP on some, but not all, behavioral responses. For example, sex-specific effects of intranasal VP have been found in regards to social communication and cooperation. VP stimulated agonistic facial motor patterns in men, but affiliative facial motor patterns in women, in response to unfamiliar same-sex faces (Thompson et al., 2006). VP also decreased perceptions of the friendliness of those faces in men, while VP increased perceptions of the friendliness of those faces in women (Thompson et al., 2006). Furthermore, in a computer game of trust, intranasal VP caused men to be more likely than women to reciprocate cooperation from human partners (Rilling et al., 2012; 2014). During cooperative interactions, VP also had sex-specific effects on brain activation. Intranasal VP increased activity in areas such as the striatum, basal forebrain, insula, amygdala, and hippocampus in men, but decreased or had no effect in women (Rilling et al., 2012; 2014). Also during reciprocated cooperation, VP increased activation in the bilateral insula and right supramarginal gyrus in males, but decreased this response in females (Feng et al., 2014). These findings suggest that VP may be modulating cooperative social interactions differently in males and females via differential activation of reward, arousal, and memory-related brain areas. On the other hand, a recent study which did include both men and women found that VP influenced empathic concern in men and women with no effect of sex (Tabak et al., 2015).
The above studies were all performed in healthy individuals. It would also be important to determine sex-specific associations of VP with psychiatric disorders of social dysfunction as this can be informative regarding the etiology and the possible sex-specific treatment strategies for these disorders. Interestingly, plasma VP showed opposite correlations with autistic-like behaviors in adolescent boys and girls. While plasma VP concentrations tended to be negatively associated with restricted and repetitive behaviors in boys with autism spectrum disorder, plasma VP was positively associated with these behaviors in girls with autism spectrum disorder (Miller et al., 2013). Furthermore, urinary VP concentrations were negatively associated with posttraumatic stress disorder (PTSD) severity in men, but not in women (Marshall, 2013). In addition, intranasal VP improved social cognition (as measured by faster attentional engagement with their partner’s expression of anger) in men with PTSD, while VP had no effect in women (Marshall, 2013). Although clearly more research is needed, these initial findings indicate that VP may play a sex-specific role in the etiology as well as the treatment of symptoms seen in these psychiatric disorders. Genetic studies of variations in the human V1aR gene (AVPR1A) provide further evidence for the involvement of V1aR in various social behaviors in humans (for reviews see Meyer-Lindenberg et al., 2011; Ebstein et al., 2010; 2012). Sex-specific influences of AVPR1A gene variants however have been limited, with most studies showing findings in both men and women, with no reports of sex differences. For example, carriers of long allele versions (327–343 bp) of the AVP1AR RS3 polymorphism, compared to carriers of the short allele versions (308–325 bp), showed higher levels of altruism as assessed by money donations in a computer game and by self-reported scales (Knafo et al., 2008). RS3 long alleles were also associated with higher AVPR1A post-mortem hippocampal mRNA expression than short RS3 alleles (Knafo et al., 2008). Men and women with the 334 bp (long version) allele for the RS3 also showed higher activation of the left amygdala compared to 20 other alleles analyzed (Meyer-Lindenberg et al., 2009). In addition, presence of the 327 allele (also referred to as the 334 bp allele, depending on the genotyping method) predicted lower cognitive empathy in men and women compared to an absence of the 327 allele (Uzefovsky et al., 2015). Finally, AVPR1A gene variants have also been associated with dance and musical aptitude (Bachner-Melman et al., 2005; Ukkola et al., 2009). In summary, these studies conclude that carriers of longer allele variations of the RS3 polymorphism show higher levels of altruism, hippocampal mRNA expression, and amygdala activation, but lower cognitive empathy. These findings suggest important variations in both behavior and brain structure and function as predicted by the AVP1AR gene.
Only a few studies have found sex-specific effects of polymorphisms in the AVPR1A gene in humans, with effects being found for pair-bonding and prepulse inhibition. In males only, those who carried the 334 pb allele for the AVPR1A RS3, opposed to men who did not carry the 334 pb allele, had lower scores on a partner bonding scale, reported more marital problems, were more likely to be unmarried, and had lower scores of marital quality as perceived by their spouse (Walum et al., 2008). Although these results are correlational and not causational, they seem consistent with the role of the V1aR in partner preference formation in male prairie voles (Winslow et al., 1993; Cho et al., 1999; Liu et al., 2001; Lim and Young, 2004). This may suggest that across species, the V1aR has evolved as an important mediator in the regulation of mammalian pair bonding. Another study found that longer as opposed to shorter AVPV1A RS3 alleles are associated with greater levels of prepulse inhibition in men and women, but the strongest association was in men (Levin et al., 2009). Prepulse inhibition is the phenomenon in which a preceding stimulus inhibits the reaction to a subsequent stronger startle stimulus. It is not necessarily related to social behavior, per se, but deficits in prepulse inhibition are observed in a variety of neuropsychiatric disorders of social dysfunction (Geyer et al., 1990; Perry et al., 2007), as well as in animal models of social dysfunction (Dieckmann et al., 2007; Koh et al., 2008). Prepulse inhibition may therefore be a reflection of not only attention and memory, but also social cognition, which may be partially regulated by AVP1A gene variants.
The AVPR1A RS3 polymorphism has also been implicated in social cognition and personality traits in chimpanzees in sex-specific ways. Specifically, while there was no effect in females, males with the DupB−/− allele (which is the shorter bp version and lacks RS3) needed more social cues to elicit an orienting response than males with the DupB+/− (which have one copy of the long allele containing RS3; Hopkins et al., 2014). In addition, while there was no effect in DupB−/− carriers, males with the DupB+/− allele had lower dominance and higher conscientiousness scores than females with the DupB+/− (Hopkins et al., 2012). Combined, these first studies suggest sex-specific links between AVPR1A polymorphisms and sociobehavioral traits across primate species.
Taken together, the VP system has been shown to regulate social behaviors in both rodents and humans often in sex-specific ways. In rodents, sex-specific regulation of partner preference in prairie voles, social recognition in rats, social play in rats, and aggression in hamsters has been found (Dantzer et al., 1987; Bluthe & Dantzer, 1990; Winslow et al., 1993; Insel & Hulihan, 1995; Ferris et al., 1997; Cho et al., 1999; Caldwell and Albers, 2004a; Gutzler et al., 2010; Veenema et al., 2012; Bredewold et al., 2014). In humans, intranasal VP had sex-specific effects during social communication and cooperation interactions (Thompson et al., 2006; Rilling et al., 2014; Feng et al., 2014), while AVP1A gene variants had sex-specific effects on pair-bondng and prepulse inhibition (Walum et al., 2008; Levin et al., 2009). Interestingly, these sex-specific effects seem to be independent of whether sex differences are found in VP and/or V1aR binding (rats; Figs. 1 and 2) or whether no sex differences are found in VP and/or V1aR binding (prairie voles: Insel et al., 1991; Smeltzer et al., 2006; humans: Loup et al., 1991). The underlying mechanisms by which the VP system regulates social behavior in sex-specific ways is therefore still in need of further investigation.
4.b. OT system
Manipulations of the OT system (mostly pharmacologically or genetically) in males and females have unveiled sex-specific actions of the OT system on social behavior in a variety of rodent species, including voles, rats, mice, and hamsters. Several of these sex-specific effects of OT on social behavior have been found after early-life OT system manipulations. Here, a single neonatal intraperitoneal (i.p.) injection of OT on postnatal day 1 facilitated partner preference in both male and female adult prairie voles, but required a high (12μg) or low (3μg) dose in males and an intermediate dose (6μ) in females (Bales & Carter, 2003a; Bales et al. 2007b). Moreover, neonatal OT facilitated the onset of mate-guarding (a component of pair bonding in which voles become more aggressive and less social towards unfamiliar voles) in female, but not male, adult prairie voles (Bales and Carter, 2003b). In contrast, a single neonatal i.p. injection of an OTR antagonist ([d(CH2)5, Tyr(Me)2, Orn8]-Vasotocin) on postnatal day 1 decreased alloparental care in male, but not female, juvenile prairie voles (Bales et al., 2004). This effect was also found in male, but not female, adult ICR mice (Mogi et al., 2014), although neonatal OTR blockade reduced social approach behavior in female, but not male, ICR mice (Mogi et al., 2014). These findings may indicate robust sex differences in the neonatal sensitivity to OT system manipulations on the development of specific social behaviors.
The sexually dimorphic effects of neonatal OT system manipulations on adult social behaviors suggest long-term and sex-specific alterations in brain parameters. Indeed, i.p. injections of either OT or OTR antagonist on postnatal day 1 induced sex-specific changes in OT, estrogen receptor alpha (ERα), and V1aR in adult prairie voles. Specifically, in females, but not males, neonatal OT and OTR antagonist both increased OT-immunoreactivity in the PVN (Yamamoto et al., 2004). Moreover, neonatal OT increased ERα-immunoreactivity in the VMH while OTR antagonist decreased ERα-immunoreactivity in the MPOA in females, but not males (Yamamoto et al., 2006). Furthermore, neonatal OT increased V1aR binding in the ventral pallidum and cingulate cortex of adult males, but decreased V1aR binding in the same regions of adult females (Bales et al., 2007a). Finally, neonatal OTR antagonist decreased V1aR binding in the BNST in both sexes, while decreasing V1aR binding in the MPOA and LS in adult males only and in the cingulate cortex in adult females only (Bales et al., 2007a). These findings clearly indicate that acute neonatal OT system manipulations have robust effects on brain systems other than the OT system. However, whether changes in these systems underlie the sex-specific behavioral changes seen in male and female prairie voles after neonatal OT manipulations is unclear.
Although OTR is present early in development in various species (Hammock, 2015), sex differences in the OTR system early in life are still to be determined. Interestingly, sex differences in OTR binding densities in specific brain regions in adult rats (Dumais et al., 2013) have also been found in the same brain regions of juvenile rats (Smith et al., 2014). These findings highlight that sex differences in the OTR may contribute, in part, to sex differences in the development of social behavior. This may be one of the mechanisms by which neonatal OT and OTR antagonist have long-lasting sex-specific effects on social behaviors and brain parameters (Bales & Carter, 2003a; 2003b; Carter et al., 2008; Mogi et al., 2014).
Despite extensive research on the role of the OT system in social behavior in diverse rodent species, only a few studies directly compared males and females within the same experimental design. Because of this limitation, we also discuss sex differences that are derived from separate studies. Overall, this research suggests that OT plays a sex-specific role in diverse social behaviors such as social avoidance, social recognition, partner preference, social play and social interest, which are briefly discussed below.
ICV OT reversed social defeat-induced social avoidance in male (Lukas et al., 2011b), but not in female (Lukas and Neumann, 2014) rats. Likewise, ICV OT improved social recognition in male (Benelli et al., 1995), but not in female (Engelmann et al., 1998) rats. However, ICV administration of an OTR antagonist impaired social recognition in both male (Lukas et al., 2013) and female (Engelmann et al., 1998) rats. It is therefore likely that the endogenous OT system plays a similar role in social recognition in both sexes, but that males may be more sensitive to the enhancing effects of exogenous OT, while females have perhaps reached a ceiling effect. If so, one would expect that males have lower release of OT during social recognition and/or lower occupation of OTR compared to females. The former is not known, but we found that males have higher OTR binding densities than females (Dumais et al., 2013) in various brain regions implicated in social behavior, such as the olfactory bulb, LS, and MPOA. Indeed, the OT system in the olfactory bulb, septum (Popik et al., 1992), and MPOA (Popik and Van Ree, 1991) plays a role in social recognition. Unfortunately, these studies were only performed in male rats. Studies in mice confirm the role of the OT system in social recognition. Here, impaired social recognition was found in male OTR KO mice (Takayanagi et al., 2005; Lee et al., 2008; Macbeth et al., 2009), male OT KO mice (Ferguson et al., 2000; Macbeth et al., 2009), and female OT KO mice (Choleris et al., 2003; Choleris et al, 2006). Together, these studies suggest a role of OT in social recognition in both sexes, but also suggest that there might be sex differences in the sensitivity to exogenous OT, with a lack of an effect in females.
Initial work in prairie voles indicated a sex-specific role of the OT system in partner preference formation, suggesting that the OT system may be more important for partner preference in females compared to males. In detail, ICV OT administration facilitated partner preference formation in female (Williams et al., 1994; Insel and Hulihan, 1995), but not in male (Winslow et al., 1993) prairie voles. Additional research indicated that the effects of OT on partner preference in females could be located in the nucleus accumbens and in the prefrontal cortex, but these studies did not include males (Young et al., 2001; Liu and Wang, 2003). However, OTR antagonist administration in the LS blocked partner preference in male prairie voles (Liu et al., 2001). These findings suggest that the OT system is involved in partner preference in both sexes, but that females may be more sensitive to exogenous OT. Interestingly, this seems the opposite to social recognition in rats, where males appear more sensitive to exogenous OT. Similar to rats, the local brain effects of OT and OTR antagonist administration on partner preference formation in prairie voles, as discussed above, have only been tested in one sex. This makes it difficult to draw any conclusions regarding the neural pathways through which OT mediates partner preference on voles and whether this is similar or different in males versus females.
We recently showed that the OT system regulates social play behavior in juvenile rats in sex-specific ways. Specifically, OT administered into the LS decreased social play behavior in females, but not in males (Bredewold et. al, 2014). OTR antagonist administration did not alter social play behavior in either sex (Bredewold et al., 2014). These findings were a bit surprising, given the typically pro-social behavior effects of OT, although recent studies suggest a role for the OTR in the LS in reducing pro-social behaviors and enhancing anxiety in rodents (Olazábal and Young, 2006; Beery et al., 2008; Beery and Zucker, 2010; Guzman et al., 2013). This underscores not only the complexity of the function of the OTR but may also provide a mechanism to explain how the OTR can be involved in such different social behaviors, namely by inducing brain region-specific effects. Obviously, this hypothesis requires further testing.
Furthermore, we found sex-specific correlations of OTR binding with social interest in adult rats. Social interest reflects the amount of time the adult rat investigates a novel juvenile rat. OTR binding in the CeA in female rats correlated negatively, while OTR binding in the MeA in male rats correlated positively with social interest (Dumais et al., 2013). These results may suggest that the OT system is involved in modulating the salience and/or valence of social stimuli differently in males and females, possibly via the activation of different neural circuits.
Although researchers are beginning to explore the sex-specific role of OT in social behavior, current findings in rodents clearly indicate the lack of comparative research regarding the role of the OT system in social behavior in males and females in a variety of species. Further research is necessary, especially regarding the mechanisms by which the OT system regulates social behaviors differently in males and females across rodent species.
Research into the role of the OT system in social behavior in both sexes in humans is slowly increasing. This may further gain momentum because of the potential of the OT system to restore social dysfunction in sex-biased psychiatric disorders (Guastella et al., 2010a; Meyer-Lindenberg et al., 2011; Cochran et al., 2013), and recent initiatives from the NIH requiring the inclusion of both sexes in preclinical research (Clayton & Collins, 2014). Below we will discuss evidence of sex-specific modulation of social behavior by OT in humans based on pharmacological (intranasal OT administration) and genetic (polymorphisms in the OTR gene) studies.
The first studies to report intranasal OT effects in humans were done in males only, showing a role for OT in trust and in the processing of socially relevant information (Kosfeld et al., 2005; Zak et al., 2007; Domes et al., 2007; Guastella et al., 2008a; Guastella et al., 2008b; Di Simplicio et al., 2009). Studies investigating the effect of intranasal OT in both men and women however have uncovered important sex-specific effects. Intranasal OT impaired recognition memory of neutral and happy faces in men, but not women (Herzmann et al., 2013), and facilitated kinship recognition in women, but not men (Fischer-Shofty et al., 2013). In addition, OT impaired accurate emotion perception ability in women, but not men (Lynn et al., 2014), and slowed reaction times in an implicit perspective taking task in men to that of the level of women (Theodoridou et al., 2013). These studies highlight the importance of emotional valence in the role of OT in perception and recognition of social stimuli, and how this may differ in men and women.
Intranasal OT was also found to increase positive behavior during couple conflict in both male and female partners (Ditzen et al, 2009). However, the underlying mechanisms may be different, because OT decreased sympathetic activity measures during couple conflict in women, but increased sympathetic activity measures in men (Ditzen et al., 2013). Similarly, women given intranasal OT reported more distress and anger in response to a social stress test, while men given OT reported less negative affect (Kubzansky et al., 2012). This suggests that OT may be playing different roles in men and women’s responses to stressful interactions. Sex differences were also found for the effect of OT on brain activation during reciprocated cooperation. Intranasal OT increased the caudate/putamen response in males, whereas it decreased this response in females (Rilling et al., 2014). Although not statistically significant in a comparison between sex, OT lowered rates of cooperation with computer partners following defection in women only (Rilling et al., 2012; 2014). This suggests that OT may differentially affect both behavior and brain processes involved in reward or salience of positive social interactions in men and women. Together, these studies demonstrate that intranasal OT can induce different behavioral responses, but even when behavioral responses are the same (Ditzen et al., 2009), the neuronal mechanisms through which OT is mediating these behavioral responses seems to be consistently different in males versus females.
Genetic studies of single nucleotide polymorphisms (SNPs) in the OTR gene provide evidence for the involvement of the OTR in various social behaviors in humans (for reviews see Meyer-Lindenberg et al., 2011; Ebstein et al., 2012), and potential sex-specific effects on human social behavior. For example, men and women carrying one or two copies of the A allele in the most common SNP, rs53576, show reduced empathy (Rodrigues et al., 2009; Uzefovsky et al., 2015), and have lower levels of optimism, mastery, and self-esteem (Saphire-Bernstein et al., 2011), whereas reduced positive affect (Lucht et al., 2009) and reward dependence (Tost et al., 2010) were found in men only. Interestingly, it was found that carriers of one or two A alleles of the same SNP were judged to be less prosocial than carriers of two G alleles, and that this was due to variations in expression of affiliative cues by these individuals (Kogan et al., 2011). Although men and women were included in this study, there was no analysis of sex (Kogan et al., 2011). Further, men and women homozygous for the A allele reported seeking less emotional social support (Kim et al., 2010). Women only were tested for their sensitivity for their toddler’s behavior which was found to be reduced in those carrying the A allele (Bakermans-Kranenburg and van IJzerdoorn, 2008). Likewise, women only were tested for social auditory processing and those carrying the A allele reported more difficulty hearing and understanding people when there was background noise present (Tops et al., 2011).
Other OTR SNPs have also been implicated in social behaviors in men and women. For example, women, but not men, carrying rs7632287A scored lower on a partner bonding scale and on a relationship quality survey (Walum et al., 2012). There were also sex-specific interactions between the rs237915 SNP, early life adversity, and emotional and peer problems in adolescents. Girls who experienced stressful life events and were CT- and TT-carriers had increased numbers of emotional problems, while boys who experienced stressful events and were TT-homozygotes had an increase in peer problems (Loth et al., 2014). Furthermore, individuals homozygous for the G allele for the SNPS rs53576 and rs2254298 exhibited higher scores on Attachment Style Questionnaire factors that are associated with depression and adult separation anxiety (Costa et al., 2009). In this study however, an analysis of sex was not reported. Participants with an A allele for SNP rs2254298 also had greater amygdala volumes compared to those homogyzous for the G allele in adolescent girls (Furman et al., 2011) and adult men and women (Inoue et al., 2010). Interestingly, however a meta-analysis of the most commonly studied SNPs rs53576 and rs2254298 did not reveal any sex effects or any general effects in the social behavior domain (Bakermans-Kranenburg and van IJzerdoorn, 2014), suggesting that it may be unlikely to expect that one SNP in the OTR gene can explain major differences in social behavior. Even so, these studies overall suggest a correlation between OTR SNPs and differences in social processing, social behaviors, and social functioning in both sexes, with some important sex differences that perhaps may be informative in understanding the potential role of the OTR in mediating sex differences in the susceptibility to develop sex-biased social disorders (Carter, 2007).
5. General Discussion
We discussed above that various rodent species show consistent sex differences in VP-immunoreactive neurons and fiber density in the brain (rats: De Vries et al., 1983; Van Leeuwen et al., 1985; Miller et al., 1989; Wang & De Vries; mice: De Vries et al., 2002; Bakker et al., 2006; Gatewood et al., 2006; Rood & De Vries, 2013; voles: Wang et al., 1995; 1996; Lonstein & De Vries, 1999; gerbils: Crenshaw et al., 1992; and European hamsters: Buijs et al., 1986). Sex differences in VP neurons and plasma VP concentrations have also been found in humans (Share et al., 1988; Asplund & Aberg, 1991; Van Londen et al., 1997; Ishinina et al., 1999; Miller et al., 2013; Graugaard-Jensen et al., 2014; however, see Fliers et al., 1985; Swaab et al., 2001; Gouin et al., 2012). In contrast, we found that only a few rodent species show sex differences in OT-immunoreactivity (CD mice: Häussler et al., 1990; mandarin voles: Qiao et al., 2014; Brandt’s voles: Xu et al., 2010; Mongolian gerbils and Chinese-striped hamsters: Wang et al., 2013). There is also a lack of sex differences in OT neuron size and number in humans (Fliers et al., 1985; Wierda et al., 1991; Ishunina and Swaab, 1999), and most human studies report a lack of sex differences in plasma OT (Zhong et al., 2012; Taylor et al., 2010; Grewen et al., 2005; Gordon et al., 2008; Gordon et al., 2010; Graugaard-Jensen et al., 2014; however see van Londen et al., 1997; Miller et al., 2013; Weisman et al., 2013). Given the robust and well-known sex differences in VP synthesis, it is surprising that there are only a few comparative studies investigating the behavioral effects of VP in males and females. In contrast, there are fewer and inconsistent sex differences in OT synthesis, yet there are several studies comparing the effects of OT on social behavior in males and females. None of these studies, however, have linked sex-specific changes in social behavior with sex differences in OT and VP synthesis, an important area for future research.
A comprehensive analysis of sex differences in V1aR in the brain is still lacking, especially in those rodent species that showed sex differences in VP-immunoreactivity (i.e., laboratory mice, hamsters, and voles). For these species, studies only analyzed a few brain regions or had only a few subjects per sex, which might explain the limited brain regions in which sex differences in V1aR binding were found (C57B6 mice: Dubois Dauphin et al., 1996; Siberian hamsters: Dubois-Dauphlin et al., 1991; golden hamsters: Delville & Ferris, 1995; voles: Bales et al., 2007a; Smeltzer et al., 2006). In rats, however, we provide new data showing robust sex differences in V1aR binding in 8 out of 21 forebrain regions analyzed (Figs. 1 and 2). Similar to V1aR, sex differences in OTR binding were found in multiple brain regions in those rodent studies that used a more comprehensive analysis (deer mice: 8 out of 20 brain regions; Insel et al., 1991; rats: 9 out of 15 forebrain regions; Dumais et al., 2013). In contrast, other rodent studies that did not find sex differences in OTR binding analyzed only a few brain regions (mandarin voles: Smeltzer et al., 2006; Cao et al, 2013, ICR mice: Tribollet et al., 2002; singing mice: Campbell et al., 2009; tuco-tuco: Beery et al., 2008). Therefore, the lack of sex differences in V1aR and OTR in some rodent species could be an artifact due to the lack of comprehensive comparative analyses.
We further discussed what is known about the sex-specific modulation of social behavior by VP and OT systems. This revealed that both neuropeptide systems often regulate social behaviors in sex-specific ways (including pair-bonding, social recognition, and social play in rodents and several aspects of social cognition in humans). Such sex-specific effects were typically reflected by having a stronger effect in one sex compared to the other sex. For example, VP plays a stronger role in social recognition in male juvenile rats compared to females (Veenema et al., 2012), and OT plays a stronger role in partner preference in female prairie voles compared to males (Winslow et al., 1993; Williams et al., 1994; Insel and Hulihan, 1995; Liu et al., 2001). Because some of these sex-specific effects were dose-dependent and required a higher dose in one sex (i.e., role of VP on partner preference in prairie voles with higher doses required in females; Cho et al., 1999), this further suggests that such sex-specific effects may be the result of sex differences in sensitivity to VP or OT systems.
Instead of a greater effect in one sex, OT and VP systems were also found to have opposing effects between the sexes. For example, VP system manipulations in the LS produced opposite effects on social play behavior in juvenile rats (Veenema et al., 2013; Bredewold et al., 2014), intranasal VP produced opposite effects on facial perception in humans (Thompson et al., 2006), and intranasal OT had opposite effects on brain activation during reciprocated cooperation in humans (Rilling et al., 2012; 2014). Furthermore, OT and VP systems were sometimes found to have an effect in one sex only. Here, ICV V1aR antagonist administration impaired partner preference in male voles only (Winslow et al., 1993; Insel & Hulihan, 1995) and OT administration into the LS altered social play in female juvenile rats only (Bredewold et al., 2014). Finally, there were occasions where OT and VP systems mediated an effect that was the same in both sexes. For example, V1aR blockade in the LS impaired social recognition in both male and female adult rats (Veenema et al., 2012), intranasal VP altered empathic concern similarly in men and women (Tabak et al., 2015), and intranasal OT increased positive behavior during couple conflict in both male and female partners (Ditzen et al, 2009).
A next step would be to determine whether these sex-specific actions (or lack thereof) of exogenously administered OT and VP and OTR and V1aR antagonists relate specifically to sex differences (or lack thereof) in the OT and VP systems. This will be an important area of research, but also a complex one, especially because the direction of sex-specific behavioral actions of the VP and OT systems can be variable, may be specific to a certain aspect of social behavior, and may depend on the species. Moreover, it may be important to look beyond sex differences in VP and OT systems and also study potential sex differences in signaling and neural pathways through which VP and OT modulate social behavior. For example, we showed that V1aR blockade in the LS increased social investigation of a familiar over a novel stimulus rat in both male and female juvenile rats, but with a stronger effect in males (Veenema et al., 2012). In contrast, V1aR blockade in the LS changed social play behavior in both male and female juvenile rats, but in opposite directions (Veenema et al., 2013). These two studies used animals of the same age, the same treatment, and the same testing conditions (home cage). The only difference was the social stimulus (3-week-old unfamiliar juvenile versus 5-week-old unfamiliar juvenile, respectively). Yet, this generated different social behaviors and different effects of manipulation of the VP system. Comparing the VP-dependent signaling and neural pathways underlying social recognition and social play behavior may gain some insights into how the VP system regulates these distinct behaviors in unique sex-specific ways.
Another unresolved issue is why sex differences in the VP (VP-immunoreactivity, V1aR) and OT (OT-immunoreactivity, OTR) systems are so highly species-specific (Tables 1–3, and 5–7). We have recently suggested that this may depend on species-specific social organizations (Dumais & Veenema, 2015). The social organization of a given species encompasses the organization of social relations within a group, including mating systems (polygamy, monogamy, promiscuity), parental systems (biparental care, maternal care), and sociality systems (the degree to which individuals live in social groups, ranging from solitary to eusocial). If indeed the presence or absence of sex differences in the brain VP and OT systems depend on the social organization of that particular species, one would predict that species in which males and females differ in one or more aspects of social organization (e.g., only females show parental care) will show more sex differences in VP and OT systems, while species in which males and females exhibit very similar social organizations (e.g., both males and females show parental care) will show less sex differences in the OT system. Alternatively, the purpose of sex differences in VP and OT systems could be to prevent, rather than induce, sex differences in behavior. This dual-function hypothesis of sex differences in the brain proposed by De Vries (De Vries, 2004) would suggest that males and females with a similar social organization may actually show sex differences in VP and OT systems in order for them to show similar behaviors. Either way, taking into account the social organization of a given species may be an important step towards understanding why certain species show sex differences in the brain VP and OT systems while other species do not.
Interestingly, in most cases discussed, VP and OT systems seem to modulate several social behaviors in a similar direction (i.e., VP and OT systems both facilitate social recognition, pair bonding, and aspects of social cognition), but they may do so by acting at distinct brain regions. For example, partner preference formation in prairie voles is modulated by the VP system acting in the LS and ventral pallidum (Liu et al., 2001; Pitkow et al., 2001; Lim & Young, 2004) and by the OT system acting in the NAc and prefrontal cortex (Young et al., 2001; Liu and Wang, 2003). This suggests that social behaviors may be regulated by a synchrony of activity of OT and VP acting at distinct brain regions, and that a balance of OT and VP activity is necessary for the modulation of a wide range of social behaviors (Neumann & Landgraf, 2012). The notion that OT and VP may be acting at distinct brain regions to modulate social behaviors in a similar direction is further supported by the distinct and non-overlapping distribution of V1aR and OTR in the brain of humans (Loup et al., 1991) and voles (Lim et al., 2004a). This lack of overlap in the distribution of V1aR and OTR in the brain is also seen in rats (Fig. 3). Notably, distinct and non-overlapping receptor distribution profiles in rats were already reported by Tribollet et al (1988), and Veinante and Freund-Mercier (1997). This could imply that VP and OT systems may modulate social behaviors in a similar way by activating distinct neural circuits. Alternatively, the distinct brain regions expressing V1aR and OTR could converge upon a common neural circuit. Further research would be required to test these hypotheses.
VP and OT systems have been implicated in sex-biased psychiatric disorders of social dysfunction, such as autism spectrum disorder and schizophrenia(Green et al., 2001; Wu et al., 2005; Jacob et al., 2007; Campbell et al., 2011; Miller et al., 2013; Di Napoli et al., 2014; Golimbet et al., 2014; Jobst et al., 2014). Therefore, a better understanding of the function of sex differences in VP and OT systems as well as sex-specific roles of these systems in the regulation of normal and impaired social behaviors is imperative, as this may provide insights into the mechanisms underlying sex biases in social dysfunction as well as be informative regarding differential treatment of social dysfunction in males versus females.
In conclusion, we found that, across species, VP and V1aR in the brain are typically higher in males than in females, and that there are fewer sex differences in OT and OTR than in VP and V1aR. The latter could be due to the lack of comprehensive analysis of sex differences in the OT system across species. In addition, we discussed that the VP and OT systems frequently mediate sex differences in social behaviors. These sex differences were highly species-specific, suggesting a role for species-specific sex differences in social organization. We conclude that the functional significance of sex differences in VP and OT systems is still largely unknown, and in need of further research.
Highlights.
VP and V1aR in the brain are typically higher in males than in females across species
Across species, fewer sex differences have been reported for OT and OTR than VP and V1aR
If sex differences are reported, OT is higher in females while OTR is higher in males
VP and OT systems very often modulate social behaviors in sex-specific ways
V1aR and OTR show distinct and largely non-overlapping expression in the rodent brain
Acknowledgments
We would like to thank Remco Bredewold, Marisa Immormino, and Sterling Karakula for technical assistance with the receptor binding. We would like to thank the Veenema lab for critically reading the manuscript. We would also like to acknowledge Jim Goodson, a dear friend and colleague who recently passed away, for his support, insights, and inspiration given over the years. This work was supported by NIMH F31MH100891 to KMD, and NSF IOS 1253386, NIMH R15MH102807, and NIMH R01MH102456 to AHV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: Relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Research. 1991;539(1):137–42. doi: 10.1016/0006-8993(91)90696-s. [DOI] [PubMed] [Google Scholar]
- Altemus M, Jacobson KR, Debellis M, Kling M, Pigott T, Murphy DL, Gold PW. Normal CSF oxytocin and NPY levels in OCD. Biol Psychiatry. 1999;45(7):931–3. doi: 10.1016/s0006-3223(98)00263-7. [DOI] [PubMed] [Google Scholar]
- Armstrong WE. Hypothalamic supraoptic and paraventricular nuclei. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Elsevier Academic; 2004. pp. 369–388. [Google Scholar]
- Asplund R, Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. Journal of Internal Medicine. 1991;229:131–4. doi: 10.1111/j.1365-2796.1991.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Bachner-Melman R, Dina C, Zohar AH, Constantini N, Lerer E, Hoch S, Sella S, Nemanov L, Gritsenko I, Lichtenberg P, Granot R, Ebstein RP. AVPR1a and SLC6A4 gene polymorphisms are associated with creative dance performance. PLoS Genet. 2005;1(3):e42. doi: 10.1371/journal.pgen.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3(2):128–34. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr Genet. 2014;24(2):45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nature Neuroscience. 2006;9(2):220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Bale TL, Davis AM, Auger AP, Dorsa DM, McCarthy MM. CNS region-specific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21(7):2546–52. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995;136(1):27–32. doi: 10.1210/endo.136.1.7828541. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2003a;117(4):854–9. doi: 10.1037/0735-7044.117.4.854. [DOI] [PubMed] [Google Scholar]
- Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Hormones and Behavior. 2003b;44:178–84. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Developmental Psychobiology. 2004;44(2):123–31. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007a;144(1):38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair bonding and alloparental care in female prairie voles. Hormones and Behavior. 2007b;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis C, Audigier S, Durroux T, Elands J, Schmidt A, Jard S. Pharmacology of oxytocin and vasopressin receptors in the central and peripheral nervous system. Ann NY Acad Sci. 1992;652:39–45. doi: 10.1111/j.1749-6632.1992.tb34344.x. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Petracca F, Dorsa DM. Autoradiography localization of specific binding sites for [3H][Arg8] vasopressin in the septum of the rat brain with tritium-sensitive film. Eur J Pharmacol. 1983;90(1):155–7. doi: 10.1016/0014-2999(83)90231-5. [DOI] [PubMed] [Google Scholar]
- Beaudet AL. Neuroscience. Preventable forms of autism?, 2012. Science. 2012;338(6105):342–3. doi: 10.1126/science.1229178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis) J Comp Neurol. 2008;507:1847–1859. doi: 10.1002/cne.21638. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169:665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Suzuki N. Neurons and circuits for odor processing in the piriform cortex. Trends Neurosci. 2013;36(7):429–38. doi: 10.1016/j.tins.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Belin V, Moos FC, Richard P. Synchronization of oxytocin cells in the hypothalamic paraventricular and supraoptic nucle in suckled rats: direct proof with paired extracellular recordings. Exp Brain Res. 1984;57:201–3. doi: 10.1007/BF00231147. [DOI] [PubMed] [Google Scholar]
- Benelli A, Bertolini A, Poggioli R, Menozzi B, Basaglia R, Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28(4):251–5. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Horm Behav. 2013;63(3):424–9. doi: 10.1016/j.yhbeh.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. 2005a. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29(3):483–93. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Young LJ. Sexual dimorphism in the vasopressin system: a lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behav Brain Res. 2005b;164(1):132–6. doi: 10.1016/j.bbr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535(2):301–4. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience. 2013;253:155–64. doi: 10.1016/j.neuroscience.2013.08.048. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124(2):439–48. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci USA. 2008;105(44):17139–44. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31(5):883–91. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pförtsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. Journal of Neuroendocrinology. 2010;22(5):420–9. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Behavioral Neuroscience. 2014;8(216):1–11. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. Long-term effects of pregnancy and parturition upon maternal responsiveness in the rat. Physiol Behav. 1975;14:245–9. doi: 10.1016/0031-9384(75)90028-1. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Parturition: its role in the long term retention of maternal behavior in the rat. Physiol Behav. 1977;24:113–7. [Google Scholar]
- Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980;207(4429):373–8. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Munte TF, Kramer U, Tempelmann C, Heldmann M. Vasopressin modulates neural responses during human reactive aggression. Soc Neurosci. 2013a;8(2):148–64. doi: 10.1080/17470919.2013.763654. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Munte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;1499:29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192(3):423–35. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- Buijs RM. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy. The Journal of Histochemistry and Cytochemistry. 1980;28(4):357–60. doi: 10.1177/28.4.6989899. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Pevet P, Masson-Pevet M, Pool CW, de Vries GJ, Canguilhem B, Vivien-Roels B. Seasonal variation in vasopressin innervation in the brain of the European hamster (Cricetus cricetus) Brain Research. 1986;371:193–6. doi: 10.1016/0006-8993(86)90829-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204(3):355–65. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Munte TF, Kramer U, Tempelmann C, Heldmann M. Vasopressin modulates neural responses during human reactive aggression. Soc Neurosci. 2013;8(2):148–64. doi: 10.1080/17470919.2013.763654. [DOI] [PubMed] [Google Scholar]
- Brunnlieb C, Munte TF, Tempelmann C, Heldmann M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013;7 (1499):29–42. doi: 10.1016/j.brainres.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261(2):237–52. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Caffé AR, van Ryen PC, van der Woude TP, van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287(3):302–25. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004a;46(4):444–9. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Albers HE. Photoperiod regulation of vasopressin receptor binding in female Syrian hamsters. Brain Res. 2004b;1002(1–2):136–41. doi: 10.1016/j.brainres.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Datta D, Jones ST, Lee EB, Sutcliffe JS, Hammock EAD, Levitt P. Association of oxytocin receptor (OXTR) gene varients with multiple phenotype domains of autism spectrum disorder. Journal of Neurodevelopmental Disorders. 2011;3:101–12. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P, Ophir AG, Phelps SM. Central vasopressin and oxytocin receptor distributions in two species of singing mice. J Comp Neurol. 2009;516(4):321–33. doi: 10.1002/cne.22116. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, Qiao X, Hao P. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor α mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Hormones and Behavior. 2013;65(1):57–65. doi: 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica MA, Schrader WT, O’Malley BW. Steroid receptor family: structure and functions. Endocri Rev. 1990;11(2):201–20. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Bales KL. Early experience and the developmental programming of oxytocin and vasopressin. Neurobiology of the Parental Brain. 2008:417–33. [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007;176 (1):170–86. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J Neuroendocrin. 2011;23(11):1113–24. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and beta-knockout mice. Proc Natl Acad Sci USA. 2003;100(10):6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes, Brain, and Behavior. 2006;5(7):528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509 (7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytoin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. 2013;21(5):219–47. doi: 10.1097/HRP.0b013e3182a75b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gaballoni P, Abelli M, Lari L, Cardini A, Muit M, Gesi C, Landi S, Galderisi S, Mucci A, Lucacchini A, Cassano GB, Martini C. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34(10):1506–14. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Crenshaw BJ, De Vries GJ, Yahr P. Vasopressin innervation of sexually dimorphic structures of the gerbil forebrain under various hormonal conditions. J Comp Neurol. 1992;332:589–98. doi: 10.1002/cne.903220412. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 1998;119:409–14. doi: 10.1016/s0079-6123(08)61584-8. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le Moal M. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology. 1987;91(3):363–8. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–8. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Progress in Brain Research. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Al-Shamma HA. Sex differences in hormonal responses of vasopressin pathways in the rat brain. J Neurobiol. 1990;21(5):686–93. doi: 10.1002/neu.480210503. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Best W, Sluiter AA. The influence of angrogens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Developmental Brain Research. 1983;8:377–380. doi: 10.1016/0165-3806(83)90019-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92(2):205–13. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Research. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain- presence of a sex difference in the lateral septum. Brain Research. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neuroscience. 2002;22(20):9005–14. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, Ferris CF. Sexual differences in vasopressin receptor binding within the ventrolateral hypothalamus in golden hamsters. Brain Res. 1995;681(1–2):91–6. doi: 10.1016/0006-8993(95)00291-w. [DOI] [PubMed] [Google Scholar]
- Delville Y, Koh ET, Ferris CF. Sexual differences in the magnocellular vasopressinergic system in golden hamsters. Brain Research Bulletin. 1994;33(5):535–540. doi: 10.1016/0361-9230(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Di Napoli A, Warrier V, Baron-Cohen S, Chakrabarti B. Genetic variation in the oxytocin receptor (OXTR) gene is associated with Asperger Syndrome. Molecular Autism. 2014;5(1):48. doi: 10.1186/2040-2392-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen PJ, Harmer CJ. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology. 2009;23(3):241–8. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Dieckmann M, Freudenberg F, Klein S, Koch M, Schwabe K. Disturbed social behavior and motivation in rats selectively bred for deficient sensorimotor gating. Schizophr Res. 2007;97:250–253. doi: 10.1016/j.schres.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Dierickx K. Immunocytochemical localization of the vertebrate cyclic nonapeptide neurohypophyseal hormones and neuophysins. Int Rev Cytol. 1980;62:119–85. doi: 10.1016/s0074-7696(08)61900-2. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Nater UM, Schaer M, Marca RL, Bodenmann G, Ehlert U, Heinrichs M. Sex-specific effects of intranasal oxytocin on autonomic nervous system and emotional responses to couple conflict. SCAN. 2013;8:897–902. doi: 10.1093/scan/nss083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychology. 2009;65:728–31. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Muraoka S, Engelmann M, Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19(6):999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Muchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biological Psychiatry. 2007;62(10):1187–90. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry. 2013;74(3):164–71. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61(6):731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Dorsa DM, Majumdar LA, Petracca FM, Baskin DG, Cornett LE. Characterization and localization of 3H-arginine8-vasopressin binding to rat kidney and brain tissue. Peptides. 1983;4(5):699–706. doi: 10.1016/0196-9781(83)90021-9. [DOI] [PubMed] [Google Scholar]
- Dorsa DM, Petracca FM, Baskin DG, Cornett LE. Localization and characterization of vasopressin-binding sites in the amygdala of the rat brain. J Neurosci. 1984;4(7):1764–70. doi: 10.1523/JNEUROSCI.04-07-01764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Barberis C, de Bilbao F. Vasopressin receptors in the mouse (Mus musculus) brain: sex-related expression in the medial preoptic area and hypothalamus. Brain Res. 1996;743(1–2):32–9. doi: 10.1016/s0006-8993(96)01019-0. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Pévet P, Barberis C, Tribollet E, Dreifuss JJ. Localization of binding sites for oxytocin in the brain of the golden hamster. Neuroreport. 1992;3(9):797–800. doi: 10.1097/00001756-199209000-00019. [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Theler JM, Zaganidis N, Dominik W, Tribollet E, Pevet P, Charpak G, Dreifuss JJ. Expression of vasopressin receptors in hamster hypothalamus is sexually dimorphic and dependent upon photoperiod. Proc Natl Acad Sci USA. 1991;88(24):11163–7. doi: 10.1073/pnas.88.24.11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Hormones and Behavior. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landrag R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Research. 2000;872(1–2):87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65(6):831–44. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Knafo A, Mankuta D, Chew SH, Lai PS. The contributions of oxytocin and vasopressin pathway genes to human behavior. Hormones and Behavior. 2012;61(3):359–79. doi: 10.1016/j.yhbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki1 K, Oishi R, Fujiwara M. New Topics in Vasopressin Receptors and Approach to Novel Drugs: Role of the Vasopressin Receptor in Psychological and Cognitive Functions. J Pharmacol Sci. 2009;109:44–49. doi: 10.1254/jphs.08r14fm. [DOI] [PubMed] [Google Scholar]
- Egashira N, Tanoue A, Higashihara F, Mishima K, Fukue Y, Takano Y, Tsujimoto G, Iwasaki K, Fujiwara M. V1a receptor knockout mice exhibit impairment of spatial memory in an eight-arm radial maze. Neurosci Lett. 2004;356(3):195–8. doi: 10.1016/j.neulet.2003.11.050. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. Journal of Neuroendocrinology. 1999;11(11):867–72. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90(1):89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55(1):145–9. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99(1):7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Fabio K, Guillon C, Lacy CJ, Lu SF, Heindel ND, Ferris CF, Placzek M, Jones G, Brownstein MJ, Simon NG. Synthesis and evaluation of potent and selective human V1a receptor antagonists as potential ligands for PET or SPECT images. Bioorg Med Chem. 2012;20(3):1337–45. doi: 10.1016/j.bmc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2014 doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature Genetics. 2000;25(3):284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience. 2001;21(20):8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Miller MA, Dorsa DM, De Vries GJ. Distribution of small vasopressinergic neurons in golden hamsters. J Comp Neurol. 1995;360(4):589–98. doi: 10.1002/cne.903600404. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interaction in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17(11):4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Shofty M, Levkovitz Y, Shamay-Tsoory SG. Oxytocin facilitates accurate perception of competition in men and kinship in women. Social Cognitive and Affective Neuroscience. 2013;8:313–7. doi: 10.1093/scan/nsr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375(2):363–7. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Fliers E, Swaab DF, Pool CW, Verwer RWH. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Research. 1985;342:45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- Fombonne E. The prevalence of autism. JAMA. 2003;289(1):87–9. doi: 10.1001/jama.289.1.87. [DOI] [PubMed] [Google Scholar]
- Freeman SM, Walum H, Young LJ. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273(12):12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Dietl MM, Stoeckel ME, Palacios JM, Richard P. Quantitative autoradiographic mapping of neurohypophysial hormone binding sites in the rat forebrain and pituitary gland-II. Comparative study on the Long-Evans and Brattleboro strains. Neuroscience. 1988;26(1):273–81. doi: 10.1016/0306-4522(88)90144-3. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Poblete VF., Jr Oxytocin and uterine function in pregnant and parturient rats. Biology of Reproduction. 1970;2:387–400. doi: 10.1095/biolreprod2.3.387. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, Gotlib IH. Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology. 2011;36(6):891–7. doi: 10.1016/j.psyneuen.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neuroscience. 2006;26(8):2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstberger R, Fahrenholz F. Autoradiographic localization of V1 vasopressin binding sites in rat brain and kidney. Eur J Pharmacol. 1989;167(1):105–16. doi: 10.1016/0014-2999(89)90752-8. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- Gilles GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299(5881):355–7. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gimpl, Fahrenholz The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;81(2):630–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Research (Part B) 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Golimbet V, Alfimova M, Abramova L, Kaleda V, Gritsenko I. Arginine vasopressin 1a receptor RS3 promoter microsatellites in schizophrenia: A study of the effect of the “risk” allele on clinical symptoms and facial affect recognition. Psychiatry Research. 2014;225(3):729–40. doi: 10.1016/j.psychres.2014.11.043. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35(3):246–65. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68:377–82. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: Associations with bonding and psychological distress. Psychophysiology. 2008;45:349–52. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grunder G, Spreckelmeyer KN. Oxytocin plasma concentrations after single intranasal oxytocin administration – a study in healthy men. Neuropeptides. 2012;46(5):211–5. doi: 10.1016/j.npep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Gouin JP, Carter CS, Pournajafi-Nazarloo H, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. Plasma vasopressin and interpersonal functioning. Biological Psychiatry. 2012;91:270–4. doi: 10.1016/j.biopsycho.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010a;67(7):692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010b;67(12):1220–2. doi: 10.1016/j.biopsych.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, Hickie IB. Arginine vasopressin selevtively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology. 2011;36(2):294–7. doi: 10.1016/j.psyneuen.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61(3):410–8. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008a;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Matthews F. Oxytocin enhances the encoding of positive social memories in humans. Biol Psychiatry. 2008b;64(3):256–8. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrain hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31(9):1655–63. 37. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- Guzman YF, Tronson NC, Jovasevic V, Sato K, Guedea AL, Mizukami H, Nishimori K, Radulovic J. Fear-enhancing effects of septal oxytocin receptors. Nat Neurosci. 2013;16:1185–1187. doi: 10.1038/nn.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot RY, Uzefovsky F, Bogopolsky H, Ebstein RP. Effects of arginine vasopressin on musical working memory. Front Psychol. 2013;4:712. doi: 10.3389/fpsyg.2013.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graugaard-Jensen C, Hvistendahl GM, Frokiaer J, Bie P, Djurhuus JC. Urinary concentration does not exclusively rely on plasma vasopressin- A study between genders. Acta Physiol. 2014;212:97–105. doi: 10.1111/apha.12337. [DOI] [PubMed] [Google Scholar]
- Green L, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biological Psychiatry. 2001;50(8):609–13. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosomatic Medicine. 2005;67:531–8. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grozhik AV, Horoszko CP, Horton BM, Hu Y, Voisin DA, Maney DL. Hormonal regulation of vasotocin receptor mRNA in a seasonally breeding songbird. Horm Behav. 2014;65(3):254–63. doi: 10.1016/j.yhbeh.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA. Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Levitt P. Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR) Neuroscience. 2012;222:20–8. doi: 10.1016/j.neuroscience.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Levitt P. Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front Behav Neurosci. 2013;7(195) doi: 10.3389/fnbeh.2013.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol Learn Mem. 2014;108:104–18. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussler HU, Jirikowski GF, Caldwell JD. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. Journal of Chemical Neuroanatomy. 1990;3:271–6. [PubMed] [Google Scholar]
- Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: regulation by stress and glucocorticoids. J Comp Neurol. 1995;363(1):15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Hermes MLHJ, Buijs RM, Masson-Pevet M, Pevet P. Seasonal changes in vasopressin in the brain of the garden dormouse (Eliomys Quercinus L.) J Comp Neurol. 1990;293:340–6. doi: 10.1002/cne.902930303. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burback JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142(4):1659–68. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Herzmann G, Bird CW, Freeman M, Curran T. Effects of oxytocin on behavioral and ERP measures of recognition memory for own-race and other-race faces in women and men. Psychoneuroendocrinology. 2013;38:2140–51. doi: 10.1016/j.psyneuen.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa A, Hashimoto K, Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur J Pharmacol. 1994;267(1):71–5. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5′ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality. Genes, Brain and Behavior. 2012;11(5):552–8. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Keebaugh AC, Reamer LA, Schaeffer J, Schapiro SJ, Young LJ. Genetic influences on receptive joint attention in chimpanzees (Pan troglodytes) Scientific Reports. 2014;4:3774. doi: 10.1038/srep03774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience. 1991;43(2–3):623–30. doi: 10.1016/0306-4522(91)90321-e. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109(4):782–9. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89(13):5981–5. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Vasopressin and oxytocin neurons of the human supraoptic and paraventricular nucleus; Size changes in relation to age and sex. The Journal of Clinical Endocrinology and Metabolism. 1999;84(12):4637–44. doi: 10.1210/jcem.84.12.6187. [DOI] [PubMed] [Google Scholar]
- Ivell R, Walther N. The role of sex steroids in the oxytocin hormone system. Molecular and Cellular Endocrinology. 1999;151(1–2):95–101. doi: 10.1016/s0303-7207(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jard S. Vasopressin receptors in mammals: relation to cyclic AMP-dependent and cyclic AMP-independent transduction mechanisms. Curr Top Membr Trans. 1983;18:255–285. [Google Scholar]
- Jard S, Barberis C, Audigier S, Tribollet E. Neurohypophyseal hormone receptor systems in brain and periphery. Progress in Brain Research. 1987;72:173–187. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- Jobst A, Dehning S, Ruf S, Notz T, Buchheim A, Henning-Fast K, Meißner D, Meyer S, Bondy B, Müller N, Zill P. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014;26(6):347–55. doi: 10.1017/neu.2014.20. [DOI] [PubMed] [Google Scholar]
- Joca L, Zuloaga DG, Raber J, Siegel JA. Long-term effects of early adolescent methamphetamine exposure on depression-like behavior and the hypothalamic vasopressin system in mice. Developmental Neuroscience. 2013;36:108–18. doi: 10.1159/000360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AE, Audigier S, Rossi F, Jard S, Tribollet E, Barberis C. Localization and characterization of vasopressin binding sites in the rat brain using an iodinated linear AVP antagonist. Brain Res. 1993;622(1–2):9–16. doi: 10.1016/0006-8993(93)90795-o. [DOI] [PubMed] [Google Scholar]
- Johnson AE, Barberis C, Albers HE. Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res. 1995;674(1):153–8. doi: 10.1016/0006-8993(95)00010-n. [DOI] [PubMed] [Google Scholar]
- Juul KV, Bichet DG, Nielsen S, Nørgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol. 2014;306(9):931–40. doi: 10.1152/ajprenal.00604.2013. [DOI] [PubMed] [Google Scholar]
- Kato Y, Igarashi N, Hirasawa A, Tsujimoto G, Kobayashi M. Distribution and developmental change in vasopressin V2 receptor mRNA in rat brain. Differentiation. 1995;59(3):163–9. doi: 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, Xu J, Chu TQ, Ryu C, Suh EM, Graham K, Taylor SE. Culture, distress, and oxytocin repceptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci USA. 2010;107(36):15717–21. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, Israel S, Darvasi A, Bachner-Melman R, Uzefovsky F, Cohen L, Feldman E, Lerer E, Laiba E, Raz Y, Nemanov L, Gritsenko I, Dina C, Agam G, Dean B, Bornstein G, Ebstein RP. Individual differences in allocation of funds in the dictator game associated with length of the arginine vasopressin 1a receptor RS3 promoter region and correlation between RS3 length and hippocampal mRNA. Genes Brain Beh. 2008;7(3):266–75. doi: 10.1111/j.1601-183X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Kogan A, Saslow LR, Impett EA, Oveis C, Keltner D, Rodrigues Saturn S. Thin-splicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. Proc Natl Acad Sci USA. 2011;108(48):19189–92. doi: 10.1073/pnas.1112658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HY, Kim D, Lee J, Lee S, Shin HS. Deficits in social behavior and sensorimotor gating in mice lacking phospholipase Cbeta1. Genes Brain Behav. 2008;7:120–8. doi: 10.1111/j.1601-183X.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Koshimizu T, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92:1813–64. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dentritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9(9):1094–5. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canadian Journal of Zoology. 2004;82(8):1194–1200. [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Hormones and Behavior. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biological Psychology. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behavior. Eur J Neurosi. 2003;18(2):403–11. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Dantzer R, Michaud B, Koob GF. Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci Lett. 1987;77(3):353–9. doi: 10.1016/0304-3940(87)90527-1. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mice line of the oxytocin receptor. Endocrinology. 2008;149(7):3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R, Heresco-Levy U, Bachner-Melman R, Israel S, Shalev I, Ebstein RP. Association between arginine vasopressin 1a receptor (AVPR1a) promoter region polymorphisms and prepulse inhibition. Psychoneuroendocrinology. 2009;34(6):901–8. doi: 10.1016/j.psyneuen.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004a;468(4):555–70. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004b;429(6993):754–7. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125(1):35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–44. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, 3rd, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92(15):6783–7. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of adult virgin prairie voles: Independence from gonadal hormones and vasopressin. Journal of Neuroendocrinology. 1999;11:441–9. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism-spectrum disorder: a meta-analysis. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.77. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, Stacey D, Cattrell A, Desrivieres S, Ruggeri B, Fritsch V, Banaschewski T, Barker GJ, Bokde AL, Muchel C, Carvalho FM, Conrod PJ, Fauth-Buehler M, Flor H, Gallinat J, Garavan H, Heinz A, Bruehl R, Lawrence C, Mann K, Martinot JL, Nees F, Paus T, Pausova Z, Poustka L, Rieschel M, Smolka M, Struve M, Feng J, Schumann G IMAGEN Consortium. Oxytocin receptor genotype modulates ventral striatal activity to social cues and response to stressful life events. Biol Psychiatry. 2014;76(5):367–76. doi: 10.1016/j.biopsych.2013.07.043. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555(2):220–32. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, Rosenberger A, Grabe HJ, Schroeder W, Völzke H, Freyberger HJ, Herrmann FH, Kroemer H, Rosskopf D. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(5):860–6. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011a;36(6):843–53. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lukas M, Neumann ID. Social preference and maternal defeat-induced social avoidance in virgin female rats: sex differences in involvement of brain oxytocin and vasopressin. Journal of Neuroscience Methods. 2014;234:101–7. doi: 10.1016/j.jneumeth.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011b;36(11):2159–68. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38(6):916–26. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Lynn SK, Hoge EA, Fischer LE, Barrett LF, Simon NM. Gender differences in oxytocin-associated disruption of decision bias during emotion perception. Psychiatry Research. 2014;14:1–6. doi: 10.1016/j.psychres.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes, Brain, and Behavior. 2009;8(5):558–67. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;23(3):452–67. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Sousa N, Cadete-Leite A, Lieberman AR, Paula-Barbosa MM. The supraoptic nucleus of the adult rat hypothalamus displays a marked sexual dimorphism which is dependent on body weight. Neuroscience. 1993;52(3):497–513. doi: 10.1016/0306-4522(93)90402-2. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinology. 2012;24(4):609–28. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AD. Posttraumatic stress disorder and partner-specific social cognition: A pilot study of sex differences in the impact of arginine vasopressin. Biological Psychology. 2013;93:296–303. doi: 10.1016/j.biopsycho.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kolachana B, Gold B, Olsh A, Nicodemus KK, Mattay V, Dean M, Weinberger DR. Genetic variants in AVPR1A linked to autism predict amygdala activation and personality traits in healthy humans. Mol Psychiatry. 2009;14(10):968–75. doi: 10.1038/mp.2008.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, Hofman MA, Swaab DF, Hoogendijk WJG. Hypothalamic oxytocin mRNA expression and melancholic depression. Molecular Psychiatry. 2007;12:118–9. doi: 10.1038/sj.mp.4001911. [DOI] [PubMed] [Google Scholar]
- Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Research. 2013;6(2):91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, De Vries GJ, Al-Shamma HA, Dorsa DM. Decline of vasopressin immunoreactvity and mRNA levels in the bed nucleus of the stria terminalis following castration. The Journal of Neuroscience. 1992;12(8):2881–7. doi: 10.1523/JNEUROSCI.12-08-02881.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Ferris CF, Kolb PE. Absence of vasopressin expression by galanin neurons in the golden hamster: implications for species differences in extrahypothalamic vasopressin pathways. Brain Res Mol Brain Res. 1999;67(1):28–35. doi: 10.1016/s0169-328x(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Miller MA, Urban JH, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Society of Biological Psychiatry. 1998;43:270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Mogi K, Ooyama R, Nagasawa M, Kikusui T. Effects of neonatal ocytocin manipulation on development of social behaviors in mice. Physiol Behav. 2014;133:68–75. doi: 10.1016/j.physbeh.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(3):251–60. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Mouillac B, Chini B, Balestre MN, Jard S, Barberis C, Manning M, Tribollet E, Trumpp-Kallmeyer S, Hoflack J, Elands J. Identification of agonist binding sites of vasopressin and oxytocin receptors. Adv Exp Med Biol. 1995;395:301–10. [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol Behav. 2008;95(1–2):182–6. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci. 2009;123(5):949–57. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–59. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after intranasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Nyuyki KD, Waldherr M, Baeumi S, Neumann ID. Yes, I am ready now: differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. Plos One. 2011;6(8):e23599. doi: 10.1371/journal.pone.0023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Gessel A, Zheng DJ, Phelps SM. Oxytocin receptor density is associated with male mating tactics and social monogamy. Horm Behav. 2012;61(3):445–53. doi: 10.1016/j.yhbeh.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero-Garcia M, Martin-Sanchez A, Fortes-Marco L, Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Extending the socio-sexual brain: arginine-vasopressin immunoreactive circuits in the telencephalon of mice. Brain Struct Funct. 2014;219(3):1055–81. doi: 10.1007/s00429-013-0553-3. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Kinney LF, Phillips KM, Lee TM. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115(6):1341–8. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, de Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. Front Behav Neurosci. 2014;8:58. doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Pearlmutter AF, Costantini MG, Loeser B. Characterization of 3H-AVP binding sites in particulate preparations of rat brain. Peptides. 1983;4(3):335–41. doi: 10.1016/0196-9781(83)90144-4. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Petracca FM, Baskin DG, Diaz J, Dorsa DM. Ontogenetic changes in vasopressin binding site distribution in rat brain: an autoradiographic study. Brain Res. 1986;393(1):63–8. doi: 10.1016/0165-3806(86)90065-9. [DOI] [PubMed] [Google Scholar]
- Phillips PA, Abrahams JM, Kelly JM, Mooser V, Trinder D, Johnston CI. Localization of vasopressin binding sites in rat tissues using specific V1 and V2 selective ligands. Endocrinology. 1990;126(3):1478–84. doi: 10.1210/endo-126-3-1478. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitaton of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–6. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1(4):555–60. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology. 1992;106(1):71–4. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- Qiao X, Yan Y, Wu R, Tai F, Hao P, Cao Y, Wang J. Sociality and oxytocin and vasopressin in the brain of male and female dominant and subordinate mandarin voles. Journal of Comparative Physiology. 2014;200:149–59. doi: 10.1007/s00359-013-0870-2. [DOI] [PubMed] [Google Scholar]
- Rhodes CH, Morrell JY, Pfaff DW. Immunocytochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin and vasopressin. J CompNeurol. 1981;198:45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–48. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–61. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106(50):21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519(12):2434–74. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ. Site of origin of sex diffrences in the vasopressin innervation of the mouse (Mus musculus) brain. J Comp Neurol. 2013;521(10):2321–58. doi: 10.1002/cne.23288. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162(4):892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GJ, De Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of oxytocin in the brain of a eusocial rodent. Neuroscience. 2008;155:809–17. doi: 10.1016/j.neuroscience.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci USA. 2011;108(37):15118–22. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33(3):368–74. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Kendrick KM, Schwering C, Noelle J, Wille A, Schläpfer TE, Maier W, Hurlemann R. Opposing effects of oxytocin on moral judgment in males and females. Human Brain Mapping. 2014 doi: 10.1002/hbm.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Audigier S, Barberis C, Jard S, Manning M, Kolodziejczyk AS, Sawyer WH. A radioiodinated linear vasopressin antagonist: a ligand with high affinity and specificity for V1 receptors. FEBS Lett. 1991;282(1):77–81. doi: 10.1016/0014-5793(91)80448-c. [DOI] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011;36(9):1378–82. doi: 10.1016/j.psyneuen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Share L, Crofton JT, Ouchi Y. Vasopressin: Sexual dimorphism in secretion, cardiovascular actions and hypertension. The American Journal of the Medical Sciences. 1988;295(4):314–9. doi: 10.1097/00000441-198804000-00017. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith CJW, Poehlmann ML, Wilkins KB, Bredewold R, Veenema AH. Society for Neuroscience abstract 832.14/NN24. Washington D.C: 2014. Age differences in te brain oxytocin system: implications for juvenile social motivation. [Google Scholar]
- Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat human. J Histochem Cytochem. 1980;28(5):475–8. doi: 10.1177/28.5.7381192. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurons and their central and vascular projections. Prog Brain Res. 1983;60:101–14. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Weindl A. Projections from the parvocellular vasopressin- and neurophysin-containing neurons of the suprachiasmatic nucleus. Am J Anat. 1978;153:391–430. doi: 10.1002/aja.1001530305. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology. 2015;51:122–34. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav. 2012;61(3):277–82. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40(2):93–8. doi: 10.1006/hbeh.2001.1682. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Pool CW, Nijveldt F. Immunofluorescence of vasopressin and oxytocin in the rat hypothalamo-neurohypophypopseal system. J Neural Transm. 1975;36(3–4):195–215. doi: 10.1007/BF01253126. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, Schneiderman N, Mendez AJ. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine. 2011;73(5):393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA, Meyer ML, Castle E, Dutcher JM, Irwin MR, Han JH, Lieberman MD, Eisenberger NI. Vasopressin, but not oxytocin, increases empathic concern among individuals who received higher levels of paternal warmth: A randomized controlled trial. Psychoneuroendocrinology. 2015;51:253–61. doi: 10.1016/j.psyneuen.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102(44):16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21(1):3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3(1):15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Mohr C. Men perform comparably to women in a perspective taking task after administration of intranasal oxytocin but not after placebo. Frontiers in Human Neuroscience. 2013;7(197):1–11. doi: 10.3389/fnhum.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Gupta S, Miller K, Mills S, Orr S. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology. 2004;29(1):35–48. doi: 10.1016/s0306-4530(02)00133-6. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103(20):7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb in involved in social recognition. Nature. 2010;464:413–7. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, van Ijzendoorn MH, Riem MM, Boksem MA, Bakermans-Kranenburg MJ. Oxytocin receptor gene associated with the efficiency of social auditory processing. Front Psychiatry. 2011;2(60) doi: 10.3389/fpsyt.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A. 2010;107(31):13936–41. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Research. 1990;511(1):129–40. doi: 10.1016/0006-8993(90)90232-z. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442(1):105–18. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Goumaz M, Raggenbass M, Dreifuss JJ. Appearance and transient expression of vasopressin and oxytocin receptors in the rat brain. J Recept Res. 1991;11(1–4):333–46. doi: 10.3109/10799899109066412. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Ueta Y, Heitz F, Marguerat A, Koizumi K, Yamashita H. Up-regulation of vasopressin and angiotensin II receptors in the thalamus and brainstem of inbred polydipsic mice. Neuroendocrinology. 2002;75:113–123. doi: 10.1159/000048227. [DOI] [PubMed] [Google Scholar]
- Uhl-Bronner S, Waltisperger E, Martínez-Lorenzana G, Condes Lara M, Freund-Mercier MJ. Sexually dimorphic expression of oxytocin binding sites in forebrain and spinal cord of the rat. Neuroscience. 2005;135(1):147–54. doi: 10.1016/j.neuroscience.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Ukkola LT, Onkamo P, Raijas P, Karma K, Jarvela I. Musical aptitude is associated with AVPR1A-haplotypes. PLoS One. 2009;4(5):e5534. doi: 10.1371/journal.pone.0005534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Edelman S, Raz Y, Mankuta D, Knafo-Noam A, Ebstein RP. Oxytocin receptor and vasopressin receptor 1a genes are respectively associated with emotional and cognitive empathy. Horm Behav. 2015;67:60–5. doi: 10.1016/j.yhbeh.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Knafo A, Ebstein RP. Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology. 2012;37(4):576–80. doi: 10.1016/j.psyneuen.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Nedocrinology. 1998;139(12):5015–33. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated salivary levels of oxytoin persist more than 7 h after intranasal administration. Front Neurosci. 2012;6(174) doi: 10.3389/fnins.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325(1–2):391–4. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Van Londen L, Goekoop JG, Godfriend M, van Kempen J, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D. Plasma levels of arginine vasopressin elevated in pateints with major depression. Neuropsychopharmacology. 1997;17(4):284–92. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermal aggression. Horm Behav. 2010;58(2):273–81. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Hormones and Behavior. 2012;61(1):50–6. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38(11):2554–61. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–76. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin- binding sites in the rat extended amygdala: A histoautoradiographic study. J Comp Neurol. 1997;383(3):305–25. [PubMed] [Google Scholar]
- Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7(2):75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- Voorhuis TA, de Kloet ER, de Wied D. The distribution and plasticity of [3H]vasopressin-labelled specific binding sites in the canary brain. Brain Res. 457(1):148–53. doi: 10.1016/0006-8993(88)90067-4. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104(42):16681–4. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsäter H, Larsson H, Westberg L. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biological Psychiatry. 2012;71(5):419–26. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Perdersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105(37):14153–6. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pan Y, Wang Z, Zhang Z. Species differences in the immunoreactive expression of oxytocin, vasopressin, tyrosine hydroxylase and estrogen receptor alpha in the brain of Mongolian gerbils (Meriones unguiculatus) and chinese striped hamsters (Cricetulus barabensis) Plos One. 2013;8(6):e65807. doi: 10.1371/journal.pone.0065807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109(2):305–11. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. Journal of Neuroendocrinology. 1995;7:827–831. doi: 10.1111/j.1365-2826.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moody K, Newman JD, Insel TR. Vasopressin and oxytocin immunoreactive neurons and fibers in the forebrain of male and female common marmosets (Callithrix jacchus) Synapse. 1997;27(1):14–25. doi: 10.1002/(SICI)1098-2396(199709)27:1<14::AID-SYN2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Wang Z, Toloczko D, Young LJ, Moody K, Newman JD, Insel TR. Vasopressin in the forebrain of common marmosets (Callithrix jacchus): studies with in situ hybridization, immunocytochemistry, and receptor autoradiography. Brain Research. 1997b;768(1–2):147–56. doi: 10.1016/s0006-8993(97)00636-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. The Journal of Comparative Neurology. 1996;366:726–37. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012;37:1582–6. doi: 10.1016/j.psyneuen.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knowkcout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6(6):540–51. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wierda M, Goudsmit E, Van der Woude PF, Purba JS, Hofman MA, Bogte H, Swaab DF. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol Aging. 1991;12(5):511–6. doi: 10.1016/0197-4580(91)90081-t. [DOI] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles in facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci. 1992;652:487–9. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994;6(3):247–50. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Letters to Nature. 1993;365:545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58(1):74–7. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Xu L, Pan Y, Young KA, Wang Z, Zhang Z. Oxytocin and vasopressin immunoreactive staining in the brains of Brandt’s voles (Lasiopodomys brandtii) and greater long-tailed hamsters (Tscherskia triton) Neuroscience. 2010;169(3):1235–47. doi: 10.1016/j.neuroscience.2010.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Shou XJ, Li J, Jia MX, Zhang JS, Guo Y, Wei QY, Zhang XT, Han SP, Zhang R, Han JS. Mothers of autistic children: lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS One. 2013;8(9):e74849. doi: 10.1371/journal.pone.0074849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125:947–55. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Carter CS, Cushing BS. Neonatal manipulation of oxytocin affects expression of estrogen receptor alpha. Neuroscience. 2006;137:157–164. doi: 10.1016/j.neuroscience.2005.08.065. [DOI] [PubMed] [Google Scholar]
- Yang SY, Cho SC, Yoo HJ, Cho IH, Park M, Kim BN, Kim JW, Shin MS, Park TW, Son JW, Chung US, Kim HW, Yang YH, Kang JO, Kim SA. Association study between single nucleotide polymorphisms in promoter region of AVPR1A and Korean autism spectrum disorders. Neurosci Lett. 2010;479(3):197–200. doi: 10.1016/j.neulet.2010.05.050. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol. 1999;11(4):291–7. doi: 10.1046/j.1365-2826.1999.00332.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J Neuroendocrinol. 2000;12(12):1179–85. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donalson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. NeuroReport. 1998;9:933–6. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Gainer H. Transgenesis and the study of expression, cellular targeting and function of oxytocin, vasopressin and their receptors. Neuroendocrinology. 2003;78(4):185–203. doi: 10.1159/000073702. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143(4):1031–9. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueng KW, Garner JP, Carson DS, Keller J, Lembke A, Hyde SA, Kenna HA, Tennakoon L, Schatzberg AF, Parker KJ. Plasma oxytocin concentrations are lower in depressed vs. healthy control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–6. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. Plos One. 2007;2(11) doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Monakhov M, Mok HP, Tong T, Lai PS, Chew SH, Ebstein RP. U-shaped relation between plasma oxytocin levels and behavior in the trust game. Plos One. 2012;7(12):1–9. doi: 10.1371/journal.pone.0051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Kempf L, Hakimi S, Rainey CA, Stein JL, Meyer-Lindenberg A. Vasopressin modulates social recognition-related activity in the left temporoparietal junction in humans. Transl Psychiatry. 2011;1:e3. doi: 10.1038/tp.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30(20):7017–22. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]