Abstract
Context
Excessive consumption of alcohol is a major problem in the United States and abroad. Despite many years of study, it is unclear why some individuals drink alcohol excessively while others do not. It has been postulated that either lower or greater acute responses to alcohol, or both, depending on the limb of the breath alcohol concentration curve, contribute to propensity for alcohol misuse.
Objective
To prospectively assess the relationship of acute alcohol responses to future binge drinking.
Design
Within-subject, double-blind, placebo-controlled, multidose laboratory alcohol challenge study with intensive follow-up. Each participant completed 3 randomized sessions examining responses to a high (0.8 g/kg) and low (0.4 g/kg) alcohol dose and placebo, followed by quarterly assessments for 2 years examining drinking behaviors and alcohol diagnoses.
Setting
Participants recruited from the community.
Participants
High-risk heavy social drinkers aged 21 to 35 years who habitually engage in weekly binge drinking (n=104) and light drinker controls (n=86).
Intervention
We conducted 570 laboratory sessions with a subsequent 99.1% follow-up (1506 of 1520).
Main Outcome Measures
Biphasic Alcohol Effects Scale, Drug Effects Questionnaire, cortisol response, Time-line Follow-Back, Drinker Inventory of Consequences–Recent, and DSM-IV alcohol abuse and dependence.
Results
Alcohol produced greater stimulant and rewarding (liking and wanting) responses and lower sedative and cortisol responses in heavy vs light drinkers. Among the heavy drinkers, greater positive effects and lower sedative effects after alcohol consumption predicted increased binge drinking frequency during follow-up. In turn, greater frequency of binge drinking during follow-up was associated with greater likelihood of meeting diagnostic criteria for alcohol abuse and dependence.
Conclusions
The widely held low level response theory and differentiator model should be revised: in high-risk drinkers, stimulant and rewarding alcohol responses even at peak breath alcohol concentrations are important predictors of future alcohol problems.
Trial Registration
clinicaltrials.gov Identifier: NCT00961792
Heavy alcohol drinking is a serious problem in young adults,1–3 and the personal, physical, familial, and financial consequences of this excessive use are enormous. Excessive drinking is the third leading preventable cause of death in the United States,4 and the cost of ethanol-related health care, loss of productivity, crime, and accidents totals more than $184 billion annually.5 However, it is unclear why some individuals escalate their consumption of alcohol over time to excessive levels while others do not. A greater understanding of the factors that contribute to the escalation and maintenance of heavy drinking, especially in young adults, is essential to guide prevention, public education, and early intervention strategies for alcohol use disorders.
One potential predictor of vulnerability to alcohol use problems is the quality and magnitude of one’s acute response to alcohol.6–8 For example, a widely cited series of studies indicates that individuals with a positive biological family history (FH) of alcoholism, who are known to be at increased risk for developing alcohol dependence,9,10 have lower responses to alcohol on several measures, including subjective alcohol intoxication ratings, body sway, stress hormones, and evoked potential responses, relative to those with a negative FH.11–16 In turn, even without regard to FH, lower level response to alcohol predicted the development of a future alcohol use disorder.8 These findings have been cited as support for the low-level response theory articulated by Schuckit,17 which posits that less intense alcohol responses may be associated with insensitivity to internal cues and warning signs to stop drinking, resulting in excessive consumption. This seminal work supports the idea that responses to an acute dose of alcohol may predict future drinking behaviors, above and beyond other personal risk factors such as FH.
However, there is also evidence inconsistent with the low-level response theory, suggesting instead that individuals with a FH of alcoholism or frequent heavy drinking experience greater alcohol-induced positive-like subjective and objective effects.7,18–25 To resolve this apparent inconsistency, Newlin and Thomson6 proposed the differentiator model, which posits that high-risk persons have greater stimulant-like effects from alcohol during the early portion of a drinking episode, when breath alcohol concentrations (BrAC) are rising, and less sedative-like effects when BrAC are decreasing. Thus, the low-level response theory proposed by Schuckit17 may be based primarily on sedative or impairing effects during descending BrAC. Although there has been initial support for the differentiator model7 and although stimulant responses to alcohol may lead to greater acute alcohol consumption,26–30 thus far studies have been limited by modest sample sizes and cross-sectional designs. In fact, the only longitudinal study examining the role of acute alcohol responses to future drinking problems was conducted more than 15 years ago by Schuckit and Smith,8 and the alcohol responses measured were primarily negative intoxicating effects.
Besides FH,9,31 there are other risk factors for the development of alcohol use disorders, including early age onset of drinking or first intoxication1,32,33 and frequent early adult “binge” drinking34—ie, consuming 5 or more drinks on 1 occasion for men, 4 or more for women.35 In terms of the latter, habitual binge drinking during adolescence and young adulthood is highly prevalent in the United States and represents a hazardous and harmful behavior in and of itself.3,36–38 For reasons that are not understood, some young adult binge drinkers progress to become problem drinkers, whereas others reduce their consumption during middle and later adulthood.39–46 Among a sample of primarily young, white, male alcohol abusers, Hasin and colleagues47 showed that several years after an initial assessment, 46% remitted and no longer met criteria for an alcohol use disorder, whereas 24% continued with alcohol abuse and 30% progressed to alcohol dependence. Preliminary cross-sectional studies19–21 have shown that young adult binge drinkers exhibit quantitatively and qualitatively different alcohol responses than lighter drinkers. However, a key unanswered question is whether positively or negatively valenced alcohol responses predict subsequent drinking trajectories and alcohol problems over time.
The present study was a within-subject, double-blind, placebo-controlled, dose-ranging alcohol challenge procedure followed by a longitudinal follow-up of alcohol drinking behaviors in high-risk young adult heavy social drinkers compared with controls who were light social drinkers. The first goal was to determine whether the groups differed on subjective and objective responses to alcohol using measures sensitive to acute positive and sedative effects across the BrAC curve. The second goal was to determine whether acute alcohol responses predicted subsequent drinking patterns and alcohol-related diagnoses during a 2-year follow-up interval in each of the groups. This design provided a systematic evaluation of the low-level response model and differentiator model in the etiology of alcohol use disorders.
METHODS
PARTICIPANTS
The Chicago Social Drinking Project was approved by The University of Chicago Institutional Review Board, and all participants provided written informed consent. Of the initial 196 participants who attended at least 1 laboratory session, 190 completed all 3 sessions and therefore comprised the sample for the study. Laboratory sessions were conducted from March 2004 to July 2006, with all follow-ups completed by July 2008. Inclusion criteria were age 21 to 35 years; a body mass index (calculated as weight in kilograms divided by height in meters squared) of 19 to 30; good general health with no current or past major medical or psychiatric disorders, including alcohol and substance dependence; and no current medications that would interact with study procedures. Participants were selected to belong to 1 of 2 distinct drinking groups on the basis of established guidelines for heavy/binge and light drinking3,35,48 and to be consistent with prior studies.21,49–51 These groups were heavy drinkers (HD; n=104 [44 women]) and light drinkers (LD; n=86 [41 women]). Criteria for the HD group were (1) consuming at least 10 but no more than 40 standard alcoholic drinks per week and (2) engaging in regular binge drinking, defined as consuming 5 or more drinks on an occasion (≥4 for women) 1 to 5 times on average per week as their predominant adult pattern. Criteria for the LD group were (1) consuming at least 1 but no more than 5 standard alcoholic drinks per week and (2) engaging in infrequent binge drinking—ie, 5 or fewer times per year, but allowing for 1 past interval of a maximum of 6 months’ duration of up to twice-weekly binge drinking (ie, typically during college-aged years). All attempts were made to match the groups on age as well as sex and race.
SCREENING AND EXPERIMENTAL PROCEDURES
Participants were recruited through advertisements in local media, on Internet Web sites, by flyers, and by word-of-mouth referrals. In-person screening included administration of the Alcohol Quantity-Frequency52 and Timeline Follow-Back53,54 interviews to assess alcohol drinking patterns during the past 3 to 6 months. In addition, the screening portion included self-report questionnaires, including the Alcohol Use Disorders Identification Test,55,56 the Beck Depression Inventory,57 and the Spielberger State-Trait Anxiety Inventory.58 Participants took part in a modified Structured Clinical Interview for DSM-IV Axis 1 Disorders–Patient Edition59 that included the screening module and specific modules for mood episodes and substance use disorders, as well as a brief physical examination and urine and blood testing. Exclusion criteria were history of major Axis I psychiatric conditions, including alcohol or substance dependence (excluding nicotine), abnormal blood chemistry or hepatic panel results (≥2 SD above the mean), positive breathalyzer or urine toxicology screen (cocaine, opiates, benzodiazepines, amphetamines, barbiturates, and phencyclidine), or positive pregnancy test. Recreational marijuana use was allowed, provided it was used no more than 3 times weekly. Participants also completed a 2-generational FH tree for alcohol use disorders and an interview of FH Research Diagnostic Criteria for drinking consequences.60 The term FH positive was defined as having at least 1 primary biological relative, or 2 or more secondary biological relatives, with an alcohol use disorder, and FH negative was defined as having no biological relatives in the past 2 generations with an alcohol use disorder. The 45 participants with either uncertain or intermediate FH were not included in analyses pertaining to FH. Participants also completed the Sensation Seeking Scale–Form V.61 As in other studies,62 we modified the disinhibition subscale to remove 3 of the 10 items pertaining to behavior associated with excessive alcohol drinking or partying.
PHASE 1: LABORATORY SESSIONS
The first phase of the study consisted of 3 randomized 5-hour laboratory sessions, each beginning between 3 and 5 PM. Subjects were tested individually, and sessions were conducted in a comfortable room and separated by at least 48 hours. Women were examined regardless of menstrual cycle phase because acute alcohol responses do not vary by menstrual phase.63 To reduce potential alcohol expectancies, participants were told that they might receive a beverage containing alcohol, a stimulant, a sedative, a placebo, or a combination of these substances. Participants were randomized to dose order and tested under double-blind conditions. In each session, they ingested a beverage containing placebo (0.0 g/kg; 1% volume of ethanol as taste mask), a low alcohol dose (0.4 g/kg), or a high alcohol dose (0.8 g/kg). The beverage was administered in clear plastic-lidded cups in 2 equal portions that were each consumed during a 5-minute interval and separated by a 5-minute interim rest. The beverages contained 190-proof ethanol prepared with water, flavored drink mix, and a sucralose-based sugar substitute. The mean total beverage volume was 471 mL (range, 327–668 mL), and doses for women were 85% of those of men to adjust for sex differences in total body water.64,65
Upon arrival, the participant completed a questionnaire to confirm compliance with 48-hour drug and alcohol abstinence instructions and 3-hour abstinence from food, caffeine, and smoking. Participants underwent several objective tests to verify compliance with pretesting instructions; these tests included carbon monoxide (<10 ppm) and alcohol breathalyzer tests and, before at least 1 session, a urine toxicology screen. Women also undertook a urinary human chorionic gonadotropin test to verify nonpregnancy before each session. After these tests, the participant consumed a low-fat snack at 20% of daily kilocalorie needs, based on a macronutrient distribution of 55% of kilocalories from carbohydrates, 10% from protein, and 35% from fat.66
Forty-five minutes after arrival, the participant completed baseline subjective and objective measures and then consumed his or her study beverage over 15 minutes (5 minutes for each portion separated by a 5-minute rest interval) in the presence of the research assistant.21,50,67 Subjective and objective measures were repeated during the ascending limb to the estimated peak BrAC (30 and 60 minutes, respectively, after beverage initiation) and the descending limb and alcohol elimination phase (120 and 180 minutes). The breathalyzer (Alco-Sensor IV; Intoximeter, St Louis, Missouri) was programmed to display readings of 0.000 mg/dL, with the actual levels later downloaded to a computer by a separate assistant. Between time points, to circumvent potential boredom, the participant was permitted to view movies or read magazines provided by the study in a comfortable, living room–like laboratory testing room. At the end of each session, when the BrAC was 0.04 mg/L (0.04%) or lower, the participant was transported home by a car service to ensure safety. At the end of the third session, the participant was debriefed and received instructions and schedule information for the follow-up phase. Participants received a $200 check for participation in the first phase ($50 per session and a $50 bonus for completing all 3 sessions).
SUBJECTIVE EFFECTS
Repeated assessments of subjective states by the Biphasic Alcohol Effects Scale68 and the Drug Effects Questionnaire69 comprised the main dependent subjective measures. The Biphasic Alcohol Effects Scale has shown good reliability and validity and yields 2 subscales, stimulation and sedation, shown to correspond to alcohol effects often observed on ascending and descending BrAC, respectively.68 As validated in a previous report,70 we modified the original Biphasic Alcohol Effects Scale instructions so as not to divulge beverage contents, and we administered the scale at predrink baseline. The Drug Effects Questionnaire consisted of 100-mm visual analog scales for the following postbeverage ratings: feel drug: “do you feel any drug effects?”; like: “do you like the effects you are feeling now?”; and want more: “would you like more of what you consumed, right now?”
OBJECTIVE EFFECTS
Saliva samples for cortisol determination were also collected at each time point using a cotton Salivette (Sarstedt AG & Company, Nümbrecht, Germany). Samples were stored in a −80°C freezer and assayed using a high-sensitivity enzyme immunoassay kit that was standardized and validated at The University of Chicago Clinical Research Center Core Laboratory. The inter- and intra-assay coefficients of variation were 6.88% and 7.12%, respectively. Other measures (eg, psychomotor performance and eye movements) were also obtained in the sessions, and these data are reported elsewhere.67,71–74
PHASE 2: FOLLOW-UP
The second phase of the study was a 2-year follow-up interval. After completion of the laboratory phase, participants were scheduled for 8 quarterly follow-ups to assess alcohol use patterns. The main dependent measure was frequency of binge drinking, defined as the percentage of days in the past month when the participant consumed at least 5 alcoholic drinks (4 for women). Binge frequency and other drinking variables (typical quantity, frequency of any drinking, and maximum quantity) were based on the Timeline Follow-Back for the 4 previous Monday-to-Sunday weeks. At 12 and 24 months, the Alcohol Use Disorders Identification Test, the Drinker Inventory of Consequences–Recent,75 and Structured Clinical Interview for DSM-IV Axis 1 Disorders–Patient Edition modules for past-year alcohol abuse and dependence were also administered. Participants were compensated with $10 gift cards for each quarterly follow-up and $40 gift cards for the annual follow-ups. They also received $10 to $20 gift card incentives if they completed their follow-ups in a timely manner.
STATISTICAL ANALYSIS
Demographic and drinking characteristics for the groups were examined by t tests and χ2 tests, as appropriate. Experimental session data raw scores were analyzed by a series of 2 group×3 dose×5 time repeated-measures analyses of variance. Analyses were then repeated controlling for sociodemographic risk factors associated with alcohol misuse, including sex, race (white vs nonwhite), FH (positive or negative), educational level, and disinhibited personality.36,39,43,46,76–78
To examine the relationship of subjective alcohol response (stimulation, like, want more, and sedation) to future drinking, change scores for these 4 responses were calculated as follows: (high dose [60-minute–baseline] – placebo [60-minute – baseline]). The main calculations were based on scores at 60 minutes (peak BrAC) and for the high dose because this time point and dose level produced consistent changes on study measures and represent clinically relevant BrAC levels, ie, the US legal limit for intoxicated driving. Change scores for the low dose at peak BrAC were also examined to determine whether there were threshold effects. In addition, change scores for ascending (15-minute) and descending (120-minute) BrAC limbs were used to examine the differentiator model in detail. For objective alcohol response (cortisol), the 180-minute time point was used in the change score calculation because this interval corresponded with the peak cortisol levels and the delay in detecting levels in saliva.79
Participants were classified into different drinking trajectory groups on the basis of their frequency of binge drinking during the follow-up interval, using a discrete mixture modeling approach, with the number of trajectory groups determined by the Bayesian Information Criterion for model selection.80 Linear trend analyses81 were conducted separately for the light drinking and heavy drinking groups to examine the relationship of alcohol response change scores to the drinking trajectory groups. These analyses controlled for the main sociodemographic risk factors associated with alcohol misuse by including them as covariates. Finally, the association of each participant’s alcohol responses to quarterly frequency of subsequent binge drinking was examined using a generalized estimation equations modeling approach82 with alcohol response score, measurement time, and their interaction as covariates. The analysis used a logit link function because of the binomial distribution of binge drinking frequency. Composite generalized estimation equations models were then conducted to examine the effects of each response after controlling for the other significant responses. Given the low frequency of binge drinking among LD, generalized estimation equations analysis using any drinking frequency as the outcome was also conducted. Finally, mediational analysis83 was conducted to examine whether the binge drinking pattern during follow-up mediated the relationship between the alcohol responses and DSM-IV alcohol use disorder diagnoses (alcohol abuse and dependence).
RESULTS
OVERVIEW
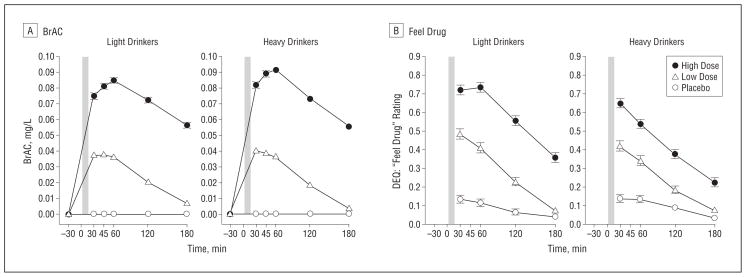
The groups were similar on most demographic characteristics (Table 1). As expected, the HD were higher than the LD on alcohol drinking and related variables, but liver enzyme levels did not differ between groups. The HD also had fewer years of education and higher disinhibited personality trait scores than the LD, both of which were included as covariates in all analyses. In the laboratory sessions, the BrAC changes over time confirmed the expected pattern of ascending and descending limbs. Although the HD exhibited higher BrAC at the high dose than did the LD (group×dose×time: F5,940=3.43; P<.01; Figure 1A), the difference between groups was small (range, 0.001–0.008 mg/L at various time points), and covarying for BrAC did not alter the main findings.
Table 1.
Participant Characteristics
| Baseline General Characteristic | Drinkersa
|
|
|---|---|---|
| Light (n=86) | Heavy (n=104) | |
| Age, y | 26.08 (0.37) | 25.28 (0.30) |
| Educational level, y | 16.66 (0.22) | 15.70 (0.14)g |
| White race, No. (%)b | 56 (65.1) | 80 (77.9) |
| Male sex, No. (%) | 44 (51.2) | 61 (58.7) |
| Spielberger trait anxiety (T score) | 44.67 (0.71) | 45.05 (0.70) |
| Beck Depression Inventory | 2.17 (0.28) | 2.87 (0.29) |
| Disinhibition scalec | 3.28 (0.21) | 4.95 (0.14)g |
| Family history positive for alcohol use disorder, No. (%)d | 32 (37.2) | 43 (41.3) |
| Baseline alcohol-related variablese | ||
| Age at first alcoholic drink, y | 17.47 (0.26) | 15.00 (0.23)h |
| AUDIT score | 3.27 (0.13) | 11.60 (0.36)g |
| No. of drinking days per month | 6.41 (0.34) | 14.22 (0.52)g |
| No. of standard drinks per drinking day | 1.69 (0.05) | 5.44 (0.32)g |
| No. of binge days per monthf | 0.12 (0.03) | 7.90 (0.33)g |
| Maximum No. of drinks consumed on 1 occasion | 2.66 (0.12) | 9.96 (0.45)g |
| Baseline liver enzyme levels, U/L | ||
| AST | 21.4 (0.63) | 23.1 (1.05) |
| ALT | 20.7 (1.34) | 22.6 (1.80) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorders Identification Test.
SI conversion factor: To convert AST and ALT to microkatal per liter, multiply by 0.0167.
Data are given as mean (SEM) unless otherwise noted.
Race was provided by participants among a list of options consistent with National Institutes of Health classifications.
Disinhibition from the Sensation Seeking Scale, modified by removing the 3 (of 10) items that pertain specifically to alcohol.
Defined as having 1 biological primary or 2 or more biological secondary relatives with an alcohol use disorder.
Drink based on standard definition of 1 drink equaling 12 oz of beer, 5 oz of wine, or 1.5 oz of liquor, and monthly average taken from Timeline Follow-Back Interview for the month preceding study enrollment.
Defined as 5 or more drinks per occasion for men and 4 or more drinks for women.
P < .01.
P < .001.
Figure 1.
Heavy and light drinkers’ breath alcohol concentrations (BrAC) (A) and Drug Effects Questionnaire (DEQ; range, 0.0–1.0) feel drug ratings (B) during the sessions. For feel drug, light drinkers had higher ratings during the high dose than did heavy drinkers at all time points; P<.001. The shaded bar indicates the alcohol drinking interval from time 0 to 15 minutes. Error bars indicate standard error.
ALCOHOL RESPONSE: HD VS LD
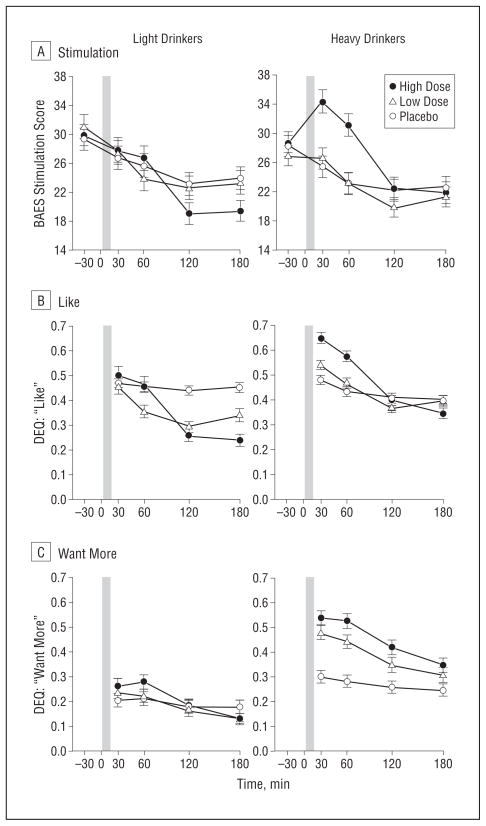
Alcohol dose-dependently increased self-report ratings of feel drug (Figure 1B), with the LD more sensitive than the HD at the high dose of alcohol (group×dose×time: F6,1128=4.57; P<.001). However, the groups differed markedly in the nature of their alcohol responses. The HD reported greater positive and rewarding subjective effects of alcohol than the LD, particularly at the high dose (Figure2A–C). High-dose alcohol significantly increased Biphasic Alcohol Effects Scale stimulation ratings in the HD vs the LD during the early, ascending limb of the BrAC (group×dose×time:F8,1504=3.21; P=.001)and both like and want more ratings throughout both limbs (group×dose: like, F2,376=15.68; P<.001; and want more, F2,376=12.04; P<.001). Furthermore, the low dose of alcohol also increased ratings for like and want more in the HD vs the LD(Figure2).
Figure 2.
Heavy and light drinkers’ subjective stimulant and rewarding responses, including the Biphasic Alcohol Effects Scale (BAES; range, 10–70) stimulation (A) and the Drug Effects Questionnaire (DEQ; range, 0.0–1.0) for like (B) and want more (C) during the sessions. Post hoc analysis of a significant group×dose×time interaction revealed that for stimulation at the high dose, heavy drinkers had higher ratings than light drinkers at 30 minutes (P<.05) and 60 minutes (P<.06). Post hoc analysis of a significant group×dose interaction for like at the high dose revealed that heavy drinkers had higher ratings than light drinkers at 30, 60, and 120 minutes (P<.01 for all), and a significant group×dose interaction for want more at the high dose and low dose revealed that heavy drinkers had higher ratings than light drinkers at all time points (P<.001 for all). The shaded bar indicates the alcohol drinking interval from time 0 to 15 minutes. Error bars indicate standard error.
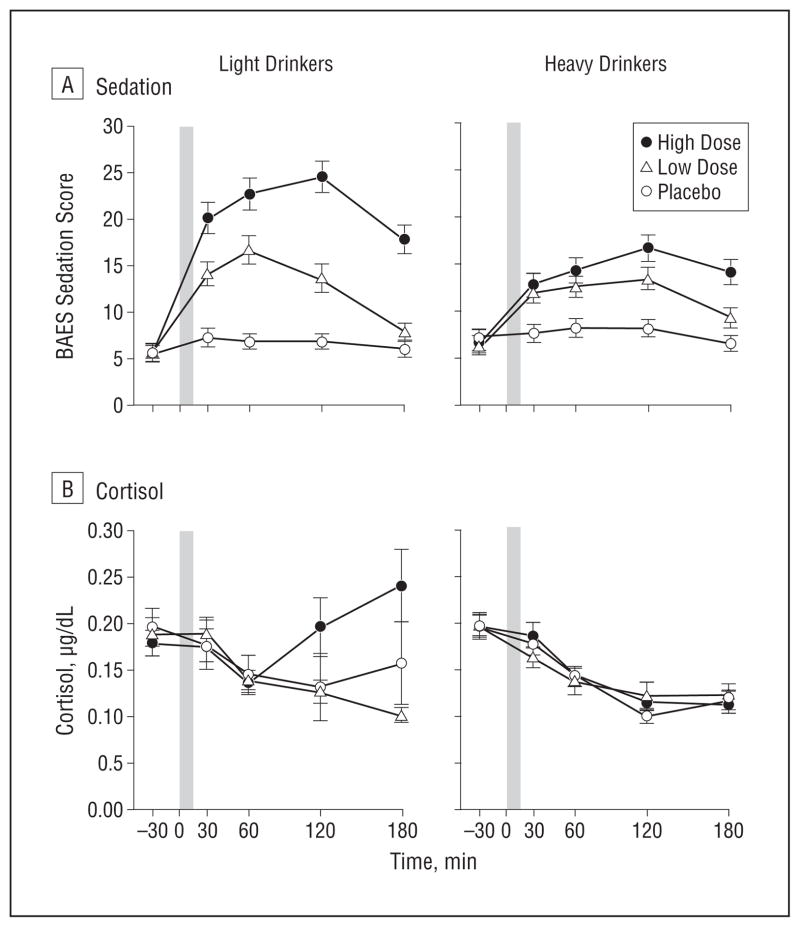
Increases in Biphasic Alcohol Effects Scale sedation were noted in both groups after alcohol consumption, with the LD reporting higher sedation than the HD throughout both BrAC limbs after the high dose (group×dose×time: F8,1504=4.87; P <.001; Figure 3A). Sedation also increased after the low dose, albeit to a lesser extent than with the high dose, with higher ratings in the LD vs the HD at peak BrAC. Increases in salivary cortisol levels were evident in the LD during the descending limb after the high dose, but there were no cortisol increases in the HD (group×dose×time: F8,1504 =6.03; P<.001; Figure 3B). Session order did not affect the main results (F ≥ 3.20 for all; P < .001 for all). The magnitude of the group differences on alcohol response change scores was moderate, with effect sizes computed by Cohen’s dequaling0.47 for stimulation, 0.46 for like, 0.53 for want more, 0.53 for sedation, and 0.36 for cortisol.
Figure 3.
Heavy and light drinkers’ Biphasic Alcohol Effects Scale (BAES; range, 10–70) sedation (A) and salivary cortisol (B) responses during the sessions. Post hoc analysis of a significant group×dose×time interaction revealed that for sedation at the high alcohol dose, light drinkers had higher ratings than heavy drinkers at 30, 60, and 120 minutes (P <.001 for all) and at the low dose, light drinkers had higher ratings than heavy drinkers at 60 minutes (P<.05). Post hoc analysis of a significant group×dose×time interaction for cortisol at the high dose revealed that the light drinkers had higher levels than heavy drinkers at 120 and 180 minutes (P<.01 for all). The shaded bar indicates the alcohol drinking interval from time 0 to 15 minutes. Error bars indicate standard error. (To convert cortisol to nanomoles per liter, multiply by 27.588).
The aforementioned differences between the HD and the LD on stimulation, like, want more, sedation, and cortisol remained significant after controlling for sex, race, educational level, FH, and disinhibited personality. There were no significant effects of FH of alcoholism (F ≤ 1.27 for all; P ≥ .27 for all) or interactions of FH and drinking group (F ≤ 1.25 for all; P ≥.26 for all) or FH and sex (F ≤ 0.93 for all; P ≥.47 for all) on alcohol response measures. Exploratory analyses examining a narrower criterion for FH—ie, men with alcoholic fathers (n=14) vs men with negative FH (n=44)—also were not significant for most alcohol response measures, including stimulation, sedation, like, and cortisol (F ≤ 1.06 for all; P > .39 for all). However, there was a trend for an interaction in these men in ratings for want more (FH×dose×time, F=2.03, P=.06), which was driven by higher ratings during placebo for men with positive FH vs men with negative FH.
DRINKING BEHAVIORS DURING THE FOLLOW-UP INTERVAL
Retention rates were very high in this study, with 1506 of a possible 1520 quarterly follow-ups (99.1%) completed. One male LD withdrew from the study without specifying the reason during the second year of follow-up. No other participants withdrew or were lost to contact during the 2-year interval. We imputed missing data (0.9%) by taking the average of values from the 2 surrounding follow-ups or the last observation carried forward, as warranted. During the 2 years of follow-up, drinking rates remained significantly different between the groups: LD consumed alcohol on 23% (range, 2%–62%) of days, whereas the HD consumed alcohol on 46% (range, 9%–89%) of days (P<.001), and LD engaged in binge drinking on 1.4% (range, 0%–8%) of days compared with 26% (range, 3%–54%) of days for HD (P<.001).
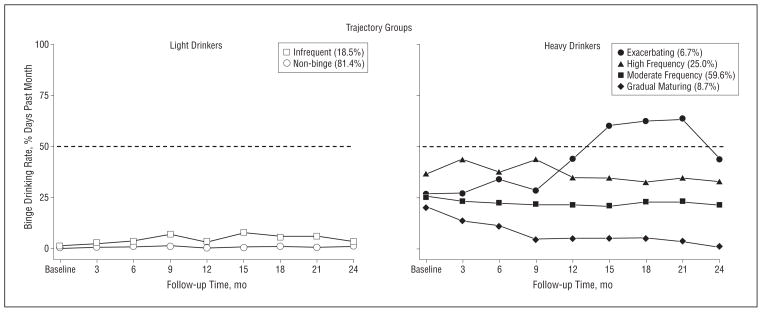
Quarterly follow-ups revealed that 4 trajectory patterns best described the binge drinking patterns among HD and 2 patterns best described the LD (Table 2). Figure 4 depicts the 2 trajectory groups for LD: non-binge (81.4%) and infrequent binge (18.6%) and, consistent with other studies,46,84–87 the 4 trajectory groups for HD: exacerbating binge (6.7%), high-frequency binge (25.0%), moderate-frequency binge (59.6%), and gradual maturing binge (8.7%). The distinction of these trajectory groups was supported by other alcohol-related outcomes during follow-up, including typical and maximal drinking quantity, alcohol-related consequences, and DSM-IV diagnoses of alcohol abuse and dependence (Table 3).
Table 2.
Bayesian Information Criterion (BIC) and Model Selection
| No. of Trajectory Groups | Drinkers
|
|||||
|---|---|---|---|---|---|---|
| Light (n=86)
|
Heavy (n=104)
|
|||||
| BIC | Null Modela | 2ln(B10)b | BIC | Null Modela | 2ln(B10)b | |
| 1 | −683 | −2729 | ||||
| 2 | −661 | 1 | 44 | −2663 | 1 | 132 |
| 3 | −669 | 2 | −17 | −2642 | 2 | 42 |
| 4 | −681 | 3 | −22 | −2632 | 3 | 20 |
| 5 | −697 | 4 | −33 | −2632 | 4 | 0 |
| 6 | −707 | 5 | −20 | −2646 | 5 | −28 |
The number of trajectory groups in the comparison model.
ln(B10) ≈ 2ΔBIC, ie, twice the difference in BIC between the 2 models, was used to determine the best model to describe quarterly frequency of binge drinking. Using 2ln(B10) ≥6 as a strong evidence for rejecting the null model,80 a 4-trajectory group model in heavy drinkers and a 2-trajectory group model in light drinkers best described the data (indicated in bold).
Figure 4.
Trajectory groups during the 2-year follow-up. Trajectory data were based on the self-reported frequency of past-month binge drinking days obtained at each quarterly interval. A discrete mixture modeling approach determined by the Bayesian Information Criterion for model selection revealed a 2-trajectory group (nonbinge and infrequent binge) model for the light drinkers and a 4-trajectory group (gradual maturing, moderate-frequency binge, high-frequency binge, and exacerbating) model for the heavy drinkers best described the data.
Table 3.
Alcohol Use Disorder Diagnoses and Other Drinking Characteristics During the 2-Year Follow-up Among the Trajectory Groupsa
| DSM-IV Diagnosesb | Light Drinker Trajectory Group
|
Heavy Drinker Trajectory Group
|
||||||
|---|---|---|---|---|---|---|---|---|
| Nonbinge (81.4%) |
Infrequent Binge (18.6%) |
P Value | Gradual Maturing (8.7%) |
Moderate Frequency (59.6%) |
High Frequency (25.0%) |
Exacerbating Binge (6.7%) |
P Value | |
| Alcohol abuse | 6 | 25 | <.05 | 0 | 63 | 81 | 86 | <.001 |
| Alcohol dependence | 0 | 0 | NS | 11 | 8 | 31 | 57 | <.001 |
| Alcohol drinking behaviorsc | ||||||||
| Dr-InC 2R score (total) | 5.16 (0.73) | 7.81 (1.25) | NS | 7.67 (1.98) | 19.44 (1.00) | 24.85 (2.19) | 33.00 (4.23) | <.001 |
| AUDIT score | 3.47 (0.24) | 5.50 (0.53) | <.001 | 4.89 (0.48) | 11.81 (0.42) | 15.50 (0.70) | 21.29 (1.08) | <.001 |
| Average No. of drinking days per month | 5.89 (0.43) | 8.85 (0.84) | <.01 | 7.13 (0.99) | 12.32 (0.49) | 14.33 (0.64) | 19.07 (1.47) | <.001 |
| Average No. of drinks per drinking day | 1.80 (0.08) | 2.58 (0.21) | <.001 | 2.67 (0.25) | 4.91 (0.21) | 5.80 (0.36) | 5.81 (0.53) | <.001 |
| Average No. of binge days per month | 0.19 (0.03) | 1.35 (0.14) | <.001 | 1.76 (0.33) | 6.27 (0.18) | 10.44 (0.25) | 12.79 (0.67) | <.001 |
| Maximum No. of drinks consumed on 1 occasion | 2.69 (0.11) | 4.98 (0.11) | <.001 | 4.81 (0.68) | 9.28 (0.33) | 11.63 (0.69) | 14.25 (1.02) | <.001 |
Abbreviations: AUDIT, Alcohol Use Disorder Identification Test; Dr-InC 2R, Drinker Inventory of Consequences–Recent.
Data are given as mean (SEM) or percentage.
DSM-IV diagnoses derived from annual Structured Clinical Interview for DSM-IV Axis 1 disorders and coded positive if criteria were met during either 12- or 24-month follow-up.
Dr-InC 2R and AUDIT are average maximum score at 12- or 24-month follow-up; number of alcohol drinking days, number of drinks per drinking day, number of binge days per month, and maximum number of drinks were the average of all 8 quarterly follow-ups. P values determined by χ2 or Fisher exact test for DSM-IV diagnoses and by linear trend analysis for alcohol drinking behaviors.
RELATIONSHIP OF ALCOHOL RESPONSE TO SUBSEQUENT DRINKING BEHAVIORS
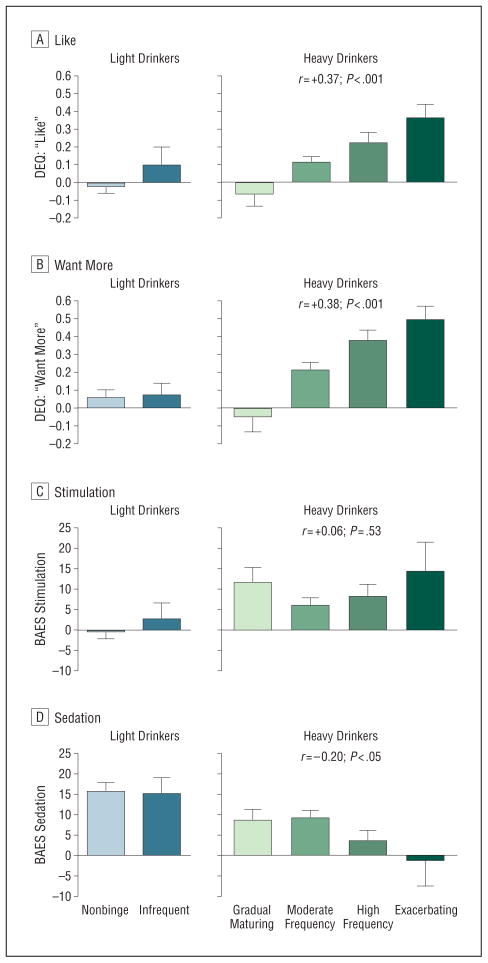
In LD, none of the alcohol response measures (peak BrAC change scores at high dose or low dose) predicted trajectory group, binge, or any drinking outcome during the follow-up. For HD at the high dose, greater ratings of like(r=+0.37; P<.001) and want more (r=+0.38; P < .001) and lower ratings of sedation(r=−0.20;P<.05)were significantly linearly associated with subsequent drinking trajectory group, indicating an overall moderate effect (Figure 5). Ratings at the low alcohol dose for like(r=+0.24;P<.05) and want more (r=+0.23; P<.05) were also predictive of drinking trajectory group, but the association was weaker than for the high dose. Cortisol response for either high or low dose did not predict drinking behavior.
Figure 5.
Heavy and light drinkers’ trajectory groups’ initial alcohol responses, including the Drug Effects Questionnaire (DEQ; range, 0.0–1.0) for like (A) and want more (B) and the Biphasic Alcohol Effects Scale (BAES; range, 10–70) for stimulation (C) and sedation (D). Alcohol responses are indicated as the mean for each trajectory group based on participants’ change score: high dose (60 minutes - baseline) – placebo (60 minutes -baseline). In light drinkers, there were no significant associations of alcohol response to trajectory group. In heavy drinkers, higher ratings for like (P <.001) and want more (P <.001) and lower ratings for sedation (P <.05) were significantly linearly associated with drinking trajectory group. Error bars indicate standard error.
Analyses using the generalized estimation equations approach confirmed that in HD, greater alcohol stimulation, greater liking, greater wanting, and lower sedation at the high dose predicted greater binge drinking frequency during follow-up (P < .001 for all). Greater liking and wanting responses to the low dose of alcohol also related to future binge drinking, but again these were not as strong as those observed with the high dose. Composite generalized estimation equations models that included all the significant alcohol responses indicated that greater alcohol stimulation (P<.05), greater liking (P<.001), and lower sedation (P<.05)for high dose remained significant predictors of future drinking. These analyses were repeated for alcohol responses during the ascending (30-minute) and descending (120-minute) BrAC limbs. On the ascending limb, alcohol wanting (P<.001)was a significant predictor of future drinking, and on the descending limb, liking (P<.001), wanting (P<.01), and less sedation (P<.01) predicted future drinking. Although disinhibited personality and FH predicted future drinking in HD, controlling for these factors did not alter the relationships between alcohol responses and subsequent drinking behavior. For HD, increases in liking(odds ratio[OR]{SE},29.81{29.42};P=.001) and want more (OR [SE],7.43 [5.32]; P=.005) were significantly associated with alcohol use disorder diagnosis during follow-up. When binge drinking pattern was included in the model, the magnitude of association for alcohol response to alcohol use disorder was reduced (like: OR [SE], 14.64 [15.04]; P<.01; and want more: OR [SE],3.51 [2.64]; P=.10), suggesting that binge drinking partially (like) or fully (want more)mediated the relationship between alcohol response and subsequent alcohol use disorder.
COMMENT
We have replicated and extended our group’s prior preliminary findings20,21 demonstrating that HD report greater acute subjective positive effects and lesser sedative and cortisol response to an intoxicating dose of alcohol than do LD. Multivariate models in the HD revealed that both the positive (more stimulation, like, and want more) and negative (less sedation) response factors were significant independent predictors of binge drinking during the subsequent 2 years. These findings were observed even after taking into account other risk factors for problem drinking, such as sex, race, FH, educational level, and disinhibited personality.2,31 Thus, young adult HD who experience heightened stimulant and rewarding and diminished sedative effects of alcohol are at risk for escalation of binge drinking over time. They also consume more alcohol overall (typical frequency, quantity, and maximum quantity) and experience clinically relevant outcomes, such as greater alcohol consequences and higher rates of DSM-IV alcohol use disorders (Table 2).
Although the finding that heavier drinkers enjoy the effects of alcohol more than lighter drinkers seems intuitive, there has been limited evidence thus far to support this notion. Indeed, the prevailing model, the low-level response theory, posits that persons who experience a lower level of response to alcohol will engage in heavier drinking over time because they do not feel the internal cues of intoxication.88 Our data partially support this theory because HD exhibited reduced subjective sedative and cortisol responses after drinking, compared with the responses of LD, and lack of sedative responses predicted future drinking. However, alcohol markedly increased positive-like effects during ascending to peak BrAC in this group, and these responses also predicted future drinking. In contrast, LD experienced significant increases in sedation during the ascending limb that was sustained for several hours, without concomitant stimulating or rewarding effects. Collectively, these responses may serve as a protective factor underlying these drinkers’ ability to “put the brakes on” and limit their drinking. Taken together, the results indicate that the low-level response theory should be revised to include heightened sensitivity to rewarding and stimulating alcohol effects as equally important predictors as lack of sedative responses in the development and maintenance of problematic drinking among at-risk persons. Furthermore, we propose a modified differentiator model to focus on stimulant, rewarding, and sedative effects without connection to a specific BrAC limb or, conversely, to simplify the model and to focus on effects observed during peak BrAC.
The present study findings are also relevant to the incentive-sensitization theory of addiction.89–91 This theory posits that sensitization and crucial neuroadaptations within mesolimbic dopamine systems92,93 may underlie the motivational reward properties (wanting) of a drug, but not the hedonic reward value (liking) of the drug, in persons with substance dependence. However, in this study, we observed that among nondependent HD, both motivational and hedonic aspects of reward were associated with increased frequency of binge drinking over time. Interestingly, in composite prediction models, wanting more alcohol during the ascending and descending limbs of the BrAC curve predicted subsequent binge drinking during follow-up. We may speculate that early in a binge drinking episode, alcohol may elicit heightened desire for alcohol, leading to impaired control and continued drinking. As modeled in this acute administration paradigm, abrupt cessation of drinking results in a sharp decrease in stimulation and liking, with sustained wanting that may underlie desire for further drinking to offset reduction of hedonic effects. Although acute responses to the high alcohol dose producing peak BrACs of 0.08% to 0.09% were more informative than responses to the lower dose, future studies of higher alcohol doses or sustained drinking for a longer interval may help discern whether positive effects decrease (ie, acute tolerance), remain elevated, or increase over time. Such studies would offer an ecologically valid model of more extreme binge episodes. Indeed, among the highly frequent and exacerbating HD binge drinkers, maximal drinking amounts were from 11 to 14 drinks on an occasion (Table 3), resulting in BrACs that would likely be 2 to 3 times the legal limit for driving.
There were several strengths of this study, including the use of a within-subjects, dose-ranging, and placebo-controlled design with intensive follow-up. Responses were obtained at baseline and repeated at several junctures during the course of the BrAC curve, expectancies were minimized by blinding beverage content, and ecological validity was maintained by using a trained research assistant to interact with the participant in a quasi-social context, albeit a paradigm that did not completely mimic all aspects of social drinking. Drinking outcomes were validated by assessment of alcohol consequences and DSM-IV diagnoses. Finally, the results were observed within a diverse sample of both men and women of various racial backgrounds, which served to increase generalizability, in contrast to prior studies focusing on white male participants.8,18,23 This latter issue may be relevant in terms of the lack of systematic FH effects observed for acute subjective and objective alcohol responses, as reported by Brumback et al67 and Roche and King.74 Given that participants were enrolled regardless of FH, the subgroup of sons of alcoholic fathers was relatively small, potentially reducing statistical power to detect FH effects. However, positive FH did predict increases in binge drinking over time, and similar to prior research findings, alcohol responses were predictive of future drinking regardless of FH classification.8 Further research using a priori stratifications of both drinking pattern and FH may provide a more thorough examination of the relationship and possible interactive effects of these risk factors to alcohol response and future drinking behaviors.
The study was limited by the legal requirement that participants be a minimum of 21 years old to be given alcohol, so it is unclear whether the findings can be generalized to even younger drinkers. This is particularly important because the ages of 18 to 20 years represent the highest prevalence of binge drinking and alcohol use disorders.36 Second, participants’ average age was 25 years, which for many HD corresponds to 5 to 7 years of regular binge drinking. Therefore, it was not possible to determine whether alcohol-induced responses were constitutional or acquired with repeated exposure to alcohol. This question may be best addressed using animal models. Finally, although the quarterly follow-up assessments after the laboratory sessions were sufficiently frequent to show fluctuations in drinking for 2 years, the length of this interval may not have been adequate for the full range of drinking and related alcohol use disorders to emerge. To address this issue, we plan to continue to follow up these participants to examine their drinking patterns over a longer time.
In summary, we propose revisions to current theories of the role of alcohol response to subsequent drinking problems in at-risk individuals, and we set forth a modified differentiator model to focus on both positive (stimulant, as well as hedonic and motivational rewarding) and sedative alcohol effects either without ties to a specific BrAC limb, or simply at peak BrAC. Heavy drinkers experience markedly different responses to alcohol than do LD. For LD, an intoxicating dose of alcohol produces tired and sluggish feelings and activates release of the stress hormone cortisol. During a 2-year interval, few of them increased their drinking. In contrast, HD showed a variety of drinking trajectories during the 2 years of follow-up, and those experiencing less positive and more sedative acute alcohol effects gradually matured out of binge drinking over time. On the other hand, HD with heightened rewarding effects of alcohol perpetuated and increased binge drinking frequency over time, thereby increasing the likelihood of meeting DSM-IV diagnoses of alcohol abuse and dependence.
Acknowledgments
Funding/Support: This study was supported by grant R01-AA013746 from the National Institute on Alcohol Abuse and Alcoholism; grant P30-CA14599 from The University of Chicago Comprehensive Cancer Center; and grant UL1 RR024999 from the National Center for Research Resources, an institute of the National Institutes of Health.
Footnotes
Author Contributions: Drs King and Cao and Mr McNamara had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: None reported.
Additional Contributions: Appreciation is extended to Sean O’Connor, MD, Henry R. Kranzler, MD, and Stephanie O’Malley, PhD, for advice during this study and for their comments on an earlier version of the paper. Thanks also to Sandra Yu Rueger, PhD, for advice on technical writing and revisions; Constantine Trela, BA, for administrative and editorial assistance; and Royce Lee, MD, for medical supervision; the staff of the Clinical and Translational Service Awards at the General Clinical Research Center; and Irene Tobis, PhD, Curt Van Riper, BS, Alyssa Epstein, PhD, Adrienne Dellinger, BS, Lauren Kemp, BS, Ty Brumback, MS, and Megan Conrad, BS, for their role in data collection and database management.
References
- 1.Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. Br J Addict. 1992;87(8):1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- 2.Naimi TS, Brewer RD, Mokdad A, Denny C, Serdula MK, Marks JS. Binge drinking among US adults. JAMA. 2003;289(1):70–75. doi: 10.1001/jama.289.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Wechsler H, Lee JE, Kuo M, Seibring M, Nelson TF, Lee H. Trends in college binge drinking during a period of increased prevention efforts: findings from 4 Harvard School of Public Health College Alcohol Study surveys: 1993–2001. J Am Coll Health. 2002;50(5):203–217. doi: 10.1080/07448480209595713. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 5.Harwood H. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Bethesda, MD: National Institutes of Health; 2000. [Google Scholar]
- 6.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 7.Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34(2):199–202. doi: 10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53(3):202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 9.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38(8):861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Arch Gen Psychiatry. 1973;28(2):238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- 11.Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10(1):69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- 12.Hill SY, Steinhauer S, Lowers L, Locke J. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biol Psychiatry. 1995;37(11):823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- 13.Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41(9):879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- 14.Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiatry. 1985;42(4):375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- 15.Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44(11):942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- 16.Schuckit MA, Gold EO, Croot K, Finn P, Polich J. P300 latency after ethanol ingestion in sons of alcoholics and in controls. Biol Psychiatry. 1988;24(3):310–315. doi: 10.1016/0006-3223(88)90199-0. [DOI] [PubMed] [Google Scholar]
- 17.Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41(3):242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- 18.Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcohol-induced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacology (Berl) 2001;157(1):20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- 19.Erblich J, Earleywine M. Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits? Alcohol Clin Exp Res. 2003;27(1):44–50. doi: 10.1097/01.ALC.0000047300.46347.CE. [DOI] [PubMed] [Google Scholar]
- 20.Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24(6):789–794. [PubMed] [Google Scholar]
- 21.King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26(6):827–835. [PubMed] [Google Scholar]
- 22.Finn PR, Pihl RO. Men at high risk for alcoholism: the effect of alcohol on cardiovascular response to unavoidable shock. J Abnorm Psychol. 1987;96(3):230–236. doi: 10.1037//0021-843x.96.3.230. [DOI] [PubMed] [Google Scholar]
- 23.Gianoulakis C, Béliveau D, Angelogianni P, Meaney M, Thavundayil J, Tawar V, Dumas M. Different pituitary beta-endorphin and adrenal cortisol response to ethanol in individuals with high and low risk for future development of alcoholism. Life Sci. 1989;45(12):1097–1109. doi: 10.1016/0024-3205(89)90167-7. [DOI] [PubMed] [Google Scholar]
- 24.Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, LeMarquand DG, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcohol Clin Exp Res. 1996;20(9):1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- 25.Morean ME, Corbin WR. Subjective response to alcohol: a critical review of the literature. Alcohol Clin Exp Res. 2010;34(3):385–395. doi: 10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 26.Corbin WR, Gearhardt A, Fromme K. Stimulant alcohol effects prime within session drinking behavior. Psychopharmacology (Berl) 2008;197(2):327–337. doi: 10.1007/s00213-007-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWit H, Pierri J, Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology (Berl) 1989;98(1):113–119. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- 28.de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11(1):52–59. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- 29.Ray LA, Miranda R, Jr, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the μ-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119(1):115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wetherill RR, Fromme K. Subjective responses to alcohol prime event-specific alcohol consumption and predict blackouts and hangover. J Stud Alcohol Drugs. 2009;70(4):593–600. doi: 10.15288/jsad.2009.70.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. Br J Psychiatry. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- 32.Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- 33.Hingson R, Heeren T, Zakocs R, Winter M, Wechsler H. Age of first intoxication, heavy drinking, driving after drinking and risk of unintentional injury among US college students. J Stud Alcohol. 2003;64(1):23–31. doi: 10.15288/jsa.2003.64.23. [DOI] [PubMed] [Google Scholar]
- 34.McCarty CA, Ebel BE, Garrison MM, DiGiuseppe DL, Christakis DA, Rivara FP. Continuity of binge and harmful drinking from late adolescence to early adulthood. Pediatrics. 2004;114(3):714–719. doi: 10.1542/peds.2003-0864-L. [DOI] [PubMed] [Google Scholar]
- 35.SAMHSA. National Survey on Drug Use and Health. Rockville, MD: Office of Applied Studies; 2005. [Google Scholar]
- 36.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 37.Oesterle S, Hill KG, Hawkins JD, Guo J, Catalano RF, Abbott RD. Adolescent heavy episodic drinking trajectories and health in young adulthood. J Stud Alcohol. 2004;65(2):204–212. doi: 10.15288/jsa.2004.65.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slutske WS. Alcohol use disorders among US college students and their non-college-attending peers. Arch Gen Psychiatry. 2005;62(3):321–327. doi: 10.1001/archpsyc.62.3.321. [DOI] [PubMed] [Google Scholar]
- 39.Bennett ME, McCrady BS, Johnson V, Pandina RJ. Problem drinking from young adulthood to adulthood: patterns, predictors and outcomes. J Stud Alcohol. 1999;60(5):605–614. doi: 10.15288/jsa.1999.60.605. [DOI] [PubMed] [Google Scholar]
- 40.Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol. 2002;70(1):67–78. [PubMed] [Google Scholar]
- 41.Dawson DA, Grant BF, Stinson FS, Chou PS. Toward the attainment of low-risk drinking goals: a 10-year progress report. Alcohol Clin Exp Res. 2004;28(9):1371–1378. doi: 10.1097/01.alc.0000139811.24455.3e. [DOI] [PubMed] [Google Scholar]
- 42.Donovan JE, Jessor R, Jessor L. Problem drinking in adolescence and young adulthood: a follow-up study. J Stud Alcohol. 1983;44(1):109–137. doi: 10.15288/jsa.1983.44.109. [DOI] [PubMed] [Google Scholar]
- 43.Gotham HJ, Sher KJ, Wood PK. Predicting stability and change in frequency of intoxication from the college years to beyond: individual-difference and role transition variables. J Abnorm Psychol. 1997;106(4):619–629. doi: 10.1037//0021-843x.106.4.619. [DOI] [PubMed] [Google Scholar]
- 44.Grant BF, Harford TC, Grigson MB. Stability of alcohol consumption among youth: a National Longitudinal Survey. J Stud Alcohol. 1988;49(3):253–260. doi: 10.15288/jsa.1988.49.253. [DOI] [PubMed] [Google Scholar]
- 45.Gruenewald PJ, Russell M, Light J, Lipton R, Searles J, Johnson F, Trevisan M, Freudenheim J, Muti P, Carosella AM, Nochajski TH. One drink to a lifetime of drinking: temporal structures of drinking patterns. Alcohol Clin Exp Res. 2002;26(6):916–925. [PubMed] [Google Scholar]
- 46.Schulenberg J, O’Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: trajectories of frequent binge drinking during the transition to young adulthood. J Stud Alcohol. 1996;57(3):289–304. doi: 10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- 47.Hasin DS, Grant B, Endicott J. The natural history of alcohol abuse: implications for definitions of alcohol use disorders. Am J Psychiatry. 1990;147(11):1537–1541. doi: 10.1176/ajp.147.11.1537. [DOI] [PubMed] [Google Scholar]
- 48.Dawson DA. US low-risk drinking guidelines: an examination of four alternatives. Alcohol Clin Exp Res. 2000;24(12):1820–1829. [PubMed] [Google Scholar]
- 49.Esterlis I, Cosgrove KP, Petrakis IL, McKee SA, Bois F, Krantzler E, Stiklus SM, Perry EB, Tamagnan GD, Seibyl JP, Krystal JH, Staley JK. SPECT imaging of nicotinic acetylcholine receptors in nonsmoking heavy alcohol drinking individuals. Drug Alcohol Depend. 2010;108(1–2):146–150. doi: 10.1016/j.drugalcdep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29(4):547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- 51.McKee SA, Harrison EL, Shi J. Alcohol expectancy increases positive responses to cigarettes in young, escalating smokers. Psychopharmacology (Berl) 2010;210(3):355–364. doi: 10.1007/s00213-010-1831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cahalan D, Cisin IH, Crossley HM. American Drinking Practices: A National Study of Drinking Behavior and Patterns. New Brunswick, NJ: Rutgers Center of Alcohol Studies; 1969. [Google Scholar]
- 53.Sobell LC, Sobell MB. Alcohol Timeline Follow-Back Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 54.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979;17(2):157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 55.Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva, Switzerland: World Health Organization; 1989. Publication WHO/MNW/DAT/89.4. [Google Scholar]
- 56.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 57.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 58.Spielberger CD, Gorsuch RL, Luchene RE. Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 59.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 60.Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- 61.Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46(1):139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]
- 62.King AC, Byars JA. Alcohol-induced performance impairment in heavy episodic and light social drinkers. J Stud Alcohol. 2004;65(1):27–36. doi: 10.15288/jsa.2004.65.27. [DOI] [PubMed] [Google Scholar]
- 63.Holdstock L, de Wit H. Effects of ethanol at four phases of the menstrual cycle. Psychopharmacology (Berl) 2000;150(4):374–382. doi: 10.1007/s002130000461. [DOI] [PubMed] [Google Scholar]
- 64.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women: the role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med. 1990;322(2):95–99. doi: 10.1056/NEJM199001113220205. [DOI] [PubMed] [Google Scholar]
- 65.Sutker PB, Tabakoff B, Goist KC, Jr, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18(suppl 1):349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- 66.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(suppl 1):5–41. [PubMed] [Google Scholar]
- 67.Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy social drinkers. Drug Alcohol Depend. 2007;91(1):10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 69.Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71(3):269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- 70.Rueger SY, McNamara PJ, King AC. Expanding the utility of the Biphasic Alcohol Effects Scale (BAES) and initial psychometric support for the Brief-BAES (B-BAES) Alcohol Clin Exp Res. 2009;33(5):916–924. doi: 10.1111/j.1530-0277.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epstein AM, Sher TG, Young MA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology (Berl) 2007;190(3):321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- 72.King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59(3):203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 73.King A, Epstein A, Conrad M, McNamara P, Cao D. Sex differences in the relationship between alcohol-associated smoking urge and behavior: a pilot study. Am J Addict. 2008;17(5):347–353. doi: 10.1080/10550490802268140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roche DJO, King AC. Alcohol impairment of saccadic and smooth pursuit eye movements: impact of risk factors for alcohol dependence. Psychopharmacology. 2010;212:33–44. doi: 10.1007/s00213-010-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. Vol. 4. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1995. [Google Scholar]
- 76.Baer JS, Kivlahan DR, Marlatt GA. High-risk drinking across the transition from high school to college. Alcohol Clin Exp Res. 1995;19(1):54–61. doi: 10.1111/j.1530-0277.1995.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 77.Dawson DA, Grant BF, Stinson FS, Chou PS. Maturing out of alcohol dependence: the impact of transitional life events. J Stud Alcohol. 2006;67(2):195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- 78.Johnstone BM, Leino EV, Ager CR, Ferrer H, Fillmore KM. Determinants of life-course variation in the frequency of alcohol consumption: meta-analysis of studies from the Collaborative Alcohol-Related Longitudinal Project. J Stud Alcohol. 1996;57(5):494–506. doi: 10.15288/jsa.1996.57.494. [DOI] [PubMed] [Google Scholar]
- 79.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 80.Jones BL, Nagin DS, Roeder KA. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. [Google Scholar]
- 81.Agresti A. An Introduction to Categorical Data Analysis. New York, NY: John Wiley & Sons, Inc; 1996. [Google Scholar]
- 82.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 83.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 84.Casswell S, Pledger M, Pratap S. Trajectories of drinking from 18 to 26 years: identification and prediction. Addiction. 2002;97(11):1427–1437. doi: 10.1046/j.1360-0443.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 85.Greenbaum PE, Del Boca FK, Darkes J, Wang CP, Goldman MS. Variation in the drinking trajectories of freshmen college students. J Consult Clin Psychol. 2005;73(2):229–238. doi: 10.1037/0022-006X.73.2.229. [DOI] [PubMed] [Google Scholar]
- 86.Tucker JS, Orlando M, Ellickson PL. Patterns and correlates of binge drinking trajectories from early adolescence to young adulthood. Health Psychol. 2003;22(1):79–87. doi: 10.1037//0278-6133.22.1.79. [DOI] [PubMed] [Google Scholar]
- 87.Windle M, Mun EY, Windle RC. Adolescent-to-young adulthood heavy drinking trajectories and their prospective predictors. J Stud Alcohol. 2005;66(3):313–322. doi: 10.15288/jsa.2005.66.313. [DOI] [PubMed] [Google Scholar]
- 88.Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- 89.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 90.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 91.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 92.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 93.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]