Abstract
Aim of the study
The estimation of the upper reference limit (URL) for autoantibodies against thyroid peroxidase (TPOAbs) is a controversial issue, because of an uncertainty associated with the criteria used to correctly define the reference population. In addition, the URL of TPOAbs is method-dependent and often arbitrarily established in current laboratory practice. The aim of this study was to determine the reference limits of TPOAbs in a male sample according to the National Academy of Clinical Biochemistry (NACB) guidelines, and to compare them with those obtained in a female group, for five third-generation commercial-automated immunoassay (IMA) platforms.
Methods
120 healthy males and 120 healthy females with NACB-required characteristics (younger than 30 years, TSH between 0.5 and 2.0 mIU/L, normal thyroid ultrasound, absence of thyroid disease and absence of other autoimmune diseases) were studied. Sera were analyzed for TPOAbs concentration using five IMA methods applied in automated analyzers: Immulite 2000 XPi (IMM); Maglumi 2000 Plus (MAG); Kryptor Compact Plus (KRY); Phadia 250 (PHA) and Liaison XL (LIA).
Results
A statistically significant difference (p < 0.05) between medians in male and female groups was observed for PHA (2.6 and 3.1 IU/mL, respectively) but not for the other four methods. Scatter plots of TPOAbs values revealed a wide dispersion with very different coefficients of variation between the five methods, varying from 48.6 % for KRY in females to 126.3 % for MAG in females. The URLs differed in males and females according to the method: 28.7 and 29.0 IU/mL for IMM, 24.6 and 25.4 IU/mL for MAG, 6.4 and 6.9 IU/mL for KRY, 8.3 and 10.0 IU/mL for PHA and 14.2 and 17.9 IU/mL for LIA, respectively. Such URLs were lower than those stated by the manufacturers except for LIA in females. The difference between URLs ranged from a minimum of 11.3 % (LIA in males) to a maximum of 66.8 % (PHA in males).
Conclusions
Differences in URLs could result from the different coating preparations of the TPO antigen (purified native or recombinant) on solid phase, which affect the proper exposure of the immunodominant epitopes recognized by the polyclonal antibodies present in serum of patients with autoimmune thyroid disease (AITD). Based on these findings, we suggest to overcome the proposal of the NACB guidelines which recommend to involve a single group of young male subjects, and propose, instead, to utilize two distinct groups: one of males and one of females. This new proposal removes the apparent contrast of an all-male reference group for a disease (such as AITD) that affects mainly females. However, in spite of the harmonization among methods provided by the use of an international standard preparation, the wide dispersion of quantitative results still observed in this study suggests the need for further efforts to better understand the cause of these discrepancies, focusing on TPO antigen preparations as the possible source of variability among different assays.
Keywords: Autoimmune thyroid disease, Reference limits, Thyroid peroxidase autoantibodies, Automation, Immunoassay, Harmonization
Introduction
Autoantibodies against thyroid peroxidase (TPOAbs) are both diagnostic hallmarks and early indicators of autoimmune thyroid disease (AITD), playing an important predictive role in healthy subjects, in pregnant women and in high-risk patients [1–3].
In recent years, refinements in autoantigen preparation and better selection of polyclonal and monoclonal antibodies have led to a new (third) generation of automated quantitative immunoassays (IMAs) with improved sensitivity and specificity for the measurement of TPOAbs [4, 5]. Despite their recently expanded use in clinical laboratories [6–8], it is yet unclear whether these more-sensitive automated third-generation IMAs have made improvements in terms of diagnostic accuracy and harmonization compared with previous methods [9, 10]. Hence, further efforts in defining the threshold values of positivity need to be made in order to avoid misclassification of patients with AITD [9].
The estimation of the upper reference limit (URL) for TPOAbs is a controversial issue, because of an uncertainty as to the criteria for correctly defining the reference population. The currently proposed direct methods are described in the National Academy of Clinical Biochemistry (NACB) guidelines, which recommend the use of a reference group composed of 120 men with the following features: younger than 30 years, biochemically euthyroid (serum TSH between 0.5 and 2.0 mIU/L), without goiter as proven by ultrasound investigation, no personal/family history of either thyroid disease or of non-thyroid autoimmune diseases [11]. These guidelines are in themselves difficult to apply or standardize. Furthermore, it is perplexing, in that it requires a male reference group for diseases (AITD) that predominantly affect females [12]. Hence, in developing commercial methods for TPOAbs quantification, manufacturers are faced with difficulties in following the NACB recommendations. Consequently, results obtained by different researchers using different analytical methods show a wide variation in URLs [8–10, 13, 14]. In addition, the definition of reference intervals for TPOAbs has limitations due to the natural history of AITD, because a large number of individuals may have specific autoantibodies in serum 10–15 years before the onset of these diseases. To overcome this problem and avoid the risk of enlisting apparently healthy subjects who may bear TPOAbs, an indirect method for the definition of reference limits was proposed by some authors [12, 15]. Using this approach, the URL values were lower than those obtained by direct methods. Also, they displayed sex-dependent differences [15]. Nevertheless, the indirect method also has limitations, being applicable only to the single method used in the population sample considered in that particular study.
Taking into account that TPOAbs reference limits are method dependent and often arbitrarily established in the current laboratory practice [9, 10, 16], the aim of this study was to determine the URL of TPOAbs in a male sample according to the NACB guidelines, and to compare it with that obtained in a female group, applying and comparing five third-generation commercial automated IMA platforms.
Methods
120 males and 120 females with NACB-required characteristics (younger than 30 years, TSH between 0.5 and 2.0 mIU/L; normal thyroid ultrasound; absence of autoimmune and non-autoimmune thyroid disease and absence of other autoimmune diseases) were enrolled in the study. They came from a large health survey called ‘Thyroid takes to the square,’ carried out in the province of Verona (Italy) from 2008 to 2013, in which 7970 subjects with no previous or current thyroid disease symptoms were screened by clinical history, thyroid function test, and thyroid ultrasound. All participants gave their informed consent.
The sera of the 120 males and 120 females were analyzed for TPOAbs concentration using five IMA methods applied in automated analyzers: Immulite 2000 XPi (IMM) (Siemens Healthcare Diagnostics, Flanders, NJ, USA); Maglumi 2000 Plus (MAG) (Shenzen New Industries Biomedical Engineering-SNIBE, Shenzen, China); Kryptor Compact Plus (KRY) (BRAHMS Thermo Scientific, Henningsdorf, Germany); Phadia 250 (PHA) (Phadia AB, Uppsala, Sweden) and Liaison XL (LIA) (DiaSorin, Saluggia, Italy).
All assays were performed according to the manufacturers’ instructions at four different laboratories in Friuli Venezia Giulia region (Italy): Pordenone (IMM and MAG), S. Vito al Tagliamento (KRY), Tolmezzo (PHA), and Udine (LIA).
The main features of the five methods are shown in Table 1.
Table 1.
The main features of the five automated immunoassay methods, as declared by the manufacturers
| System | Method | Tracer | Antigen | Imprecision (%): intra-assay; between-assay | Imprecision (%): total | LoD (IU/mL) | LoQ (IU/mL) |
|---|---|---|---|---|---|---|---|
| IMM | CLIA | Luminescence: adamantyl dioxetane phosphate | Native TPO | 4.9–6.3; nr | 6.1–8.1 | 5.0 | nr |
| MAG | CLIA | Luminescence: N-(aminobutyl)-N-(ethyl)-isoluminol | Native TPO | 6.8–7.3; 7.4–7.6 | nr | 0.38 | nr |
| KRY | FIA | Fluorescence: europium cryptate/XL 665 | Native TPO | 2.1–7.6; 5.8–16.0 | nr | 1.8 | 9.0 |
| PHA | FEIA | Fluorescence: β-D-galactosidase; 4-methyl-umbelliferyl-β-D-galactoside | Recombinant TPO | 4.1–4.7; 2.5–4.8 | nr | nr | nr |
| LIA | CLIA | Luminescence: isoluminol derivative | Recombinant TPO | 3.6–6.2; 4.7–6.9 | nr | 0.6 | 1.0 |
CLIA chemiluminescence immunoassay, FEIA fluorescence enzyme immunoassay, FIA fluoroimmunoassay, IMM Immulite 2000 XPi, KRY Kryptor Compact Plus, LIA Liaison XL, LoD limit of detection, LoQ limit of quantitation, MAG Maglumi 2000 Plus, nr not reported, PHA Phadia 250, TPO thyroid peroxidase
The Immulite 2000 XPi was a continuous random-access system designed around a proprietary assay tube that allowed for efficient washing of an integral antibody- or antigen-coated polystyrene beads as solid phase. This assay used an alkaline phosphatase enzyme label that was quantified with a sensitive chemiluminescent substrate (adamantyl-dioxetane phosphate) by creation of an unstable adamantyl-dioxetane anion. The breakdown of this unstable anion generated a prolonged glow of light [17]. The TPOAbs test was based on a sequential chemiluminescent IMA (CLIA), which used native purified TPO antigen coated on the solid phase. As stated by the manufacturer, the intra-assay imprecision was 4.9–6.3 %; the total imprecision was 6.1–8.1 %; the limit of detection (LoD) of TPOAbs assay was 5.0 IU/mL and the limit of quantitation (LoQ) was not declared.
The Maglumi 2000 Plus was a continuous random-access IMA system that used N-(aminobutyl)-N-(ethyl)-isoluminol (ABEI) as luminescence substrate and magnetic particles serving both the solid phase and the separator in a liquid phase, with two different monoclonal antibodies labeled with either ABEI or fluorescein-5-isothiocyanate [18]. The TPOAbs test was a sandwich CLIA with native TPO coated to nanomagnetic microbeads. According to the manufacturer, the intra-assay imprecision was 6.8–7.3 %; the between-assay imprecision was 7.4–7.6 %; the LoD of the method was 0.38 IU/mL and the LoQ was not declared.
The Kryptor Compact Plus was an IMA system, based on time-resolved amplified cryptate emission technology (TRACE), which measured the signal emitted from an immunocomplex with a time delay. The basis of the TRACE technology was non-radioactive energy transfer from a donor to an acceptor. The donor was a cage-like structure with an europium ion in the center (cryptate); the acceptor was part of a chemically modified, light-collecting algal protein (XL 665). The proximity of the donor to the acceptor intensified the fluorescent signal of the cryptate and extended the life-span of the acceptor signal, permitting the measurement of temporarily delayed fluorescence [19]. The TPOAbs test was a competitive fluorescence IMA (FIA) that used enzymatically active native purified TPO. As stated by the manufacturer, the intra-assay imprecision was 2.1–7.6 %; the between-assay imprecision was 5.8–16.0 %; the LoD was 1.8 IU/mL and the LoQ was 9.0 IU/mL.
The Phadia (previously known as UniCAP, then ImmunoCAP) 250 was a fully integrated analyzer designed for the testing of allergy and autoimmune diseases. The reagents for the autoimmune diagnostics were based on the well-established EliA, normal ELISA-type-coated wells, that used β-D-galactosidase-labeled antibodies as tracer and 4-methyl-umbelliferyl-β-D-galactoside as developing agent, forming a fluorescent product (4-methyl-umbelliferone) fluorescence enzyme immunoassay (FEIA) [20]. The EliA TPOAbs test was a sandwich FEIA method that used human recombinant TPO antigen. As stated by the manufacturer, the intra-assay imprecision was 4.1–4.7 %; the between-assay imprecision was 2.5–4.8 %; the LoD and the LoQ were not declared.
The Liaison XL was a fully automated CLIA analyzer, which adopted a flash technology with paramagnetic microparticles as solid phase [15, 21]. The anti-TPO Liaison XL 2-step assay was a sandwich CLIA that used directly coated magnetic microparticles with recombinant TPO as solid phase and a conjugate based on an isoluminol derivate. As declared by the manufacturer, the intra-assay imprecision was 3.6–6.2 %; the between-assay imprecision was 4.7–6.9 %, the LoD was 0.6 IU/mL and the LoQ was 1.0 IU/mL.
Statistical analysis
All methods were standardized with the reference preparation MRC 66/387 and used international units (IU), except for KRY whose results were initially expressed in arbitrary units and subsequently corrected in IU (conversion factor = 0.175). To compare the data obtained by the five systems, the results were expressed as a median and the URL was established at the 99.0th ‰. The non-parametric Mann–Whitney U test was used to compare TPOAbs levels in males and females within the same method. A two-sided value of p < 0.05 was considered statistically significant. The difference between the manufacturer’s URL and the experimental URL was expressed as the ratio between them in percentage (Δ). The correlation studies were performed on all samples across all the five methods.
GraphPad Prism, version 4.0 (GraphPad Prism Software, San Diego, CA, USA) and MedCalc, version 13.3.1 (MedCalc Software, Ostend, Belgium) were used for statistical analysis.
Results
Value distributions were not Gaussian with a positive skew in both males and females for all the five methods.
A statistically significant difference (p < 0.05) between medians in male and female groups was observed for PHA (2.6 and 3.1 IU/mL, respectively) but not for the other four methods.
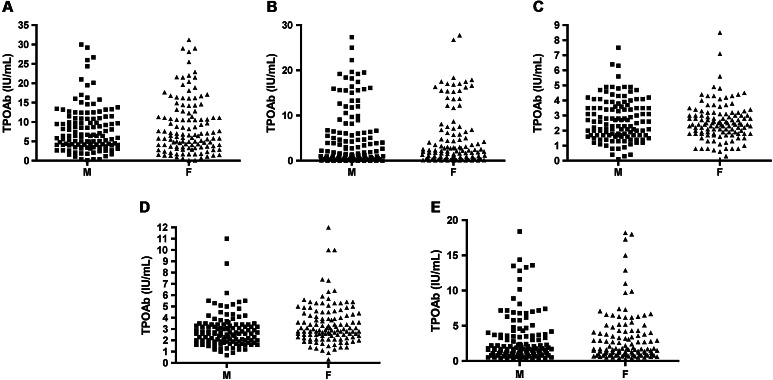
The scatter plots of TPOAbs values revealed a wide dispersion with very different coefficient of variation (CV) between methods, varying from 48.6 % for KRY in females to 126.3 % for MAG in females (Fig. 1).
Fig. 1.
Values of thyroid peroxidase autoantibodies, in males and females, measured by the five automated methods. a Immulite 2000 XPi, b Maglumi 2000 Plus, c Kryptor Compact Plus, d Phadia 250, e Liaison XL. M males, F females
URLs differed in males and females according to the method (Table 2): 28.7 and 29.0 IU/mL for IMM, 24.6 and 25.4 IU/mL for MAG, 6.4 and 6.9 IU/mL for KRY, 8.3 and 10.0 IU/mL for PHA, and 14.2 and 17.9 IU/mL for LIA, respectively. Such URLs were lower than those stated by the manufacturers except for LIA in females. The Δ between URLs ranged from a minimum of 11.3 % (LIA in males) to a maximum of 66.8 % (PHA in males).
Table 2.
The main statistical parameters of thyroid peroxidase autoantibodies, in males and females, measured by the five automated immunoassay methods
| Sex | No. | Mean (CI 95 %) | SD | CV | Median | p | eURL | mURL | Δ |
|---|---|---|---|---|---|---|---|---|---|
| IMM | |||||||||
| Males | 120 | 8.2 (7.1–9.3) | 6.0 | 73.6 | 6.4 | 0.8 | 28.7 | 35.0 | 18.0 |
| Females | 120 | 8.9 (7.6–10.1) | 7.0 | 79.6 | 6.7 | 29.0 | 17.1 | ||
| MAG | |||||||||
| Males | 120 | 5.5 (4.3–6.6) | 6.5 | 119.8 | 2.7 | 0.8 | 24.6 | 30.0 | 18.0 |
| Females | 120 | 4.9 (3.8–6.1) | 6.2 | 126.3 | 2.2 | 25.4 | 15.3 | ||
| KRY | |||||||||
| Males | 120 | 2.7 (2.5–2.9) | 1.4 | 50.6 | 2.6 | 0.6 | 6.4 | 10.5 | 39.0 |
| Females | 120 | 2.6 (2.3–2.8) | 1.2 | 48.6 | 2.4 | 6.9 | 34.3 | ||
| PHA | |||||||||
| Males | 120 | 2.8 (2.6–3.1) | 1.4 | 50.9 | 2.6 | <0.05 | 8.3 | 25.0 | 66.8 |
| Females | 120 | 3.7 (3.1–3.8) | 1.8 | 51.8 | 3.1 | 10.0 | 60.0 | ||
| LIA | |||||||||
| Males | 120 | 3.2 (2.6–3.9) | 3.4 | 106.0 | 1.9 | 0.8 | 14.2 | 16.0 | 11.3 |
| Females | 120 | 3.2 (2.6–3.9) | 3.9 | 111.3 | 1.7 | 17.9 | 11.9 | ||
CV and Δ are expressed in percentage; Mean, Median, SD, eURL and mURL are expressed in IU/mL. eURL = 99.0th ‰; Δ = ∣mURL − eURL∣/mURL × 100
CI confidence intervals, CV coefficient of variation, eURL experimental upper reference limit, IMM Immulite 2000 XPi, KRY Kryptor Compact Plus, LIA Liaison XL, MAG Maglumi 2000 Plus, mURL manufacturer’s upper reference limit, PHA Phadia 250, SD standard deviation
Correlation analysis showed that all the five methods did not compare well with each other (Table 3), suggesting discrepancies between them.
Table 3.
Method comparison
| Methods | n | r (95 % CI) |
|---|---|---|
| IMM | ||
| MAG | 240 | 0.0011 (−0.1269 to 0.1291) |
| KRY | 240 | −0.0235 (−0.1513 to 0.1050) |
| PHA | 240 | 0.0008 (−0.1266 to 0.1282) |
| LIA | 240 | 0.0707 (−0.0576 to 0.1966) |
| KRY | ||
| MAG | 240 | −0.0845 (−0.2108 to 0.0445) |
| LIA | 240 | −0.0988 (−0.2247 to 0.0304) |
| PHA | 240 | −0.0078 (−0.1362 to 0.1208) |
| LIA | ||
| MAG | 240 | 0.2628 (0.1390 to 0.3786) |
| PHA | 240 | 0.3679 (0.2521 to 0.4734) |
| MAG | ||
| PHA | 240 | 0.3226 (0.2028 to 0.4330) |
CI confidence intervals, IMM Immulite 2000 XPi, KRY Kryptor Compact Plus, LIA Liaison XL, MAG Maglumi 2000 Plus, PHA Phadia 250, r correlation coefficient
Discussion
To our knowledge, this is the first study on the definition and comparison of TPOAbs URLs between five different commercially available automated methods.
The study was designed to accurately define and harmonize TPOAbs URLs in order to avoid misclassification of patients in the process of AITD diagnosis. Thus, according to the NACB recommendations, we evaluated TPOAbs concentrations and URLs in a reference male group comparing them with a female one.
The first relevant result of this study was the demonstration of a significant difference between the TPOAbs URLs obtained with the five different automated IMA methods (both in males and females) and the results expected according to the corresponding package insert. With the exception of the LIA method in females, URLs values obtained in the present study were lower than those predicted in the insert, with Δ varying from 11.3 % (LIA in males) to 66.8 % (PHA in males) (Table 2). This finding was in contrast to the conclusion of a recent study which showed that the established URLs were very similar to those recommended by seven different manufacturers [14]. The difference between that study and the present one resides in the population sample: in the study by Springer et al. TPOAbs were measured in women in the first trimester of pregnancy; in the present study, TPOAbs were determined in 120 healthy males and in 120 healthy females. The discrepancies between the experimental and the manufacturer’s URLs, as found in the present study, could be linked to any of several factors. First of all, there are racial differences in autoantibody concentrations measured in different populations over the world: in most cases, studies, that were sponsored by the test’s manufacturer and were conducted in the geographical area of the production line, may be not reproducible in other geographical settings. An additional aspect was the lack of strict criteria in the selection of subjects for the reference group. In fact, the possibility of enrolling apparently healthy individuals with subclinical AITD and high levels of TPOAbs, whose prevalence is estimated around 3.3–25.8 % in the general population, raises the 99th ‰ of the reference value distribution [12, 13, 15, 22, 23] and produces an increase in the proportion of subjects classified as negative for TPOAbs. On the other hand, the demonstration of a significant difference between the TPOAbs URLs obtained with the five different automated IMA methods both in males and females, confirms the data of a previous study on the same topic, using indirect methods [15].
The second relevant consideration emerging from this study was the dependence of the URLs on the method used. A decade ago, when third-generation methods were introduced the differences between the URL values were on the order of 4–5 times (from 20.0 to 100.0 IU/mL) [8, 9]. In this study, the differences of the experimental URLs were similar, but at concentration levels that are four times lower than those reported in the past, ranging from 6.4 to 28.7 IU/mL in males and from 6.9 to 29.0 IU/mL in females (Table 2).
The discrepancy of URLs between methods was associated with the dispersion of the results obtained by each method in both the male and female reference groups. Indeed, the dispersion expressed as CV % showed widely divergent and fluctuating values, from 48.6 % for KRY to 126.3 % for MAG (Table 2; Fig. 1). There are no clear explanations for these discrepancies. In fact, over the years, there has been an improvement in the harmonization among methods, due to automation of the analytical procedures and the use of the same reference preparation (MRC 66/387) [10]. Moreover, it does not seem that the intra-method analytical imprecision contributes to these differences, since the precision performances declared by the individual manufacturer are essentially overlapping (Table 1). In our opinion, these differences could lie in the different coating preparations of the TPO antigen (purified native or recombinant) on solid phase, which affect the proper exposure of the immunodominant epitopes recognized by the polyclonal antibodies present in serum of AITD patients (epitopic fingerprint), with the consequent lack of recognition of some of them [24–26].
Another important result to be noted was the difference between genders: medians were not statistically significant different between males and females for IMM, KRY, MAG, and LIA, while they were different for PHA.
Based on these results, we suggest to overcome the proposal of NACB guidelines [11], which recommend to involve a single group of young male subjects in favor of using two distinct groups, male and female ones. This new proposal removes the apparent incongruity of using a male reference group for a disease (such as AITD) affecting mainly females.
Moreover, in spite of the harmonization among methods, the wide dispersion of quantitative results, still observed in this study, suggests the need for further studies to better understand the cause of these discrepancies, focusing on TPO antigen preparation as the possible source of variability among different assays.
Acknowledgments
The authors thank all the companies and laboratories that contributed to the study.
Compliance with ethical standards
Conflict of interest
The authors state that there are no conflicts of interest regarding the publication of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Czarnocka B, Cocks Eschler D, Godlewska M, et al. Thyroid antibodies: thyroid peroxidase and thyroglobulin antibodies. In: Shoenfeld Y, Meroni PL, Gershwin ME, et al., editors. Autoantibodies. 3. Amsterdam: Elsevier; 2014. pp. 365–373. [Google Scholar]
- 2.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the America Thyroid Association. Endocr Pract. 2012;18:988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 3.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and post-partum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tozzoli R, Bonaguri C, Melegari A, et al. Current state of diagnostic technologies in the autoimmunology laboratory. Clin Chem Lab Med. 2013;51:129–138. doi: 10.1515/cclm-2012-0191. [DOI] [PubMed] [Google Scholar]
- 5.Bizzaro N, Tozzoli R, Villalta D. Autoimmune diagnostics: the technology, the strategy and the clinical governance. Immunol Res. 2015;61:126–134. doi: 10.1007/s12026-014-8587-z. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez C, Garcia-Berrocal B, Talavan T, et al. Clinical evaluation of a microsphere bead-based flow cytometry assay for the simultaneous determination of anti-thyroid peroxidase and anti-thyroglobulin antibodies. Clin Biochem. 2005;38:966–972. doi: 10.1016/j.clinbiochem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.La’ulu SL, Slev PR, Roberts SL. Performance characteristics of 5 automated thyroglobulin and thyroperoxidase autoantibody assays. Clin Chim Acta. 2007;376:88–95. doi: 10.1016/j.cca.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair D. Analytical aspects of thyroid antibodies estimation. Autoimmunity. 2008;41:46–54. doi: 10.1080/08916930701619466. [DOI] [PubMed] [Google Scholar]
- 9.Tozzoli R, Bizzaro N, Tonutti E, et al. Immunoassay of anti-thyroid autoantibodies: high analytical variability in second generation methods. Clin Chem Lab Med. 2002;40:568–573. doi: 10.1515/CCLM.2002.098. [DOI] [PubMed] [Google Scholar]
- 10.D’Aurizio F, Tozzoli R, Villalta D, et al. Immunoassay of thyroid peroxidase autoantibodies: diagnostic performance in automated third generation methods. Clin Chem Lab Med. 2015;53:415–421. doi: 10.1515/cclm-2014-0545. [DOI] [PubMed] [Google Scholar]
- 11.Baloch Z, Carayon P, Comte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 12.Jensen EA, Petersen PH, Blaabjerg O, et al. Establishment of reference distributions and decision values for thyroid antibodies against thyroid peroxidase (TPOAb), thyroglobulin (TgAb) and the thyrotropin receptor (TRAb) Clin Chem Lab Med. 2006;44:991–998. doi: 10.1515/CCLM.2006.166. [DOI] [PubMed] [Google Scholar]
- 13.Zophel K, Saller B, Wunderlich G, et al. Autoantibodies to thyroperoxidase in a large population of euthyroid subjects: implication for the definition of TPOAb reference intervals. Clin Lab. 2003;49:591–600. [PubMed] [Google Scholar]
- 14.Springer D, Bartos V, Zima T. Reference intervals for thyroid markers in early pregnancy determined by 7 different analytical systems. Scan J Lab Investig. 2014;74:95–101. doi: 10.3109/00365513.2013.860617. [DOI] [PubMed] [Google Scholar]
- 15.Tozzoli R, Giavarina D, Villalta D, et al. Definition of reference limits for autoantibodies to thyroid peroxidase and thyroglobulin in a large population of outpatients using an indirect method based on current data. Arch Pathol Lab Med. 2008;132:1924–1928. doi: 10.5858/132.12.1924. [DOI] [PubMed] [Google Scholar]
- 16.Tozzoli R, Villalta D, Bizzaro N, et al. Laboratory diagnosis of autoimmune thyroid disease. Recent Prog Med. 2001;92:609–617. [PubMed] [Google Scholar]
- 17.Babson AL. Immulite 2000 and Immulite XPi. In: Wild DG, editor. The Immunoassay Handbook. 4. Amsterdam: Elsevier; 2013. pp. 575–578. [Google Scholar]
- 18.D’Aurizio F, Villalta D, Metus P, et al. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2014;14:363–369. doi: 10.1016/j.autrev.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Taieb J, Sarnel C, Benattar C, et al. A new technique for measuring 17beta-estradiol using Kryptor: utilization to monitor ovulation stimulation. Ann Biol Chem (Paris) 2000;58:71–79. [PubMed] [Google Scholar]
- 20.Gore A, Evans G, Rilven M. Phadia Laboratory Systems. In: Wild DG, editor. The Immunoassay Handbook. 4. Amsterdam: Elsevier; 2013. pp. 617–619. [Google Scholar]
- 21.van Helden J, Weiskirchen R. Experience with the first fully automated chemiluminescence immunoassay for the quantification of 1α, 25-dihydroxy-vitamin D. Clin Chem Lab Med. 2015;53:761–770. doi: 10.1515/cclm-2014-0698. [DOI] [PubMed] [Google Scholar]
- 22.Kaloumenou I, Mastorakos G, Alevizaki M, et al. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage and maternal thyroid autoimmunity. Thyroid. 2008;18:747–754. doi: 10.1089/thy.2007.0370. [DOI] [PubMed] [Google Scholar]
- 23.Taubner K, Schubert G, Pulzer F, et al. Serum concentrations of anti-thyroid peroxidase and anti-thyroglobulin antibodies in children and adolescents without apparent thyroid disorders. Clin Biochem. 2014;47:3–7. doi: 10.1016/j.clinbiochem.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Godlewska M, Gora M, Buckle AM, et al. A redundant role of human thyroid peroxidase propeptide for cellular, enzymatic and immunological activity. Thyroid. 2014;24:371–382. doi: 10.1089/thy.2013.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M-M, Li Q, Zhao L-L, et al. Glycosylation of recombinant human thyroid peroxidase ectodomain of insect cell origin has little effect on recognition by serum thyroid peroxidase antibody. Chin Med J. 2013;126:2907–2911. [PubMed] [Google Scholar]
- 26.Nielsen CH, Brix TH, Gardas A, et al. Epitope recognition patterns of thyroid peroxidase autoantibodies in healthy individuals and patients with Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 2008;69:664–668. doi: 10.1111/j.1365-2265.2008.03245.x. [DOI] [PubMed] [Google Scholar]