Abstract
Objective(s):
In this study, we aimed at evaluation of electrophysiological and histopathalogical characteristics of statin-induced muscle injury as well as clinical features of patients who develop this condition in terms of frequency and pattern of evolution.
Materials and Methods:
Forty patients (age 39-74 years) including 25 subjects with type 2 diabetes mellitus, 9 with cardiovascular diseases and 6 with hyperlipidemia, who were receiving atrovastatin 40 mg/day for variable period, were studied. Thirty three healthy subjects (age 31-74 years) served as control group. Creatine phosphokinease level, thyroid function, motor unit potential parameters and muscle fiber conduction velocity of biceps brachii and tibialis anterior muscles were measured.
Results:
Creatine phosphokinase level was elevated in statin users, particularly in those with diabetes mellitus. Less than 50% of statin users experienced symptoms related to muscle injury. Muscle fiber conduction velocity of the biceps brachii muscle was significantly reduced. Statin users with diabetes mellitus showed significant changes in electrophysiological parameters as compared to those with cardiovascular diseases and hyperlipidemia. Muscle biopsies showed muscle fiber variation in size, fibrosis and mild inflammatory cell infiltration. Immunohistochemical evaluation of muscle biopsies showed positive expression of Bcl-2 and one patient showed positive P53 immunohistochemical expression with elevated level of creatine phosphokinase.
Conclusion:
Atorvastatin increased average creatine kinase, statins produce mild muscle injury even in asymptomatic subjects. Diabetic statin users were more prone to develop muscle injury than others. Muscle fiber conduction velocity evaluation is recommended as a simple and reliable test to diagnose statin-induced myopathy instead of invasive muscle biopsy.
Keywords: Statin, EMG, MFCV, Histopathology, Immune histochemistry, Muscle injury
Introduction
Statins are considered to be safe, well-tolerated efficient drugs for the treatment of hypercholes-terolemia; Therefore, they are frequently prescribed (1, 2). The most severe adverse effects that are seen following statins administration are various forms of myotoxicity including myopathy, myalgia, myositis and rhabdomyolysis (3). Symptoms usually develop within four weeks but can be delayed up to four years after starting statins therapy (4, 5).
According to the US National Lipid Association Statin Safety Assessment Task Force, myopathy, defined as muscle symptoms and creatine phosphor-kinase (CPK) levels 10 times normal, occurs in five patients per 100,000 person-years (6, 7).
Myalgia with or without CPK elevation affects 7-20% of patients, and asymptomatic CPK elevation up to 10 times normal is noted in 11-63% of patients (8, 9) whereas normal CPK values do not exclude statin- induced myopathy (10).
Statin-induced muscle weakness is dose and time-related and can be accompanied by myopathic electromyographic (EMG) changes. In this regard, usually muscle necrosis is observed in muscle biopsy (11, 12). Myopathic changes are usually seen in the proximal muscles and a normal EMG does not exclude statin-induced myopathy, because it primarily affects type II muscle fibers (13).
In certain circumstances, when statins trigger toxic myopathy, they cause clinical weakness, fibrillations, positive sharp waves, and even myotonic discharges with early recruitment of short-duration motor unit action potentials (MUAPs) become apparent in weak muscles (14, 15).
The objective of our study was to assess electro-physiological and histopathalogical characteristics and clinical features of statin-induced muscle injury in patients, in term of frequency and pattern of evolution.
Materials and Methods
Forty patients aging 54.0±10.42 years old (39–74 years old) were recruited from those attending the outpatient medical clinic or those who were hospitalized in Al-Imamian Al-Kadhimiyian Medical City, Baghdad, Iraq between January and May 2012.
The study was approved by the Institute Review Board of the College of Medicine, Al-Nahrain University. The protocol was carefully explained to each subject before entering the study and their written consent was obtained.
Twenty-five (62.5%) patients had type 2 diabetes mellitus (DM) and took statin according to ADA guidelines (above 40 years with multiple risk factors for cardiovascular disease (CVD) or low density lipoprotein (LDL) >100 mg/dl) (16), 9 (22.5%) patients had CVD (acute coronary syndrome and history of ischemic heart disease) and 6 (15%) patients had unclassified hyperlipidemia (hypercholesterolemia).
All patients received atorvastatin 40 mg/day for a period of one to seven months. 10 (25%) patients received the drug for two months, 9 (22.5%) for three or four months and only 1 (2.5%) patient for seven months.
Patients with documented history of muscle disorder, renal impairment, liver diseases, thyroid dysfunction, and those who had long-term steroids use, alcohol consumption and concurrent treatment with other lipid-lowering agents (fibrates, bile acid sequestrates and niacin) were excluded. Patients who were treated with amiodarone and proton pump inhibitors, or those who had electrolytes abnormalities, surgery or trauma in the past 6 months or recent intramuscular injection were also excluded. Exclusion criteria were chosen based on the fact that the aforementioned conditions or diseases are considered as risk factors to increase the tendency for myopathies (17).
Participants were asked if they had pain. Those who answered “yes” were asked to specify the severity and location of their pain. We assessed them using functional activity score (18) and the patients were scored as follows:
No limitation: the patient’s activity is not limited by pain.
Mild limitation: the patient’s activity is mild to moderately limited by pain.
Severe limitation: the patient ability to do the activity is severely limited by pain.
For practical issues and because of difficult interpretation by some illiterate patients, some modifications were applied as mild (for grade A), moderate (for grade B), severe (for grade C) and not specified.
Thirty three volunteers (18 females and 15 males) aging 31 to 74 years old (mean±SD =52.0±9.22) were enrolled in the current study as the control group.
Biochemical tests
Serum CPK was measured using commercially available kits purchased from Abbot Laboratories (Kit No. 7D63-21, ARCHITECT plus-c4000, USA). CPK was considered abnormal if it exceeded the range of 30-200 IU/l in male patients and 29-168 IU/l in female patients.
Thyroid function test (T3, T4 and TSH) was done by antigen-antibody reaction using Mini VIDAS equipment (SN. ivd30003599, France). VIDAS130111-0(SN. 16140) kit was used for T3, VIDAS 130111-0 (SN. 35373) for T4, and VIDAS Code no.121215-0 for TSH. The expected normal values were 0.95-2.5 nmol/l for T3, 60-120 nmol/l for T4, and 0.25-5.0 μIU/ml for TSH.
Electrophysiological examination
This was done using Micromed, 8-channel electromyography (SN. GH17H9NW315431 B, model 1715, code GH17ESSM/EDC, Italy).
The biceps brachii and tibialis anterior muscles of the left side were chosen for all studies. The subjects were comfortably lying prone with both the arms and the legs extended and held fixed in an abducted position. The skin over the muscle was thoroughly treated with antiseptic solution. The examination room temperature was roughly maintained at 24-25 °C.
Electromyographic activity was analyzed using monopolar needle EMG electrodes (Micromed code DIN42802). Another electrode which served as ground (Micromed code 337003) was fitted across the muscle belly. Twenty MUPs were analyzed for duration, amplitude and polyphasia during minimal volitional effort. The setup used in this test was as follows: gain at 200 microvolt/cm, sweep speed 10 msec/cm, and filter setting 20 Hz to 10 KHz.
The method of Troni et al (19) was adopted for measurement of muscle fiber conduction velocity (MFCV) using the same monopolar needle electrode as an active recording electrode and surface silver cup-shaped disc electrode (Micromed Code tpco) as reference recoding electrode. The muscle stimulated at distal position from the recording electrode by means of the same monopolar needle electrode. The latency, amplitude, and CV were estimated. The setup of the test was as follows: stimulation insured to be supramaximal, band pass filter 500Hz-10 kHz and the time base varied between 5 to 10 msec per division.
Muscle biopsy
Under local or general anesthesia, muscle biopsies were taken from the tibialis anterior muscle of six patients and examined using clamp fixation method. Four out of six samples were symptomatic with elevated CPK and the other two patients were asymptomatic. Each sample had two sections namely cross sectional and longitudinal section. The biopsy
specimen was put in 10% formalin solution for Hematoxylin and Eosin test and further immune-histochemical studies. The immune-histochemical staining was done using Bcl-2 (Dako, code NO887, Denmark) and p53 (Dako Cytomation IHC kit LSAB2 System-HRP, code K0679, Denmark). All samples were examined by histopathology consultant.
Statistical analysis
All statistical analyses were done using SPSS software version 17 and Microsoft office Excel 2007. The values were presented as (mean±SD), median and range. The comparison between patient group and control group was made using t-test for parametric variables whereas Chi square and Mann-Whitney U test were employed for non-parametric variables. Comparison between more than two groups was made by using ANOVA and Kruskal-Wallis tests. P-value less than 0.05 was considered significant.
Results
Demographic data
No difference was observed between control subjects and statin users concerning their age, gender, weight, and thyroid function indices, whereas CPK level was significantly higher among statin users (Table 1).
Table 1.
Demographic features of the statin users vs control subjects
| Parameters | Control subjects N= 33 | Statin users N= 40 | |
|---|---|---|---|
| Gender | Males | 15 (45.46%) | 20 (50)% |
| Females | 18 (54.54%) | 20 (50%) | |
| Thyroid function test | T3 | 1.861 ± 0.47 | 1.751 ± 0.35 |
| T4 | 90.861 ± 16.97 | 85.281 ± 14.05 | |
| TSH | 2.021 ± 0.89 | 2.311 ± 1.12 | |
| Age (years) | 52.0 ± 9.22 | 54.01±10.42 | |
| Weight (Kg) | 70.081 ± 10.46 | 70.551 ± 13.1 | |
| CPK range (U/l) | 70 (33-139) | 98.5 (30-1300)* | |
=P<0.001 (Mann-Whitney U test), CPK= creatine phosphokinase, T3= Triiodothyronine, T4= Triiodothyronine, TSH= Thyroid stimulating hormone
Age and weight were not different between the control subjects and statin users with different underlying pathologies while CPK level was significantly higher (P<0.001) in diabetic statin users as compared to the control subjects (Table 2). ANOVA showed that age, weight and the duration of statin treatment (3.4±1.66 months; 4.17±1.94 months; and 3.67±1.0 months, respectively) were not different among statin users with various underlying pathologies. Similarly, Kruskal-Wallis test showed that CPK was not different among statin users with various underlying pathologies.
Table 2.
Comparison of age and weight among control subjects and statin users with different pathologies
| Parameter | Control Subjects N=33 | Statin users N=40 | ||
|---|---|---|---|---|
| DM N=25 | CVD N=9 | HL N=6 | ||
| Age (Year) | 52.0±9.22 | 53.24+10.48 | 56.78+11.5 | 53.0+9.53 |
| Weight (Kg) | 70.55+13.1 | 70.08+10.46 | 73.89+13.25 | 76.33+14.65 |
| CPK (U/l) | 75.39±27.06 (33-139) | 120.13±63.65 (67-1300)* | 98.67±44.83 (30-228) | 118.33±68.8 (50-370) |
= P<0.001 (Mann-Whitney U test), CPK= creatine phosphokinase, DM= diabetes mellitus, CVD= cardiovascular disease, HL= hyperlipidemia
Clinical data
Seventeen statin users (42.5%) showed symptoms related to myopathy while the rest of subjects (57.5%) were symptoms free. None of the patients in the present study presented signs and symptoms of rhabdomyolysis. Symptomatic statin users had different degrees of muscle pain severity as shown in Figure 1A. Different parts of the body had muscle pain as demonstrated in Figure 1B.
Figure 1.
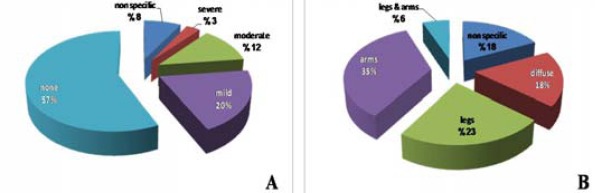
Percentage of statin users showing the severity (A) and location (B) of muscle pain
Neurophysiologic data
Here, 240 MUPs were analyzed in the patient’s group and 210 in the control group. In the biceps brachii muscle, the amplitude and duration of the MUPs were significantly reduced in patients as compared to the control subjects (P< 0.05 and P<0.001, respectively) but this was not seen in the tibialis anterior muscle (Table 3). Moreover, no difference was noticed between symptomatic and non-symptomatic statin users regarding MUPs parameters in the muscles tested.
Table 3.
MUP parameters in statin users vs control subjects
| MUP parameter | Control subjects N= 210 | Statin users N= 240 | |
|---|---|---|---|
| Biceps brachii | Amplitude (mV) | 1.85 ± 0.61 | 1.37±0.39* |
| Duration (ms) | 13.87±4.1 | 12.37±4.76** | |
| Tibialis anterior | Amplitude (mV) | 1.73±1.02 | 1.62±0.58 |
| Duration (ms) | 12.75±4.39 | 13.73±4.42 | |
P < 0.05,
P <0.001, (student t test), MUP=Motor unit potential
Table 4 illustrates the number of MUPs phases (polyphasia) in statin users and control subjects. Starin users had significantly increased polyphasia (more than 5 phases) as compared to the control group in the biceps brachii and tibialis anterior muscles (P<0.05). Furthermore, no difference was noticed between symptomatic and non- symptomatic statin users concerning the number of MUPs phases in examined muscles.
Table 4.
MUPs phases in statin users vs control subjects
| Group | Biceps brachii MUPs phases | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Control group | Frequency | 42 | 62 | 69 | 31 | 6 | 0 | 0 |
| % | 20.0 | 29.53 | 32.86 | 14.76 | 2.86 | 0.0 | 0.0 | |
| Statin users | Frequency | 58 | 61 | 55 | 37 | 26 | 2 | 1 |
| % | 24.16 | 25.42 | 22.92 | 15.42 | 10.83* | 0.83 | 0.42 | |
| Tibialis anterior MUPs phases | ||||||||
| Control group | Frequency | 24 | 41 | 42 | 22 | 1 | 0 | - |
| % | 18.46 | 31.54 | 32.31 | 16.92 | 0.77 | 0.0 | ||
| Statin users | Frequency | 29 | 49 | 43 | 33 | 13 | 3 | - |
| % | 17.06 | 28.82 | 25.29 | 19.41* | 7.65* | 1.77 | ||
P=< 0.05 (Chi square test), MUP=Motor unit potential
The MUP amplitude recorded from biceps brachii muscle was significantly reduced (P<0.05) in statin users with DM and CVD as compared to the control subject.
Similarly, MUPs duration recorded from biceps brachii and tibialis anterior muscles was significantly different (P<0.05) in statin users with DM as compared to the control subjects (Table 5). Using ANOVA test, the MUP amplitude and duration recorded from the two muscle samples were not different among statin users with various underlying pathologies.
Table 5.
MUP parameters of biceps brachii and tibialis anterior muscles among control subjects and statin users with different underlying pathologies
| Parameter | Control subjects N=33 | Statin users N=40 | |||
|---|---|---|---|---|---|
| DM N=25 | CVD N=9 | HL N=6 | |||
| Biseps | Amplitude (mV) | 1.85 ± 0.61 | 1.33 ± 0.36‡ | 1.4 ± 0.4‡ | 1.5 ± 0.48† |
| Brachii | Duration (ms) | 13.87 ± 4.1 | 12.69 ± 5.06* | 12.69 ± 4.46 | 13.02 ± 4.11 |
| Tibialis | Amplitude (mV) | 1.73 ± 1.02 | 1.64 ± 0.58 | 1.55 ± 0.58 | 1.65 ± 0.58 |
| Anterior | Duration (ms) | 12.75 ± 4.39 | 14.07 ± 4.5** | 12.87 ± 4.04 | 14.15 ± 4.74 |
= P<0.05,
= P<0.01,
= P<0.0005,
= P<0.0001 (ANOVA test), (statin users vs control subjects), MUPs= Motor unit potentials, DM= diabetes mellitus, CVD= cardiovascular disease, HL= hyperlipidemia
The latency of evoked muscle fiber response was not different between statin users and control subjects whether it was measured in the biceps brachii or tibialis anterior muscles. On the other hand, the recorded amplitude of the biceps brachii and tibialis anterior muscles showed significant decrement in patients in comparison with the control subjects (P<0.05). In addition, CV of the biceps brachii muscle was significantly reduced in statin users as compared to the control subjects (P<0.05), whereas it showed non-significant reduction in the tibialis anterior muscle (Table 6). The amplitude recorded from tibialis anterior muscle was the only parameter that was significantly reduced (P<0.05) in the symptomatic vs asymptomatic statin users.
Table 6.
Muscle fiber parameters in statin users vs control subjects
| Parameters | Control subjects N=33 | Statin users N=40 | |
|---|---|---|---|
| Biceps brachii | Latency (msec) | 9.91±1.46 | 10.82±2.54 |
| Amplitude (mv) | 1.75±0.57 | 1.31±0.42** | |
| CV (m/s) | 5.15±0.75 | 4.69±0.8* | |
| Tibialis anterior | Latency (msec) | 10.34±2.04 | 11.18±1.95 |
| Amplitude (mv) CV (m/s) | 1.8±0.67 | 1.29±0.49** | |
| CV (m/s) | 4.94±0.88 | 4.59±0.76 | |
= P<0.05,
= P<0.001, (student t test), CV= conduction velocity
The amplitude of muscle fiber response evoked from biceps brachii was significantly reduced (P<0.05) in statin users with DM and CVD as compared to the control subjects. Likewise, the latency and CV were also significantly reduced (P<0.05) in statin users with DM in comparison with the control subjects. The amplitude of muscle fiber response evoked from tibialis anterior muscle was significantly reduced (P<0.05) in statin users with DM when compared to the control subjects (Table 7). Furthermore, using ANOVA test, neither the latency nor the amplitude or CV was different among statin users with different underlying pathologies.
Table 7.
Muscle fiber response parameters among control group and statin users with different pathologies
| Muscle Parameter | Control subjects N=33 | Statin users N=40 | |||
|---|---|---|---|---|---|
| DM N=25 | CVD N=9 | HL N=6 | |||
| Biceps brachii | Latency (ms) | 9.91±1.46 | 11.53±2.73* | 9.33±2.02 | 10.09±1.09 |
| Amplitude (mV) | 1.75±0.57 | 1.28±0.41‡ | 1.52±0.49† | 1.14±0.23 | |
| CV (m/s) | 5.15±0.75 | 4.53±0.91** | 4.91±0.44 | 5.02±0.55 | |
| Tibialis anterior | Latency (ms) | 10.34±2.04 | 11.35±2.18 | 11.06±1.47 | 10.72±1.76 |
| Amplitude (mV) | 1.8±0.67 | 1.21±0.42† | 1.59±0.53 | 1.17±0.6 | |
| CV (m/s) | 4.94±0.88 | 4.55±0.83 | 4.59±0.6 | 4.77±0.78 | |
= P<0.05,
= P<0.01,
= P<0.0005,
= P<0.0001 (ANOVA test), (statin users vs control subjects), MUPs= Motor unit potentials, DM= diabetes mellitus, CVD= cardiovascular disease, HL= hyperlipidemia
A significant inverse correlation was demonstra-ted between CPK level and MFCV of the tibialis anterior muscle (r= -0.445 and P= 0.012).
Histopathalogical analysis
Hematoxylin-eosin study
Light microscope examination reveals polygonal myofibers fitting against each other with little interposing endomysial connective tissue in between (Figure 2A). Histopathological changes were noticed in 5 out of the 6 specimens. The changes ranged from myopathic changes like muscle fiber disorientation, fibrosis, mild inflammatory cell infiltration and muscle fiber variation in size, fibrosis and perivascular infiltration of inflammatory cells (Figure 2B) to muscle fiber destruction and vaculation (adipose tissue replacement) as shown in Figure 2C.
Figure 2.
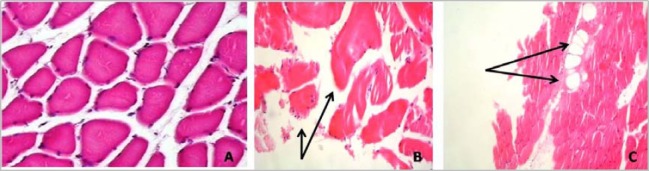
. (A) Normal muscle biopsy (x40), (B) Muscle fiber disorientation, variation in size, fibrosis and mild inflammatory cell infiltrate (x100), (C) Destruction and vaculation of muscle tissue (x100) (H&E staining)
Evaluation of Bcl-2 reaction
Three biopsy specimens belonging to statin users with elevated CPK level showed positive Bcl-2 immun-histochemical reaction while the other three biopsies showed negative Bcl-2 immunohistochemical reaction in subjects with normal CPK level (Figure 3).
Figure 3.
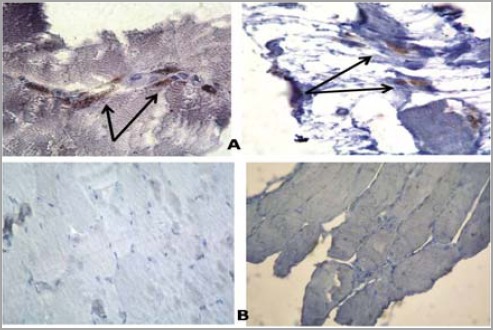
Immune histochemical staining with Bcl-2 (A) positive reaction (B) negative reaction (x100)
Evaluation of p53 reaction
Regarding this type of immunhistochemical expression, only one muscle biopsy showed positive reaction. The same statin user showed positive P53 reaction with elevated CPK level. The other biopsies (five samples) showed negative reaction (Figure 4).
Figure 4.
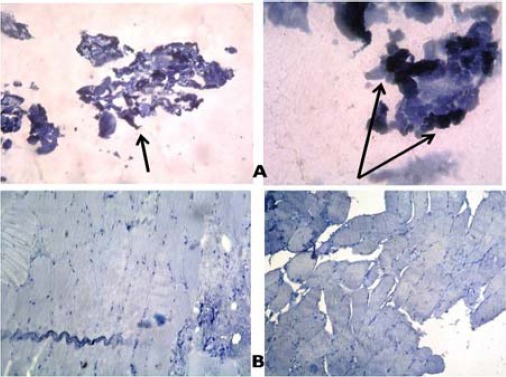
Immune-histochemical staining with p53 (A) positive reaction (B) negative reaction (x100)
Discussion
Clinical data
Serum CPK level was normal in the majority of patients enrolled in the current study when taken individually. This finding was in agreement with other studies (20, 21). However, as a whole, CPK level was significantly elevated in diabetic statin users. Moreover, symptomatic statin users show elevated CPK level as compared to those who were symptoms free. In statin-induced myopathies, CPK level may be normal or there may be asymptomatic CPK increase (8, 22). Some estimates suggest that up to 5% of all treated patients have hyper-creatinine kinaseaemia (23, 24).
In the present study, one patient with CPK level exceeding 1300 U/l showed significant structural muscle fiber abnormalities ranging from myopathic changes like muscle fiber disorientation, fibrosis and mild inflammatory cell infiltration to muscle fiber variation in size, fibrosis and perivascular infiltration of inflammatory cells (Figure 2B and B). Karas et al also noticed one patient out of 44 statin users documented 10 times increment in CPK level with structural abnormalities in the muscle fibers (24).
Fortunately, rhabdomyolysis is a rare complication (25) and none of statin users in this study experienced it. It is worth to say that the minority of our statin users were symptomatic while the majority were symptoms free; this finding was also reported by others (26, 27).
The risk of statin-associated myopathy has been shown to increase as the statin dose increases (28). That is why the dose was fixed to 40 mg/day for all users in this study in an attempt to minimize differences in clinical as well as subsequent neurophysiological data.
Muscle pain was the prominent complaint of the studied group. The presence, severity and location of this pain (Figure 1) were different from the findings of Hansen et al (29) who studied 45 patients with statin-associated myopathy. This difference could be attributed to different system that was adopted for functional activity scoring.
Muscle symptoms frequently begin within several months after initiation of therapy (26). Mantel-Teeuwisse et al (30) stated that symptoms usually begin two to three months after starting statin therapy. In a small retrospective study by Hansen et al, it was found that the mean duration of therapy before onset of symptoms was 6.3 months (29). Moreover, Wight et al found that the average time of onset is about six months (31). These findings were noticed in the current study as the time of onset of symptoms for the majority of statin users ranged from two to four months and to a lesser extent to six months.
Neurophysiological data
In statin users, the MUPs were of short duration, low amplitude and polyphasic. The short-duration MUPs are main characteristic and are often seen in primary muscle diseases in which the muscle fibers show random loss of their components ranging from necrosis, variation of size, degeneration or atrophy (32).
The frequency of polyphasic MUPs were significantly increased in stain users, a finding which has also been reported by Strommen et al (33) and Hanaoka et al (34). When the fibers fire asynchronously, the number of phases (or turns) increases. This may occur as a result of relative asynchrony from drop-out of muscle fibers or differences in MFCVs in the MU (35). The changes in MUAP configuration was proven electrophysiologically and histopathologically by changes seen in Figure 2.
Direct measurement of the MFCV is not a standard technique in clinical EMG, and is only used for research purposes. In statin users, muscle fiber response parameters changes significantly to variable degrees from the control subjects. Since MFCV is the speed of the depolarization wave along muscle fibers, it can be considered indicative of sarcolemmal excitability (36). Furthermore, MFCV is related to the diameter of muscle fibers (because diameter determines cytoplasmic resistance of a fiber) and muscle fiber type (type II fibers present greater values of MFCV than type I fibers due to their bigger diameter) (37).
Possible explanation for the observed reduction in MFCV is changes in sarcolemmal permeability that may occur due to decreased function of proteins regulating transsarcolemmal electrolyte balance which ultimately leads to decreased sarcolemmal excitability (38). Also, the other factors are muscle fiber atrophy and muscle fiber diameter variation which had been proven in histopathological sections (Figure 2). To the best of our knowledge, no data concerning MFCV in statin-treated patients has been reported so far.
Statins have been shown to induce apoptosis in skeletal myocytes in vitro in a concentration-dependent manner (39, 40). This apoptosis is thought to be influenced by the net contribution of pro- vs anti-apoptotic members in the B-cell CLL/lymphoma 2 (Bcl-2) family of proteins. Down-regulation of the anti-apoptotic Bcl-2 protein was proved in the present study by the positive reaction to Bcl-2 in three statin users (Figure 3). Furthermore, high levels of DNA damage may exceed the cellular repair capacity, generating mutations and triggering apoptosis. It has been shown that tumor suppressor p53 is an important regulator of the cellular response to reactive oxygen species-induced DNA damage. Severe reactive oxygen species (ROS) stress and high levels of DNA damage cause persistent accumulation/activation of p53 which leads to induction of apoptosis in the damaged cells (41). In this study, one of statin users who had high CPK level showed positive reaction to p53 (Figure 4). This finding was in agreement with the findings of other investigators (42, 43).
Hyperlipidemics can also provoke vacuolar lesion characterized by the presence of vacuoles with increased lysosomal activity. Vacuolated fibers could represent an early stage of necrosis fiber (44). This was also proven in the current study by the presence of muscle fiber destruction and vaculation (Figure 2).
Diabetic patients in the current study were more prone to develop statin-induced muscle injury. Patients with co-existing medical conditions such as DM, renal dysfunction, hepatic disease or subjects on concomitant medications like fibrates and macrolides were more likely to develop myopathy than other (45, 46). In addition, it could be attributed to a possible reduction in clearance of statin lactone (47) especially if it is complicated by early stages of renal impairment where the renal function parameters were not yet altered. In addition, other mechanisms like deficiency of relevant compounds like mevalonate and ubiquinone lead to mitochondrial dysfunction or prenylated protein causing altered intracellular messaging which induces vacuolation of the myofibers, degeneration and swelling of organelles and eventually results in apoptosis (48).
Conclusion
Absence of symptoms in statin users does not exclude muscle damage. Diabetic statin users are more prone to develop muscle injury than others. Statin users had abnormal electromyographic results and in particular, the MFCV which is recommended as a simple and reliable test to diagnose statin-induced myopathy instead of invasive muscle biopsy.
Acknowledgment
We would like to thank Professor Alaa Ghani Hussain who examined and interpreted muscle biopsy slides. The results described in this paper were part of student thesis. No specific grant from any funding agency in the public, commercial or not-for-profit sector was received.
References
- 1.Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373–387. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 2.Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banach M, Serban C, Sahebkar A, Ursoniu S, Rysz J, Muntner P, et al. Effects of coenzyme Q10 on statin-induced myopathy: A Meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24–34. doi: 10.1016/j.mayocp.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sulaiman AA, Al-Khamis FA. Statin-induced myopathy: a clinical perspective. Bahrain Med Bull. 2009:31. [Google Scholar]
- 5.Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150:858–68. doi: 10.7326/0003-4819-150-12-200906160-00009. [DOI] [PubMed] [Google Scholar]
- 6.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. National lipid association statin safety assessment task force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Tomaszewski M, 3Stępień KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63:859–66. doi: 10.1016/s1734-1140(11)70601-6. [DOI] [PubMed] [Google Scholar]
- 8.Paganoni S, Amato A. Electrodiagnostic Evaluation of myopathies. Phys Med Rehabil Clin N Am. 2013;24:193–207. doi: 10.1016/j.pmr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baer AN, Wortmann RL. Myotoxicity associated with lipid-lowering drugs. Curr Opin Rheumatol. 2007;19:67–73. doi: 10.1097/BOR.0b013e328010c559. [DOI] [PubMed] [Google Scholar]
- 10.Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Findling O, Meier N, Sellner J, Nedeltchev K, Arnold M. Clinical reasoning: rhab- domyolysis after combined treatment with simvastatin and fluconazole. Neurology. 2008;71:e34–e37. doi: 10.1212/01.wnl.0000327566.57661.09. [DOI] [PubMed] [Google Scholar]
- 12.Radcliffe KA, Campbell WW. Statin myopathy. Curr Neurol Neurosci Rep. 2008;8:66–72. doi: 10.1007/s11910-008-0011-4. [DOI] [PubMed] [Google Scholar]
- 13.Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol. 2002;40:163–71. doi: 10.1097/00005344-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Huynh T, Cordato D, Yang F, Choy T, Johnstone K, Bagnall F, et al. HMG CoA reductase-inhibitor-related myopathy and the influence of drug interactions. Intern Med J. 2002;32:486–490. doi: 10.1046/j.1445-5994.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- 15.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes - 2014. Diab Care. 2014;37:S1. [Google Scholar]
- 17.Ahmad Z. Statin intolerance. Am J Cardiol. 2014;113:1765–1767. doi: 10.1016/j.amjcard.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Gloth FM, III, Scheve AA, Stober CV, Chow S, Prosser J. The functional pain scale: reliability, validity, and responsiveness in an elderly population. J Am Med Dir Assoc. 2001;2:110–114. [PubMed] [Google Scholar]
- 19.Troni W, Cantello R, Rainero I. Conduction velocity along human muscle fibers in situ. Neurology. 1983;33:1453–1459. doi: 10.1212/wnl.33.11.1453. [DOI] [PubMed] [Google Scholar]
- 20.Wald JJ. The effects of toxins on muscle. Neurol Clin. 2000;18:695–717. doi: 10.1016/s0733-8619(05)70219-x. [DOI] [PubMed] [Google Scholar]
- 21.Sieb JP, Gillessen T. Iatrogenic and toxic myopathies. Muscle Nerve. 2003;27:142–156. doi: 10.1002/mus.10244. [DOI] [PubMed] [Google Scholar]
- 22.Dalakas MC. Toxic and drug-induced myopathies. J Neurol Neurosurg Psychiat. 2009;80:832–838. doi: 10.1136/jnnp.2008.168294. [DOI] [PubMed] [Google Scholar]
- 23.Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644–650. doi: 10.1097/BOR.0b013e32833f0fc7. [DOI] [PubMed] [Google Scholar]
- 24.Karas RH, Mohaupt MG, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, et al. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:E11–E18. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valiyil R, Christopher-Stine L. Drug-related myopathies of which the clinician should be aware. Curr Rheumatol Rep. 2010;12:213–220. doi: 10.1007/s11926-010-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients –the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 27.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 28.Chatzizisis Y, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy. Drug Saf. 2010;33:171–187. doi: 10.2165/11319380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin associated myopathy. Arch Intern Med. 2005;165:2671–2676. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 30.Mantel-Teeuwisse AK, Klungel OH, Herings RM, van Puijenbroek EP, Porsius AJ, de Boer A. Myopathy due to statin/fibrate use in the Netherlands. Ann Pharmacother. 2002;36:1957–1960. doi: 10.1345/aph.1C097. [DOI] [PubMed] [Google Scholar]
- 31.Wright RS, Murphy JG, Bybee KA, Kopecky SL, LaBlanche JM. Statin lipid-lowering therapy for acute myocardial infarction and unstable angina: efficacy and mechanism of benefit. Mayo Clin Proc. 2002;77:1085–1092. doi: 10.4065/77.10.1085. [DOI] [PubMed] [Google Scholar]
- 32.Rubin DI, Daube JR. Application of clinical neurophysiology: assessing peripheral neuromuscular symptom complexes. In: Daube JR, Rubin DI, editors. Linical Neurophysiology. 3rd ed. Chapter 47. Oxford University Press; 2009. p. 827. [Google Scholar]
- 33.Strommen JA, Johns JS, Kim CT, Williams FH, Weiss LD, Weiss JM, et al. Neuromuscular rehabilitation and electrodiagnosis 3. Diseases of muscles and neuromuscular junction. Arch Phys Med Rehabil. 2005;86:S18–27. doi: 10.1016/j.apmr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Hanaoka BY, Peterson CA, Horbinski C, Crofford LJ. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat Rev Rheumatol. 2012;8:448–457. doi: 10.1038/nrrheum.2012.85. [DOI] [PubMed] [Google Scholar]
- 35.Gutiérrez GG, Lopez CB, Navacerrada F, Martínez AM. Use of electromyography in the diagnosis of inflammatory myopathies. Reumatol Clin. 2012;8:195–200. doi: 10.1016/j.reuma.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Andreassen S, Arendt-Nielsen L. Muscle fiber conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. J Physiol. 1987;391:561–571. doi: 10.1113/jphysiol.1987.sp016756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blijham PJ, Ter Laak HJ, Schelhaas HJ, van Engelen BG, Stegeman DF, Zwarts MJ. Relation between muscle fiber conduction velocity and fiber size in neuromuscular disorders. J Appl Physiol. 2006;100:1837–1841. doi: 10.1152/japplphysiol.01009.2005. [DOI] [PubMed] [Google Scholar]
- 38.Minetto MA, Botter A, Lanfranco F, Baldi M, Ghigo E, Arvat E. Muscle fiber conduction slowing and decreased levels of circulating muscle proteins after short-term dexamethasone administration in healthy subjects. J Clin Endocrinol Metab. 2010;95:1663–1671. doi: 10.1210/jc.2009-2161. [DOI] [PubMed] [Google Scholar]
- 39.Sacher J, Weigl L, Werner M, Szegedi C, Hohenegger M. Delineation of myotoxicity induced by 3-hydroxy-3-methylglutaryl CoA reductase inhibitors in human skeletal muscle cells. J Pharmacol Exp Ther. 2005;314:1032–1041. doi: 10.1124/jpet.105.086462. [DOI] [PubMed] [Google Scholar]
- 40.Matzno S, Yasuda S, Juman S, Yamamoto Y, Nagareya-Ishida N, Tazuya-Murayama K, et al. Statin-induced apoptosis linked with membrane farnesylated Ras small G protein depletion, rather than geranylated Rho protein. J Pharm Pharmacol. 2005;57:1475–1484. doi: 10.1211/jpp.57.11.0014. [DOI] [PubMed] [Google Scholar]
- 41.Achanta G, Huang P. Role of p53 in sensing oxidative DNA damage in response to reactive oxygen species generating agents. Cancer Res. 2004;64:6233–6239. doi: 10.1158/0008-5472.CAN-04-0494. [DOI] [PubMed] [Google Scholar]
- 42.Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005;26:883–891. doi: 10.1093/carcin/bgi036. [DOI] [PubMed] [Google Scholar]
- 43.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 44.Alzira A, Carvalho S, Poti Lima Ü W, Valiente RA. Statin and fibrate associated myopathy: Study of eight patients. Arq Neuropsiquiatr. 2004;62:257–261. doi: 10.1590/s0004-282x2004000200013. [DOI] [PubMed] [Google Scholar]
- 45.Law M, Rudnicka AR. Statin safety: A systematic review. Am J Cardiol. 2006;97:52c–60c. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Josan K, Majumdar SR, McAlister FA. The Efficacy and safety of intensive statin therapy: A Meta-analysis of randomized trials. CMAJ. 2008;178:576–84. doi: 10.1503/cmaj.070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dostalek M, Sam WJ, Paryani KR, Macwan JS, Gohh RY, Akhlaghi F. Diabetes mellitus reduces the clearance of atorvastatin lactone: results of a population pharmacokinetic analysis in renal transplant recipients and in vitro studies using human liver microsomes. Clin Pharmacokinet. 2012;51:591–606. doi: 10.2165/11632690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy. J Am Coll Cardiol. 2007;49:2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]


