Abstract
Objective(s):
In this study the effect of oral administration of honey on serum glucose, lipids, stress oxidative markers, and morphology of langerhans islets in noise induced hyperglycemic rats was investigated.
Materials and Methods:
Male Wistar rats were divided into control, hyperglycemic, honey treated control, and honey treated hyperglycemic groups. For induction of hyperglycemia, noise stress was used. Serum glucose, triglyceride (TG), total cholesterol, low density lipoprotein (LDL), and high density lipoprotein (HDL)-cholesterol levels were determined before the study and at 4th and 8th weeks after the study. Markers of oxidative stress in brain were also measured. Morphology of langerhans islets in four groups was evaluated using Gomori staining method.
Results:
Treatment of noise induced hyperglycemic rats with honey produced a hypoglycemic effect and appropriate changes regarding serum lipids in treated diabetic group at 4th and 8th weeks as compared to the control group. Meanwhile, honey treatment significantly ameliorated the increased malondialdehyde (MDA) content and reduced the activity of superoxide dismutase (SOD) in brain. Histology of langerhans islets in hyperglycemic group showed a lower number and granularity of beta cells; honey treatment produced beneficial change in this respect.
Conclusion:
Oral administration of honey in experimental model of diabetes showed a significant hypoglycemic effect and led to appropriate changes in serum lipid profiles.
Keywords: Beta cell, Diabetes mellitus, Glucose, Honey, Lipid, Noise, Rat
Introduction
Noise pollution is increasing nowadays especially in industrial countries. Prevalence of noise is implicated in various human diseases and it is responsible for increased morbidity associated with modern life style (1). There are several physiological effects of noise exposure including hypertension (2), ischemic heart disease (3), annoyance (4), sleep disturbance, and decreased school enactment. Moreover, changes in the immune system and birth defects have been also attributed to noise exposure, in addition to altered serum lipid, plasma viscosity, platelet amount, glucose, and reduced motor efficiency (5). Beyond these physical effects, elevated noise levels create stress, increase workplace accident rate, and stimulate violence and other antisocial behaviors. Noise can originate from environment, work place, and even from leisure activities such as personal stereos (6).
Hydrogen peroxide (H2O2) and reactive oxygen species (ROS) account for production of free radical factors that cause degeneration of pancreas tissue cells and decreased insulin secretion (7). Free radicals arising from either the normal metabolism or induced by environmental sources interact continuously in the biological systems. Oxidants/-antioxidants must be kept in balance to minimize cellular, molecular, and tissue impairment (8). ROS such as superoxide, hydrogen peroxide, and hydroxyl radicals are formed in the course of cellular metabolism. A series of antioxidant compounds present in the cells, react with oxidizing agents and disarm them (9). Several studies have indicated the effects of different types of stress on the antioxidant system and induction of lipid peroxidation in the brain of various stress exposed models (10, 11). Honey as an antioxidant has been used by physicians throughout the history. Honey is utilized in both disease and health conditions, because of its anti-microbial and antioxidant properties (12, 13). Honey is full of enzymatic and non-enzymatic antioxidants such as: catalase, alkaloids, ascorbate, and flavonoids that act against oxidative agents to prevent from combination with non-saturated fatty acids and lipoprotein oxidation (14-17). Honey is rich in fructose, glucose, and minerals such as magnesium, potassium, calcium, phosphate, sulfur, ferrous, and sodium chloride as well as vitamins such as B1, B2, B5, B6, and C (18).
This study is aimed to investigate the effect of noise of (90-120 dB, 350 Hz) on plasma blood glucose concentration and lipid profile with involvement of oxidative stress in the rats. The effect of honey as an antioxidant in compensating the noise induced oxidative damages in pancreas tissue is also investigated here.
Materials and Methods
Animals
In this study a total of 32 mature male Wistar rats with 200±25 g weight have been purchased from Laboratory Animal Reproduction and Breeding Center, Ahvaz, Iran. Procedures involving animals and their care were conducted in conformity with the NIH guidelines for the care and use of laboratory animals. Rats were separated to four groups with eight members in each, randomly. Group 1 (control) was kept in natural condition without any stress. Group 2 received 3 ml/kg 5% honey solution by gavage method, 3 times a day for 8 weeks. Groups 3 and 4 were exposed to 90-120 dB noise with 350 Hz frequency, from 7 PM to 7 AM for 60 days. Group 3 was exposed to the noise without any complementary material and group 4 was exposed to noise along with 3 ml/kg 5% honey solution by gavage method administered 3 times a day for 8 weeks.
Stress procedure
Two groups of rats were exposed to white noise (90-120 dB, 350 Hz). Exposure was started in morning of the first day for the period of 2 month, from 7 PM to 7 AM, intermittently (6 sound-hours per day). Each sound-hr consisted of a programmed variable intensity (intermittent noise) from low to high dB every 2-3 min by a noise generator device. The noise generator was off automatically after one hr and then restarted one hr later.
Determination of brain malondialdehyde concentration
After 8 weeks, the rats were anesthetized with ketamine (100 mg/kg), decapitated, and the brains were removed, dried, and weighed. Then, they were made into 10% tissue homogenate in ice-cold 0.9% saline solution and centrifuged (1000 ×g, 4 °C, and 10 min) to remove particulates. The obtained supernatant was aliquot, then stored at -80 °C until assay. The malondialdehyde (MDA) concentration (thiobarbituric acid reactive substances, TBARS) in the supernatant was gauged as described before (19). Briefly, trichloroacetic acid and TBARS reagent were added to the supernatant, then blended, and incubated at 100 °C for 80 min. After refrigerating on ice, the samples were centrifuged at 1000 ×g for 20 min and the absorbance of the supernatant was read at 532 nm. TBARS results were articulated as MDA equivalents using tetraethoxypropane as standard.
Measurement of brain superoxide dismutase activity
The supernatant of brain homogenate was obtained as described earlier. Superoxide dismutase (SOD) activity measurement was based on the previous works (19). Briefly, supernatant was incubated with xanthine and xanthine oxidase in potassium phosphate buffer (pH 7.8 at 37 °C) for 40 min and nitro blue tetrazolium was added. Blue formazan was then checked spectrophotometrically at 550 nm. The amount of protein that inhibited nitro blue tetrazolium reduction by 50% of maximum was defined as 1 nitrite unit of SOD activity.
Measurement of serum glucose and lipid profile
Changes in body weight, food consumption, and water intake were regularly observed during the experimental period. In addition, serum triglyceride (TG), total cholesterol, and HDL cholesterol levels were spectrophotometrically measured using appropriate kits (Parsazmoon, Iran). LDL and VLDL cholesterol levels were calculated by the following formula:
VLDL= TG/5
Pancreas histology
At the end of the 8th week, rats were killed subsequent of total deep anesthesia and their pancreas gland tissue was segregated. After washing, tissues were put in the normal saline and 10% formalin; paraffin blocks were obtained after several tissue processing levels, and tissue sections were made by microtome (Lyka, Germany) at 5 µm. The blocks were carried to lams and stained by Gomory staining method. At final, the samples were observed by light microscope at 400 magnitudes. Langerhans islands diameter, distribution pattern, and β cells condensation were calculated and compared between all four groups. For tissue assessment we used image tools (version3) software.
Honey solution preparation
Honey was provided from a bee keeping center of Urmia city, Iran, and was made as 5% solution and given to rats by gavage method.
Statistical analysis
All data are presented as mean±SEM. The significance of differences among different groups was assessed by one-way ANOVA followed by Tukey post-hoc test. The approval level of significance was P<0.05. Data was evaluated by SPSS for windows version 12.0.1, with 95% confidence interval level.
Results
General considerations
One rat was excluded at final weeks of the study due to severe weight loss and immobility. Meanwhile, 82% of rats were made diabetic following noise induction and had a serum glucose level higher than 250 mg/dl. The data for drinks intake was significantly increased in the vehicle treated noise induced rats (140±15 ml/rat/day) compared to control group (42±8 ml/rat/day) (P<0.001). This data in honey treated group was (100±7 ml/rat/day). After eight weeks, the weight of the vehicle treated noise induced rats was significantly decreased as compared to control rats (175±16) (P<0.01); honey treatment caused a less significant decrease in diabetic rats as compared to noise induced diabetic rats (226±11.9), (P<0.01), (Figure 1). In addition, noise induced rats had also an elevated serum glucose level over those of control rats (401±25.9) P<0.001); treatment of these rats with honey caused a significant decrease in the serum glucose (208±34.6) (P<0.01), relative to noise induced group. Meanwhile, honey treatment of control rats did not produce any significant change regarding serum glucose level (112.8±16.4) (Figure 2).
Figure 1.
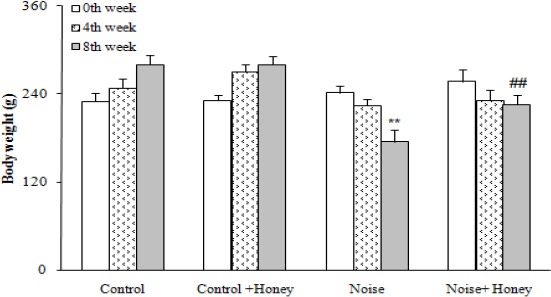
Body weight in weeks 0 (baseline), 4, and 8, (means±SEM) ** P<0.01 (as compared to week 0 in the same group); ## P<0.01 (as compared to noise group in the same week)
Figure 2.
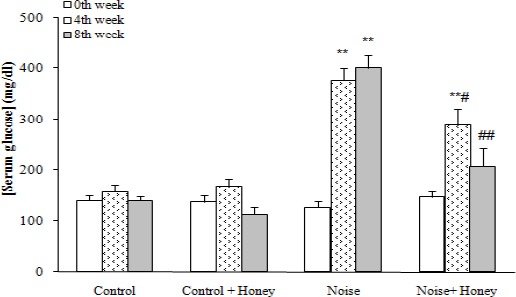
Serum glucose concentration in weeks 0, 4, and 8, (means±SEM). ** P<0.01 (as compared to week 0 (baseline) in the same group); # P<0.05, ## P<0.01 (as compared to noise group in the same week)
Regarding serum lipids, noise induction caused a significant increase in total cholesterol (94.7±4.5) (P<0.05), TG (104.1±5.5) (P<0.01), and LDL-cholesterol (47.9±2.9) (P<0.005) after eight weeks and a significant reduction in HDL-cholesterol (25.9±2.5) (P<0.01) concentrations compared to baseline data; honey treatment improved all of these changes. Comparing honey treated and untreated noise induced groups showed a significant difference between them only after eight weeks regarding serum TG, HDL, and LDL-cholesterol level. In addition, honey treatment of control group non-significantly improved serum lipid profile compared to baseline data (Figures 3-6).
Figure 3.
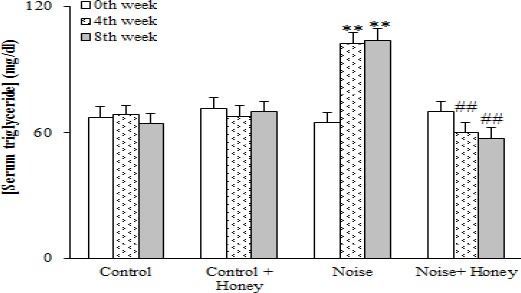
Serum triglyceride concentration in weeks 0 (baseline), 4, and 8, (means±SEM). ** P< 0.01 (as compared to week 0 in the same group); ## P<0.01 (as compared to noise group in the same week)
Figure 4.
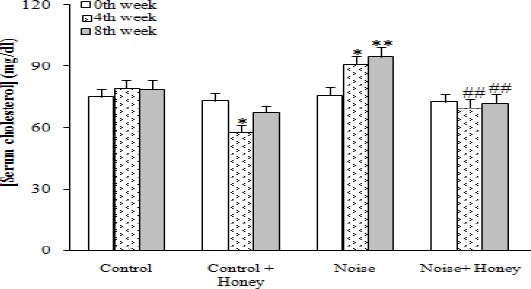
Serum cholesterol concentration in weeks 0, 4, and 8, (means±SEM). * P<0.05, ** P<0.01 (as compared to week 0 in the same group); ## P<0.01 (as compared to hyperglycemics in the same week)
Figure 5.
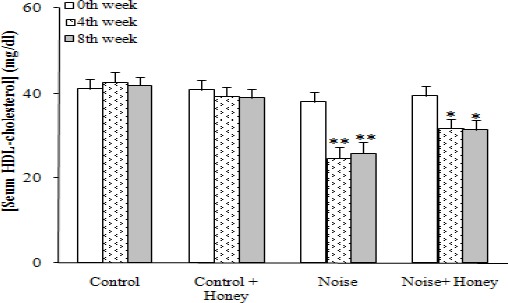
Serum HDL-cholesterol concentration in weeks 0, 4, and 8, (means±SEM). * P<0.05, ** P<0.01 (as compared to week 0 in the same group)
Figure 6.
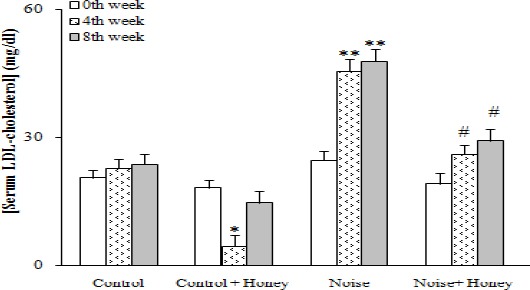
Serum LDL-cholesterol concentration in weeks 0, 4, and 8, (means±SEM). * P<0.05, **P<0.01 (as compared to week 0 in the same group); # P<0.05 (as compared to hyperglycemics in the same week)
Markers of oxidative stress: Regarding brain lipid peroxidation and oxidative stress markers (Figures 7, 8), noise-induced diabetes resulted in significant elevation of MDA content (7.03±0.29) (P<0.001) and significant reduction of SOD activity (4.46±0.14) (P<0.001); treatment of noise induced rats with honey altered increment of MDA content (5.98±0.25) (P<0.01) and reduction of SOD activity (5.77±0.18) (P<0.01). However, level of SOD was significantly higher than it in honey treated diabetics as compared to diabetic group.
Figure 7.
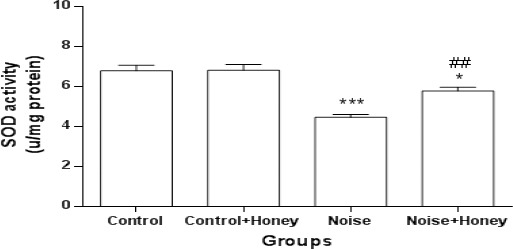
Superoxide dismutase activity in whole brain homogenate in different groups (means±SEM). * P<0.05, *** P<0.001 (as compared to control group); ## P<0.01 (as compared to hyperglycemic group)
Figure 8.
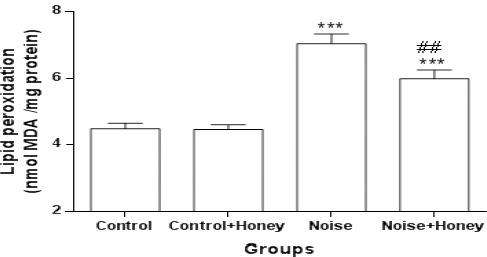
MDA concentration in whole brain homogenate in different groups (means±SEM)
*** P<0.001 (as compared to control group); ## P<0.01 (as compared to hyperglycemic group)
Histology of pancreas gland
The pancreas tissue in control group showed langerhans islands with well borders and beta cells with clear margins and abundant excretory granules, but noise induced diabetic rats showed granules with atrophic and wrinkle changes. In addition, the β cells were degranulated and diminished. In noise induced diabetic rats the number of β cells in each island was significantly decreased and treatment with honey had an intense effect in increasing pancreatic β cells. Treatment in control group did not change the number of β cells in each island. (Table 1, Figure 9).
Table 1.
Circumference on langerhans island in different groups, (means±SEM) (n=8). *P<0.05 (as compared to control group)
| Group | Diameter of islands (µm) |
|---|---|
| Control | 16/5 ± 475/3 |
| Control + Honey | 36/5± 481 |
| Noise | * 19/4 ± 217/4 |
| Noise + Honey | * 28/5 ± 309/2 |
Figure 9.
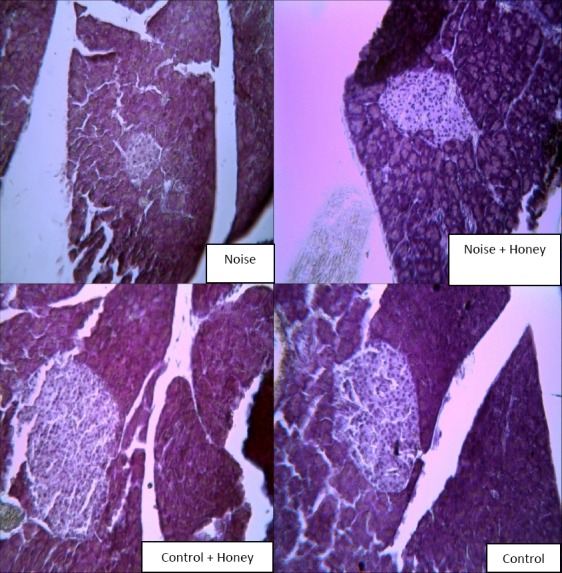
Cells of the pancreas were present in their normal proportions. The islet cells are seen embedded within the acinar cells and surrounded by a fine capsule. (Gomory staining, magnification 400x)
Discussion
In this study, development of diabetic indicators in noise stress induced rats was confirmed after two months. The present study observed the effect of noise on blood glucose and lipids and revealed the elevation of the plasma blood glucose and lipids in chronic noise exposure group as compared to control. According to our results chronic noise stress caused significantly lower body weight in stressed rats, on the 4th and 8th weeks of the experiment; however, treatment with honey had protective effects on these abnormal indicators.
Increasing activity of corticotrophin releasing hormone (CRH), as an anorexigenic neuropeptide, following to stress exposure, led to decrement of food intake and body weight in the stressed rats (23, 24). However it has also been proposed that glucocorticoids provoke a dose-related increment in total caloric intake (25). In the chronically stressed rats, even if food intake is not altered (26) or increased (27), reduction in body weight gain is observed. This may be a result of stress activation of the rich sympathetic innervations of brown adipose tissue, which is possibly increased in stressed rats (26). Thus our results regarding the weights of the animals were consistent with previous studies. Other investigations have shown a protein in brain and pancreas called brain–pancreas relative protein (BPRP) which is affected with many of stressors. It has been revealed that pancreatic BPRP expression was decreased significantly in stress induced diabetic rats in which glucose levels can reach to 40 mmol/l. Different response to the hyperglycemia and hypoinsulinemia suggests that the BPRP in pancreas is highly sensitive to the changes in levels of glucose and insulin and these changes may play a role in onset of diabetic condition (28).
Increased level of MDA and reduced activity of SOD were observed in noise induced diabetic rats in our study which might be due to the increased lipid peroxidation and overgenesis of ROS, which is in compatible with the previous report (27). The results of the present study also showed that honey administration can exert an antioxidant effect in noise induced diabetic rats, not in control normoglycemic rats. Increased free radical mediated toxicity has also been well documented in clinical diabetes (27) and STZ-diabetic rats (29). The elevated level of toxic oxidants during diabetic state may be due to the processes such as glucose oxidation and lipid peroxidation (27). Based on our results, oral administration of honey for two months produced a significant protecting effect at tissue pancreas in noise induced diabetic rats. One of the possible mechanisms that could partially explain the beneficial property of honey may be attributed to its hypoglycemic and antioxidant effects (7, 30-33).
A similar result has been obtained with flavonoids, which have almost no effects on glycemic status and oxidative stress markers in control rats, but show major effects these parameters in STZ-treated rats (34). According to previous research finding in diabetic rats induced by alloxan or STZ, increase in blood glucose level indirectly increases the serum TG, LDL-cholesterol, VLDL-cholesterol, and decreases HDL-cholesterol serum level (35). These finding confirm that undesirable changes of lipid serum levels are exist in noise induced diabetic rats in this study. Diabetes induce condition by some material like STZ and alloxan in rodents like rat is associated with degenerative changes in the pancreas island of langerhans and prominent undesirable changes in the lipid serum and lipoprotein level of plasma. According to this, some tissues like liver have essential roles in absorption of blood free fatty acid, oxidation, and metabolic exchange to other material, cholesterol, phospholipids exceeding and exertion of some kind of lipoprotein into the blood (35).
Conclusion
The results of the present study demonstrate that chronic noise stress increases fasting plasma glucose, lipid profile, and MDA levels and decreases SOD and body weight levels, and degenerates pancreas tissue; honey feeding ameliorates these parameters over 60 days. The most interesting finding of this study is that pancreatic β cells from noise induced diabetic group fed with honey demonstrate an increased intensively compared to controls. However, this finding should be considered in future studies on the destructive effects of noise stress.
Acknowledgment
The authors wish to appreciate the sincere collaboration of head of Physiology Research Center of Jundishapur University of Medical Sciences, Ahvaz, Iran, for his great technical assistance.
References
- 1.Mahmood R, Khan GJ, Alam S, Safi AJ. Effect of 90 decibel noise of 4000 hertz on blood pressure in young adults. J Ayub Med Coll Abbottabad. 2003;16:30–33. [PubMed] [Google Scholar]
- 2.Babisch W. The noise/stress concept, risk assessment and research needs. Noise Health. 2002;4:1. [PubMed] [Google Scholar]
- 3.Babisch W. Epidemiological studies of the cardiovascular effects of occupational noise-a critical appraisal. Noise Health. 1998;1:24. [PubMed] [Google Scholar]
- 4.Theebe MA. Planes, trains, and automobiles: the impact of traffic noise on house prices. J Real Estate Finance and Economics. 2004;28:209–234. [Google Scholar]
- 5.Van Kempen EE, Kruize H, Boshuizen HC, Ameling CB, Staatsen BA, de Hollander AE. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ Health Perspect. 2002;110:307. doi: 10.1289/ehp.02110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maschke C, Harder J, Ising H, Hecht K, Thierfelder W. Stress hormone changes in persons exposed to simulated night noise Noise Health. 2002;5:35. [PubMed] [Google Scholar]
- 7.Gate L, Paul J, Ba GN, Tew K, Tapiero H. Oxidative stress induced in pathologies: the role of antioxidants. Biomed Pharmacol. 1999;53:169–180. doi: 10.1016/S0753-3322(99)80086-9. [DOI] [PubMed] [Google Scholar]
- 8.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant [alpha]-lipoic acid. Free Radic Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 9.Fridovich I. Superoxide anion radical (O• ?2), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 10.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharm. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 11.Van Campen LE, Murphy WJ, Franks JR, Mathias PI, Toraason MA. Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res. 2002;164:29–38. doi: 10.1016/s0378-5955(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 12.Molan PC. The antibacterial activity of honey: 2 Variation in the potency of the antibacterial activity. 1992 [Google Scholar]
- 13.Molan P. Honey as an antimicrobial agent. Bee Products: Springer; 1997. pp. 27–37. [Google Scholar]
- 14.Beretta G, Granata P, Ferrero M, Orioli M, Maffei Facino R. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 2005;533:185–191. [Google Scholar]
- 15.Nagai T, Inoue R, Kanamori N, Suzuki N, Nagashima T. Characterization of honey from different floral sources. Its functional properties and effects of honey species on storage of meat. Food Chem. 2006;97:256–262. [Google Scholar]
- 16.Martos I, Ferreres F, Yao L, D'Arcy B, Caffin N, Tomás-Barberán FA. Flavonoids in monospecific Eucalyptus honeys from Australia. J Agric Food Chem. 2000;48:4744–4748. doi: 10.1021/jf000277i. [DOI] [PubMed] [Google Scholar]
- 17.Machha A, Mustafa MR. Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J Cardiovasc Pharmacol. 2005;46:36–40. doi: 10.1097/01.fjc.0000162769.83324.c1. [DOI] [PubMed] [Google Scholar]
- 18.Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Tox. 2008;46:3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 19.Roghani M, Baluchnejadmojarad T. Chronic epigallocatechin-gallate improves aortic reactivity of diabetic rats: underlying mechanisms. Vascul Pharmacol. 2009;51:84–89. doi: 10.1016/j.vph.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Roghani M, Baluchnejadmojarad T. Hypoglycemic and hypolipidemic effect and antioxidant activity of chronic epigallocatechin-gallate in streptozotocin-diabetic rats. Pathophysiology. 2010;17:55–59. doi: 10.1016/j.pathophys.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Roghani M, Baluchnejadmojarad T. Mechanisms underlying vascular effect of chronic resveratrol in streptozotocin?diabetic rats. Phytother Res. 2010;24:S148–S54. doi: 10.1002/ptr.3032. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Euro J Pharm. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 24.Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology. 1999;70:160–167. doi: 10.1159/000054472. [DOI] [PubMed] [Google Scholar]
- 25.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–2185. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 26.Gao B, Kikuchi-Utsumi K, Ohinata H, Hashimoto M, Kuroshima A. Repeated immobilization stress increases uncoupling protein 1 expression and activity in Wistar rats. Jpn J Physiol. 2003;53:205–214. doi: 10.2170/jjphysiol.53.205. [DOI] [PubMed] [Google Scholar]
- 27.Akana SF, Strack AM, Hanson ES, Horsley CJ, Mulligan ED, Bhatnagar S, et al. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–46. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- 28.Lin YH, Liu AH, Xu Y, Tie L, Yu HM, Li XJ. Effect of chronic unpredictable mild stress on brain–pancreas relative protein in rat brain and pancreas. Behav Brain Res. 2005;165:63–71. doi: 10.1016/j.bbr.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Toleikis PM, Godin DV. Alteration of antioxidant status in diabetic rats by chronic exposure to psychological stressors. Pharmacol Biochem Behav. 1995;52:355–366. doi: 10.1016/0091-3057(95)00117-f. [DOI] [PubMed] [Google Scholar]
- 30.Gill-Sharma M, D'Souza S, Parte P, Balasinor N, Choudhuri J, Majramkar D, et al. Effect of oral tamoxifen on semen characteristics and serum hormone profile in male bonnet monkeys. Contraception. 2003;67:409–413. doi: 10.1016/s0010-7824(03)00018-0. [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Ghani AS, Dabdoub N, Muhammad R, Abdul-Ghani R, Qazzaz M. Effect of palestinian honey on spermatogenesis in rats. J Med Food. 2008;11:799–802. doi: 10.1089/jmf.2008.0085. [DOI] [PubMed] [Google Scholar]
- 32.Siti A. Honey and reproductive hormones. Malays J Med Sci. 2007;14:105–106. [Google Scholar]
- 33.Kenjerić D, Mandić ML, Primorac L, Bubalo D, Perl A. Flavonoid profile of i>Robinia /i>honeys produced in Croatia. Food Chem. 2007;102:683–690. [Google Scholar]
- 34.Mirshekar M, Roghani M, Khalili M, Baluchnejadmojarad T, Moazzen SA. Chronic oral pelargonidin alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: involvement of oxidative stress. Iran Biomed J. 2010;14:33. [PMC free article] [PubMed] [Google Scholar]
- 35.Yanardağ R, Bolkent Ş, Özsoy?Saçan Ö, Karabulut?Bulan Ö. The effects of chard (Beta vulgaris L. var. cicla) extract on the kidney tissue, serum urea and creatinine levels of diabetic rats. Phytother Res. 2002;16:758–761. doi: 10.1002/ptr.1041. [DOI] [PubMed] [Google Scholar]


