Abstract
Objective(s):
Neuropathic pain remains a clinical problem and is poorly relieved by conventional analgesics. This study was designed to determine whether maprotiline administration was effective in alleviating symptoms of neuropathic pain and whether the antinociceptive effect of maprotiline mediated through the opioid system.
Materials and Methods:
Neuropathic pain was induced by chronic constriction injury (CCI) of the sciatic nerve in rats, which resulted in thermal hyperalgesia, and mechanical and cold allodynia. Maprotiline (10, 20 and 40 mg/kg, IP) was administered on the 7th and 14th days after surgery. To study the role of the opioid system in the antinociceptive effects of maprotiline, maprotiline (20 mg/kg, IP) was administered in combination with naloxone (1 mg/kg, SC) on the 7th post-surgery day. Behavioral tests were done at 45 min after drug injections on the 7th and 14th days after surgery.
Results:
Systemic administration of maprotiline blocked heat hyperalgesia, cold allodynia and reduced mechanical allodynia. Also antihyperalgesic effect of maprotiline was reversed by pretreatment with naloxone.
Conclusion:
Our results suggest that maprotiline can be considered a potential therapeutic for the treatment of neuropathic pain, and the opioid system may be involved in the antihyperalgesic effects of maprotiline.
Keywords: Maprotiline, Naloxone, Neuropathic pain, Opioids, Rat
Introduction
Neuropathic pain as consequence of nerve injury that affects the somatosensory system is one of the most difficult challenges in pain treatment (1). The management of chronic pain and, mainly, neuropathic pain, is still a major challenge to clinicians because of its unresponsiveness to most of the currently used analgesic drugs (2).
The etiology and underlying mechanisms of such pains are poorly understood and the existing treatments have limited benefits and are associated with significant drawbacks (3). Hence, it is important to elucidate the mechanisms underlying these surprisingly common disorders and identify new strategies for the development of effective therapies (4).
Antinociceptive effects of some antide- pressants have been demonstrated (5, 6). Maprotiline is an antidepressant drug with an atypical tetracyclic structure differing from the conventional antidepressants such as amitriptyline and imipramine. This drug is well tolerated with fewer side effects (7, 8).
Maprotiline is a strong norepinephrine reuptake inhibitor with only weak effects on serotonin and dopamine reuptake and weak blocking effect at the muscarinic (mACh) postsynaptic receptors (9, 10). There are a few reports that explain the analgesic properties of maprotiline (11, 12).
One published randomized, double-blind crossover trial has reported that maprotiline relieved post herpetic neuralgia (PHN) in many patients (13).
Obata et al have reported that intrathecal administration of maprotiline did not produce antiallodynic effects in a rat model of neuropathic pain (14). However Nakajima and his colleagues have shown the analgesic effects of maprotiline as a selective norepinephrine reuptake inhibitor. Their results showed that maprotiline produced an antiallodynic effect, and the observed effect was reversed by intrathecal idazoxan administration, an alpha2-adrenoceptor antagonist (9).
Some studies also have reported that maprotiline enhances analgesic effects of morphine and delayed tolerance development to morphine (15, 16). But until now the effects of maprotiline in inhibition of peripheral neuropathy and also underlying mechanisms that may be involved in its antinociceptive effects have not been fully examined.
Therefore, the aims of the present study were to determine the effects of systemic maprotiline administration in an animal model of peripheral neuropathic pain and the possible involvement of the opioidergic system in the antinociceptive effects of maprotiline.
Materials and Methods
Chemicals
Maprotiline and naloxone were obtained from Darupakhsh pharmaceutical Co, Iran. Acetone was bought from Iran Kaveh Co, Iran. Maprotiline was dissolved in 0.9% NaCl solution.
Animals and housing conditions
The experiments were performed on male Sprague–Dawley rats (200–250 g). They were housed under standard environmental conditions (12/–12 hr light/dark cycle at 22 °C), with free access to food and water.
Neuropathic pain model
The rats were anesthetized with ketamine (50 mg/kg IP) and xylazine (10 mg/kg IP). Common sciatic nerve was exposed and four ligatures (4.0 chromic gut) with a 1–1.5 mm interval between ligatures were tied loosely around the nerve. Sham-operated rats had the same surgery, the left sciatic nerve was exposed but no ligation was made. All experiments followed the ethical standards for investigation of experimental pain in animals and were also approved by the Research and Ethics Committee of Kashan University of Medical Sciences, Kashan, Iran (17).
Behavioral tests of neuropathic pain
Behavioral scores of the neuropathic pain were determined as thermal hyperalgesia, cold and mechanical allodynia. All experiments were performed between 8:00 AM and 1:00 PM to prevent response fluctuation. After cage exploration and major grooming activities ceased, the behavioral tests were performed. The behavioral scores of neuropathic pain were determined 45 min after the drug injection on the 7th and 14th post-operative days (18).
Thermal hyperalgesia (Plantar test)
By using a Plantar test apparatus (Ugo Basile, Varese, Italy), hyperalgesia to the thermal stimulus was measured. Rats were placed in a Plexiglass container on a transparent glass floor and allowed to fully adapt. An infrared beam was moved beneath the mid-plantar surface of the hind paw through the glass window. Time (seconds) between the irradiation onset and paw withdrawal was defined as paw withdrawal latency. A cut-off time of 22 sec was allowed to avoid tissue damage. Each animal was tested three times with 5 min intervals between stimulations to avoid sensitization of the hind paw. Mean time of the withdrawal latency for ipsilateral (operated) paws were calculated separately (19).
Cold allodynia (acetone test)
Cold allodynia was performed by using the acetone spray test (evaporation-evoked cooling). This test was performed by applying one drop of acetone bubble touched with the plantar surface of hind paw. This test was repeated five times with one minute intervals. If the animal withdrew or shook its hind paw in response to acetone drop, response was considered positive. The frequency of paw withdrawal was expressed as a percentage (the number of paw withdrawals/number of trials×100) (20).
Mechanical allodynia (von Frey filament stimulation)
Paw withdrawal threshold to mechanical stimuli was measured using von Frey filaments (Stoelting, Wood Dale, IL, USA) in the following order: 0.6, 1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, 26.0 and 60 g. Rats were placed on a mesh wire (0.8×0.8 cm cell) floor, on an elevated transparent Plexiglass box (18×18×25 cm). After acclimatization, filaments in an increasing manner from weakest to strongest force were perpendicularly touched to the plantar surface of ipsilateral hind paw. The smallest filament size which induced at least 3 withdrawal responses during 5 consecutive applications was considered as withdrawal threshold (21).
Treatment
Day 7 was chosen as the start day of treatment because previous studies have indicated that the neuropathic pain is fully established and stable by this time (22). Maprotiline (10, 20 and 40 mg/kg IP) was administered on the 7th and 14th days after surgery 45 min before behavioral tests, in each group of rats (6). The vehicle groups were given saline injections according to the same schedule.
Evaluation of the role of opioid receptors
To study the role of the opioid system in the antinociceptive effects of maprotiline, this drug (20 mg/kg IP) was administered in combination with naloxone (1 mg/kg SC) on the 7th post-surgery day.
Statistical analysis
Data were compared by one-way analysis of variance (ANOVA) followed by Fisher LSD post hoc test for multiple comparisons. Time-course analysis of behavioral data was compared by ANOVA repeated measures for each experimental group.
Results
Behavioral tests of neuropathic pain
The rats did not show any signs of autotomy after the sciatic nerve ligation. Paw gesture of the ipsilateral paw was slightly altered, but this did not interfere with the normal activity of the rats.
Cold allodynia
As shown in Figure 1A the ipsilateral paw of nerve ligated animals became much more sensitive to acetone application (P<<0.001). Sham operation did not produce any alteration of the nociceptive response. Maprotiline (10, 20 and 40 mg/kg IP) treatment significantly reduced the withdrawal frequency in comparison with CCI group (P<<0.01) (Figure 1B).
Figure 1.
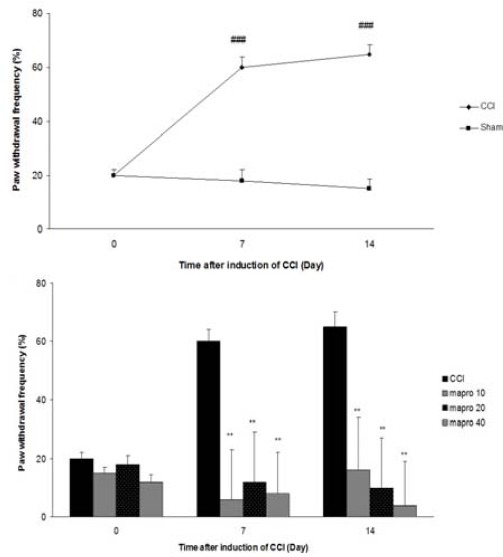
Cold allodynia after CCI, (A) Time course of cold allodynia (B), Effect of maprotiline (10, 20 and 40 mg/kg IP) on cold allodynia The results are expressed as Mean ± SEM, ###P<<0.001 versus sham group, **P<<0.01 versus CCI group. n=6 in all groups
Thermal hyperalgesia
Sciatic nerve ligation decreased paw withdrawal latency to the thermal stimulus in ipsilateral paw, but sham operation did not produce any significant change in the paw withdrawal latency (Figure 2A). Acute administration of maprotiline blocked thermal hyperalgesia on the 7th and 14th days after surgery (P<<0.001) (Figure 2B).
Figure 2.
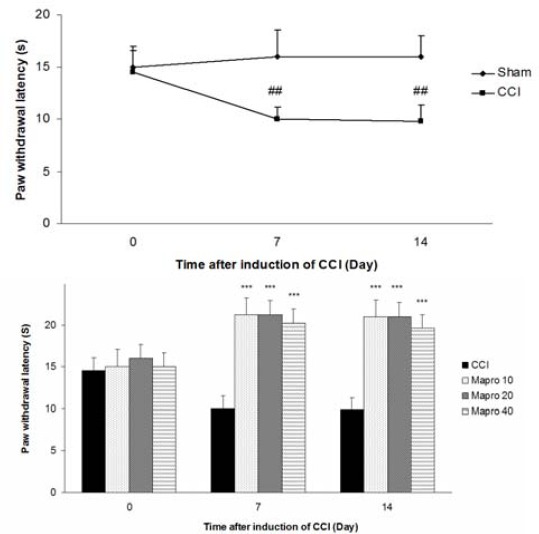
Heat hyperalgesia after CCI, (A) Time course of heat hyperalgesia, (B) Effect of maprotiline (10, 20 and 40 mg/kg IP) on heat hyperalgesia. The results are expressed as Mean ± SEM, n=6 in all groups. ***P<<0.001 versus CCI group, ##P<<0.01 versus sham group
Mechanical allodynia
Sciatic nerve ligation led to a significant decrease of withdrawal threshold of ipsilateral paw in comparison with sham operated group (P<0.001). Treatment with maprotiline (20 and 40 mg/kg IP) significantly modified paw withdrawal threshold (P<<0.01) (Figure 3B).
Figure 3.
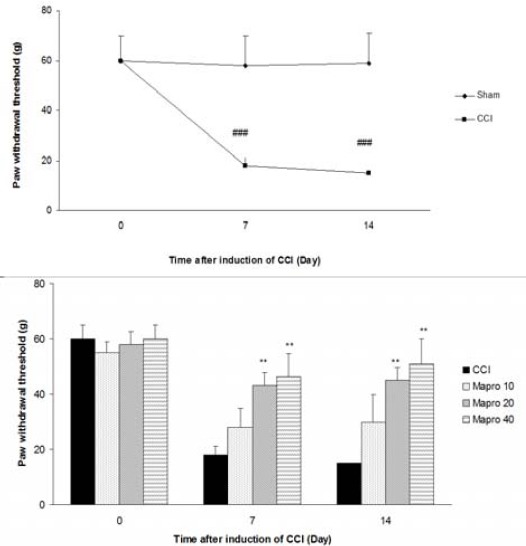
Mechanical allodynia after CCI, (A) Time course of mechanical allodynia, (B) Effect of maprotiline (10, 20 and 40 mg/kg IP) on the mechanical allodynia. The results are expressed as Mean ± SEM, n=6 in all groups. **P<<0.01 versus CCI group, ###P<<0.001 versus sham group
The effects of naloxone on maprotiline-induced effects
Figure 4 shows the effects of naloxone on the thermal anti-hyperalgesic effect of maprotiline (20 mg/kg IP). Naloxone significantly prevented antihyperalgesic effect of maprotiline in the radiant heat Plantar test. However could not significantly change the effect of maprotiline in von Frey filament or acetone test. Also naloxone (1 mg/kg SC) did not induce any significant change in the nociceptive thresholds (Figure 4).
Figure 4.
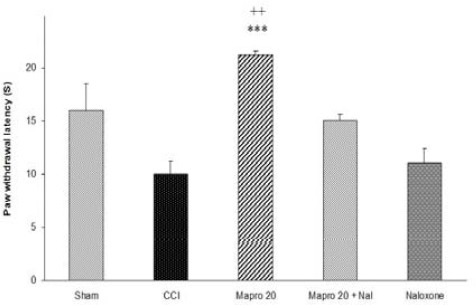
The effect of naloxone (1 mg/kg SC) on the antihyperalgesic effect of acute treatment with maprotiline (20 mg/kg IP). Mapro 20+ Nal: Rats treated with maprotiline (20 mg/kg IP) and naloxone (1 mg/kg SC). The results are expressed as Mean ± SEM, n=6 in all groups. ***P<<0.001 versus CCI group, ++P<<0.01 versus Mapro 20 + Nal
Discussion
In this study our results showed that systemic administration of maprotiline on the 7th and 14th days after surgery blocked heat hyperalgesia, cold allodynia and reduced mechanical allodynia. Rats did not show any sign of ataxia due to maprotiline (10, 20 and 40 mg/kg IP) administration. Also it has been reported that maprotiline only at higher doses (150 and 300 mg/kg PO) produced toxic effects in rats (23).
The increasing body of evidence has demonstrated the antinociceptive effects of anti-depressants particularly those with selective norepinephrine reuptake inhibition property (24-26). Maprotiline is an antidepressant drug with strong norepinephrine reuptake inhibition property (7). Considering our results maprotiline attenuated pain related behaviors in the neuropathic pain.
It could be suggested that maprotiline, by inhibiting norepinephrine reuptake, triggered the activation of the descending noradrenergic inhibitory pathway which produced antinociceptive effects (9). However, further studies are needed to confirm this claim.
Moreover maprotiline has a potent anti-inflammatory effect, and it i’s believed that this effect is mediated via central and peripheral mechanisms. Recent studies have demonstrated that injury of the peripheral nerve initiates an inflammatory cascade that induces activation of immune cells, therefore mediators such as histamine, IL-1β and TNF-α, which stimulate nociceptors, are released (27). These mediators enhanced inflammatory responses in the injured nerve and contribute to the neuropathic pain development (28).
Multiple lines of studies also have shown the interaction between maprotiline and the opioid system. It has been reported that maprotiline significantly increased the intensity and duration of morphine antinociceptive activity (15, 16). On the other hand studies on the animal model of neuropathic pain have demonstrated that peripheral nerve injury down-regulates the mu-opioid receptor (MOR) in the dorsal horn of the spinal cord (29, 30). This loss of spinal MOR is linked with behavioral exhibition of the neuropathic pain and the deficiency of opioids (31, 32). Our results showed that naloxone inhibits the antihyperalgesic effect of maprotiline in the radiant heat Plantar test. Hence, it can be suggested that opioid system may be involved in the antihyperalgesic effects of maprotiline. Previously it has been reported that naloxone also reversed antinociceptive effects of lithium in neuropathic pain (19). Also naloxone could not reverse the antinociceptive effects of maprotiline in cold and mechanical allodynia.
This difference is probably due to different nociceptors and neuronal pathways involved in different sensory modalities. Non-noxious tactile stimulus is transmitted chiefly through low-threshold, large diameter, myelinated Aβ fibers, while cold stimulus is transmitted to the spinal cord through high-threshold, thin unmyelinated primary C-fiber nociceptors (33). In particular, that painful reaction to innocuous thermal stimuli seems not to be due to a simple decrease in nociceptor thresholds. The increasing body of evidence demonstrated the involvement of distinctive warm and cold receptors in thermal allodynia (34). Recent studies on transient receptor potential melastatin 8 (TRPM8) knockout mice indicate the involvement of cold and menthol-sensitive receptor TRPM8 in cold allodynia while it does not have any involvement in mediation of heat or mechanical pain (35).
Conclusion
Our results showed that maprotiline reduces behavioral scores of the neuropathic pain and the opioid system may be involved in the antihyperalgesic effects of maprotiline. Other mechanisms also may be involved in the antinociceptive effect of maprotiline, particularly in attenuation of cold and mechanical allodynia. Further investigations are required to clarify the other possible mechanisms of maprotiline in the treatment of neuropathic pain.
Acknowledgment
The authors are grateful to Vice Chancellor for Research, Kashan University of Medical Science, Kashan, Iran, for financial support.
Footnotes
Conflict of interest statement
The authors declare no conflicts of interest.
References
- 1.Zhuo M. Neuronal mechanism for neuropathic pain. Mol Pain. 2007;3:1–9. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ro L S, Jacobs J M. The role of the saphenous nerve in experimental sciatic nerve mononeuropathy produced by loose ligatures: a behavioral study. Pain. 1993;52:359–369. doi: 10.1016/0304-3959(93)90170-T. [DOI] [PubMed] [Google Scholar]
- 3.Guindon J, Hohmann AG. Recent advances in the pharmacological management of pain. Drugs. 2007;67:2121–2133. doi: 10.2165/00003495-200767150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bennett G, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Zychowska M, Rojewska E, Makuch W, Przewlocka B, Mika J. The influence of microglia activation on the efficacy of amitriptyline, doxepin, milnacipran, venlafaxine and fluoxetine in a rat model of neuropathic pain. Eur J Pharmacol. 2015;749:115–123. doi: 10.1016/j.ejphar.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A, Abed A. Antinociceptive effects of venlafaxine in a rat model of peripheral neuropathy: Role of alpha2-adrenergic receptors. Eur J Pharmacol. 2014;5:230–236. doi: 10.1016/j.ejphar.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 7.Ahles S, Gwirtsman H, Halaris A, Shah P, Schwarcz G, Hill MA. Comparative cardiac effects of maprotiline and doxepin in elderly depressed patients. J Clin Psychiatry. 1984;45:460–465. [PubMed] [Google Scholar]
- 8.Gruter W, Poldinger W. Maprotiline. Mod Probl Pharmacopsychiatry. 1982;18:17–48. [PubMed] [Google Scholar]
- 9.Nakajima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressants after peripheral nerve injury in the rat. Pain. 2012;153:990–997. doi: 10.1016/j.pain.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Yokogawa F, Kiuchi Y, Ishikawa Y, Otsuka N, Masuda Y, Oguchi K, et al. An investigation of monoamine-receptorsinvolved in antinociceptiveeffects of antidepressants. Anesth Analg. 2002;95:163–168. doi: 10.1097/00000539-200207000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Korzeniewska-Rybicka I, Plaznik A. Supraspinally mediated analgesic effect of antidepressant drugs. Pol J Pharmacol. 2000;52:93–99. [PubMed] [Google Scholar]
- 12.Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindstrom T, Thorell LH. A comparison of amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clin J Pain. 1997;13:313–323. doi: 10.1097/00002508-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Watson CP, Chipman M, Reed K, Evans RJ, Birkett N. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomized, double-blind, crossover trial. Pain. 1992;48:29–36. doi: 10.1016/0304-3959(92)90128-X. [DOI] [PubMed] [Google Scholar]
- 14.Obata H, Saito S, Koizuka S, Nishikawa K, Goto F. The monoamine-mediated antiallodyniceffects of intrathecally administered milnacipran, a serotonin noradrenaline reuptake inhibitor, in a rat model of neuropathic pain. Anesth Analg. 2005;100:1406–1410. doi: 10.1213/01.ANE.0000149546.97299.A2. [DOI] [PubMed] [Google Scholar]
- 15.Pettersen VL, Zapata-Sudo G, Raimundo JM, Trachez MM, Sudo RT. The synergistic interaction between morphine and maprotiline after intrathecal injection in rats. Anesth Analg. 2009;109:1312–1317. doi: 10.1213/ane.0b013e3181b16ff5. [DOI] [PubMed] [Google Scholar]
- 16.Lee RL, Spencer PS. Effect of tricyclic antidepressants on analgesic activity in laboratory animals. Postgrad Med J. 1980;1:19–24. [PubMed] [Google Scholar]
- 17.Verdi J, Jafari-Sabet M, Mokhtari R, Mesdaghinia A, Banafshe HR. The effect of progesterone on expression and development of neuropathic pain in a rat model of peripheral neuropathy. Eur J Pharmacol. 2013;699:207–212. doi: 10.1016/j.ejphar.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Amin B, Hajhashemi V, Hosseinzadeh H, Abnous Kh. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience. 2012;224:15–25. doi: 10.1016/j.neuroscience.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 19.Banafshe HR, Mesdaghinia A, Arani MN, Ramezani MH, Heydari A, Hamidi GA. Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacol Biochem Behav. 2012;100:425–430. doi: 10.1016/j.pbb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Banafshe H, Hamidi GA, Noureddini M, Mirhashemi M, Mokhtaria M, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202–206. doi: 10.1016/j.ejphar.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Hamidi GA, Ramezani MH, Arani MN, Talaei SA, Mesdaghinia A, Banafshe HR. Ethosuximide reduces allodynia and hyperalgesia and potentiates morphine effects in the chronic constriction injury model of neuropathic pain. Eur J Pharmacol. 2012;674:260–264. doi: 10.1016/j.ejphar.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Hajhashemi V, Sadeghi H, Minaiyan M, Movahedian A, Talebi A. Central and peripheral anti-inflammatory effects of maprotiline on carrageenan-induced paw edema in rats. Inflamm Res. 2012;59:1053–1059. doi: 10.1007/s00011-010-0225-1. [DOI] [PubMed] [Google Scholar]
- 23.Darcym P, Dredge K, Kellehir P, Kelly JP, Leonard BE, Chambers PL. Acutetoxicityprofile of maprotiline in the rat. Pharmacol Toxicol. 1999;85:276–281. doi: 10.1111/j.1600-0773.1999.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 24.Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20:449–458. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 25.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Evidence-based data from animal and human experimental studies on pain relief with antidepressants: a structured review. Pain Med. 2000;1:310–316. doi: 10.1046/j.1526-4637.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 26.McQuay JH, Tramer M, Nue BA, Carroll D, Wiffen PJ, Moore RA. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217–227. doi: 10.1016/s0304-3959(96)03140-5. [DOI] [PubMed] [Google Scholar]
- 27.Jia HB, Wang XM, Qiu LL, Liu XY, Shen JC, Ji Q, et al. Spinal neuroimmune activation inhibited by repeated administration of pioglitazone in rats after L5 spinal nerve transection. Neurosci Lett. 2013;543:130–135. doi: 10.1016/j.neulet.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynapthic Ca²? channel complex. Nat Med. 2011;17:822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff JR, Burkey AR, Goff DJ, Jasmin L. Reorganization of the spinal dorsal horn in models of chronic pain: correlation with behaviour. Neuroscience. 1998;82:559–574. doi: 10.1016/s0306-4522(97)00298-4. [DOI] [PubMed] [Google Scholar]
- 30.Porreca F, Tang QB, Bian D, Riedl M, Elde R, Lai J. Spinal opioid mu receptor expressionin lumbar spinal cord of rats following nerve injury. Brain Res. 1998;795:197–203. doi: 10.1016/s0006-8993(98)00292-3. [DOI] [PubMed] [Google Scholar]
- 31.Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributesto the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- 32.Back SK, Lee J, Hong SK, Na HS. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain. 2006;123:117–126. doi: 10.1016/j.pain.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 34.Gautron M, Jazat F, Ratinahirana H, Hauw JJ, Guilbaud G. Alterations in myelinated fibres in the sciatic nerve of rats after constriction: possible relationships between the presence of abnormal small myelinated fibres and pain-related behavior. Neurosci Lett. 1990;111:28–33. doi: 10.1016/0304-3940(90)90339-b. [DOI] [PubMed] [Google Scholar]
- 35.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]


