Abstract
Objective(s):
N-myc downstream regulated gene 2 (NDRG2) is a candidate gene for tumor suppression. The expression of NDRG2 is down-regulated in several tumors including lung cancer. The aim of this study was to explore the effect of NDRG2 overexpression on invasion, migration, and enzymatic activity of matrix metalloproteinase-2 (MMP-2) and -9 (MMP-9) in human lung adenocarcinoma A549 cells.
Materials and Methods:
A recombinant plasmid encoding green fluorescent protein (GFP)-tagged NDRG2 (pCMV6-AC-NDRG2-GFP) was used to overexpress GFP-tagged NDRG2 in A549 cells. The cells in the experimental group and those in the control group were transfected with pCMV6-AC-NDRG2-GFP and a control plasmid without NDRG2 (pCMV6-AC-GFP), respectively. Fluorescent microscopy and flowcytometry analysis of GFP expression were used to evaluate the cellular expression of GFP-tagged NDRG2 and the efficiency of transfection. The effects of NDRG2 expression on cell invasion and migration were evaluated using transwell filter migration assay. The gelatinase activity of secreted MMP-2 and MMP-9 was measured by gelatin zymography.
Results:
Our results demonstrated the expression of GFP-tagged NDRG2 in the cytoplasm and nucleus of A549 cells. The findings of transwell assay showed that NDRG2 overexpression reduced migration and invasion of A549 cells compared to control cells. Gelatin zymography analyses revealed that NDRG2 overexpression decreased the gelatinase activity of secreted MMP-2 and MMP-9.
Conclusion:
These findings suggest that NDRG2 may be a new anti-invasion factor in lung cancer that inhibits MMPs activities.
Keywords: Invasion, Lung cancer, Matrix metalloproteinase, Migration, N-myc downstream-regulated gene 2
Introduction
Lung cancer is one of the major causes of cancer related death in the world (1). The incidence and mortality rate of this malignancy is increasing annually. Lung cancer is often diagnosed in the late stages, when the tumor cells have been metastasized (2, 3). Currently, platin-based cytotoxic chemotherapy and removal of metastatic tumors by surgery are available therapeutic options. Resistance to chemotherapy and recurrence of metastatic tumors are major limitations of these treatments. Metastasis is a complex and multistep process which includes degradation of the extracellular matrix, migration of tumor cells into blood or lymph circulation, and invasion to other tissues (4). Understanding the molecular mechanisms involved in these processes is very crucial because it could lead to development of new therapeutic strategies.
N-myc downstream regulated gene 2 (NDRG2) belongs to NDRG family and encodes a 41KD cytoplasmic protein (5). It is normally expressed in several tissues and involved in multiple biological processes, including cell growth, differentiation, and apoptosis (5-7). Increasing evidence indicates the role of NDRG2 as a tumor suppressor in the control of cell growth and metastasis of tumor cells (5, 8, 9). Reduced expression of NDRG2 has been shown in several cancers including breast (10), colon (11), renal cell carcinoma (12), glioblastom (13), liver (14), and lung (15). On the other hand, over-expression of NDRG2 leads to inhibition of proliferation, invasion, and metastasis of tumor cell lines (16-19). Li et al (15) has recently reported down-regulation of NDRG2 gene in human lung cancer which was negatively correlated with the stage of tumor and survival time of the patients.
However, the effects of NDRG2 overexpression on the migration and invasion of lung tumor cells remain unknown. Matrix metalloproteinases (MMPs) are a large family of zinc-dependent peptidases involved in metastasis of various tumors through degradation of the extracellular matrix proteins (20). MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are among the most important MMPs highly expressed in the lung tumor cells and their expression is correlated with invasiveness of these cells (5, 20). In the current study, the effects of NDRG2 overexpression on the invasion and migration of A549 cell line were investigated. Furthermore, the effects of NDRG2 overexpression on the enzymatic activities of MMP-2 and -9 were also evaluated.
Materials and Methods
Cell culture and reagents
The human lung adenocarcinoma cell lines A549 was purchased from Pasteur Institute of Iran (NCBI code: C137). The cells were grown in RPMI (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum and 100 units/ml penicillin-streptomycin (Gibco/Invitrogen, Carlsbad, CA) at 37 °C in a humidified 5% CO2 incubator. Lipofectamine™ 2000 and Plasmid Filter Purification Kit were from Invitrogen.
Plasmid amplification and purification
A plasmid encoding C-terminal green fluorescent protein (GFP)-tagged NDRG2 (pCMV6–AC–GFP-NDRG2) and a negative control pCMV6–AC-GFP plasmid without NDRG2 (mock plasmid) were purchased from OriGene (OriGene, USA). The competent Escherichia coli strains DH5α were used for proliferation of plasmid constructs. For each transformation, 100 ng of DNA was added to 25 μl of competent cells and incubated on ice for 30 min, followed by heat shock at 42 °C for 2 min and incubation on ice for 2 min. The cells were allowed to recover in 1 ml Luria-Bertani (LB) broth and then incubated for 60 min at 37 °C with shaking. Cells were plated on LB-agar plate containing 100 μg/ml ampicillin (plasmids encoded ampicillin resistance) and incubated at 37 °C overnight to select the transformants. After overnight culture, one colony of each plasmid was transferred to 3 ml of LB broth supplemented with ampicillin (50 μg/ml) for 5 hr of pre-culture at 37 °C before transfer to 500 ml LB broth for a further overnight of incubation in a rotating incubator. The overnight culture was centrifuged at 5000 g for 10 min, and the resulting pellet was used to extract plasmid DNA using PureLink™ HiPure plasmid filter Purification Kit (Invitrogen, UK) as per manufacturer’s instructions. The concentration of the DNA extracted was measured using the NanoDrop ND-100 spectrophotometer.
Overexpression of the NDRG2 gene in A549 cells
A549 cells were transfected with NDRG2 plasmid or mock plasmid using lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. After 48 hr, the transfected cells were detached with EDTA (10 mM in PBS), washed, and resuspended in cold PBS buffer. Fluorescent microscopy and flowcytometry analysis were then used to monitor the cellular expression of GFP-tagged NDRG2 and to measure the efficiency of transfection. Fluorescence microscopy was performed on an inverted microscope (Hund, Germany) with filter sets designed for GFP.
Flowcytometric analysis was conducted using FACSAria flowcytometer (BD Biosciences, USA) equipped with a water cooled 488 nm argon-ion laser. Green fluorescence (FL1 detector) was detected using 530/30 filter. The data were analyzed with Cell Quest software (BD Biosciences, USA). For each sample, 20,000 events were collected.
Migration and invasion assays
Invasion and migration assays were performed using a 24 well transwell insert (8 µm pore filters, BD Bioscience, Bedford, MA) with and without matrigel-coated membrane, respectively. Briefly, for migration assays, after filling the lower part of the transwell with RPMI plus 10% FBS, A549 cells (5×103) suspended in serum-free RPMI were added to the upper part of the transwell, and incubated for 6 hr at 37 °C. The cells were allowed to migrate to the bottom of the membrane. After incubation, the cells migrated to the lower surface of the transwell were fixed with methanol for 5 min and stained with 0.1% crystal violet. For invasion assays, the transwell was coated with 100 µl (1 mg/ml) matrigel (BD Bioscience, Bedford, MA). A549 cells (5×103) were plated onto the upper part of the matrigel-coated transwell chamber and incubated for 24 hr. The invaded cells were then fixed with methanol, stained and counted. The number of invading or migrating cells was determined by counting five high-power fields (400) on each membrane and was calculated as the mean number of cells per field (21).
Determination of MMP-2 and MMP-9 activities by gelatin zymography
The enzymatic activities of MMP-2 and MMP -9 were analyzed by gelatin zymography. Briefly, serum-free culture supernatant fractions were collected and mixed at a 1:1 ratio with non-reducing sample buffer (2% sodium dodecyl sulfate, 50 mM Tris-HCl (PH 6.8), 10% glycerol, and 0.001% bromophenol blue for preparing samples. The samples were electrophoresed on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) containing 0.1% gelatin. After separation of proteins, the gel was washed twice with washing buffer (50 mM Tris-HCl at pH 7.5, 100 mM NaCl, and 2.5% triton X-100) and then treated with incubation buffer (50 mM Tris-HCl at PH 7.5, 150 mM NaCl, 10 mM CaCl2, and 0.02% NaN3) at 37 °C for 24 hr. After incubation, the gels were stained (0.5% coomassie blue, 10% metanol, and 5% acetic acid) and then destained (20% methanol and 10% acetic acid). MMPs were detected as a clear zone on the blue background. The gels were scanned using a densitometer (Bio-Rad GS-800) and the density of the bands was measured using Image J software (National Institutes of Health, Bethesda, MD, USA) (22-24).
Statistical analysis
All statistical analyses were performed using SPSS software (version 15.0; SPSS, Inc., Chicago, IL, USA). The normal distribution of variables was assessed using the Shapiro Wilk test (P<0.05). Comparisons of variables between NDRG2 group and the control group were made using two-tailed Students t-test (for normally distributed invasion assay data) or Mann-Whitney U-test (for non-normally distributed migration assay data). All data are represented by the mean±standard deviation of at least three independent experiments. P-value< 0.05 was considered as the significant difference.
Results
Overexpression of NDRG2 in A549 cells
For overexpression of NDRG2 in A549 cells, the cells were transfected with a plasmid encoding C-terminal GFP-tagged NDRG2. The expression of GFP-tagged NDRG2 was confirmed by fluorescence microscopy (Figure 1). NDRG2 was localized in the cytoplasm and nucleus of the cells. Flowcytometry analysis showed that the efficiency of transfection was more than 60% (Figure 2).
Figure 1.
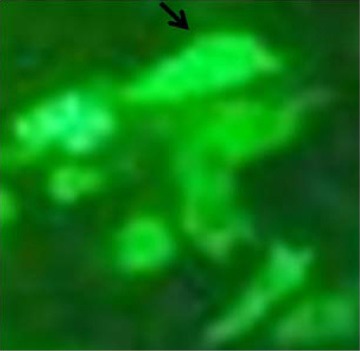
Overexpression of GFP-tagged N-myc downstream regulated gene 2 in the A549 cells
Green fluorescence represented the successful transfection and overexpression of GFP-tagged NDRG2. The exogenously introduced NDRG2 was localized in the cytoplasm of the A549 cells
Figure 2.
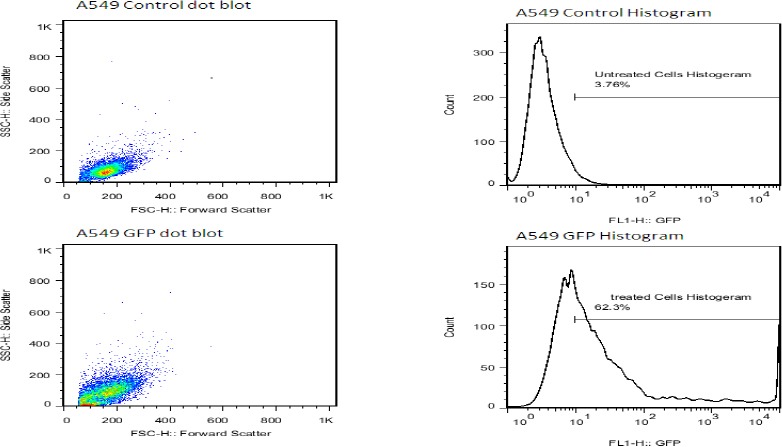
Determination of transfection efficiency of pCMV6-AC-GFP vector using flowcytometry. Right: detection of transfection efficiency by flowcytometry. Transfection efficiency was maintained at 60%, 48 hr post-transfection. Left: dot blot flowcytometry after 48 hr. High efficiency of transfection with fluorescent GFP (green) in A549 cells was easily identified for 48 hr post-transfection (×100). Green fluorescence intensities (FL1-H) are indicated on the x axis, and cell counts are indicated on the y axis. The cytometric analysis was performed for 2 × 104 events
NDRG2 gene inhibits the invasion and migration of human lung cancer cells in vitro
To evaluate the effect of NDRG2 overexpression on metastatic activity, we performed in vitro transwell migration assays and matrigel coated transwell invasion assays with human lung cancer cell line A549. Representative micrographs (Figure 3) were taken from the lower surface of the transwell filter, and the cells that migrated or invaded were counted. As shown in Figure 3, the number of migrated cells was considerably reduced in NDRG2 overexpressing cells compared to the control cells (P<0.02). Furthermore, overexpression of NDRG2 in the cells significantly decreased the number of invaded cells compared to the controls (P<0.02; Figure 4).
Figure 3.
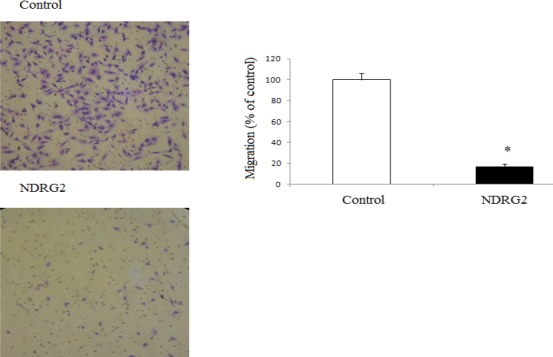
Effects of NDRG2 overexpression on the migration of A549 cells in an in vitro assay. A fixed number of cells were plated onto the upper part of the transwell chamber. After incubation for 6 hr, invasive cells were counted at the lower part of transwell filter. Representative micrographs from the lower surface of the transwell filter. Overexpression of NDRG2 significantly suppressed their migration through the transwell. *P< 0.001 (values are compared with control cells)
Figure 4.
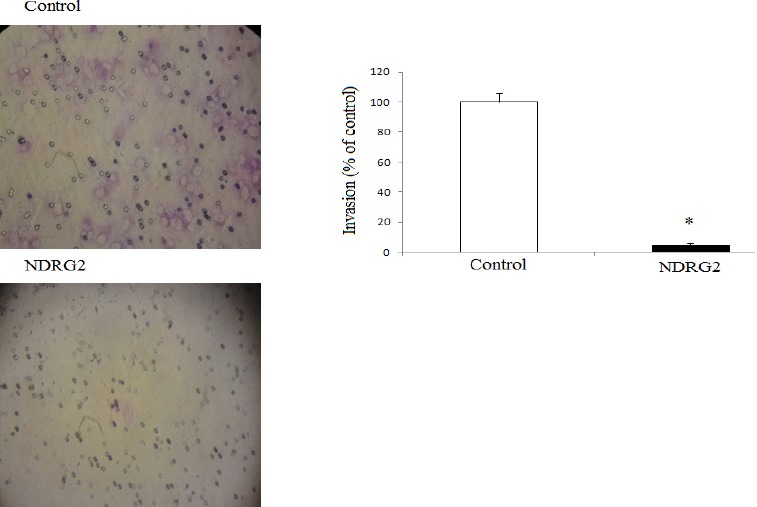
Effects of N-myc downstream regulated gene 2 overexpression on the invasion of A549 cells. A fixed number of cells were plated onto the upper part of the matrigel-coated transwell chamber. After incubation for 24 hr, invasive cells were counted at the lower part of transwell. *P<0.002 compared with control cells
NDRG2 transfection suppresses the expression of MMP-2, MMP-9 in A549 cells
Gelatin zymography was used to evaluate the effects of NDRG2 overexpression on MMP-2 and MMP-9 activity. As can be seen in Figure 5 A, A549 control cells express and secret MMP-2 and MMP-9. Both MMP-2 and MMP-9 were represented as pro- and active forms in the gel. Analysis of the gel for the effect of NDRG2 overexpression on MMP-2 and MMP-9 activity showed that both pro- and active forms of MMP-2 and MMP-9 significantly were reduced in NDRG2- transfected cells compared to the control cells (Figure 5 B).
Figure 5.
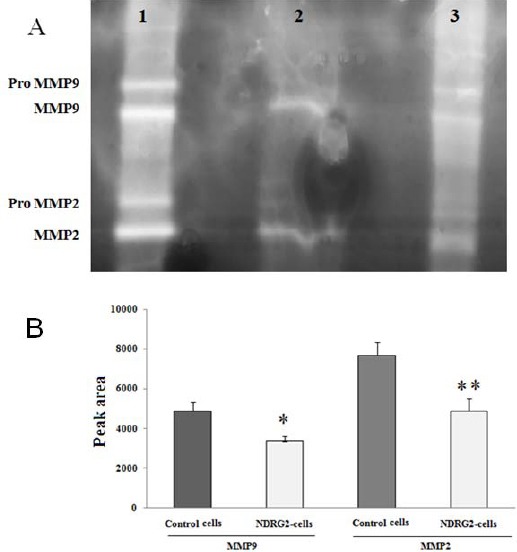
Effects of N-myc downstream regulated gene 2 overexpression on matrix metaloproteinase-2 and -9 activities. (A) A representative gelatin zymograph showing MMP-2 and MMP-9 from cell culture supernatant of A549 control cells (lane 1), NDRG2 overexpressing A549 cells (lane 2). A serum sample (contain secreted MMP-2 and -9) was used as the positive control (lane 3). (B) The density of MMP-2 and -9 bands obtained in gelatin-zymographic gel (10% SDS-PAGE containing 0.1% gelatin). The activities of MMP-2 and MMP-9 were decreased in NDRG2 overexpressed cells compared to the control cells. **P< 0.01 and *P< 0.05 vs. control
Discussion
A growing body of evidence has documented the role of human NDRG2 gene as a tumor suppressor in a variety of cancers (5, 8, 9). However, the anti-invasion effect of NDRG2 in the lung tumor cells was unclear. In this study, GFP-tagged human NDRG2 gene was overexpressed in a highly invasive A549 lung tumor cell line to investigate its effect on cell invasion. Our data showed that NDRG2 was increased significantly in the nucleus and cytoplasm of the cells. In vitro invasion and migration assays showed that NDRG2 inhibited migration and invasion of A549 cells. Moreover, the activities of the secreted form of MMP-2 and MMP-9 were decreased, upon overexpression of NDRG2. To the best of our knowledge, it is the first study about the effect of NDRG2 on invasion of the lung tumor cell line, suggesting that NDRG2 may act as an anti-invasion factor in lung cancer.
It has been demonstrated that under basal condition the expression of NDRG2 is low and it is mainly localized in the cytoplasm of cells (7). When the cells are activated by stimulation factors, such as ischemia or hypoxia, NDRG2 is overexpressed and translocated to the nucleus. Thus, it appears that nucleolar localization of NDRG2 observed in our study may be related to the overexpression of NDRG2. This finding is consistent with the results of previous studies which showed the presence of NDRG2 in the cytoplasm and nucleus of other cells (7, 12). The presence of nuclear localization signal (NLS) sequence is required for translocation of many proteins to the nucleus. NLS sequence has not been shown in the primary structure of NDRG2 and it has been suggested that helix α6 in the structure of NDRG2 may be responsible for its translocation to the nucleus (5).
Tumor progression and metastasis are dependent on the ability of tumor cells to invade the surrounding tissues. The findings of our study revealed that NDRG2 significantly suppressed migration and invasion of A549 cells. Similar results have been reported about the effects of NDRG2 on invasion and migration of prostate (16), breast (25), and hepatocarcinoma (19) cancer cell lines, suggesting the role of NDRG2 as an anti-invasion factor. Degradation of the extracellular matrix by MMPs is required for cancer cell invasion. MMP-2 and MMP-9 are among the most important MMPs which are highly expressed in the lung tumor cells and its expression is correlated with invasiveness of these cells (20). The synthesis and secretion of MMP-2 and MMP-9 from A549 cells have been previously reported (18). We used SDS-PAGE gelatin zymography to measure the activities of MMP-9 and MMP-2. This technique is based on degradation of gelatin by MMP-9 and MMP-2. It is a repeatable and sensitive method that can detect picogram levels of MMP-9 and MMP-2. Furthermore, it can discriminate between MMP-2 and MMP-9 and their respective pro-enzymes (pro-MMP-2 and pro-MMP-9). Here, we showed a decreased level of MMP-2 and MMP-9 activities upon NDRG2 overexpression, suggesting that NDRG2 probably affects cell invasion via inhibition of MMP-2 and MMP-9. The activities of MMPs can be influenced by several factors including the rate of their expression. We did not evaluate the effect of NDRG2 on MMPs gene expression. It is a limitation of our study; thus, our results should be reconfirmed in studies employing real time PCR in order to define the effect of NDRG2 on MMPs gene expression. Despite this limitation, our results indicate that NDRG2 inhibited the invasion and reduced the activities of MMP-2 and -9.
The exact molecular mechanisms that are behind the NDRG2 effects in A549 cells have remained unknown. Up-regulation of TGF-b1 (9), nuclear factor kappa B (NF-kB) (5), and IL-8 (26) are among mechanisms previously suggested for the effects of NDRG2 on other cells. Further studies are required to explore the role of these pathways in the mechanism of NDRG2 effects in A549 cells.
Conclusion
In summary, our data support the view that overexpression of NDRG2 inhibits migration and invasion of A549 cells. The findings also showed the inhibition of MMP-2 and MMP-9 activities upon NDRG2 overexpression. It may be a molecular mechanism for anti-invasion effects of NDRG2. However, further studies are needed to explore the potential mechanisms through which NDRG2 inhibits invasion of A549 cells.
Acknowledgment
The results described in this paper were part of MSc thesis of Seyed Nooredin Faraji and was supported by grant number 90-5971 from the Vice-Chancellor for research affairs of Shiraz University of Medical Sciences, Shiraz, Iran. We are also grateful to all staff of Diagnostic Laboratory Sciences and Technology Research Center and Shiraz Institute for Cancer Research Center for technical assistance in this work.
References
- 1.Yang J, Li Y, Wu L, Zhang Z, Han T, Guo H, et al. NDRG2 in rat liver regeneration: role in proliferation and apoptosis. Wound Repair Regen. 2010;18:524–531. doi: 10.1111/j.1524-475X.2010.00614.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, et al. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer. 2005;10(116):52–61. doi: 10.1002/ijc.20997. [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Ferreri AM, Guerra F, Orlandi M. Anti-invasive effects and proapoptotic activity induction by the rexinoid IIF and valproic acid in combination on colon cancer cell lines. Anticancer Res. 2012;32:2855–2862. [PubMed] [Google Scholar]
- 4.Umesalma S, Nagendraprabhu P, Sudhandiran G. Antiproliferative and apoptotic-inducing potential of ellagic acid against 1,2-dimethyl hydrazine-induced colon tumorigenesis in Wistar rats. Mol Cell Biochem. 2014;388:157–172. doi: 10.1007/s11010-013-1907-0. [DOI] [PubMed] [Google Scholar]
- 5.Hwang J, Kim Y, Kang HB, Jaroszewski L, Deacon AM, Lee H, et al. Crystal structure of the human N-Myc downstream-regulated gene 2 protein provides insight into its role as a tumor suppressor. J Biol Chem. 2011;8(286):12450–12460. doi: 10.1074/jbc.M110.170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu XL, Liu XP, Deng YC, Lin SX, Wu L, Zhang J, et al. Expression analysis of the NDRG2 gene in mouse embryonic and adult tissues. Cell Tissue Res. 2006;325:67–76. doi: 10.1007/s00441-005-0137-5. [DOI] [PubMed] [Google Scholar]
- 7.Okuda T, Kokame K, Miyata T. Differential expression patterns of NDRG family proteins in the central nervous system. J Histochem Cytochem. 2008;56:175–182. doi: 10.1369/jhc.7A7323.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enayat S, Banerjee S. The ethanolic extract of bark from Salix aegyptiaca L. inhibits the metastatic potential and epithelial to mesenchymal transition of colon cancer cell lines. Nutr Cancer. 2014;66:999–1008. doi: 10.1080/01635581.2014.936949. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Yoon SY, Kim JT, Song EY, Lee HG, Son HJ, et al. NDRG2 expression decreases with tumor stages and regulates TCF/beta-catenin signaling in human colon carcinoma. Carcinogenesis. 2009;30:598–605. doi: 10.1093/carcin/bgp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorentzen A, Lewinsky RH, Bornholdt J, Vogel LK, Mitchelmore C. Expression profile of the N-myc Downstream Regulated Gene 2 (NDRG2) in human cancers with focus on breast cancer. BMC Cancer. 2011;11:14. doi: 10.1186/1471-2407-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Xie Y, Zhang H, Wu Y. Down-regulation of NDRG2 gene expression in human colorectal cancer involves promoter methylation and microRNA-650. Biochem Biophys Res Commun. 2011;25(406):534–538. doi: 10.1016/j.bbrc.2011.02.081. [DOI] [PubMed] [Google Scholar]
- 12.Ma J, Jin H, Wang H, Yuan J, Bao T, Jiang X, et al. Expression of NDRG2 in clear cell renal cell carcinoma. Biol Pharm Bull. 2008;31:1316–1320. doi: 10.1248/bpb.31.1316. [DOI] [PubMed] [Google Scholar]
- 13.Zhou B, Tang Z, Deng Y, Hou S, Liu N, Lin W, et al. Tumor suppressor candidate gene, NDRG2 is frequently inactivated in human glioblastoma multiforme. Mol Medicine Rep. 2014;10:891–896. doi: 10.3892/mmr.2014.2237. [DOI] [PubMed] [Google Scholar]
- 14.Lee DC, Kang YK, Kim WH, Jang YJ, Kim DJ, Park IY, et al. Functional and clinical evidence for NDRG2 as a candidate suppressor of liver cancer metastasis. Cancer Res. 2008;68:4210–4220. doi: 10.1158/0008-5472.CAN-07-5040. [DOI] [PubMed] [Google Scholar]
- 15.Li SJ, Wang WY, Li B, Chen B, Zhang B, Wang X, et al. Expression of NDRG2 in human lung cancer and its correlation with prognosis. Med Oncol. 2013;30:421. doi: 10.1007/s12032-012-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L, Wu GJ, Liu XW, Zhang R, Yu L, Zhang G, et al. Suppression of invasion and metastasis of prostate cancer cells by overexpression of NDRG2 gene. Cancer Lett. 2011;1(310):94–100. doi: 10.1016/j.canlet.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS, Kim JH, et al. NDRG2 suppresses cell proliferation through down-regulation of AP-1 activity in human colon carcinoma cells. Int J Cancer. 2009;1(124):7–15. doi: 10.1002/ijc.23945. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Yu C, Jiang F, Gao L, Li J, Wang Y, et al. Overexpression of N-Myc downstream-regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PloS one. 2013;16(8):e76689. doi: 10.1371/journal.pone.0076689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Li Y, Yang J, Liu Q, Shi M, Zhang R, et al. NDRG2 inhibits hepatocellular carcinoma adhesion, migration and invasion by regulating CD24 expression. BMC cancer. 2011;251:1–9. doi: 10.1186/1471-2407-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009;385:198–203. doi: 10.1016/j.bbrc.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Gui Q, Chen W, Wu L, Sun W, Zhang N, et al. Small interference RNA targeting tissue factor inhibits human lung adenocarcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2011;30:63. doi: 10.1186/1756-9966-30-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung LW, Leung PC, Wong AS. Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res. 2006;66:10902–10910. doi: 10.1158/0008-5472.CAN-06-2217. [DOI] [PubMed] [Google Scholar]
- 23.Dong W, Li H, Zhang Y, Yang H, Guo M, Li L, et al. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim Biophys Sin (Shanghai) 2011;43:840–848. doi: 10.1093/abbs/gmr085. [DOI] [PubMed] [Google Scholar]
- 24.Ikari A, Sato T, Takiguchi A, Atomi K, Yamazaki Y, Sugatani J, et al. Claudin-2 knockdown decreases matrix metalloproteinase-9 activity and cell migration via suppression of nuclear Sp1 in A549 cells Life Sci. 2011;286:628–633. doi: 10.1016/j.lfs.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Liu Q, Li Y, Yang J, Ma J, Yu F, et al. NDRG2 expression regulates CD24 and metastatic potential of breast cancer cells. Asian Pac J Cancer Prev. 2010;11:1817–1821. [PubMed] [Google Scholar]
- 26.Cao W, Zhang JL, Feng DY, Liu XW, Li Y, Wang LF, et al. The effect of adenovirus-conjugated NDRG2 on p53-mediated apoptosis of hepatocarcinoma cells through attenuation of nucleotide excision repair capacity. Biomaterials. 2014;35:993–1003. doi: 10.1016/j.biomaterials.2013.09.096. [DOI] [PubMed] [Google Scholar]
- 27.Gautron M, Jazat F, Ratinahirana H, Hauw JJ, Guilbaud G. Alterations in myelinated fibres in the sciatic nerve of rats after constriction: possible relationships between the presence of abnormal small myelinated fibres and pain-related behavior. Neurosci Lett. 1990;111:28–33. doi: 10.1016/0304-3940(90)90339-b. [DOI] [PubMed] [Google Scholar]
- 28.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]


