Abstract
Objective(s):
Use of biological scaffolds and automating the cells directing process with materials such as growth factors and glycosaminoglycans (GAGs) in a certain path may have beneficial effects in tissue engineering and regenerative medicine in future. In this research, chondroitin sulfate sodium was used for impregnation of the scaffolds. It is a critical component in extracellular matrix and plays an important role in signaling pathway; however, little is known about its role within mammalian development and cell linage specification.
Materials and Methods:
Due to its porous and appropriate structure and for putting cells in 3D space, the kidney of BALB/c mouse was selected and decellulalized using physical and chemical methods. After decellularization, the scaffold was impregnated in chondroitin sulfate solution (CS) for 24 hr. Then, 60×105 human adipose-derived mesenchymal stem cells were seeded on the scaffold to assess their behavior on day 5, 10, 15, 20, and 25.
Results:
After 48 hr, DAPI staining approved completed decellularized kidney by 1% SDS (sodium dodecyl sulfate). Migration and establishment of a number of cells to the remaining area of the glomerulus was observed. In addition, cell accumulation on the scaffold surface as well as cells migration to the depth of kidney formed an epithelium-like structure. Up to the day 15, microscopic study of different days of seeding showed the gradual adhesion of large number of cells to the scaffold.
Conclusion:
Glycosaminoglycan could be a right option for impregnation. It is used for smartification and strengthening of natural scaffolds and induction of some behaviors in stem cells.
Keywords: Acellular scaffold, Chondroitin sulfate, Mesenchymal stem cell, Cell communication, Cell interaction, Extracellular matrix
Introduction
The term tissue engineering was firstly used in 1988 and defined as the application of engineering and biological principles and methods in fundamental understanding of performance-structure relationship in natural and damaged tissues and evolution of biological alternatives for healing, maintenance, or improvement of tissue performance. Although significant advances have been made in this field, a serious lack is existed in comprehensive understanding of natural principles of developmental biology and their application for the final goal of regenerative medicine, which is structural and functional healing of natural body parts. Advances in tissue and organ healing depend specifically on cell composition, scaffolds, and biological molecules. However, complicated issues such as 3D spatial organization of cells, nerve formation, formation of vascular network and lymph nodes and immune system for external particles, in cases of successful clinical applications should be implemented, and these challenges are not trivial (1).
In recent years, extracellular matrix (ECM), which is derived from various tissues, has been used as scaffolds for applications in regenerative medicine. Special composition and more importantly 3D structure of these scaffolds and extracellular matrix provide a highly appropriate level for cells, which are capable of healing and reconstruction (1). The existing molecules of these scaffolds have been arranged in a unique 3D pattern (2). Decellularized tissues of natural scaffolds are useful in that they maintain the extracellular matrix structure as well as the cell-ECM binding domains critical in promoting cell attachment, migration, and proliferation (3). Furthermore, confining attention to regenerative medicine and tissue engineering and conducting basic researches on abovementioned issues are the matter of the utmost importance(4).
ECM could affect several mechanical and biochemical processes simultaneously, work as adhesive substrate, and create some routes for directing immigrant cells (5). The compositions of ECM including proteoglycans, glycosaminoglycan (GAGs), colognes, and other ECM glycoproteins work as the regulators for development of the mammary glands, salivary glands, kidneys, gastrointestinal tract and lungs (6). The role of ECM in morphogenesis is not limited to structural support. ECM components, by linking to cell surface receptors can induce cascades of induction and lead to translation of some significant growth factors (7). Cellular messaging through ECM can determine the cell fate and lead to proliferation and cell stability (6). Moreover, by providing stimulating or preventive signals of distinctions, it can play an important role in specialization of the cell fate (8). Additionally, it can affect differentiation by indirect arrangement of the cell shape. It is shown that using modeled substrates of ECM, which impose various forms to the cultivated cells, the cell fate can be influenced by the cell shape (9). Natural and synthetic polymers cannot create the spatial structure of complicated tissues, like kidney. Therefore, decellularized tissues are used based on which extracellular matrix maintains its natural structure, and also on the basis of specific molecules that increase cell migration and proliferation.
Human adipose mesenchymal stem cells were recognized as cells, which can be differentiated to adipocyte, osteoblast, chondrocyte, and myocyte (10). In addition, ADSCs can be easily extracted from adipose tissue (11). The combination of these salient features was an important milestone in ADSCs researches of the recent decade (12), which have mostly focused on tissue engineering and regenerative medicine(13). Since the ECM of different tissues has various compositions, the evaluation of multipotent cells’ behavior in this divergent scaffolds can be the subject of many future researches. Regarding their complicated and porous structure, these scaffolds can be used for smartification and directing the differentiation of multipotent cells.
Glycosaminoglycans are important mixtures of ECM and have significant role in trapping and maintenance of growth factors and cytokines, water conservation, and ECM gel-like property in in vitro condition. Binding property of GAGs to many cell receptors and growth factors makes them appropriate for regenerative scaffolds (14). GAGs play significant role in the control of signaling pathways, but very little is known about their role during mammalian development and cell linage specification (15). The aim of this study was to create scaffold from decellularized kidney tissue of BALB/c mice and to evaluate its effect on the behavior of human adipose mesenchymal stem cells in the presence of a kind of GAGs.
Materials and Methods
Removal of mouse’s kidney tissue
All animal protocols were approved before implementation by the Animal Research Ethical Committee of Tehran University of Medical Sciences. Kidneys were collected from adult BALB/c mice. All tissues were placed in normal saline.
Decellularization process
A combination of physical and chemical method was used for decellularization of mouse kidney (16). After kidney removal through physical decellularization, samples were washed in normal saline and stored for one week at -4 °C. After thawing and washing in normal saline, snap freezing and thawing was used. To this end, for snap freezing samples were soaked in liquid nitrogen for 2 min, and then soaked in distilled water for 5 min for rapid thawing. Afterward, samples were washed in phosphate-buffered saline (PBS) at 37 °C (17). The next step was chemical decellularization method, in which the samples were soaked for 48 hr in 1% sodium dodecyl sulfate (SDS, Merck), and after the treatment procedure with detergent, samples were soaked in PBS solution for 24 hr. SDS destroys the cell membrane and removes the proteins from DNA (18).
The provided scaffolds were put in 70% ethanol for 30 min for sterilization (16). This procedure was performed under sterile conditions (laminar hood, Pars Pajouhesh, Iran). To eliminate the ethanol form scaffold, samples were washed with distilled water and were soaked in sterile PBS solution for 1 hr (19). In the last step, samples were put in 6 well plates (Orange Scientific, Belgume) containing 2 ml of culture medium, DMEM (Dulbecco’s Modified Eagle’s Medium), and 10% fetal bovine serum (FBS, biosera), and incubated at 37 °C with 5% CO2. Finally, scaffolds were ready for cell culture.
Impregnation of scaffolds with chondroitin sulfate sodium
Scaffold was impregnated with CS (Chondroitin sulfate A sodium salt from bovine trachea, sigma). The solution was made by using sterile distilled water and filtered. In this step, scaffolds were put in 6 well plates, and then the CS solution was added, and incubated for 24 hr at 37 °C.
Cell seeding
HAD-MSCs (Human Adipose-drived Mesenchymal Stem Cells) carrying GP gene were provided from Institute of Biotechnology of Ferdowsi University (20). In order for cell cultivation on sterile scaffolds, a cell suspension with a density of 60×105 cells in 50 µl was provided for each kidney scaffold (0.8×0.5×0.3 cm).
Scaffolds were divided into three groups: control group 1 with no cell and no CS, control group 2 with cell and no CS, and test group with cell and CS. Five kidney scaffolds were used in each group to be fixed with owen and paraformaldehyde fixator in different time intervals. The abovementioned experiments were repeated for 3 times. 60×105 cells (in 50 microliter) were seeded in each scaffold and then incubated. One hour after seeding, 2 µml culture medium was added to these cells. Their culture medium was every 1 or 2 days.
Histological studies
Samples were fixed with Bowen and Paraformaldehyde fixators and then were prepared for staining by Hematoxylin and Eosin (H& and E), periodic acid–Schiff (PAS)-Hematoxylin, Picro fuchsin and DAPI.
To observe green cells with fluorescence microscope, scaffolds were provided by paraformaldehyde fixators underwent cleaning and hydration processes with 50% alcohol. When slides were dried, scaffolds were analyzed with the fluorescence microscope.
Statistical analysis
Three slides were selected each day, and the numbers of cells were counted from part of the slides randomly by 400X magnification. After data collection, one-way analyses of variance (ANOVA), then Tukey’s (T-test) were used to determine the significance of time on number of cells using GraphPad InSat statistical software. P <0.05 was considered as significant level, and related diagram was designed using Excel software.
Results
The obtained results were analyzed in two sections: range of cellular omission and maintenance of matrix components and analysis of mesenchymal stem cells in no CS/CS impregnated scaffolds in day 5, 10, 15, 20, and 25. Histological analysis was performed using various staining methods. Results of hematoxylin and eosin staining suggest complete removal of cells from mouse’s kidneys after several physical and chemical processes (Figure 1).
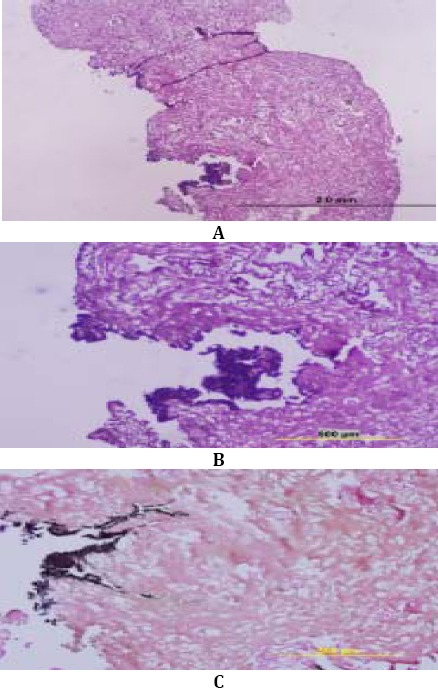
Figure 1.

The Figure indicate mouse’s kidney before and after decellularization using PAS- hematoxylin. Figure A indicates nuclei by violet and basal membrane by purple colors. Figure C indicates Glomerular after decellularization and cell removal
DAPI staining was used to confirm the complete removal of nuclei from scaffold. Results showed that using 1% SDS for 48 hr completely removed the cells from scaffolds (Figure 2).
Figure 2.

Kidney analysis before and after decellularization using DAPI staining. Figure A indicates the kidney before decellularization and Figure B shows kidney scaffold after decellularization. The Figure illustrates complete removal of nuclei from scaffolds. Figure C indicated the whole kidney after decellularization
Analysis of AD-MSCs on no CS scaffolds
Day 5 of cultivation
Histological studies of no CS scaffolds in day five by using H& and E staining indicated adhesion in the number of cells and their penetration to scaffolds and glomerulus, cell arrangement around scaffold, and cell deformation in some areas (Figure 3).
Figure 3.

Figure A: cells inside and on the scaffold. Figure B: cells adhesion to each other and scaffold. Figure C: cell placement in rest of glomerulus. Figure D: formation of cellular arrangement inside the scaffold. Figure E: Observation of cell mass inside scaffold and formation of Epithelium-like structure on scaffold. Figure F: Further magnification of previous image. Figures G and H indicate cell placement inside scaffold cavities which are thin and covered the cavity with a certain arrangement
Day 10 of cultivation
Histological studies indicated considerable increase in the number of adhered cells to the scaffold and creation of cell mass, which regularly placed in some areas in layers and produced an epithelium-like structure. A number of these cells, moreover, are about to migrate to some inner parts of the scaffold (Figure 4).
Figure 4.

Human Adipose Mesenchymal Stem Cells behavior in no CS scaffolds during the tenth day with H& and E staining. Figure A. indicates cells placement in a number layers and creation of cell mass and Figure B and C show their penetration to inner parts of the scaffold
Day 15 of cultivation
Analysis of scaffolds in day 15 suggested the aggregation of number cells on the scaffold and their inclination to penetrate inside the scaffold. Active nuclei, cell order, and pressure, as well as the formation of epithelium-like structure, which are obvious from cell mass side on scaffold, are important (Figure 5).
Figure 5.

Human Adipose Mesenchymal Stem Cells behavior in no CS scaffolds on day 15. A cell observation on scaffold, B cell pressure and their penetration inside scaffold, and C the formation of Epithelium-like structure and narrowing of cells in outermost layer with picro fuchsin staining
Day 20 of cultivation
During thisIn day 20, cells with low density were observed on scaffold. The nucleus of most cells in this day, furthermore, was more condensed than the previous day. Additionally, it seemed that cells have begun to be destroyed (Figure 6).
Figure 6.

Human Adipose Mesenchymal Stem Cells behavior in no CS scaffolds on day 20 with H& and E staining. Images indicate the decrease in cell density, and flash shows cellular debris, which are increasing in this day
Day 25 of cultivation
Scaffolds analysis has illustrated drastic decrease of cells density. During the day 25, cells were placed in small areas on the scaffold. Some destructions were observed in the scaffold, which possibly was in the place of existence and the path of cells’ penetration. The condensing and fragmentation of nuclei was probably indicator of cells progress to death (Figure 7).
Figure 7.

Human Adipose Mesenchymal Stem Cells behavior in no CS scaffolds on day 20 with H& and E staining. Figures A-C illustrate dramatic decrease of cell density with H& and E staining. Flash in Figure A, shows probable destroyed areas by cells. D is nuclei fragmentation and E indicates their condensing. F illustrates controlling scaffold of no cell after 25 days
Analysis of AD-MSCs behavior in CS impregnated scaffolds
Day 5
In day 5, dividing cells are obvious in the cell mass, which is being created (Figure 8).
Figure 8.

Human Adipose Mesenchymal Stem Cells behavior in CS impregnated scaffolds in day 5. A: H& and E staining indicates cell division (Anaphase). B: cell mass with picro fuchsin staining, Figure C, PAS-hematoxylin-picrofuschin-indigocarmin. The purple parts in Figure C indicate positive PAS areas with PAS staining impregnated the scaffold by CS
Day 10
During day 10, histological studies of scaffolds illustrated considerable increase in the number of cells adhered to the scaffold in mass. In some areas, small cell aggregations were migrated inside the scaffold and glomerulus (Figure 9).
Figure 9.

Human Adipose Mesenchymal Stem Cells behavior in CS impregnated scaffolds on day 10 with H& and E staining. Figure A represents cell mass and Figure B and C, show cells penetration to inner parts of scaffold especially inside glomerulus
Day 15
By analyzing the scaffolds in day 15, cell growth and penetration were obvious on and inside of the scaffold. It is noteworthy that in this group cells have active nuclei. Images indicate cell migration and epithelium-like structures on the scaffold (Figure 10).
Figure 10.

Human Adipose Mesenchymal Stem Cells behavior in CS impregnated scaffolds on day 15 with H& and E staining. Figures A-C show epithelium-like structure on the scaffold and cell migration inside the scaffold with H& and E staining
Day 20
analysis in day 20 illustrated decrease of cellular density in most parts of the scaffold. In this group, cell debris is observable, as well. Figures of decrease of cell density and cell debris were indicated by H& and E, picro fuchsin and, PAS-hematoxylin-picrofuschin-indigocarmin staining (Figure 11).
Figure 11.

Human Adipose Mesenchymal Stem Cells behavior in CS impregnated scaffolds on day 20. Decrease of cell density and cellular debris with H& and E (A), picro fuchsin staining (B), purple parts of positive PAS areas in PAS-hematoxylin-picrofuschin-indigocarmin staining
Day 25
In day 25, substantial decrease of cells density and cell condensing were obvious. Images illustrated decrease of density and cell size in scaffolds with H& and E, and picro fuchsin staining (Figure 12).
Figure 12.
Human Adipose Mesenchymal Stem Cells behavior in CS impregnated scaffolds on day 25. Decrease of density and cell size are shown in scaffolds by H& and E (A-B) and picro fuchsin (C) staining
Florescent
Those scaffold, which were fixed with paraformaldehyde fixator were analyzed using fluorescence microscope, then stained with DAPI, and afterward two pictures merged together (Figure 13, 14). These pictures indicate cell existence and adhesion to control and test group in different days and confirm the presence of cells containing GFP gene (Figure 13, 14). GFP positive cells were used in this research because it was simple track them through fluorescence microscope in kidney scaffold, and also it can be proved that these are not the remaining kidney cells.
Figure 13.

Cells presence and adhesion to no CS scaffolds. First row: cells with no staining under the fluorescence microscope. Second row: cells with DAPI staining. Third row: combination of the two pictures
Figure 14.

Cells presence and adhesion to CS scaffolds. First row: cells with no staining under the fluorescence microscope. Second row: cells with DAPI staining. Third row: combination of the two pictures
Statistical results
In the resultant graph of cellular counting and density, each day comparison with the same day in the control group and CS impregnated group did not indicate any significant difference (P>0.05). In each group, however, there was a significant difference (P<0.001) between days 20 and 25 with days 5, 10, and 15. There was a significant difference in the test group between days 15 with 5, which indicate significant increase (P<0.01) in cellular density. This difference was also obvious in day 15 of control group and the day 5 (P<0.01).
Diagram 1.

Average number of cells in each scaffold in different days of cultivation
The Diagram indicates significant decrease in both groups in days 20, and 25 in comparison to days 5, 10, and 15. There was no significant difference between range of cellular density between no CS scaffolds and scaffolds impregnated to CS (P>0.05). In day 15, significant difference can be observed in both group comparing with day 5 of the same group (P<0.01). Data were provided as Mean±SD. P<0.01, P<0.001
Discussion
Methods of scaffold preparation from ECM affect surface morphology and mechanical characteristics of the scaffold (21). The objective of the present study was whole organ decellularization, in a way that matrix signals remain for differentiation of multipotent stem cells (22). Decellularized tissues are ideally natural scaffolds in that they preserve the natural extracellular matrix structure, and by keeping cell-matrix domains increase the cell adhesion, migration, and proliferation (23). The effect of icing-thawing process on mechanical characteristics of cells is little (16). Tissue freezing will bring about cell crystallization, membrane destruction, and cell content leakage (18). Compared with other detergents such as Triton X-100, SDS is more effective in removing cell debris from tissue (24).
Sullivan et al used 0.25% and 0.5% densities along with 1% Triton for decellularization of a mature pig kidney. These materials indicated remarkable decellularization; nonetheless, 0.5% SDS was more effective (25).
Nakayama et al used monkey’s kidney for basic structures of tissue engineering with the aim of producing decellularized kidney scaffold with required structural, and mechanical, and physiological characteristics. Using SDS and 1% Triton, the kidney was decellularized separately. In addition, H and E staining was used for indicating cell components removal from scaffold. Results showed that SDS was more effective than Triton X-100 for nuclei removal from condensed tissues, such as kidney, while it preserves the tissue mechanics (3). By injecting 1% SDS and 1% Triton X-100 to a rat kidney, Lin et al could completely decellularized the tissue. Results have shown that, no cell was observable by optic microscope. Scanning Electron Microscope has shown the reticulated structure, residual basal membrane and collagen with normal cellular structure, and DAPI staining approved decellularization, as well (26).
Using immunohistochemistry method, Nak- ayama et al could observe ECM proteins including heparan sulfate, fibronectin, collagen I, and IV and laminin in decellularized kidney of a monkey (3).
After decellularization of a rat’ kidney using a method included SDS, Ross et al approved the perseverance of laminin and collagen IV by immunohistochemistry method (22).
Walker reports indicated the role of collagen and laminin in cell regeneration, proliferation, migration, revitalization of normal performance, and kidney regeneration after injury (27).
One of the salient functions of ECM is to provide paths as an adhesive substrate for cell migration. Different existed molecules in ECM could have various effects on adhesion and pace of migration of different kinds of cells (5).
After decellularization of a monkey’s kidney, Nakayama et al cultivated it in the vicinity of an embryonic kidney. The obtained results have illustrated that cells were migrated from kidney tissue toward decellularized scaffold and penetrated in it (3).
Findings of Li and his colleagues indicated that collagen considerably facilitated the migration of embryonic stem cells via adhesion and increased the efficiency of solid organ transplants aided by stem cells (28).
It was also demonstrated that derivative products of matrix will modify the migration and proliferation of endothelial and precursor cells in in vitro and also motivate reconstruction and modification in in vivo (29).
Accordingly, cell adhesion, proliferation and migration inside the scaffold in days 5, 10, and 15 were probably due to cells interaction with existing molecules in extracellular matrix of kidney. 3D scaffolds of collagen were used for cultivation of various stem cells in tissue engineering. Researches have indicated that collagen scaffolds preserve distinguished applications and induce the differentiation in cultivated cells (30). Studies have shown that embryonic stem cells of monkey could be differentiated as neural phenotype or endothelial cells (31). When combined with proper factors and cultivated in collagen scaffolds, human embryonic stem cells have the potentiality of differentiation to blood vessels (32). Similar results have been yielded using 3D collagen scaffolds for producing hepatocytes by embryonic stem cells (33). In a research that embryonic stem cells have been used in decellularized scaffold of kidney in the presence of collagen and laminin, it was approved that cells express PAX2, which is an indicator of epithelial cells (22).
Proteoglycans are part of extracellular matrix, which participate in molecular incidents such as cell adhesion, migration, and proliferation. Many researches have indicated that proteoglycans could have a direct effect on these kinds of cellular behaviors through ligands or receptors. These kinds of studies support the role of proteoglycans as effective factors of cellular processes involved in development and disease (34).
As a similar product to ECM, collagen – glycosaminoglycans (CG) scaffolds have been used for skin restoration and lately for nerve regeneration. CG scaffolds have also applications in orthopedic and regenerative medicine. Recently, some scaffolds with certain shapes of pores and structure have been applied for the analysis of cells behavior (35).
Neurogenesis and functional improvement was observed in a study after CG matrix links to an injured brain. These results have suggested that using these kinds of scaffolds could be considered as a tool for clinical applications in regenerative medicine and functional improvement in brain injuries (36).
The role of CG scaffolds and density of these two materials on osteoblasts behavior and activities have been analyzed. In these scaffolds, density of collagen and GAG are effective in survival potentiality, proliferation and spatial development of osteoblast (37).
The results of an in vitro study has shown that the use of type II collagen in CG scaffolds is effective on chondrogenesis (38).
Regarding conducted studies, impregnation of scaffolds by GAGs have had substantial role in observed behaviors. However, the issue is subjected to further research.
Statistical analysis of scaffolds in days 20 and 25 after cultivation has shown reduction in cell density on scaffold. Frisch et al have indicated that disconnection of epithelial cells and ECM induces apoptosis (39). In addition, Sugiyama et al have shown that fresh and natural ECM will save the mesenchymal cells from apoptosis (40). Makino et al claimed that matrix of basal membrane will prevent mesenchymal cells apoptosis (41). In 2010, Nakayama et al using PCR in his primary studied scaffold reported the expression of apoptotic genes after placement of embryonic kidney near monkey’s kidney (3). After cultivation of embryonic stem cells in scaffold of a rat kidney, Ross et al have shown that cells that were not connected to basal membrane of matrix may experience apoptosis (22). In response to substrate, migrating cells activate various kinds of enzymes including proteases and metalloproteinases or increase their expression (42). It is believed that, mesenchymal cells destroy ECM proteins through proteases secretion and open a new path for migration in extracellular matrix (43), which is probable cause of scaffold destruction in the place of cell existence.
Conclusion
The obtained results indicated that this kind of scaffold is an appropriate candidate for in vitro applications, and its role in the induction of cell behavior such as adhesion, migration, and proliferation of AD-MSCs has shown that further confirmation of these behaviors need more accurate molecular analyses. Results achieved from using CS impregnated scaffolds and analysis of AD-MSCs interactions with this scaffold indicated that in given density and form, CS could probably have a positive effect on adhesion and proliferation of AD-MSCs. However, this process is dependent on using of various CS densities and more accurate techniques for confirmation.
Yielded results could provide valuable information in next in vitro researches in the field of tissue engineering and regenerative medicine, which is in relation to scaffolds application and their optimization for directing totipotent, multipotent and differentiated cells. The main focus of the next researches could be on molecular processes involved in interactions and clinical application based on animal models.
Acknowledgment
Many thanks to Institute of Biotechnology of Ferdowsi University of Mashhad for providing required facilities of the research and deep appreciation from Dr Maryam Moghadam Matin and Dr Ahmadreza Bahrami, member of academic board of Ferdowsi University of Mashhad, Iran, for their invaluable cooperation and consultations. The results described in this paper were part of student thesis.
References
- 1.Badylak SF. Regenerative medicine and developmental biology: the role of the extracellular matrix. Anat Rec B New Anat. 2005;287:36–41. doi: 10.1002/ar.b.20081. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Engineering Part A. 2010;16:2207–16. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mardani M, Hashemibeni B, Ansar MM, Zarkesh Esfahani SH, Kazemi M, Goharian V, et al. Comparison between chondrogenic markers of differentiated chondrocytes from adipose derived stem cells and articular chondrocytes in vitro. Iran J Basic Med Sci. 2013;16:763–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Strachan LR, Condic ML. Neural crest motility and integrin regulation are distinct in cranial and trunk populations. Developmental Biology. 2003;259:288–302. doi: 10.1016/s0012-1606(03)00187-8. [DOI] [PubMed] [Google Scholar]
- 6.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Developmental Biology. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development. 2007;134:2501–2509. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neu R, Adams S, Munz B. Differential expression of entactin-1/nidogen-1 and entactin-2/nidogen-2 in myogenic differentiation. Differentiation. 2006;74:573–582. doi: 10.1111/j.1432-0436.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 9.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009 Jul 2;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology Of The Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: Their identity and uses in clinical trials, an update. World J Stem Cells. 2011;3:25–33. doi: 10.4252/wjsc.v3.i4.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadzadeh E, Nikravesh MR, Jalali M, Fazel A, Ebrahimi V, Ebrahimzadeh-Bideskan AR. Immunohistochemical study of type III collagen expression during pre and post-natal rat skin morphogenesis. Iran J Basic Med Sci. 2014;17:196–200. [PMC free article] [PubMed] [Google Scholar]
- 14.Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2:209–217. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- 15.Prinz RD, Willis CM, van Kuppevelt TH, Kluppel M. Biphasic role of chondroitin sulfate in cardiac differentiation of embryonic stem cells through inhibition of Wnt/beta-catenin signaling. PLoS One. 2014;9:e92381. doi: 10.1371/journal.pone.0092381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naderi S, Khayat Zadeh J, Mahdavi Shahri N, Nejad Shahrokh Abady K, Cheravi M, Baharara J, et al. Three-dimensional scaffold from decellularized human gingiva for cell cultures: glycoconjugates and cell behavior. Cell J. 2013;15:166–175. [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Mahdavi Shahri N, Baharara J, Takbiri M, Khajeh Ahmadi S. In vitro decellularization of rabbit lung tissue. Cell J. 2013;15:83–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Haddad-Mashadrizeh A, Bahrami AR, Matin MM, Edalatmanesh MA, Zomorodipour A, Gardaneh M, et al. Human adipose-derived mesenchymal stem cells can survive and integrate into the adult rat eye following xenotransplantation. Xenotransplantation. 2013;20:165–176. doi: 10.1111/xen.12033. [DOI] [PubMed] [Google Scholar]
- 21.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 22.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc of Nephrol. 2009;20:2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Renal tissue engineering with decellularized rhesus monkey kidneys: age-related differences. Tissue Engin Part A. 2011;17:2891–2901. doi: 10.1089/ten.tea.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cebotari S, Tudorache I, Jaekel T, Hilfiker A, Dorfman S, Ternes W, et al. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif Organs. 2010;34:206–210. doi: 10.1111/j.1525-1594.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DC, Mirmalek-Sani SH, Deegan DB, Baptista PM, Aboushwareb T, Atala A, et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials. 2012;33:7756–7764. doi: 10.1016/j.biomaterials.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Liu CX, Liu SR, Xu AB, Kang YZ, Zheng SB, Li HL, et al. Preparation of whole-kidney acellular matrix in rats by perfusion. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:979–982. [PubMed] [Google Scholar]
- 27.Walker PD. Alterations in renal tubular extracellular matrix components after ischemia-reperfusion injury to the kidney. Lab Investig. 1994;70:339–345. [PubMed] [Google Scholar]
- 28.Li HY, Liao CY, Lee KH, Chang HC, Chen YJ, Chao KC, et al. Collagen IV significantly enhances migration and transplantation of embryonic stem cells: involvement of alpha2beta1 integrin-mediated actin remodeling. Cell Transplant. 2011;20:893–907. doi: 10.3727/096368910X550206. [DOI] [PubMed] [Google Scholar]
- 29.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. doi: 10.1016/j.matbio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michelini M, Franceschini V, Sihui Chen S, Papini S, Rosellini A, Ciani F, et al. Primate embryonic stem cells create their own niche while differentiating in three-dimensional culture systems. Cell Prolifer. 2006;39:217–229. doi: 10.1111/j.1365-2184.2006.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab investig. 2003;83:1811–1820. doi: 10.1097/01.lab.0000106502.41391.f0. [DOI] [PubMed] [Google Scholar]
- 33.Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol. 2006;50:645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 34.Wight TN, Kinsella MG, Qwarnstrom EE. The role of proteoglycans in cell adhesion, migration and proliferation. Curr Opin Cell Biol. 1992;4:793–801. doi: 10.1016/0955-0674(92)90102-i. [DOI] [PubMed] [Google Scholar]
- 35.Brendan AC, Lorna J. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Engin J. 2008;137:102–121. [Google Scholar]
- 36.Huang KF, Hsu WC, Chiu WT, Wang JY. Functional improvement and neurogenesis after collagen-GAG matrix implantation into surgical brain trauma. Biomaterials. 2012;33:2067–2075. doi: 10.1016/j.biomaterials.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 37.Tierney CM, Jaasma MJ, O'Brien FJ. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J Biomed Mater Res A. 2009;91:92–101. doi: 10.1002/jbm.a.32207. [DOI] [PubMed] [Google Scholar]
- 38.Vickers SM, Squitieri LS, Spector M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: dynamic pore reduction promotes cartilage formation. Tissue Engin. 2006;12:1345–1355. doi: 10.1089/ten.2006.12.1345. [DOI] [PubMed] [Google Scholar]
- 39.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugiyama H, Kashihara N, Maeshima Y, Okamoto K, Kanao K, Sekikawa T, et al. Regulation of survival and death of mesangial cells by extracellular matrix. Kidney Int. 1998;54:1188–1196. doi: 10.1046/j.1523-1755.1998.00116.x. [DOI] [PubMed] [Google Scholar]
- 41.Makino H, Sugiyama H, Kashihara N. Apoptosis and extracellular matrix-cell interactions in kidney disease. Kidney Int. 2000;77:S67–75. [PubMed] [Google Scholar]
- 42.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]


