Abstract
Objective(s):
Estrogen is a sexual hormone that has prominent effects on reproductive and non-reproductive tissues. The aim of this study is to evaluate the effects of estrogen on the proliferation and neural differentiation of human adipose derived stem cells (ADSCs) during neurogenic differentiation.
Materials and Methods:
Isolated human ADSCs were trans-differentiated in neural induction medium containing neurobasal medium, N2 and B27 with or without 17β-estradiol (E2) treatment. Proliferation rate and neural differentiation of human ADSCs were assessed using MTT assay, immunostaining and real time RT- PCR analysis, respectively.
Results:
Analysis of data show that estradiol treatment can significantly increase proliferation rate of differentiated cells (P<0.05). Immunocytochemical and real time RT-PCR analysis revealed that the expression of precursor and mature neuronal markers (nestin and MAP2) was significantly higher in the E2 treated cell cultures when compared to the untreated cell cultures (P<0.05).
Conclusion:
According to our findings, estrogen can promote proliferation and neuronal differentiation of human ADSCs.
Keywords: Adipose derived stem cells, Cell proliferation, Neurogenic differentiation, 17β-estradiol
Introduction
Mesenchymal stem cells can be a safe stem cell source, and their therapeutic effects have been shown in the treatment of neurodegenerative diseases (1, 2). Previous studies demonstrated that stem and progenitor cells from adult adipose stromal tissue retain the capacity to differentiate toward mesodermal and non-mesodermal lineages (3-5).
Estrogen as a female sexual steroid hormone also is important in modifying the activities of non-reproductive tissues. One of the most important targets for estrogen is brain. It has neurotrophic and neuroprotective effects on brain (6-8). Estradiol (E2), one of the major estrogens in the central nervous system, can influence neurogenesis by increasing the ratio of neurons to glial cells during differentiation of neural stem cells (NSCs) (9-11). Effect of E2 on neural differentiation can be exerted through neurotrophic factor secretion. E2 enhances the expression of brain derived neurotrophic factor (BDNF) as well as nerve growth factor (NGF) in the frontal cortex and hippocampus of rats (12, 13). Furthermore, it can increase the BDNF, neurotrophin-3 (NT-3), and NGF production in the olfactory cortex of ovariectomized rats (14). In addition, E2 stimulates the level of glial derived neurotrophic factor expression in developing hypothalamic neurons and peripheral organs (15). Therefore, E2 treatment can increase elongation and branching of neurites in cortical culture (16).
E2 influences the neuronal differentiation in human umbilical cord blood mesenchymal stem cells as well as in embryonic stem cells and NSCs (17, 18). Kang et al, believed that it might be due to neurotrophic factor expression (17).
It has been reported that ADSCs secret some growth factors such as BDNF, insulin-like growth factor, and fibroblast growth factor (FGF), which are important for nerve regeneration during peripheral nerve injury (19, 20). In addition, previous study indicated that ADSCs-induced repair may act through cell differentiation as well as secreting trophic factors (21). These results suggest that E2 via neurotrophic factor modulation might participate proliferation and neurogenic differentiation of human ADSCs.
We focused our attention on human adipose derived stem cells (ADSCs) because these cells can be obtained by less invasive method and cultured with a greater proliferation rate than other mesenchymal stem cells (5). However, ADSCs can be obtained easily and expanded in vitro for autologous transplantation. Therefore, ADSCs appear to be an appropriate source for cell-based therapy.
On the other hand, the effect of E2 on the proliferation and neural differentiation of ADSCs are unclear. Therefore, we examined the effects of E2 on the proliferation and neural differentiation of human ADSCs in vitro.
Materials and Methods
Isolation and culture of human ADSCs
All procedures were approved by the Ethics Committee of Isfahan University of Medical Sciences. Human adipose tissue was obtained from three elective lipoaspirate samples of abdominal fat from female donors (age range: 20–45 years old), after receiving informed consent and cultured as previously described (22). Briefly, samples were washed extensively with sterile phosphate-buffered saline (PBS) to remove contaminating debris and red blood cells. Washed aspirates were treated with 0.075% collagenase type I in PBS for 30 min at 37°C with gentle agitation. Later, the collagenase I was inactivated with an equal volume of Dulbecco’s Modified Eagle’s Medium (DMEM)/10% fetal bovine serum (FBS) and then, the supernatant was centrifuged for 10 min at 800 rpm. The cellular pellet was resuspended in DMEM/10% FBS and plated in DMEM: F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin. After 24 hr, the non-adherent cells were removed and expansion of adherent ADSCs was acquired by serial passage for accessing the pure population of ADSCs. The primary cells were cultured in 25 cm2 flasks for 4–5 days until they reached a confluency of approximately 80% in a 37°C humidified incubator with a 5% carbon dioxide (CO2) environment. They were defined as passage 0. At confluence, the cells were detached with 0.25% trypsin/0.02% ethylene diamine tetraacetic acid (Gibco, BRL, Paisley, UK) at a ratio of 1:3 in each passage. The cells used in the present study were from passages 3 to 5. All chemicals, except where specified otherwise, were purchased from Sigma-Aldrich, St. Louis, MO, USA.
In order to determine stemness of isolated cells, human ADSCs within 3–5 passages were harvested by trypsinization, and then the cells were washed twice with 1% bovine serum albumin/PBS and incubated with antibodies against CD90, CD44, CD105, CD34, CD14, and CD45 (Chemicon, Temecula, CA, USA) for 30 min. Primary antibodies were directly conjugated with fluorescein isothiocyanate (FITC) and phycoerythrin. For isotype control, nonspecific FITC-conjugated IgG was substituted for the primary antibodies. Flowcytometry was performed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA).
Induction of neurogenic differentiation
The transdifferentiation procedure was carried out according to previous study (23). Human ADSCs within 3–5 passages were induced into neurospheres. Briefly, we dissociated human ADSCs (80–90% confluence) with 0.25% trypsin (Gibco, BRL, Paisley, UK) and then plated them on plastic dish at a concentration of 1-2 × 105/cm2 in DMEM/F12 supplemented with 20 ng/ml human epidermal growth factor (EGF), 20 ng/ml basic FGF and 2% B27 (1:50, Gibco) at 37 °C in 5% CO2. Fresh medium was added every 3–4 days. After 7 days, neurospheres were triturated using a fire-polished Pasteur pipette and re-plated in fresh medium. The triturated neurospheres and 2×104 singled cells re-plated in 24 well plate in neurobasal medium supplemented with 5% FBS, 1% penicillin/strepto-mycin, 1% L-glutamine, 1% N2, 1% none essential amino acids, 2% B27, for 7 days. To determine the effect of E2 on neural differentiation, according to previous study we added E2 10 nM to the neural induction medium approximately every day in treated culture, whereas in control culture E2 was absent (17).
MTT assay
To examine the viability of differentiated cells (3×10³ cells/well) in the E2 presence 7 days post induction, 5 mg of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was dissolved in 1 ml of PBS. The stock solution was added to the culture medium at a dilution of 1:10. The plates were incubated at 37°C for 4 hr. The medium was then aspirated and 200 µl of dimethyl sulfoxide was added to extract the MTT formazan. The absorbance of each well was detected by a microplate reader (Hiperion MPR 4+, Germany) at the wavelength of 540 nm.
The percentage of cell viability was obtained using the following equation:
% Cell Viability= (treated cell OD-treated blank OD)/(untreated cell OD-untreated blank OD) × 100
Immunocytochemistry
After fixation with 4% paraformaldehyde (PFA)/PBS, cells were treated with blocking solution (PBS containing 4% goat serum and 0.1% triton X-100) for 45 min at room temperature. Then, cells were incubated in primary antibodies in PBS/0.1% triton X-100 and 1% goat serum overnight at 4°C. Anti-nestin (1:300, Abcam, UK), anti-microtubule-associated protein 2 (MAP-2) (1:300, Abcam, UK), and anti-glial fibrillary acidic protein (GFAP) (1:300, Abcam, UK) were used. After washing with PBS, the slides were exposed to secondary antibodies and rabbit anti-mouse FITC-conjugated (1:500; Abcam, UK). They were incubated at room temperature for 1 hr. Diamidino-2-phenylindole (DAPI, 1:1000) was used for nuclear counterstaining. For negative controls, primary antibody was omitted from the reaction series in each experiment. Cells were observed using a fluorescence microscope (Olympus BX51, Japan). To perform quantitative analysis, the number of positive cells was counted on each slide image acquired by ImageJ 1.42 (NIH), and the ratio to the number of nuclei was analyzed for each antigen. The number of immunopositive cells was counted in a minimum of 200 cells per slide. All immunocytochemical experiments were repeated twice.
Real time RT-PCR
Total ribonucleic acid (RNA) was extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. Equal amounts of total RNA were reverse transcribed into complementary deoxyribonucleic acid (cDNA) using oligo-dT and RevertAid First Strand cDNA Synthesis Kit (Fermentas). Real-time polymerase chain reaction (PCR) was performed using a thermal cycler (Rotor-Gene 6000, QIAGEN), with 12.5 μl SYBR Green PCR Master Mix (QIAGEN), 5 pM of each of forward and reverse primers, and 1.5-2 µl cDNA (50 ng/μl cDNA) for each reaction in final volume of 20 μl. Cycle conditions were carried out according to the manufacturer’s instructions (QIAGEN). Relative gene expression was analyzed using the comparative Ct method (2−ΔΔCt). All samples were normalized to levels of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), which was used as the internal control. All measurements were done in triplicates. The list of primers used in this study are depicted in Table 1.
Table 1.
The primer sequences (forward, reverse) which were used in the real-time RT-PCR technique
| Gene | Forward (top) Reverse (bottom) | Size (bp) | Real time RT-PCR program |
|---|---|---|---|
| Nestin | 5’-AACAGCGACGGAGGTCTCTA-3’ 5’-TTCTCTTGTCCCGCAGACTT-3’ | 220 | 94 °C – 20 sec, 59 °C – 30 sec, 72 °C – 30 sec 35 cycles |
| MAP2 | 5’-TCAGAGGCAATGACCTTACC-3’ 5’-GTGGTAGGCTCTTGGTCTTT-3’ | 321 | 94 °C – 20 sec, 57 °C – 30 sec, 72 °C – 30 sec 45 cycles |
| GFAP | 5’-CCTCTCCCTGGCTCGAATG-3’ 5’-GGAAGCGAACCTTCTCGATGTA-3’ | 161 | 94 °C – 20 sec, 59 °C – 30 sec, 72 °C – 30 sec 40 cycles |
| GAPDH | 5’-ACCACAGTCCATGCCATCAC-3’ 5’-TCCACCACCCTGTTGCTGTA-3’ | 452 | 94 °C – 20 sec, 60 °C – 30 sec, 72 °C – 30 sec 25 cycles |
Statistical analysis
Data are presented as mean ± standard error from 4 to 5 independent cell cultures. Kruskal-Wallis one-way analysis of variance and Dunn’s multiple-comparison tests were used to determine the statistical significance between data. Statistical significance was considered when P<0.05.
Results
Morphological features and characterization of isolated human ADSCs
After initial plating of human ADSCs, the primary culture appeared to be a mono-layer of flat cells. As the cells approached confluency, they assumed a more spindle-shaped, fibroblastic morphology (Figure 1A). To further characterize these cells, cell surface markers were examined by flow cytometry. Flowcytometry analysis of human ADSCs within 3–5 passages showed that ADSCs CD90, CD105, and CD44 were positive, but CD45, CD34, and CD14 were negative, as previously described (22).
Figure 1.
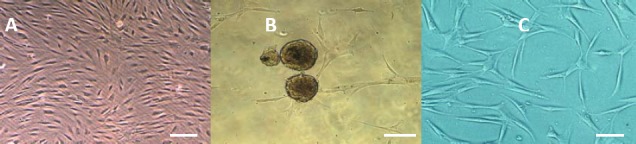
Phase contrast image of (A) stem cells derived from human adipose tissue, (B) neurospheres formation, (C) differentiated cells derived from human adipose derived stem cells 14 days after neural induction in untreated cell cultures. It shows bipolar and multipolar cells with elongated processes, Scale bars denote in A= 150 µm, B= 200 µm and in C= 100 µm
Viability of differentiated ADSCs
We examined the viability of differentiated ADSCs using MTT assay. The mean percentage of cell viability in the E2 treated cell culture (186.7 ± 21) was significantly increased as compared to the untreated group (124.1 ± 25) (P< 0.05).
Differentiation to neuron-like cells phenotype
In preinduction medium human ADSCs were spheres of floating cells (Figure 1B). Whereas, in terminal induction medium differentiated human ADSCs showed cytoplasmic retraction and ramified shapes (Figure 1C).
To characterize neural differentiation, induced cells (in the presence or absence of E2) were stained with the markers against nestin (neuronal progenitor cell), GFAP (astrocyte), and MAP-2 (mature neuronal cell) and cell nuclei were counterstained with DAPI. The mean percentage of positive cells for neural markers of nestin, GFAP, and MAP-2 were evaluated 7 days after terminal differentiation. As shown by immunostaining, the mean percentage of nestin positive cells of differentiated cells was significantly increased in treated cell cultures (84.3% ± 11.0) when compared with untreated cultures (64.7% ± 9.2) (P<0.05). The mean percentage of MAP-2 positive cells was increased in the presence of E2 (36.0%±5.5) compared with the cultures without E2 treatment (31.0% ± 6.8). Moreover, the mean percentage of GFAP positive cells was increased in E2 treated cultures (83.25 ± 2.80) when compared to untreated cultures (82% ± 8.6) (Figure 2).
Figure 2.
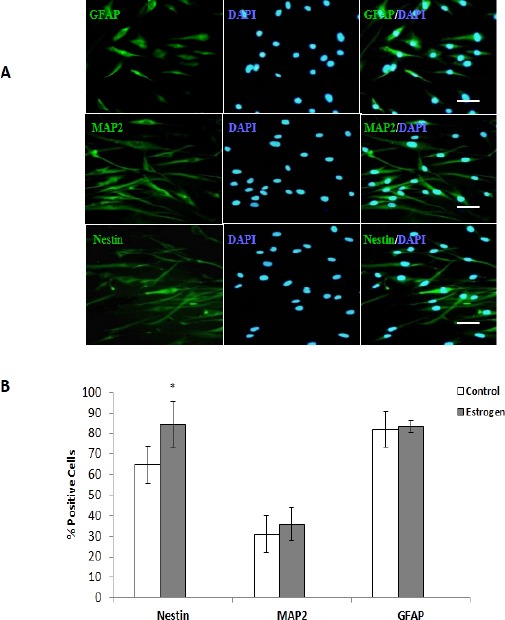
Immunocytochemistry of neural differentiated cells from human adipose derived stem cells (ADSCs) in the presence or without estradiol (E2). Cells were positive for glial fibrillary acidic protein (GFAP), microtubule-associated protein 2 (MAP-2), and nestin markers. Cell nuclei were counterstained with diamidino-2-phenylindole (blue). Scale bars: nestin, MAP-2, and GFAP= 50 µm. Comparative analysis between the mean percentages of immunoreactive positive cells for nestin, MAP-2, and GFAP markers in differentiated cells derived from human ADSCs with or without E2 treatment. A significant mean difference in the expression of nestin positive cells was observed (*P<0.05)
Real-time reverse transcriptase (RT)-PCR provided further evidence for neurogenic differentiation of human ADSCs. Our results show that E2 significantly up-regulated the expression level of MAP-2 compared to the untreated cultures (P<0.05). While, the expression of nestin gene, marker of neural precursor cells was significantly down-regulated in E2 treated cell cultures as compared to untreated cell cultures (P<0.05). While, the level of expression of glial fibrillary acidic protein gene was similar in both of differentiated cell cultures (Figure 3).
Figure 3.
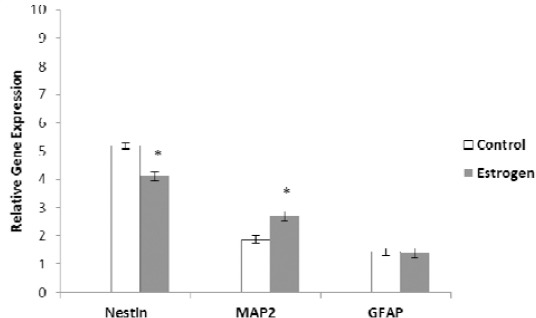
Comparative analysis of some neural markers in treated and untreated cultures examined by real-time reverse transcriptase polymerase chain reaction. The expression of nestin gene, marker of neural precursor cells was significantly down-regulated in estradiol (E2) treated cell cultures compared to untreated cell cultures (*P<0.05), but the expression of microtubule-associated protein 2 (MAP-2) was significantly up-regulated in E2 treated cell cultures relative to untreated cell cultures (*P<0.05). While, the level expression of glial fibrillary acidic protein gene was equal in both of differentiated cell cultures
Discussion
In the current study, we assessed the potential of E2 to assume selective typical features of neuronal cells during neurogenic differentiation of hADSCs. Using neurosphere formation protocol, human ADSCs induced to neuron-like cells and expressed some neural markers 7 days post-induction. We added E2 with 10 nM concentration, in neurogenic induction medium every day in the treated culture consistent with previous study (17), but some studies have used a different concentration (10−11 –10−3 M) of E2 (18, 24).
Immunocytochemical analysis of the present study revealed that in the E2 treated cell culture, the differentiated ADSCs had higher expression of the neuronal specific markers (nestin and MAP-2 markers) following neural differentiation. In addition, real-time RT-PCR results show that in the presence of E2, the differentiated ADSCs expressed more MAP-2 markers than the control group, whereas, the expression of nestin was down regulated in the E2 treated cell cultures as compared to untreated cell cultures.
Moreover, the results of MTT assay show that the mean percentage of cell viability and proliferation rate in the E2 treated cell cultures are higher than others. It may be due to presence of EGF and b-FGF as mitogenic factors in the preinduction medium. Also, addition of E2 to cell cultures could lead to rise in the number of neuronal differentiated cells in treated cell cultures when compared to non-treated cell cultures.
Some evidences show that estrogen acts not only on reproductive organs, but also on brain function by affecting mood, cognition and emotional behavior (25, 26). Hormone therapy via estrogen can improve learning, memory, and protect against cerebral stroke (27, 28).
Specific effects of estrogen are mediated by estrogen receptors (ERs) and act as ligand-activated transcription factors to regulate the expression of estrogen-responsive genes (29, 30). Estrogen has two types of receptors including ERα and ERβ. They are differentially expressed in various tissues with special function (31, 32). Expression of both of these receptors in the brain was known (33). Moreover, expression of ERs in embryonic and mesenchymal stem cells was reported, and it suggests that estrogen may modify the function of stem cells (34, 35).
Wong et al (2003) found that chronic exposure to 17β-E2 had anti-mitotic, but neuroprotective effects; although, pulsed 17β-E2 treatments significantly increased mitogenesis of granular cells. They reported that neurotoxicity effect of pulsed 17β-E3 was mediated through mitogen-activated protein kinase (MAPK) pathway, but the neuroprotective effects of chronic E2 exposure were MAPK-independent. Therefore, the same concentration of E2 can induce either neuroprotection or neurotoxicity, depending on the time of the exposure. Thus, duration and concentration of E2, were two important factors in the mitogenesis effect and viability of cells (36).
Previous studies demonstrated that estrogen treatment can increase expression of some neurotrophic factors in the central nervous system (12-15). On the other hand, human ADSCs act as a source of variety of neurotrophic factors with neuroprotective activity, which may prevent neuronal degeneration and are involved in neuronal development (23, 37). Therefore, estrogen can promote proliferation and neuronal differentiation of human ADSCs during neural induction through production of neurotrophic factors.
Conclusion
It could be concluded that estrogen may improve proliferation rate and neuronal differentiation of human ADSCs by production of neurotrophic factors and supports the survival and regeneration of nervous tissue in the neurodegenerative diseases. However, further studies are required to confirm this potential.
Acknowledgment
The authors are grateful to Isfahan University of Medical Sciences for financial support (Grant no. 187128) and Dr Freshteh Haghighat for providing human adipose tissue.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological decits by intracerebral transplant of human adipose-derived stem cells after ischemia in rats. Exp Neurol. 2003;83:355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 2.Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006;15:583–594. doi: 10.1089/scd.2006.15.583. [DOI] [PubMed] [Google Scholar]
- 3.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 6.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Segura LM, Azcoitia I, DonCarlos LL. DonCarlos, Neuroprotection by estradiol. Prog. Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog. Neurobiol. 2003;71:31–41. doi: 10.1016/j.pneurobio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Gerhold LM, Böttner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 11.Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 12.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovariectomy reduces ChAT activity and NGF mRNA levels in the frontal cortex and hippocampus of the female Sprague–Dawley rat. Soc Neurosci Abstr. 1993;19:254. [Google Scholar]
- 13.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague–Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 14.Bimonte-Nelson CA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov T, Karolczak M, Beyer C. Estradiol stimulates GDNF expression in developing hypothalamic neurons. Endocrinology. 2002;143:3175–3178. doi: 10.1210/endo.143.8.8794. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Chang YH, Barker JL, Hu Q, Maric D, Li BS, Rubinow DR. Testosterone and estrogen affect neuronal differentiation but not proliferation in early embryonic cortex of the rat: the possible roles of androgen and estrogen receptors. Neurosci Lett. 2000;231:57–60. doi: 10.1016/s0304-3940(99)00942-8. [DOI] [PubMed] [Google Scholar]
- 17.Kang JH, Lee CK, Kim JR, Yu SJ, Jo JH, Do BR, Kim HK, Kang SG. Estrogen stimulates the neuronal differentiation of human umbilical cord blood mesenchymal stem cells (CD34-) Neuroreport. 2007;18:35–38. doi: 10.1097/WNR.0b013e3280123192. [DOI] [PubMed] [Google Scholar]
- 18.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 19.Okada M, Murase K, Makino A, Nakajima M, Kaku T, Furukawa S, Furukawa Y. Effects of estrogens on proliferation and differentiation of neural stem/progenitor cells. Biomed Res. 2008;29:163–70. doi: 10.2220/biomedres.29.163. [DOI] [PubMed] [Google Scholar]
- 20.Razavi S, Razavi MR, Zarkesh Esfahani H, Kazemi M, Mostafavi FS. Comparing brain-derived neurotrophic factor and ciliary neurotrophic factor secretion of induced neurotrophic factor secreting cells from human adipose and bone marrow-derived stem cells. Dev Growth Differ. 2013;55:648–55. doi: 10.1111/dgd.12072. [DOI] [PubMed] [Google Scholar]
- 21.Wei X, Zhao L, Zhong J, Gu H, Feng D, Johnstone BH, March KL, Farlow MR, Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci Lett. 2009;462:76–79. doi: 10.1016/j.neulet.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 22.Razavi S, Mardani M, Kazemi M, Esfandiari E, Narimani M, Esmaeili A, Ahmadi N. Effect of leukemia inhibitory factor on the myelinogenic ability of Schwann-like cells induced from human adipose-derived stem cells. Cell Mol Neurobiol. 2013;33:283–289. doi: 10.1007/s10571-012-9895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razavi S, Razavi MR, Kheirollahi-Kouhestani M, Mardani M, Mostafavi FS. Co-culture with neurotrophic factor secreting cells induced from adipose-derived stem cells: promotes neurogenic differentiation. Biochem Biophys Res Commun. 2013;25(440):381–387. doi: 10.1016/j.bbrc.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 24.Su JD, Qiu J, Zhong YP, Li XY, Wang JW, Chen YZ. Expression of estrogen receptor (ER)-alpha and -beta immunoreactivity in hippocampal cell cultures with special attention to GABAergic neurons. J Neurosci Res. 2001;65:396–402. doi: 10.1002/jnr.1166. [DOI] [PubMed] [Google Scholar]
- 25.Pilgrim C, Hutchison JB. Developmental regulation of sex differences in the brain: Can the role of gonadal steroids be redefined? Neuroscience. 1994;60:843–855. doi: 10.1016/0306-4522(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 26.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: Implications for cognition and aging. Neurology. 1997;48:S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 27.Lafferty FW, Fiske ME. Postmenopausal estrogen replacement: A long-term cohort study. Am J Med. 1994;97:66–77. doi: 10.1016/0002-9343(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 28.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631– 652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERαand ERβat AP1 sites. Scienc. e1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 31.Patrone C, Pollio G, Vegeto E, Enmark E, de Curtis I, Gustafsson JA, Maggi A. Estradiol induces differential neuronal phenotypes by activating estrogen receptor alpha or beta. Endocrinology. 2000;141:1839–1845. doi: 10.1210/endo.141.5.7443. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 33.Patrone C, Andersson S, Korhonen L, Lindholm D. Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci U S A. 1999;96:10905–10910. doi: 10.1073/pnas.96.19.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- 35.Hong SH, Nah HY, Lee YJ, Lee JW, Park JH, Kim SJ, Lee JB, Yoon HS, Kim CH. Expression of estrogen receptor- alpha and –beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 2004;18:320–325. [PubMed] [Google Scholar]
- 36.Wong JK, Le HH, Zsarnovszky A, Belcher SM. Estrogens and ICI182, 780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci. 2003;23:4984–4995. doi: 10.1523/JNEUROSCI.23-12-04984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114:2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]


