Abstract
Objective(s):
Propofol (2, 6-diisopropylphenol) is an intravenous anesthetic that is commonly used for the general anesthesia. It is well known that the spinal cord is one of the working targets of general anesthesia including propofol. However, there is a lack of investigation of the effects of propofol on spinal dorsal horn which is important for the sensory transmission of nociceptive signals. The objective of this study was to investigate the effects of increasing dosage of propofol on the release of glutamate (Glu), γ-aminobutyric acid (GABA) and glycine (Gly) in the spinal dorsal horn.
Materials and Methods:
The efflux of Glu, GABA or Gly in the spinal dorsal horn of rats was detected using transverse spinal microdialysis under an awake condition and various depths of propofol anesthesia. The infusion rates of propofol were, in order, 400 µg/(kg·min), 600 µg/(kg·min) and 800 µg/(kg·min), with a 20 min infusion period being maintained at each infusion rate.
Results:
Propofol decreased the glutamate efflux within spinal dorsal horn in a dose-dependent manner, and the maximum decrease was 56.8 ± 6.0% at high-dose propofol infusion producing immobility. The inhibitory GABA and Gly efflux was also decreased about 15–20% at low-dose propofol infusion only producing sedation, but did not continue to drop with higher doses of propofol.
Conclusion:
Propofol decreased both excitatory and inhibitory amino acids efflux in spinal dorsal horn, and the preferential suppression of the excitatory amino acid might be associated with the analgesic effect of propofol.
Keywords: Amino acids, Anesthetics, Dorsal hornm, Microdialysis, Propofol
Introduction
Propofol (2, 6-diisopropylphenol) is an IV anesthetic that is widely used for the induction and maintenance of general anesthesia. It is well known that the spinal cord is one of the working targets of general anesthetics including propofol. In the spinal cord, the anesthetics might suppress the sensory transmission of nociceptive signals within the dorsal horn and the neuronal activity in the ventral horn (1). While neurons in the spinal ventral horn seem to be more susceptible to propofol (2), the dorsal horn neurons still serve as a potent target for anesthetics to suppress the processing of sensory afferent information (3).
It was shown that general anesthetics produced a profound suppression of the activity of dorsal horn neurons induced by peripheral noxious (4). Previous study reported that propofol, in anesthetic doses, directly depressed neuronal activity in the lumbar dorsal horn with minimal indirect supraspinal effects in the intact goat (5). Takechi and his colleagues demonstrated that the topical application of propofol inhibited the responses of lumbar dorsal horn wide dynamic range (WDR)-type neurons to ipsilateral noxious thermal stimulation, and suppressed the sensitization of WDR neurons induced by the allyl isothiocyanate (6).
Upon exposure to the noxious stimuli several neurotransmitters, including glutamate (Glu), γ-aminobutyric acid (GABA) and glycine (Gly), are released from the sensory afferents and nocisponsive neurons in the dorsal horn, thus integrating the peripheral nociceptive information and forwarding it to the supraspinal brain regions for pain sensation. Currently the differential effect of propofol on the neurotransmission in dorsal horn remains unclear. In this study, we applied transverse spinal microdialysis to delineate the accurate profile of propofol at different doses to affect the release of these neurotransmitters in dorsal spinal cord in the rats.
Material and Methods
Animals
Fifteen adult male Wistar rats (supplied by the medical experimental animal center in Chinese People Liberation Army General Hospital) weighing 280–350 g were used in the study. All animals were housed at a constant temperature (24 ± 0.5 °C) and relative humidity (60±2%) on a light-controlled schedule (light on between 6:00 AM and 6:00 PM), and had free access to food and water. The experimental protocols were approved by the Animal Care and Use Committee of the Chinese People Liberation Army (PLA) General Hospital (Beijing, China), and were conducted under the guidelines for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of the People’s Republic of China.
Propofol administration and the assessment of the anesthesia states of rats from behavioral signs
A heparinized saline–filled polyethylene catheter (OD= 0.8 mm) was inserted into the right internal jugular vein of rats under the isoflurane anesthesia for the following propofol infusion. The catheter was set under subcutaneous tissues, and the end of the catheter was secured to the nape of the neck with sutures for infusion of propofol. The rats received the propofol infusion twice with 2 days apart. The first infusion was scheduled 24 hr after catheterization to assess the effect of propofol on the anesthesia states of the rats, and the second infusion was scheduled 24 hr after the placement of the microdialysis probe to determine the effect of propofol on the neurotransmitter release in the dorsal horn.
The rats were transferred to a bowl-shape chamber 24 hr after the catheterization. Propofol (10 g/l) was continuously infused through the polyethylene catheter with a 3500 syringe pump (Graseb Medical, UK) following the schedule shown in Figure 1. The infusion rates were, in order, 400 µg/(kg·min), 600 µg/(kg·min) and 800 µg/(kg·min), and a 20 min infusion period was maintained at each infusion rate (Figure 1). Every 2 min before changing the infusion rate to the next level, vibrissal stroking test, right flex test and tail pinch test were conducted in turn to assess the anesthetic states of rats at the current time point. The rats were defined as sedate with the loss of vibrissa stroking reflex, unconsciousness with the loss of righting reflex, or immobile with the loss of tail pinch reflex. If it was assessed using vibrissal stroking test that the rat was under sedative state, the righting reflex test would then be done to evaluate the state of consciousness of the rat; if the rat was in unconscious state, the tail pinch test would then be done to evaluate if the rat was in analgesic state (immobility). Throughout the studies, the rats had spontaneous ventilation.
Figure 1.
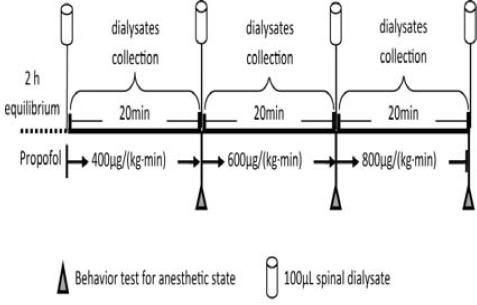
Protocol of administration of propofol and collection of dialysate
Microdialysis procedure
Microdialysis probes were placed as previously described (7) 24 hr after the first propofol administration. Briefly, the rat was anesthetized with chloral hydrate (350 mg/kg, intraperitoneally), and an incision along the dorsal midline from T9 to L3 was made. The T13 vertebra was cleared of muscle and two small holes were drilled into the lateral aspect on each side to expose a small portion of the spinal cord. A linear microdialysis probe (BAS MD-2005, 5 mm membrane window, 6/pkg, USA) was inserted transversely across the dorsal horn of the spinal cord through the two holes and then fixed to the bone with dental cement (S.1). The polyethylene catheters at the inflow side and the outflow side of the probe were fixed at the neck via piercing the tunnel beneath the back skin. The animals were allowed to recover for 24 hr. Only the rats without significant weight loss and limb paralysis or impaired movement proceeded for further experiments.
Twenty four hours after the placement of the microdialysis probe, the rats were placed in the U-shaped chamber which is a part of a system for freely moving animals. The linear microdialysis probes were perfused with artificial cerebrospinal fluid (ACSF: 140.0 mM NaCl, 3.0 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 1.5 mM Na2HPO4, 0.27 mM NaH2PO4, and pH 7.4 adjusted with 0.1 M NaOH). After a period at a rate of 5 µµl/min with an infusion microvolume pump (CMA/100, CMA, Stockholm, Sweden), samples of dialysate in polyethylene vials in the refrigerated fraction collector (4°C, HoneyComb™, BAS, U.S.A) via connecting tubes (MF-5366, 0.025OD×0.005 ID, BAS, USA) were collected. Dialysate samples were collected every 20 min (100 µµl) for 1 hr prior to infusion of propofol to establish the baseline level, and then propofol (10 g/l) was continuously infused. The infusion rates were, in order, 400 µg/(kg·min), 600 µg/(kg·min) and 800 µg/(kg·min), and a 20 min infusion period was maintained at each infusion rate. During the propofol anesthesia, samples were collected every 20 min (100 µµl) for 1 hr. The collected samples were transferred immediately to a -80 °C freezer for amino acid detection. At the end of the experiment, all rats were killed with an overdose of pentobarbital, and the spinal cord was removed and postfixed in 10% formalin to verify the microdialysis fiber placement site.
Detection of amino acids in the dialysates
The concentrations of Glu, GABA and Gly in the dialysates were measured by OPA-β-mercaptoethanol precolumn derivatization, reversed phase gradient elution and fluorescence detection. The HPLC employed buffer A: 0.1 M NaH2PO4 buffer (adjusted to pH 6.8): chromatographic pure methanol=70:30; and buffer B: HPLC-grade methanol. Buffer A was ultrasonically degassed, buffer B was filtered and degassed through a 0.2 mm nitrocellulose membrane. The above two-buffer HPLC system (HP1100, Agilent, U.S.A) was coupled to a fluorescent detector (1100, Agilent, USA). Separation was achieved on a C18 column (Hypersil AA-ODS, 5 µm, 2.1×200 mm, Agilent, U.S.A). The o-phthaldialdehyde derivatizing reagent was prepared by dissolving 25 mg o-phthaldialdehyde (Sigma, USA) in absolute methanol (0.5 ml) and adding 2-mercaptoethanol (10 μl) and borate buffer (0.4 mol/l, pH 9.4-9.5, and 5 ml). Two microliters dialysate and 10 µµl OPA derivating fluid were allowed to react for 3 min at room temperature, and then the reaction mixture was injected into the column and separated with a gradient from A: B (100: 0) to 60% B within 30 min. The flow rate was set to 0.5 ml/min. Excitation wavelength was set as 340 nm, and emission wavelength was 450 nm. Detector temperature was set at 28°C.
Measurement of hemodynamic parameters
Another cohort of rats (n = 6) was used to investigate the hemodynamic changes during the propofol anesthesia. Twenty four hours before the propofol administration (same as mentioned above), the rats were anesthetized with 1.8% isoflurane, and a heparinized saline–filled polyethylene catheter was inserted into the femoral artery for the measurement of mean arterial blood pressure (MAP) and heart rate (HR) during propofol infusion.
Statistical analysis
All microdialysis data were presented as percentage ± SEM of basal values. Hemodynamic parameters were shown as mean ± SEM. Repeated measures analysis of variance was performed on amino acid levels under anesthesia with different dosages of propofol, “within” factor being time and “between” factor being different dosage of propofol treatment. Multiple comparisons were performed using LSD. Hemodynamic parameters were analyzed with one-way ANOVA. A value of P < 0.05 was considered statistically significant. All data were analyzed using the statistical software SPSS13.0.
Results
Enrolled rats
Two rats showing neurological deficits of paralysis and allodynia following the catheterization for spinal microdialysis were excluded from the experiment. The catheter in one rat was not placed in the intended spinal area. Finally, the data of twelve rats were adopted for the statistical analysis.
The anesthesia state assessment of rats from behavioral signs
After 20 min infusion of propofol at 400 µg/(kg·min), all rats were in sedative state, and one rats showed the absence of righting reflex; after 20 min infusion at 600 µg/(kg·min), all rats showed the loss of righting reflex while keeping tail pinch reflex; after 20 min infusion at 800 µg/(kg·min), all rats showed the loss of tail pinch reflex.
Hemodynamic parameters
As shown in Table 1, the heart rate was significantly decreased at 600 µg/(kg·min) or 800 µg/(kg·min) propofol infusion (P < 0.05 vs baseline). The mean arterial blood pressure (MAP) was decreased at 800 µg/(kg·min) propofol infusion for 20 min (P < 0.05 vs baseline). There was no statistically significant difference in PaO2 between basal condition and propofol infusion at different doses (P > 0.05 vs baseline), while the PaCO2 was increased at 800 µg/(kg·min) propofol infusion for 20 min compared with baseline.
Table 1.
Effects of increasing doses of propofol on systemic hemodynamics in rats
| Baseline | 400 µg/(kg·min) | 600 µg/(kg·min) | 800 µg/(kg·min) | |
|---|---|---|---|---|
| HR | 367±47 | 374±37 | 329±39* | 323±35* |
| MAP | 121±8 | 120±7 | 110±5 | 99±4* |
| PaO2 | 104±9 | 102±8 | 103±9 | 98±5 |
| PaCO2 | 31±1 | 30±1 | 34±2 | 35±1* |
Data are expressed as mean ±. (n = 6). HR: heart rate, MAP: mean arterial blood pressure. Data analyzed by one-way ANOVA for comparison
P<0.05 as compared to baseline
Amino acids release in the spinal dorsal horn under various dosages of propofol anesthesia
GluGlu, Gly and GABA retention times were 3.5, 10 and 14.5 min, respectively. The representative chromatograms for three amino acids are shown in Figure 2. As shown in Figure 3A, propofol decreased the Glu efflux in spinal dorsal horn in a dose-dependent manner (P < 0.00; n = 15).
Figure 2.
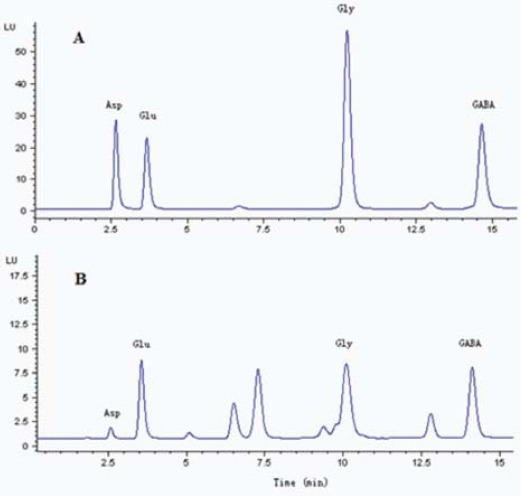
The representative chromatograms of standard glutamate (Glu), glycine (Gly) andγ-aminobutyric acid (GABA) after derivatization (A) adding a microdialysis sample (B). The abscissa indicates the time after sample injection (min)
Figure 3.
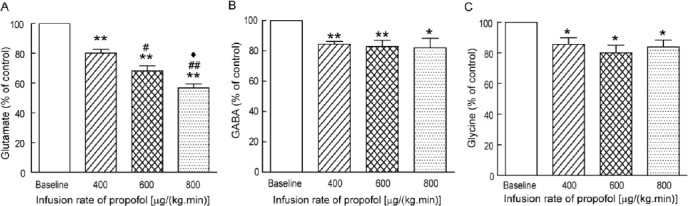
Effects of increasing doses of propofol on the glutamate (Glu) (A), γ-aminobutyric acid (GABA) (B) and glycine (Gly) (C) efflux in the spinal cord dorsal horns of rats. Glutamate, glycine and GABA were shown as percent compared with the data before administration of propofol. Baseline levels of glutamate, glycine and GABA were set as 100%. N = 15; Values are percentage ± SEM. *P < 0.05, **P < 0.01 as compared to baseline; #P < 0.05, ##P < 0.01 as compared to 400 µg/(kg·min) propofol infusion; ♦P < 0.05 as compared to 600 µg/(kg·min) propofol infusion
The release of Glu was decreased to 80.1 ± 5.5% at 400 µg/(kg·min) propofol infusion (P = 0.006 vs baseline; n = 15), and 56.8 ± 6.0% at 800 µg/(kg·min) propofol infusion (P<0.001 vs baseline; n= 15). Meanwhile, the GABA efflux in the spinal dorsal horn was decreased to 84.6±3.9% at 400 µg/(kg·min) propofol infusion (P<0.001 vs baseline; n=15), 83.0±9.7% at 600 µg/(kg·min) infusion (P = 0.008 vs baseline; n=15), and 82.2±15.1% at 800 µg/(kg·min) infusion (P=0.035 vs baseline; n=15) (Figure 3B). As shown in Figure 3C, The Gly efflux was decreased to 85.7±10.2% (P=0.02 vs baseline; n=15) and 80.1 ± 12.1% (P= 0.01 vs baseline; n= 15) at 400 µg/(kg·min) and 600 µg/(kg·min) propofol infusion, respectively, and 83.9±10.8% at 800 µg/(kg·min) propofol infusion (P= 0.015 vs baseline; n=15).
Discussion
Using a transverse microdialysis, we investigated the effects of increasing dosage of propofol on the Glu, GABA and Gly efflux in spinal dorsal horn. It was revealed that propofol had inhibitory action on the efflux of both excitatory and inhibitory amino acids in spinal dorsal horn, and the inhibition on the excitatory amino acids was stronger than that on the inhibitory ones. We speculated that the preferential suppression on the release of excitatory amino acids may be associated with the analgesia effect of propofol.
In the current study, propofol inhibited the efflux of Glu in spinal dorsal horn in a dose-dependent manner. Glu is contained in primary afferent fibers (PAFs) and dorsal horn neurons (8, 9), and the majority of the primary afferent synapses such as the C-fiber afferent synapses are glutamatergic synapses. The release of Glu from PAFs is inhibited (presynaptic inhibition) mainly via presynaptic opioid receptors and GABA receptors in dorsal horn (10). Activation of GABAA receptor induces the hyperpolarization of primary afferents, and produces a decrease in the amplitude of presynaptic action potentials and, consequently, the amount of transmitter released (11). GABAA and GABAB receptors have been found on the central terminals of the nociceptive fine PAFs in superficial dorsal horn, and the activation of the presynaptic GABAA and GABAB receptors reduces the release of neurotransmitters such as Glu and substance P (12, 13). Propofol is thought to exert its anesthetic action by augmenting GABAA receptor-mediated chloride current (14). Therefore, propofol might enhance GABAA receptors-mediated presynaptic inhibition at PAFs and then attenuate the release of Glu. Previous in vivo experiment reports that propofol (1 mg/kg) enhances GABAA receptor-mediated presynaptic inhibition in PAFs in human spinal cord (15). In our study, the Glu level was decreased to 80% during the low-dose propofol infusion, and the maximum decrease was about 45% at high-dose propofol infusion. The dose-response effects on the Glu release may be associated with the different actions of propofol on GABA receptors.
The present study also showed that the GABA and Gly efflux was decreased about 15–20% during the low-dose propofol infusion, but did not continue to drop with the increased dosage of propofol.
Yamakura et al demonstrated that he enhancement of GABAergic and glycinergic neurotransmission by propofol is responsible for its general anesthetic properties (16). However, this research about the molecular mechanism of general anesthesia focused on the effects of propofol on the receptors, especially the GABAA receptor which is the selective target of propofol. And the release of excitatory and the inhibitory amino acids in the spinal dorsal horn of the living rats is the global result of the action of propofol on the GABAA receptor and other potential targets. The exact mechanism for this inhibition of GABA and Gly efflux is not clear. The release of inhibitory amino acids in the spinal cord was related to the tone of a descending pain modulation pathway (17). The facilitation or inhibition of descending pain modulation pathway might influence the release of excitatory and inhibitory amino acids in the spinal cord. The decrease of GABA and Gly release may result from disinhibition of descending supraspinal inhibitory systems induced by propofol.
Conclusion
The current study demonstrates that propofol has inhibitory action on both excitatory and inhibitory amino acids efflux in dorsal spinal cord. Moreover, the inhibition on Glu release was stronger than that on GABA and Gly release. The imbalance of excitation and inhibition within the dorsal spinal cord might contribute to the anesthetic effects of propofol.
Acknowledgment
This work was supported financially by the National Natural Science Foundation of China (Grant No. 2012FC-CXYY-4044). We thank Bihua Bie for the excellent advice on manuscript writing. The results reported in this paper were part of a student thesis.
References
- 1.Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: Contributions to the state of general anesthesia. Trends Neurosci. 1995;18:549 –553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- 2.Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108:1531–1537. doi: 10.1213/ane.0b013e31819d9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takasusuki T1, Yamaguchi S, Hamaguchi S, Yaksh TL. Effects of general anesthetics on substance P release and c-Fos expression in the spinal dorsal horn. Anesthesiology. 2013;119:433–442. doi: 10.1097/ALN.0b013e31829996b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antognini JF, Wang XkW, Piercy M, Carstens E. Propofol directly depresses lumbar dorsal horn neuronal responses to noxious stimulation in goats. Can J Anaesth. 2000;47:273–279. doi: 10.1007/BF03018926. [DOI] [PubMed] [Google Scholar]
- 6.Takechi K, Carstens MI, Klein AH, Carstens E. The antinociceptive and antihyperalgesic effects of topical propofol on dorsal horn neurons in the rat. Anesth Analg. 2013;116:932–938. doi: 10.1213/ANE.0b013e31827f560d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skilling SR, Smullin DH, Beitz AJ, Larson AA. Extracellular amino acid concentrations in the dorsal spinal cord of freely moving rats following veratridine and nociceptive stimulation. J Neuroehem. 1988;51:127–132. doi: 10.1111/j.1471-4159.1988.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 8.Sluka KA, Westlund KN. An experimental arthritis model in rats: dorsal horn aspartate and glutamate increases. Neurosci Lett. 1992;145:141–144. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- 9.Valtschanoff JG, Phend KD, Bernardi PS, Weinherg RJ, Rustioni A. Amino acid immunocytochemistry of primary afferent terminals in the rat dorsal horn. J Comp Neurol. 1994;346:237–252. doi: 10.1002/cne.903460205. [DOI] [PubMed] [Google Scholar]
- 10.Ataka T1, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Adelta-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. doi: 10.1016/S0304-3959(00)00255-4. [DOI] [PubMed] [Google Scholar]
- 11.Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa T, Marsala M, Sakabe T, Yaksh TLl. Characterization of spinal amino acid release and touch-evoked allodynia produced by spinal glycine or GABA (A) receptor antagonist. Neuroscience. 2000;95:781–786. doi: 10.1016/s0306-4522(99)00461-3. [DOI] [PubMed] [Google Scholar]
- 13.Kleschevnikov AM, Belichenko PV, Gall J, George L, Noshenv MT. Increased efficiency of the GABAA and GABAB receptor-mediated neurotransmission in the Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2012;45:683–691. doi: 10.1016/j.nbd.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manuel NA, Davies CH. Pharmacological modulation of GABA (A) receptor-mediated postsynaptic potentials in the CA1 region of the rat hippocampus. Br J Pharmacol. 1998;125:1529–1542. doi: 10.1038/sj.bjp.0702237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu M, Yamakura T, Tobita T, Okamoto M, Ataka T, et al. Propofol enhances GABAA receptor-mediated presynaptic inhibition in human spinal cord. Neuroreport. 2002;13:357–360. doi: 10.1097/00001756-200203040-00021. [DOI] [PubMed] [Google Scholar]
- 16.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Lü N, Han M, Yang ZL, Wang YQ, Wu GC, Zhang YQ. Nociceptin/Orphanin FQ in PAG modulates the release of amino acids, serotonin and norepinephrine in the rostral ventromedial medulla and spinal cord in rats. Pain. 2010;148:414–425. doi: 10.1016/j.pain.2009.11.025. [DOI] [PubMed] [Google Scholar]


