Abstract
The controlled assembly and organization of multi-cellular systems to mimic complex tissue structures is critical to the engineering of tissues for therapeutic and diagnostic applications. Recent advances in micro-scale technologies to control multi-cellular aggregate formation typically require chemical modification of the interface between cells and materials and lack multi-scale flexibility. Here we demonstrate that simple physical entrapment of magnetic microparticles within the extracellular space of stem cells spheroids during initial formation enables scaffold-free immobilization, translocation and directed assembly of multi-cellular aggregates across multiple length and time scales, even under dynamic suspension culture conditions. The response of aggregates to externally applied magnetic fields was a direct function of microparticle incorporation, allowing for rapid and transient control of the extracellular environment as well as separation of heterogeneous populations. In addition, spatial patterning of heterogeneous spheroid populations as well as individual multi-cellular aggregates was readily achieved by imposing temporary magnetic fields. Overall, this approach provides novel routes to examine stem cell differentiation and tissue morphogenesis with applications that encompass the creation of new model systems for developmental biology, scaffold-free tissue engineering strategies and scalable bioprocessing technologies.
Introduction
Mammalian tissues are comprised of individual cells assembled in a hierarchical manner to form multi-cellular units that span micron to millimetre scales in order to coordinately contribute to tissue function.1 The engineering of tissues ex vivo seeks to emulate the complexity of native tissues and organs by generating 3D multi-cellular constructs capable of serving as models of healthy and diseased tissues for use as novel diagnostic and drug-screening platforms2 and potentially for organ replacement or regenerative therapies.3 Construction of 3D multi-cellular neo-tissues requires directed assembly of both homogeneous and heterogeneous populations of cells. Current approaches to control the assembly and patterning of cells in 3D rely on intrinsic adhesive properties of the cells or engineering cellular interactions. Intercellular adhesion is mitigated through natural cell-cell adhesion mechanisms, such as cadherins,4,5 or can be engineered by chemically modifying cell surface molecules to promote and stabilize multi-cellular assembly.6-8 In some instances, otherwise nonadhesive cell types can be induced to form homotypic cell aggregates; however, engineering of aggregates with multiple cell types requires a step-wise building process which has thus far been limited to the formation of small aggregates comprised of at most 2–4 cell layers.6 One particular concern associated with engineering cell surface adhesion properties of stem cells is the possibility of attenuating or inhibiting signaling pathways important to differentiation that rely upon dynamic transmembrane binding events, including cell-cell and cell-ECM interactions. An alternative approach to controlling larger scale cell aggregation is to entrap cells within biomaterials, such as hydrogels, which can then be assembled by engineering the chemical and physical properties of the cellladen material building blocks.9,10 Unlike modification of individual cells, cell encapsulation technologies can control the spatial assembly of multi-cellular modules (100s of μm to mm in size) into composite structures. However, cell encapsulation limits the interaction of cells in different modules and properties of hydrogel materials such as degradability, stiffness and ligand density can independently and cooperatively influence cell phenotype,11-13 thereby requiring that cell-specific design criteria potentially be considered for different stem and progenitor cell populations. In either case, engineering cell membrane properties and cell entrapment both require a priori design of parameters including complementary cell adhesion mechanisms or design of material properties based on the cell type and desired construct geometry. Thus, a robust method capable of geometrically controlled multi-scale assembly for a variety of cell types without the need for cell modification or a priori materials design would provide a novel and flexible route to study the biology of multi-cellular structures in 3D.
Magnetic forces can be broadly applied across multiple length scales to direct individual cells at the micro-scale (i.e. microtweezers14 and microfluidics15) and have long been used for larger scale applications including technologies for separation and sorting of cell populations.16,17 In general, approaches to magnetically label and direct cells have required surface modification of the magnetic particles and/or the cell plasma membranes in order to permit stable association between the materials and the cells.18 Alternatively, the directed endocytosis of magnetic material can be utilized to induce magnetic sensitivity of individual cells;19 however, endocytosis of sub-micron magnetic material can interfere with intracellular signaling and viability.20,21 In contrast, magnetic manipulation of multi-cellular aggregates with larger(>1 μm diameter) paramagnetic material incorporated within the extracellular space does not require cellular modification or material internalization and remains an attractive route to control cell behavior because of the inherently flexible and spatiotemporally controlledmanner in which magnetic fields can be applied. Recently our lab has demonstrated that biomaterial microparticles (MPs) of varying sizes (1–20 μm) can be efficiently incorporated in a dose-dependent and homogeneous manner within the extracellular space of multi-cellular spheroids to engineer physicochemical properties of the 3D stem cell microenvironment.22-24 However, to date, controlling the spatial incorporation and distribution of MPs or populations of different cells within multi-cellular aggregate environments has proven difficult. Thus, we aimed to incorporate magnetic microparticles (MPs) as a means to spatially control homogeneous and heterogeneous multicellular aggregates, without adversely impacting cell viability or phenotype.
We report here that magnetic MPs (magMPs) can be efficiently and stably incorporated in a dose-dependent manner in the extracellular environment of 3D stem cell aggregates without the need for biochemical modification of the cell membrane or material adhesive properties. MagMP entrapment within multi-cellular aggregates enables the directed movement and assembly of spheroids in suspension culture with an externally applied magnetic field to yield complex organization of 3D constructs across broad length and time scales. Therefore, this method can be used to address a variety of questions of relevance to stem cell and developmental biology, tissue engineering and cell bioprocessing.
Materials and methods
Spheroid formation and magMP incorporation
D3 murine embryonic stem cells (ESCs) were propagated on 0.1% gelatin-coated tissue culture dishes in DMEM media containing 15% FBS and 103 U/ml leukemia inhibitory factor (LIF; Millipore, Billerica, MA). Embryoid bodies (EBs) were formed using a single-cell suspension by forced aggregation in AggreWell™ 400 inserts (Stem Cell Technologies, Vancouver, CA). Briefly 1.2 × 106 cells in 0.5 ml of medium were inoculated into AggreWell inserts, containing approximately 1200 wells per insert, and centrifuged at 200 RCF for 5 min to pellet cells in the wells. Magnetic, polystyrene microparticles with a diameter of 4 μm (SpheroTech, Lake Forest, IL) were washed 3x with ddH2O, diluted in 0.1 mL DMEM media, and subsequently added at a 1 : 10, 1 : 3, 1 : 1, and 3 : 1 microparticleto-ESC ratio. To pellet microparticles in the wells, either a second centrifugation was performed at 200 RCF for 5 min or a magnet (10 lb pull strength) was applied below the plate for 5 min. All magnets used to create external magnetic fields were purchased from Gaussboys Super Magnets (Portland, OR). After 24 h of culture, aggregates were removed from the wells using gentle pipetting and transferred to a suspension culture on a rotary orbital shaker (45 RPM) to maintain the homogeneous populations of spheroids.25
Quantification of magnetic microsphere incorporation in EBs
After 24 h of culture, EBs containing magnetic microparticles were collected and transferred to a 48 well plate where they were first counted and then lysed in a 5% SDS solution. The magMPs remaining were counted using a Coulter Counter (Beckman Coulter, Brea, CA) using a collection window of 3.5–4.5 μm for each sample. The resulting count was divided by the number of EBs lysed to calculate the average incorporation of magMPs per EB.
Histological analysis and cell viability
Spheroids cultured for 2 days were collected and rinsed in PBS, fixed in 10% formalin and embedded in Histogel (Thermo Scientific), processed and paraffin-embedded. Sections of 5 μm in thickness were deparaffinized before staining with Fast Green. Histological samples were imaged using an 80i upright microscope (Nikon) and an SPOT Flex camera (15.2 64 MP Shifting Pixel, Diagnostic Instruments). Cell viability was analyzed after 2 days of EB differentiation using LIVE/DEAD staining (Invitrogen, Carlsbad, CA). Samples were incubated in serum-free, phenol red-free medium containing 1 mM calcein AM and 2 mM ethidium homodimer I at room temperature for 30 min. Spheroids were then washed three times with PBS and imaged using an LSM 510 confocal microscope (Carl Zeiss, Inc).
Spheroid translocation
Spheroids formed from 1 : 10 and 1 : 3 magMP: cell seed ratios were aligned linearly in a 100 mm suspension culture Petri dish using a bar magnet (65 lb pull strength) placed below the dish. The magnet was removed from directly below the magMP laden spheroid population and placed under the dish at a distance of 4 mm away from the linear array of cell aggregates. The movement of individual spheroids was recorded at 3 frames per second using an SPOT Flex camera mounted on an Elipse TE2000U phase microscope (Nikon).
Cell labeling
Adherent ESC monolayers were rinsed in PBS and incubated with a 5 μM solution of CellTracker™ (Blue, Red or Green, Invitrogen) in serum-free medium for 30 min. The CellTracker solution was aspirated and cells were incubated with serum containing medium for 30 min prior to trypsinization and spheroid formation.
Magnetic manipulation
4 mm × 1 mm nickel plated neodymium magnets were used to spatially confine the location of spheroids in rotary orbital suspension culture. The desired configurations of magnets were created by magnets held in place on opposing sides of the culture lid. The ability of the magnets to pattern the spheroids was dependent on the distance of the magnets to the level of culture medium, which could be adjusted by stacking magnets on top of each other to increase the thickness of the magnet layer. After the magnets were configured, the dish was placed on a rotary orbital shaker at 40–50 RPM. In order to reversibly manipulate the translocation of individual spheroids between paramagnetic elements, iron rods were embedded in a PDMS gel approximately 4 mm in thickness such that the ends were spaced 2–3 mm apart and the opposite end of each iron element protruded at least 2 cm from the PDMS gel near the outside edge of the dish. Individual rods were transiently magnetized by contacting the free end of each with a 10 lb pull force bar magnet in a sequential manner such that after removing the magnet, one of the other rods could be magnetized. Individual spheroids were added in 1 ml of medium on top of the PDMS gel and imaged continuously as the rods were sequentially magnetized and demagnetized.
Spheroid merging
Spheroids were first formed from ESC populations labeled with CellTracker fluorescent dyes. For multi-aggregate assembly patterning, a 15 mm × 2 mm nickel plated neodymium magnet was applied underneath cell culture dishes to control spheroid location. Populations of spheroids were then added to the dish in small volumes of medium (100–500 μL) in the order shown in Fig. 4A and D. The EBs were then guided to a closely packed formation by swirling the magnet around the area occupied by the spheroids. After a population of spheroids was closely packed, spheroids labeled with a different color were added to the culture. For a stacked Venn diagram configuration, spheroids were sequentially added to the side of magnet each time, whereas for “bullseye” configuration, the spheroids were added in a circle around the magnet each time.
Fig. 4.
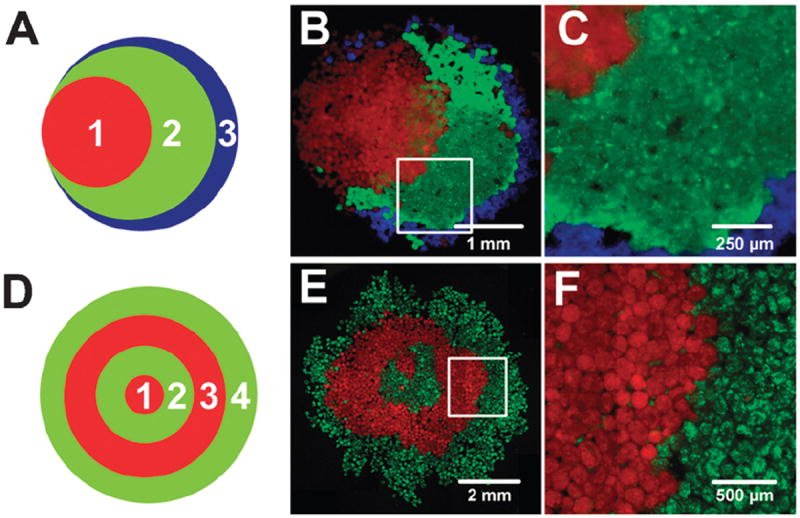
Large populations of magMP spheroids were manipulated to form macroscopic patterns including a “stacked Venn diagram” shape (a–c) or a “bullseye” pattern (d–f). Populations of CellTracker labeled EBs were added sequentially as shown in panels a and d and were aggregated using a magnet applied underneath the Petri dish. Mixing between the layers was limited as demonstrated in panel c and f, magnified from the boxed areas in b and e. Scale bars 1mm (b), 250 μm (c), 2 mm (e), 500 μm (f).
For merging of individual aggregates, single spheroids labeled green, red or blue were transferred from batch suspension culture to a 30 mm suspension culture Petri dish containing 500 μl of medium. A 3 mm × 9 mm nickel plated neodymium cylindrical magnet positioned beneath a Petri dish was used to direct the spheroids to the desired position. Merging of two spheroids was monitored at 37 °C via time-lapse microscopy (Biostation IM, Nikon) with images taken every 3 min for a period of up to 15 h. Merging of 3 and 4 spheroids composed of fluorescently labeled cells was performed similarly by adding different colored individual spheroids (one blue, red, green and no fluorescent label) sequentially to a culture dish. Each spheroid was then magnetically guided to the desired position and allowed to sit for 10 min before other spheroids were added. After spheroids were in contact with each other, the plate was placed in a cell culture incubator overnight and imaged using epifluorescent microscopy after 18–24 h.
Statistical analysis
Experimental values are reported as mean ± standard deviation (n = 6). Statistical analysis was determined using one way ANOVA coupled with Tukey’s post hoc analysis using Systat (v12, Systat Software Inc.). p-values < 0.05 were considered statistically significant.
Results
Magnetic microparticle incorporation in cell spheroids
Mouse ESCs and magMPs (4 μm diameter) were sequentially seeded in PDMS microwells through successive centrifugation or a combination of centrifugation and magnetic pull-down (Fig. 1A). Immediately after centrifugation or magnetic pull-down, magMPs were clearly observed on top of the cell layer (Fig. 1Bi, arrows) and were primarily visible along the edges of the wells (Fig. 1Bii, arrows). Within 18–24 h, multi-cellular aggregates formed within the wells (Fig. 1Biii) with magMPs distributed throughout the entirety of the spheroids (Fig. 1Biv). Time-lapse microscopy analysis indicated that cell movement during spheroid formation corresponded with displacement of the magMPs, but no significant differences were observed in the kinetics of spheroid formation with or without the introduction of magMPs (Supplemental Video 1). The number of magMPs incorporated within cell spheroids significantly (p < 0.01) increased in a dose-dependent manner as a function of the microparticle-to-cell seed ratio (Fig. 1C). After transferring the multi-cellular aggregates from the microwells to suspension culture, the resulting spheroid populations exhibited comparable sizes and shapes, and the magMPs (observed as opaque regions) remained readily apparent in contrast to the translucent cells (Fig. 1D). EBs formed with a 1 : 10 and 1 : 3 seed ratio were sufficiently sensitive to magnetic manipulation; therefore, further analysis on the effects of magMP incorporation on EB morphology and cell viability were performed with these groups. MagMPs in histological sections were visibly brown, due to their iron composition, and were clearly identifiable in the extracellular space of the cell spheroids in proportion to the respective seed ratio. The addition of magMPs (1 : 10 or 1 : 3) did not alter the cellular organization within EBs or affect the morphology of the cells in direct contact with the magMPs (Fig. 1E). Additionally, no difference in cell viability was observed between EBs with or without magMPs as assessed by LIVE/DEAD® staining after 2 days of culture. Differentiation of EBs, with and without magMPs, was assessed by gene expression analysis of pluripotent and germ layer specific markers over seven days of culture in serum containing medium. The expression of pluripotent markers, Nanog and OCT4, decreased with differentiation time and temporal expression patterns of markers from each of the three germ lineages (Pax6 – ectoderm, Brachyury-T – mesoderm, and Gata4 – endoderm) appeared as expected, with no significant differences between the experimental groups indicating that the presence of magMPs did not interfere with the normal differentiation process (Supplemental Fig. 1). MagMPs were also similarly incorporated within spheroids of human mesenchymal stem cells (Supplemental Fig. 2) and human induced pluripotent cells, indicating the broad applicability of this technique.
Fig. 1.
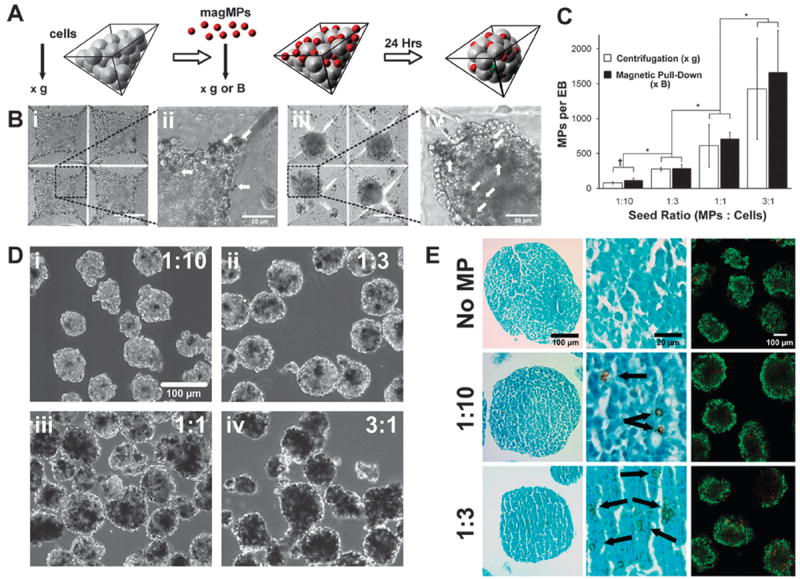
Magnetic MPs are incorporated in a dose dependent manner. (a) MagMPs were incorporated within stem cell spheroids within PDMS microwells (b) MagMPs (arrows) were observed after ESC and magMP centrifugation (i,ii) as well as throughout the spheroids that were formed after 18–24 h of culture (iii,iv). Scale bars, 200 μm (i,iv) and 50 μm (ii,iii) (c) Spheroids were formed using centrifugation or magnetic pull-down and the number of MPs incorporated per spheroid was determined after 24 h of culture. *p ≤ 0.05 Comparison of loading ratios, †p ≤ 0.05 comparison between pull-down and centrifugation. (d) EBs formed at cell seeding ratios of 1 : 10 (i), 1 : 3 (ii), 1 : 1 (iii), 3 : 1 (iv) were of similar shape and size and dark regions of magMPs increased with the seeding ratio. Scale bar 100μm. (e) Histological sections stained with Fast Green stain revealed that magMP incorporation did not disrupt cellular arrangement or morphology. No differences in cell viability were observed for any of the groups (LIVE/DEAD assay, dead cells labeled red and live cells labeled green). Scale bars 100 μm (first and third columns) and 20 μm (middle column).
Spatially directed translocation of EBs containing magMPs
Having demonstrated robust incorporation of magMPs within stem cell spheroids, the ability to manipulate the spatial positioning and translation of stem cell spheroids was examined in static and dynamic culture systems using externally applied magnetic fields. Under static culture conditions, entire fields of individual spheroids could be concentrated, translated and rotated based upon the relative location, direction and manner in which external magnetic fields were applied (Supplemental Video 2). As expected, spheroids containing a higher density of magMPs were more sensitive to applied magnetic fields (increased acceleration towards the magnet) compared to spheroids with a lower density of magMPs. In order to demonstrate the quantitative relationship between magMP incorporation and magnetic field strength, the positions of individual spheroids were tracked over time as they moved towards a magnet placed at a lateral distance of 4 mm (Fig. 2A and D). Spheroids formed at a ratio of 1 : 3 (magMPs : cells) were translated (Fig. 2B) at an average speed of 451 ± 96 μm s−1, whereas spheroids formed at a ratio of 1 : 10 moved at an average speed of 144 ± 80 μm s−1 in response to an identical magnetic field (500 Gauss). Spheroids with a lower seed ratio (1 : 10) demonstrated increased variability in their response to the magnet, presumably a consequence of the greater variability of magMPs per spheroid with lower seeding ratios. As expected, weaker or stronger magnets (10–65 lb pull strength) resulted in slower or faster translocation, respectively, of the same magMP-spheroid populations (data not shown). It was notable that the velocities of the distinct spheroid populations did not overlap (Fig. 2B and C), suggesting that complex mixtures of different types of spheroids could be sorted and organized rapidly based simply on the number of magMPs incorporated in distinct multicellular aggregate populations.
Fig. 2.
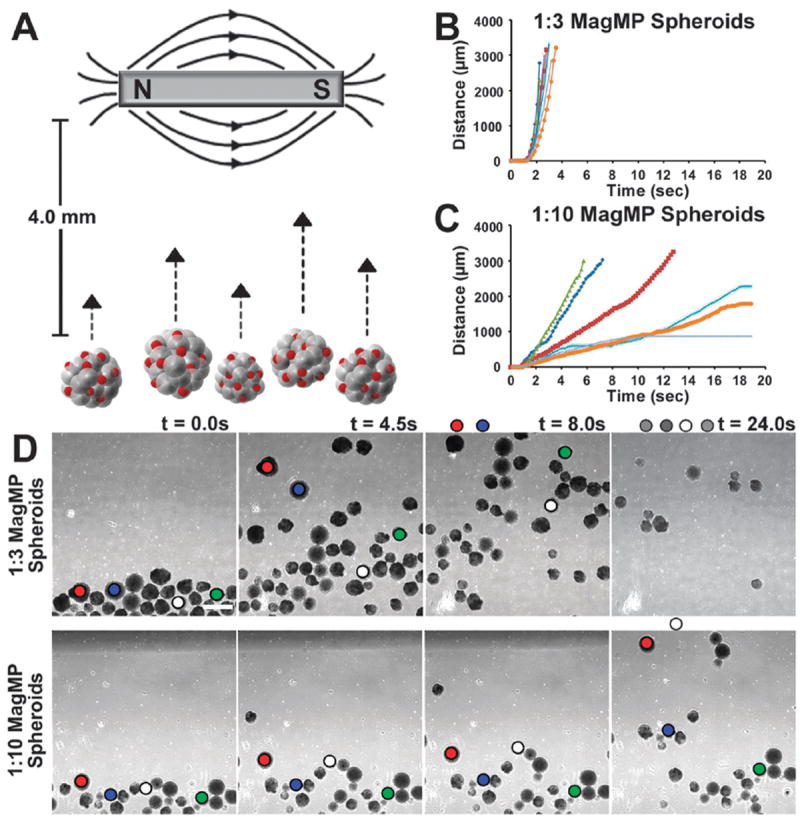
Spheroids with magMPs respond in a dose dependent fashion to magnetic fields. (a) Spheroids can be directed to move in a dish by an externally applied magnetic field. (b,c) Spheroids formed with a higher ratio (1 : 3) of magMPs to cells move faster and more uniformly relative to spheroids formed with a 1 : 10 seed ratio. (d) The distance moved between frames was tracked and suggests that the populations could be sorted based on magnetic sensitivity alone. Scale bar 500 μm.
Magnetic control of hydrodynamic suspension cultures
Bioreactor culture is often utilized or envisioned in stem and progenitor cell culture as a means to produce high yields of cells thought to be required for large-scale biomanufacturing and clinical efficacy.26,27 The inability to control the spatial position of cells and aggregates under hydrodynamic conditions becomes problematic because shear forces, which can affect stem cell differentiation, vary as a function of position within individual mixed/stirred systems.28,29 As a simplified example, we examined the use of patterned magnetic fields to constrain spheroid location within rotary orbital culture. Our lab and others have previously reported on the use of rotary orbital shaker culture of ESCs to create and maintain homogeneous populations of EBs in suspension culture conditions.25,30,31 In rotary culture, spheroids generally occupy a focused region of the dish (Fig. 3A) and are subjected to a range of shear forces depending on their precise location, which changes slightly with each revolution of the culture. By adhering magnets in a circular pattern on the lid of a Petri dish (Fig. 3B), we observed that the distance of the EBs to the center of the dish could be confined exclusively to the region below the magnetic pattern, which constrains the range of shear forces experienced by the cell spheroids. Other patterns could also be imposed on dynamically cultured spheroids including discrete islands across the dish (Fig. 3Bii), a “four leaf clover” pattern (Fig. 3Biii) or a straight line (Fig. 3iv) by altering the configuration of the magnets on the lid of the Petri dish (shown in the insets of Fig. 3B). Asymmetric geometries (Fig. 3C) could also be formed and maintained by simply altering the externally applied magnetic field via the configuration of the magnets on the lid of the dish.
Fig. 3.
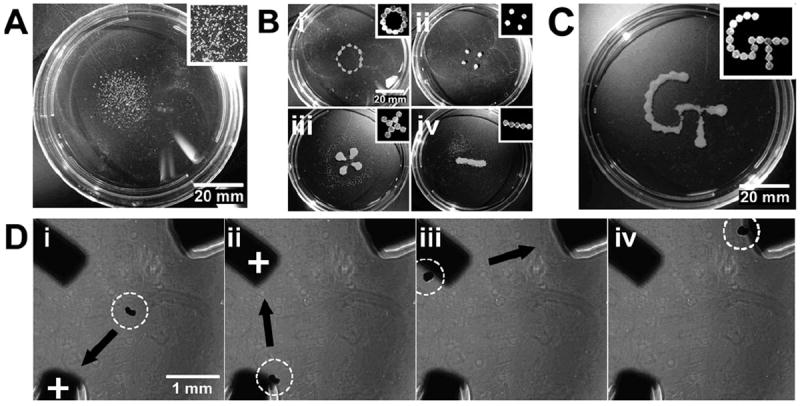
Spheroid location can be controlled in dynamic suspension culture. (a) Without magnets, spheroids cultured on a rotary orbital shaker (45 RPM) cluster in the center of the dish. Scale bar 20 mm. (b) The placement magnets on the lid of the dish (configuration shown in insets) could be used to confine the spheroids to (i) a defined radius, (ii) distinct islands, (iii) a four-leaf clover, (iv) or a line configuration. Scale bar 20 mm. (c). More complicated patterns, including the Georgia Tech logo, could be made at slower culture speeds. Scale bar 20 mm. (d) In addition, the location of a single spheroid could be manipulated back and forth between iron pillars embedded in PDMS. The iron pillars were alternately magnetized (demonstrated by a “+” label), resulting in attraction of the spheroid to the magnetized pillar (i–iv). Scale bar 1 mm.
The transient application of magnetic fields was also investigated as a method to control the spatiotemporal localization and translocation of individual spheroids. A triangular configuration was created with iron rods embedded in a thin layer of PDMS approximately 1 mm apart (Fig. 3D) and individual spheroids containing magMPs were placed in the center of the configuration on top of the PDMS (Fig. 3Di). In the absence of an external magnetic field, a spheroid remained free-floating in the center of the configuration, but as soon as one of the iron rods was magnetized, the spheroid was immediately attracted by the magnetic field to the end of that particular rod (Supplemental Video 3). By sequentially magnetizing other rods, the spheroid could be rapidly and specifically translocated from point-to-point, demonstrating the ability to direct multi-cellular movement and location in suspension culture purely by alternating magnetic fields of paramagnetic elements (Fig. 3Dii–iv).
Multi-scale complex assembly of spheroid units
The sequential assembly of bulk populations of magMP containing spheroids (hundreds to thousands of aggregates) was next examined using external magnetic fields in static culture environments. A single population of fluorescently labeled spheroids, indicated with a “1” in Fig. 4A and D, was added to a Petri dish and aggregated into a circle using a magnet 5 mm in diameter. When subsequent populations, labeled “2”, “3” were added only on one side of the magnet, the spheroids aggregated into a stacked Venn diagram pattern (Fig. 4B). At the interfaces between the different spheroid populations little mixing was observed, demonstrating that geometry could be controlled with good pattern fidelity (Fig. 4C). When subsequent aggregate populations were added around the entire circumference of a fixed magnet, large numbers of spheroids could be arranged in a “bullseye” pattern of concentric circles (Fig. 4E). Again, higher magnification analysis revealed that the interface between different spheroid populations was largely conserved even in a construct as large as 5 mm in diameter (Fig. 4F).
Spatial patterning of magnetically sensitive spheroids can be applied to various cell types which can be grown as aggregates but embryonic stem cell spheroids are prone to E-cadherin mediated agglomeration during early stages of differentiation. 29 In general, agglomeration increases the heterogeneity of culture and is avoided; however, controlled agglomeration may yield a platform for local control of the spheroid microenvironment. In order to test this hypothesis, individual spheroids, pre-labeled with CellTracker™ Red, Blue or Green (or not labeled), were magnetically guided within close proximity of each other (Fig. 5Ai) and monitored continuously thereafter with time-lapse microscopy. After 5 h of static culture, the individual spheroids remained in contact and began to merge along the interface adjoining adjacent aggregates (Fig. 5Aii). After an additional 5 h, the initially distinct spheroids continued to merge to form an oblong, oval structure (Fig. 5Aiii) that eventually became a fully conjoined aggregate by ~15 h (Fig. 5Aiv, Supplemental Video 4). Interestingly, throughout the entirety of the merging process, the fluorescently labeled populations of cells remained largely in the same hemispheres corresponding to the initial relative location of their original respective spheroids. Based on the ability to assemble spheroids that retained the relative position of the original sub-units, the same process was applied to the stepby- step construction of more complex multi-spheroid aggregates (Fig. 5B). Two spheroids were magnetically oriented adjacent to one another (Fig. 5Bi–ii) before introducing a third (Fig. 5Biii) and fourth spheroid (Fig. 5Biv) on opposite sides. Within 18 h the individual spheroids had merged to form a multi-quadrant aggregate, whose contributing multi-cellular units could be identified and distinguished by epifluorescent microscopy (Fig. 5C).
Fig. 5.
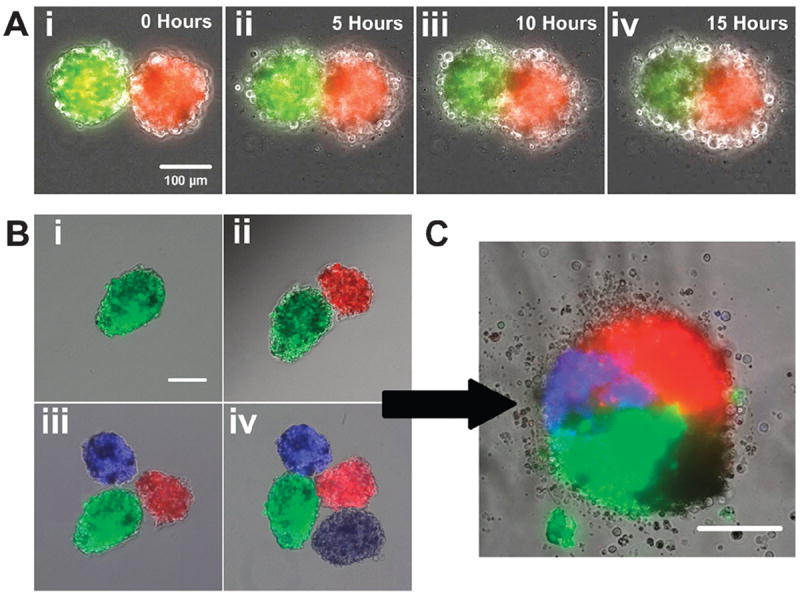
Merging of EBs can be spatially controlled on the single aggregate level. (a) The merging process of two spheroids was visualized over a period of 15 h using time lapse microscopy. Fluorescently labeled stem cell spheroids were brought in contact with one and other using a magnetic probe (i) and began to merge after 5 h of culture (ii). The spheroids continued to merge after 10 h (iii) and became more rounded after 15 h (iv). Scale bar 100 μm. (b) Merging of more than two spheroids could also be controlled by magnetic manipulation. Spheroids were drawn together magnetically in a sequential manner (i–iv) which maintained the position of the spheroids relative to each other. Scale bar 100 μm. (c) After 24 h of culture 4 spheroids merged to create a single construct consisting of four distinct quadrants of fluorescence. Scale bar 100 μm.
Discussion
In these studies, we have shown that magnetic manipulation of stem cells in 3D cultures provides a simple approach to control the aggregation and design of multi-cellular geometries for neo-tissue constructs. The method described herein relies upon high-throughput formation of magnetically sensitive stem cell spheroids, which differs significantly from other lowerthroughput methods,19,32 in part because it is not dependent on engineered cell or material properties. Entrapment of magnetic microparticles can be conducted with various types of stem cells that are commonly grown as spheroids including embryonic,33 induced pluripotent,34 mesenchymal35,36 and neural stem cells.37,38 Magnetizing cells or cell spheroids generally requires internalization of sub-micron material or conjugation to the cell surface which can impede cell motility and induce cell death.20 By simply physically entrapping magMPs within the extracellular space of stem cell spheroids, magnetic fields can be applied to induce multi-cellular aggregates to rapidly assemble into higher order structures without directly perturbing intracellular machinery and signaling pathways. In addition, the patterns that we report in these studies could all be created in static suspension culture using a relatively weak (10x weaker than a 3 tesla magnetic resonance imaging electromagnet) and transiently (1min or less) appliedmagnetic field. In such case where a continuous magnetic field may be necessary, the effects of magnetic field strength on stem cell differentiation could be assayed, though adverse effects on viability are not expected, based on prior reports of ESCs and neural stem cells cultured in the continuous presence of a magnetic field for 48–72 h.39,40
Patterning of spheroids in dynamic suspension culture systems, such as bioreactors, can be used for more uniform control of the extracellular environment and therefore offers advantages for stem cell bioprocessing. Spheroids with incorporated magMPs could be strictly confined to specific locations within dynamic suspension culture suggesting a possible route to study confined regions of shear forces for individual EBs. The distinct position of spheroids could also be controlled by magnetizing paramagnetic elements that could be physically integrated into the culture vessel design. This method introduces the possibility of automating electromagnetic field patterning for precise control of spheroid locations within a bioreactor to better direct stem cell differentiation in batch culture processes.
Another distinct advantage of incorporating magMPs within multi-cellular aggregates is the ability to manipulate their movement over multiple length scales, thereby providing new ways to study interactions of heterogeneous cell populations or homogeneous cells engineered within heterogeneous microenvironments. Distinct types of cells or preformed spheroids can be conjoined with control over the ratios of different cells, as well as their spatial organization with respect to one another in order to create more complex 3D spatial patterns.41 The sensitivity of the spheroids in this study to magnetic manipulation was dependent on the number of magMPs incorporated. Therefore the magnetic sensitivity can be determined during the formation of spheroids and populations can be self-sorted in a rapid and scalable manner to form complex, organized structures from mixed populations of different types of spheroids. Magnetic field patterns could be specifically created with this purpose in mind to form hierarchical tissue architectures with multiple cell types from a mixed population of multi-cellular aggregates.
The magMPs incorporated within multi-cellular aggregates are currently used for magnetic control of the spheroids; however, recent work from our lab and others has shown that MPs can also be used to deliver morphogenic factors locally within cell spheroids.23,42-44 Dual incorporation of magMPs and morphogen-laden MPs within pluripotent stem cell aggregates could be used to engineer the types of gradients observed during embryonic morphogenesis, a novel and powerful approach to study fundamental principles of developmental biology in a more controlled and tractable manner. This approach could also be complemented by encapsulation of individual 3D cell aggregates, like EBs, in engineeredmicrogel niches to expose cells to patterned, heterogeneous environmental signals.45 Specifically, a spheroid containingMPs loaded with a single morphogen could be merged together with three other spheroids each containing MPs loaded with complementary or antagonist molecules, as in Fig. 5C, to pattern pluripotent cells in a polarized manner similar to what is observed in pre-gastrulation stage embryos. As such, spatiotemporal effects of controlled morphogen and inhibitor delivery could be studied to model aspects of human or mouse embryonic development and potentially be applied to the production of tissues from stem cells ex vivo.
Conclusions
The incorporation of magnetically sensitive microparticles within the extracellular environment of stem cell spheroids provides a facile means to control the subsequent spatial location of spheroids in dynamic and suspension cultures using transiently applied magnetic fields. The number of magMPs incorporated could be controlled during the aggregate formation stages and determined the magnitude of aggregate response to applied magnetic fields. The method described herein was applied to single aggregates as well as large populations of spheroids without a priori design of materials or alterations to the cellular membrane. This work is suited to the construction of neo-tissues comprised of aggregates made from different cell types in which the spatial orientation is important to tissue function. In addition, combining magMPs with morphogen loaded MPs for localized delivery of multiple molecules within a single aggregate could be used for spatially controlled heterogeneous differentiation. Altogether, the incorporation of magMPs within stem cell spheroids represents an easily implemented method to further address significant questions of interest to the fields of stem cell biology, tissue engineering and cell bioprocessing.
Supplementary Material
Insight, innovation, integration.
In this report, we describe a facile approach to incorporate magnetic microparticles within the extracellular space of stem cell spheroids to enable spatial patterning of multicellular aggregates in 3D dynamic and suspension culture systems. Dose-dependent control of microparticle incorporation during multicellular aggregate formation in PDMS microwells was achieved and dictated the relative sensitivity of aggregates to transiently applied magnetic fields. This approach permits spatially organized assembly of complex aggregates and neo-tissues from spheroids comprised of different cell types and/or different engineered microenvironments over multiple length scales (μm–mm). Thus, this method enables a new route to investigate principles of stem cell and developmental biology and spatially control multicellular assembly for tissue engineering and cell bioprocessing technologies.
Acknowledgments
We are grateful to Dr Priya Baraniak for culturing and providing MSCs. Financial support for this work was provided by funding from the National Science Foundation (CBET 0651739, CBET 0939511), the National Institutes of Health (R01 GM088291) and the Georgia Tech/Emory/PKU seed grant program. ABL was supported by an NIH training grant (T32 GM008433) as well as funding from the Goizueta Foundation. RLC was supported by a GAANN fellowship from the Center of Drug Design, Development and Delivery.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ib00064k
References
- 1.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–6. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–52. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 5.Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–22. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 6.Gartner ZJ, Bertozzi CR. Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc Natl Acad Sci U S A. 2009;106:4606–10. doi: 10.1073/pnas.0900717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bank PA, Kellam B, Kendall DA, Shakesheff KM. Surface engineering of living myoblasts via selective periodate oxidation. Biotechnol Bioeng. 2003;81:800–8. doi: 10.1002/bit.10525. [DOI] [PubMed] [Google Scholar]
- 8.Gothard D, Roberts SJ, Shakesheff KM, Buttery LD. Controlled embryoid body formation via surface modification and avidin-biotin cross-linking. Cytotechnology. 2009;61:135–44. doi: 10.1007/s10616-010-9255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci U S A. 2008;105:9522–7. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;103:11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khetan S, Burdick JA. Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials. 2010;31:8228–34. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 13.King WJ, Jongpaiboonkit L, Murphy WL. Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading. J Biomed Mater Res A. 2010;93:1110–23. doi: 10.1002/jbm.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alenghat FJ, Fabry B, Tsai KY, Goldmann WH, Ingber DE. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem Biophys Res Commun. 2000;277:93–9. doi: 10.1006/bbrc.2000.3636. [DOI] [PubMed] [Google Scholar]
- 15.Pamme N. Magnetism and microfluidics. Lab Chip. 2006;6:24–38. doi: 10.1039/b513005k. [DOI] [PubMed] [Google Scholar]
- 16.Melville D. Direct magnetic separation of red cells from whole blood. Nature. 1975;255:706. doi: 10.1038/255706a0. [DOI] [PubMed] [Google Scholar]
- 17.Molday RS, Yen SP, Rembaum A. Application of magnetic microspheres in labelling and separation of cells. Nature. 1977;268:437–8. doi: 10.1038/268437a0. [DOI] [PubMed] [Google Scholar]
- 18.Ho VH, Muller KH, Barcza A, Chen R, Slater NK. Generation and manipulation of magnetic multicellular spheroids. Biomaterials. 2010;31:3095–102. doi: 10.1016/j.biomaterials.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS, Bronk LF, Ananta JS, Mandelin J, Georgescu MM, Bankson JA, Gelovani JG, Killian TC, Arap W, Pasqualini R. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5:291–6. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry CC, Wells S, Charles S, Aitchison G, Curtis AS. Cell response to dextran-derivatised iron oxide nanoparticles post internalisation. Biomaterials. 2004;25:5405–13. doi: 10.1016/j.biomaterials.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 21.Pisanic TR, 2nd, Blackwell JD, Shubayev VI, Finones RR, Jin S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials. 2007;28:2572–81. doi: 10.1016/j.biomaterials.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials. 2011;32:48–56. doi: 10.1016/j.biomaterials.2010.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30:2507–15. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenedo RL, Seaman SA, McDevitt TC. Microsphere size effects on embryoid body incorporation and embryonic stem cell differentiation. J Biomed Mater Res A. 2010;94:466–75. doi: 10.1002/jbm.a.32710. [DOI] [PubMed] [Google Scholar]
- 25.Carpenedo RL, Sargent CY, McDevitt TC. Rotary Suspension Culture Enhances the Efficiency, Yield and Homogeneity of Embryoid Body Differentiation. Stem Cells. 2007;25:2224–34. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 26.Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–81. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Randle WL, Cha JM, Hwang YS, Chan KL, Kazarian SG, Polak JM, Mantalaris A. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng. 2007;13:2957–70. doi: 10.1089/ten.2007.0072. [DOI] [PubMed] [Google Scholar]
- 28.Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, McDevitt TC. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105:611–26. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- 29.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–42. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 30.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 31.Singh H, Mok P, Balakrishnan T, Rahmat SN, Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165–79. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Lin RZ, Chu WC, Chiang CC, Lai CH, Chang HY. Magnetic reconstruction of three-dimensional tissues from multicellular spheroids. Tissue Eng Part C. 2008;14:197–205. doi: 10.1089/ten.tec.2008.0061. [DOI] [PubMed] [Google Scholar]
- 33.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–9. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–11. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 38.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czyz J, Nikolova T, Schuderer J, Kuster N, Wobus AM. Non-thermal effects of power-line magnetic fields (50 Hz) on gene expression levels of pluripotent embryonic stem cells-the role of tumour suppressor p53. Mutat Res. 2004;557:63–74. doi: 10.1016/j.mrgentox.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Song M, Kim YJ, Kim YH, Roh J, Kim SU, Yoon BW. Using a neodymium magnet to target delivery of ferumoxide-labeled human neural stem cells in a rat model of focal cerebral ischemia. Hum Gene Ther. 2010;21:603–10. doi: 10.1089/hum.2009.144. [DOI] [PubMed] [Google Scholar]
- 41.Elbert DL. Bottom-up tissue engineering. Curr Opin Biotechnol. 2011;22:674–80. doi: 10.1016/j.copbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahoney MJ, Saltzman WM. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat Biotechnol. 2001;19:934–9. doi: 10.1038/nbt1001-934. [DOI] [PubMed] [Google Scholar]
- 43.Ferreira L, Squier T, Park H, Choe H, Kohane DS, Langer R. Human Embryoid Bodies Containing Nano- and Microparticulate Delivery Vehicles. Adv Mater. 2008;20:2285–2291. [Google Scholar]
- 44.Solorio LD, Fu AS, Hernandez-Irizarry R, Alsberg E. Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-beta1 from incorporated polymer microspheres. J Biomed Mater Res A. 2010;92:1139–44. doi: 10.1002/jbm.a.32440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi H, Du Y, Wang L, Kaji H, Bae H, Khademhosseini A. Patterned differentiation of individual embryoid bodies in spatially organized 3D hybrid microgels. Adv Mater. 2010;22:5276–81. doi: 10.1002/adma.201002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


