Development of the service
The advent of targeted therapies for idiopathic pulmonary hypertension, with an initial focus on prostaglandins and endothelin antagonists, mandated the planning and development of a service that could evaluate and manage patients with this orphan disease in the United Kingdom (UK). Accordingly, a small group of interested physicians met to debate the model of service delivery that was best suited to the UK and its National Health Service (NHS) at the Royal College of Physicians in London. There was a uniform view, given the rarity of the disorder, its complexity and the anticipated cost of therapy to the NHS, that a model of a limited number of specialist centres working in a collaborative way would offer best value and quality care for patients.
Although such a model would inevitably lead to patients having to travel to receive expert care, advantages included the ability to set standards of service and service delivery that designated specialist centres must meet. Furthermore the group recognized the value of setting up a national registry so that each centre would be responsible for collecting a standard data set which in time would provide information of value for audit, equable access for patients, service planning and research. A major factor in the arguable success of the UK model was the strong sense of collegiality and confidence in working together to achieve these common aims. Furthermore it was recognized that in addition to adult centres there was a need to establish a paediatric centre best operating on a hub and spoke fashion with satellite centers to meet the need of paediatric patients.
The group realized at an early stage that it was important to meet with commissioners of care and establish jointly agreed service specifications and policies that were both affordable, based on the anticipated growth of evidence in terms of improved patient outcomes and responsive to that evidence base. The proposed development of the service was discussed at a National Commissioning level and whilst it was decided that full funding would not via the National Specialist Commissioning Advisory Group budget, the appropriate committee at the time, monies to pump prime the service were made available. Moreover there was agreement from the locality commissioning groups to top slice funding for pulmonary hypertension and operate in a risk sharing model. This ensured that patients could be investigated and treated without the need for a complex slow administrative process leading to delays in our ability to treat patients.
Adult centres were established in Glasgow, Newcastle, Sheffield, Cambridge and London. London comprised centres at Hammersmith, Royal Brompton and Royal Free Hospitals and the paediatric centre at Great Ormond Street Hospital. All centres met a set of standards of service delivery and were led by physicians with expertise and training in patients with pulmonary hypertension.
Regular audits and reviews of practice were instigated on an annual basis and an agreed management policy was achieved via consensus based on available evidence. The formation of a patient's association – the Pulmonary Hypertension Association (PHA) - was strongly supported and close relationships developed between the PHA and the service providers to ensure the patient voice was heard and that the service was responsive to the patient's needs.
A good example of how this helped the service model is that patients were happy and preferred to travel distances to the centres to receive expert care rather than access local service providers, which - though more convenient - were less expert. The size of the UK obviously facilitates this model because distances are not so great. As the service has matured and developed, all of the centres have established satellite clinics so that expert follow up care can be provided more locally for patients throughout the UK, further evidence of a true service with patient care at its centre.
The advent of pulmonary endarterectomy (PEA) surgery for patients with chronic thromboembolic pulmonary hypertension led to discussion and a decision to establish a single national centre for this service in Cambridge. The decision was based on the benefits to maximize the speed of the surgical “learning curve” by concentrating surgical activity in one centre. It was recognized that, in time, consideration of development of a second centre could be entertained if demand could not be met from this single centre. The results have certainly justified this decision and Cambridge has recently undertaken the 1000th PEA with excellent overall results.
Currently a review of patient experience and demand is being undertaken to consider whether our single centre service provider model continues to represent the most suitable approach for patients in the UK.
The willingness of all centres to participate in review and audit on a national basis and provide combined national statistics is an undoubted further strength of the UK model.
The national service provides an overview and audit of all patients within the service on an annual basis which are available for public scrutiny.
Moreover we have recently developed dashboards based on four outcomes so that each centre can be compared and outlying potential variation in markers of service quality be recognized. This then prompts review of reasons for any variance to be undertaken and the sharing of best practice to improve the quality of health care provision.
The radical change in the NHS brought about by the Health and Social Care Act of 2012 led to an increased focus on the patient at the centre of all that we do, and on the quality of outcomes as indicators of quality in health care provision. Arguably the National PH service was already well set up to meet the challenges laid down and has acted as an exemplar in this regard. Certainly the establishment of clinical reference groups to provide advice for specialized commissioning was very easy for the UK PH community, and the development of national protocols, service specification and policy could be introduced via minor changes in what already existed.
Currently the UK PH service remains a Nationally Commissioned service and whilst designation no longer is a feature in NHS England, delivery of the service via the original service providers remains the universally preferred option for patients, providers and commissioners of health care.
The National Registry
The National Registry of Pulmonary Hypertension is a nationwide audit of the specialised pulmonary hypertension centres in the United Kingdom. The registry extends to the Channel Islands, the Isle of Man and Gibraltar. The purpose of the audit is to describe clinical practice in the United Kingdom, provide epidemiological information for future service planning, and measure clinical outcomes. The seven hospitals that were designated more than 14 years ago to diagnose and treat pulmonary hypertension in adults and the one hospital for children, are shown in Fig. 1.
Figure 1.
The UK and Ireland National PH Collaboration.
The data presented in this review is that from the year 1 April 2013 to 31 March 2014 and covers all patients seen at the specialised centres. This audit includes England, Scotland, Wales, Northern Ireland, the Channel Islands, Gibraltar and the Isle of Man.
The specialised centres follow current European Society of Cardiology / European Respiratory Society and UK guidelines and are responsible for making an accurate diagnosis, initiating disease–targeted drug therapy and, where appropriate, undertaking the long term management of patients with diverse causes of pulmonary hypertension. The specialised centres provide care at their own hospital sites as well as undertaking shared care and outreach clinics with more distant hospitals. All reporting of data in the audit is the responsibility of the centre irrespective of where the activity occurred.
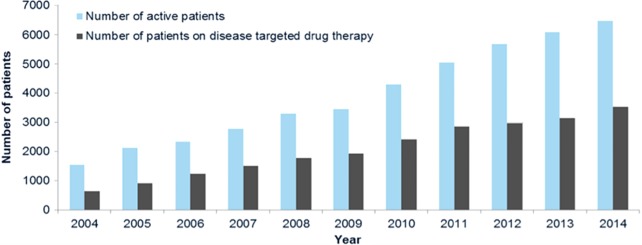
The treatment of pulmonary hypertension with the disease-targeted drug therapies is controlled by the NHS England drug policies on pulmonary hypertension drugs which were last updated in May 2014 (Figure 2, Table 1). Scotland, Wales and Northern Ireland largely follow these policies. These policies do not apply to children. These drug policies must be borne in mind when interpreting the data collected about drug prescriptions.
Figure 2.
Number of patients active under the service and number of patients on disease targeted therapies for pulmonary hypertension on 31st March 2004–2014.
Table 1.
Total number of patients active under the service 2010-2014, broken down by centres.
| Specialised pulmonary hypertension centre | Total 2010 | Total 2011 | Total 2012 | Total 2013 | Total 2014 |
| Golden Jubilee National Hospital | 291 | 329 | 375 | 428 | 494 |
| Great Ormond Street Hospital for Children | 300 | 319 | 244 | 361 | 350 |
| Imperial College Healthcare | 664 | 764 | 914 | 942 | 936 |
| Papworth Hospital, Cambridge | 591 | 625 | 716 | 777 | 809 |
| Royal Brompton and Harefield | 492 | 640 | 776 | 968 | 902 |
| Royal Free London | 581 | 774 | 997 | 980 | 1,049 |
| Sheffield Teaching Hospitals | 1,074 | 1,291 | 1,393 | 1,212 | 1,489 |
| Newcastle upon Tyne Hospitals | 294 | 320 | 318 | 422 | 455 |
| Total patients seen at specialized centres | 4,287 | 5,062 | 5,733 | 6,090 | 6,484 |
Diagnoses are reported according to the 2008 Dana Point clinical classification. The recent changes made to the NICE 2013 clinical classification were minor and given the amount of work to reclassify patients in the database the Pulmonary Hypertension Outcomes Group decided to maintain the Dana Point classification.
Research
Research plays a major focus for the UK collaboration and currently there are major collaborative trials involving careful clinical phenotyping to identify patients with idiopathic pulmonary hypertension and l collection of biological samples. We are involved in whole exome sequencing of this cohort to identify genes responsible for expression of the disease phenotype in addition to BMPR2 and undertaking studies of environmental exposure. We are shortly to commence an experimental medicine study of an anti-inflammatory biological. The strength of our cohorts is underpinned by our collaboration such that all UK patients are invited to participate
Conclusions
The UK PH collaboration represents a unique National resource that has enabled us to review our clinical results, overall performance and undertake research. We believe it represents an exemplar for service design for patients with rare disorders.