Abstract
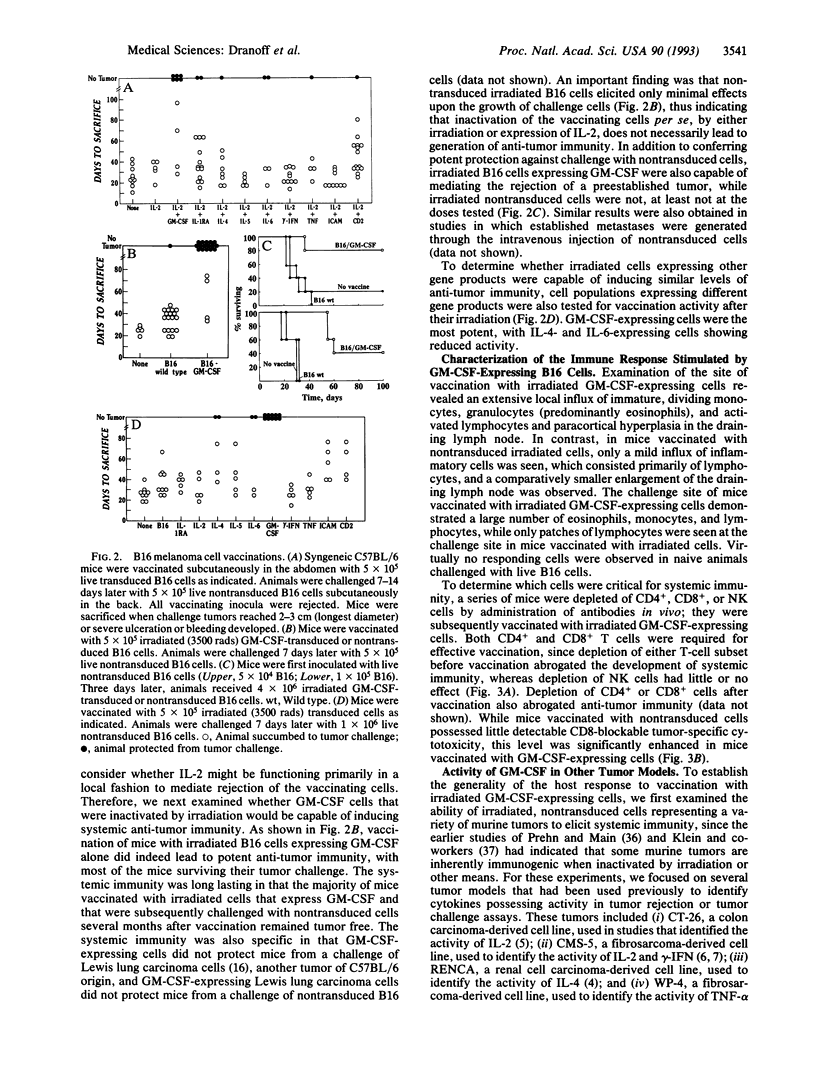
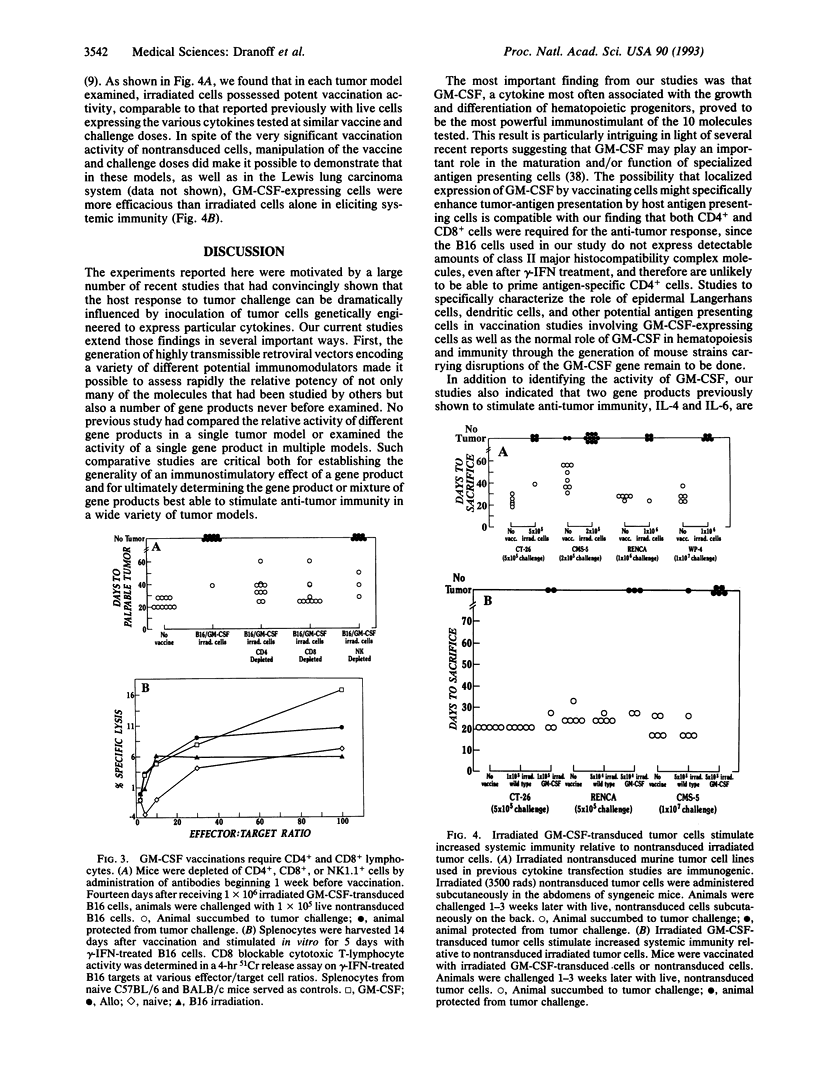
To compare the ability of different cytokines and other molecules to enhance the immunogenicity of tumor cells, we generated 10 retroviruses encoding potential immunomodulators and studied the vaccination properties of murine tumor cells transduced by the viruses. Using a B16 melanoma model, in which irradiated tumor cells alone do not stimulate significant anti-tumor immunity, we found that irradiated tumor cells expressing murine granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulated potent, long-lasting, and specific anti-tumor immunity, requiring both CD4+ and CD8+ cells. Irradiated cells expressing interleukins 4 and 6 also stimulated detectable, but weaker, activity. In contrast to the B16 system, we found that in a number of other tumor models, the levels of anti-tumor immunity reported previously in cytokine gene transfer studies involving live, transduced cells could be achieved through the use of irradiated cells alone. Nevertheless, manipulation of the vaccine or challenge doses made it possible to demonstrate the activity of murine GM-CSF in those systems as well. Overall, our results have important implications for the clinical use of genetically modified tumor cells as therapeutic cancer vaccines.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki T., Tashiro K., Miyatake S., Kinashi T., Nakano T., Oda Y., Kikuchi H., Honjo T. Expression of murine interleukin 7 in a murine glioma cell line results in reduced tumorigenicity in vivo. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3850–3854. doi: 10.1073/pnas.89.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher A. L., Mulé J. J., Kasid A., Restifo N. P., Salo J. C., Reichert C. M., Jaffe G., Fendly B., Kriegler M., Rosenberg S. A. Murine tumor cells transduced with the gene for tumor necrosis factor-alpha. Evidence for paracrine immune effects of tumor necrosis factor against tumors. J Immunol. 1991 May 1;146(9):3227–3234. [PMC free article] [PubMed] [Google Scholar]
- Blankenstein T., Qin Z. H., Uberla K., Müller W., Rosen H., Volk H. D., Diamantstein T. Tumor suppression after tumor cell-targeted tumor necrosis factor alpha gene transfer. J Exp Med. 1991 May 1;173(5):1047–1052. doi: 10.1084/jem.173.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. D., Sanderson C. J., Wang Y., Hort Y., Martinson M. E., Tucker W. Q., Stellwagen A., Strath M., Young I. G. Isolation, structure and expression of cDNA and genomic clones for murine eosinophil differentiation factor. Comparison with other eosinophilopoietic lymphokines and identity with interleukin-5. Eur J Biochem. 1988 Jun 1;174(2):345–352. doi: 10.1111/j.1432-1033.1988.tb14104.x. [DOI] [PubMed] [Google Scholar]
- Chiu C. P., Moulds C., Coffman R. L., Rennick D., Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. P., Ferrari G., Stoppacciaro A., Parenza M., Rodolfo M., Mavilio F., Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991 Apr 1;173(4):889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Gansbacher B., Bannerji R., Daniels B., Zier K., Cronin K., Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990 Dec 15;50(24):7820–7825. [PubMed] [Google Scholar]
- Gansbacher B., Zier K., Daniels B., Cronin K., Bannerji R., Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990 Oct 1;172(4):1217–1224. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golumbek P. T., Lazenby A. J., Levitsky H. I., Jaffee L. M., Karasuyama H., Baker M., Pardoll D. M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science. 1991 Nov 1;254(5032):713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum C. H., Wilcox C. J., Arend W. P., Joslin F. G., Dripps D. J., Heimdal P. L., Armes L. G., Sommer A., Eisenberg S. P., Thompson R. C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990 Jan 25;343(6256):336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hock H., Dorsch M., Diamantstein T., Blankenstein T. Interleukin 7 induces CD4+ T cell-dependent tumor rejection. J Exp Med. 1991 Dec 1;174(6):1291–1298. doi: 10.1084/jem.174.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horley K. J., Carpenito C., Baker B., Takei F. Molecular cloning of murine intercellular adhesion molecule (ICAM-1). EMBO J. 1989 Oct;8(10):2889–2896. doi: 10.1002/j.1460-2075.1989.tb08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Koo G. C., Dumont F. J., Tutt M., Hackett J., Jr, Kumar V. The NK-1.1(-) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986 Dec 15;137(12):3742–3747. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. Q., Diamantstein T., Blankenstein T. Lack of tumorigenicity of interleukin 4 autocrine growing cells seems related to the anti-tumor function of interleukin 4. Mol Immunol. 1990 Dec;27(12):1331–1337. doi: 10.1016/0161-5890(90)90039-3. [DOI] [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Katz A., Vadai E., Revel M., Feldman M., Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992 Jul 1;52(13):3679–3686. [PubMed] [Google Scholar]
- Rollins B. J., Sunday M. E. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Mol Cell Biol. 1991 Jun;11(6):3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Teng M. N., Park B. H., Koeppen H. K., Tracey K. J., Fendly B. M., Schreiber H. Long-term inhibition of tumor growth by tumor necrosis factor in the absence of cachexia or T-cell immunity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3535–3539. doi: 10.1073/pnas.88.9.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kuribayashi K., Miyatake S., Nishihara K., Nakayama E., Taniyama T., Sakata T. Exogenous expression of mouse interferon gamma cDNA in mouse neuroblastoma C1300 cells results in reduced tumorigenicity by augmented anti-tumor immunity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9456–9460. doi: 10.1073/pnas.86.23.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Jefferson D. M., Chowdhury J. R., Novikoff P. M., Johnston D. E., Mulligan R. C. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Okumura K., Nakauchi H. Molecular cloning of the murine homologue of CD2. Homology of the molecule to its human counterpart T11. J Immunol. 1988 Feb 15;140(4):1321–1326. [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]


