Abstract
On October 7, 2011, the United States Preventive Services Task Force (USPSTF) released their evidence statement and grade D recommendation against prostate-specific antigen (PSA)-based prostate cancer screening. Using a time series design, we assessed the effect of this recommendation upon evaluations for elevated PSA levels and prostate biopsies in our large urology group practice. We found that, despite a 24.1% increase in total visits, the 32 urologists in our practice completed 16.4% fewer evaluations for elevated PSA levels (317 fewer evaluations per month; P = .017) and 21.4% fewer prostate biopsies (42 fewer biopsies per month; P = .001) in the 2 years following the USPSTF grade D recommendation.
Key words: Prostate-specific antigen, Prostate cancer screening, Prostate biopsies, United States
Prostate cancer is the most common noncutaneous malignancy in American men. In the United States in 2015, approximately 220,800 men will be diagnosed with prostate cancer and 27,540 men will die from the disease.1
In 1986, the US Food and Drug Administration approved prostatespecific antigen (PSA) testing for monitoring disease progression in men previously diagnosed with prostate cancer.2 In 1991, Catalona and colleagues3 published their findings that, when coupled with digital rectal examination and ultrasound, serum PSA measurement improved the detection of prostate cancer.
Aiming to clarify the effect of PSA-based prostate cancer screening upon prostate cancer mortality, two large randomized trials of screening matured in 2009: the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO)4 and the European Randomized Study of Screening for Prostate Cancer (ERSPC).5 After 7 to 10 years of follow-up, the PLCO trial found no difference in prostate cancer mortality between men randomized to annual PSA testing and digital rectal examination versus usual care.4 In the ERSPC trial, PSA-based screening reduced the rate of death from prostate cancer by 20% at 9 years median follow-up. This reduction in prostate cancer mortality was associated with a high risk of over-diagnosis: 1410 men needed screening and 48 additional cases of prostate cancer required treatment to prevent 1 death from prostate cancer.5
Using these two large studies as their evidence foundation for the benefits of early detection and treatment of prostate cancer, the United States Preventive Services Task Force (USPSTF) determined that the harms of PSA-based prostate cancer screening outweighed the benefits. On October 7, 2011, the USPSTF published their evidence statement and draft recommendation against PSA-based prostate cancer screening. 6 Extensive media coverage and national discussion ensued, with many publically disagreeing with the Task Force’s draft recommendation. 7–9 In May 2012, the USPSTF finalized their grade D recommendation: PSA-based prostate cancer screening should be discouraged.10
Although multiple screening guidelines exist that differ from those of the USPSTF,11–13 primary care physicians are historically most influenced by the USPSTF recommendations. 14 In a study of primary care providers from Johns Hopkins Community Physicians, a university-affiliated practice including 26 outpatient sites in 11 Maryland counties, following release of the USPSTF draft recommendation against PSA-based prostate cancer screening, fewer than 50% agreed with the new recommendation, suggesting the change may encounter significant barriers to adoption.15 Consistent with this observation, various effects of the USPSTF recommendation upon the number of PSA tests performed, 16–19 evaluations for elevated PSA levels,20 and prostate biopsies completed20–22 have been reported in the literature since 2012. Based on our clinical observations, we hypothesized that the number of evaluations for elevated PSA levels and number of prostate biopsies performed in our community-based, large urology group practice would decrease significantly following the publication of the USPSTF draft recommendation against prostate cancer screening.
Methods
Population
Our large urology group practice of 32 physicians services a population of nearly 1.7 million people in a geographic coverage area of approximately 2500 square miles. Within our coverage systems, postgraduate education is provided to several hundred interns and residents in three graduate medical education systems. This dynamic, coupled with our service to a population that is wealthier and better educated than the national average,23 results in the rapid dissemination of new policy and practices from academia into the community. The announcement of the USPSTF grade D evidence review and draft recommendation against PSA-based prostate cancer screening on October 7, 20116 was extensively reviewed and publicized in our metropolitan area.
Study Design
We used an interrupted time series study design, with October 2011 as our interruption point, to understand the effect of the USPSTF grade D recommendation upon our practice. We queried our electronic medical record at the administrative level for total patient visits, evaluations for elevated PSA levels, and completed prostate biopsies. We defined total practice volume as all office visits to 1 of our 32 physician providers during sequential 12-month periods preceding and directly following release of the USPSTF grade D draft recommendation. October 2010 to September 2011 served as our baseline; October 2011 to September 2012 and October 2012 to September 2013 served as our evaluable periods following the USPSTF draft recommendation release. Using both Current Procedure Terminology codes and International Classification of Diseases-9 diagnosis and procedure codes to identify men evaluated for elevated PSA levels and prostate biopsies, we collected monthly practice totals over the same time period, again using October 2010 to September 2011 as our baseline and October 2011 to September 2013 as our effect period.
Statistical Analysis
We first calculated the percentage change in total practice office visits, evaluations for elevated PSA levels, and prostate biopsies performed, comparing our baseline period of October 2010 to September 2011 with our two follow-up periods of October 2011 to September 2012 and October 2012 to September 2013. These calculations were performed using Microsoft Excel version 14.5.4 (Microsoft, Redmond, WA).
We then conducted an interrupted time series analysis. We assessed the Durbin-Watson statistic to identify potential autocorrelation among successive time points. We used the Prais-Winsten method to estimate the segmented regression of monthly evaluations for elevated PSA levels and prostate biopsies performed. From this model, we estimated a monthly trend change in the number of practice evaluations for elevated PSA levels and prostate biopsies performed. We used an a priori level of statistical significance of P < .05. Analyses were performed using Stata 13.1 (Stata Corp., College Station, TX).
Results
Practice Volume
In the year preceding the USPSTF grade D draft recommendation release (October 2010–September 2011), our 32 physicians completed 103,600 patient encounters (Table 1). Following the new draft recommendation release on October 7, 2011, annual total practice volume increased: 114,163 (+ 10.2%) patient encounters were completed from October 2011 to September 2012 and 128,531 (+ 12.6%) encounters were completed from October 2012 to September 2013 (Table 1 and Figure 1). From our baseline study period to the conclusion of the second year following the draft recommendation release, total practice volume increased 24.1%.
Table 1.
Stratification of Total Practice Volume, Evaluations for Elevated PSA Level, and Prostate Biopsies Performed in the Year Preceding the USPSTF Grade D Recommendation and the 2 Years Following Recommendation Release
| Baseline Period: October 2010-September 2011 | Post-USPSTF Grade D: October 2011-September 2012 | Post-USPSTF Grade D: October 2012-September 2013 | |
|---|---|---|---|
| Patient volume | |||
| Yearly total | 103,600 | 114,163 | 128,531 |
| Annual % change | N/A | +10.2% | +12.6% |
| Evaluations for elevated PSA level | |||
| Yearly total | 28,698 | 25,065 | 24,002 |
| Monthly range | 1919–3008 | 1599–2551 | 1602–2284 |
| Annual % change | N/A | −12.7% | −4.2% |
| Prostate biopsies performed | |||
| Yearly total | 2465 | 1988 | 1929 |
| Monthly range | 155–250 | 113–207 | 130–196 |
| Annual % change | N/A | −19.4% | −3.0% |
PSA, prostate-specific antigen; USPSTF, United States Preventive Services Task Force.
Figures 1.
Number of practice office visits per year from October 2010 to September 2013. Over this time period, visits increased from 103,600 (October 2010-September 2011) to 114,163 (October 2011–September 2012) to 128,531 (October 2012–September 2013), representing annual increases of 10.2% and 12.6%, respectively. USPSTF, United States Preventive Services Task Force.
Evaluations for Elevated PSA Levels
From October 2010 to September 2011, the year preceding release of the USPSTF grade D draft recommendation, our 32 physicians evaluated 28,698 men for an elevated PSA level (Table 1). From October 2011 to September 2012 and October 2012 to September 2013, the evaluation periods following draft recommendation release, 25,065 and 24,002 men were evaluated for an elevated PSA level, decreases of 12.7% and 4.2%, respectively, were observed. From our baseline study period to the conclusion of the second year following the draft recommendation release, evaluations for elevated PSA levels decreased a total of 16.4% in our practice.
When visualized graphically, a downward trend in the number of evaluations for elevated PSA levels occurred over our study period, beginning with a sharp decrease in November 2011, the month following the USPSTF grade D draft guideline release (Figure 2). In the following 2 years, evaluations for elevated PSA levels decreased by 317 visits per month (95% confidence interval [CI]: −573, −61; P = .017).
Figures 2.
Monthly total of evaluations for elevated PSA performed from October 2010 to September 2013 at Delaware Valley Urology, LLC (Voorhees, NJ). In the 2 years following release of the USPSTF grade D recommendation, evaluations for elevated PSA decreased by 317 visits per month (95% CI: −573, −61; P = .017). CI, confidence interval; PSA, prostate-specific antigen; USPSTF, United States Preventive Services Task Force.
Prostate Biopsies
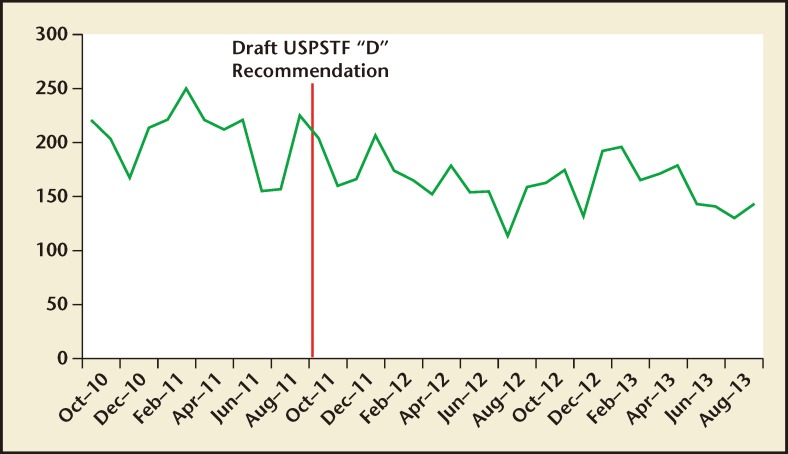
From October 2010 to September 2011, 2465 men underwent a prostate biopsy by 32 physicians in our large urology group practice (Table 1). From October 2011 to September 2012, the first year following release of the USPSTF grade D draft recommendation, our practice performed 1988 biopsies, a decrease of 19.8%. From October 2012 to September 2013, our 32 physicians performed 1929 biopsies, a further decrease of 3.0% and a decrease of 21.7% from our baseline study period.
When visualized graphically, a downward trend in monthly prostate biopsies performed in our large urology group practice also occurred during our study period (Figure 3). The effect of the USPSTF grade D draft recommendation was a decrease of 42 prostate biopsies per month (95% CI, −64, −19; P = .001).
Figures 3.
Monthly totals of prostate biopsies performed from October 2010 to September 2013 at Delaware Valley Urology, LLC (Voorhees, NJ). In the 2 years following release of the USPSTF grade D recommendation, prostate biopsies decreased by 42 biopsies per month (95% CI: −64, −19; P = .001). USPSTF, United States Preventive Services Task Force.
Discussion
The USPSTF grade D draft recommendation and evidence statement against PSA-based prostate cancer screening published on October 7, 20116 challenged the status quo of prostate cancer screening practices in the United States. Although the screening guidelines of other societies differ from those of the USPSTF,11–13 the influence of the government task force’s various recommendations, particularly among primary care providers, is strong.14 The effects of the grade D recommendation upon prostate cancer mortality in the United States will take years to develop. To understand the policy’s early impact, we assessed available proxy measures: evaluations for elevated PSA levels and prostate biopsies performed in our large urology group practice. Although total practice volume increased 24.1% during our study period, evaluations for elevated PSA levels decreased 16.4% and our prostate biopsy volume decreased 21.7% in the 2 years following release of the USPSTF draft recommendation against PSA-based prostate cancer screening. Following the draft guideline release, evaluations for elevated PSA decreased by 317 visits per month.
The significant decrease in evaluations for elevated PSA levels that occurred in our large urology group practice following the USPSTF grade D draft recommendation is a novel finding. Perez and colleagues20 similarly assessed evaluations for elevated PSA levels following the policy change, though used the USPSTF final statement in May 201210 as their cutpoint for analysis. In contrast to our findings, Perez and colleagues20 found a small increase (n 5 11; 5.2%) in the number of men referred to their tertiary care center for an elevated PSA level following publication of the USPSTF grade D recommendation. They attributed this increase to an overall increase in referrals during their study period and noted screening practices among primary care physicians in their catchment area are likely unchanged.
Although we recorded a significant increase in overall patient visits during our study period, the evaluations for elevated PSA levels plummeted, suggesting primary care providers in our geographic area altered their prostate cancer screening practices in response to the USPSTF grade D recommendation. Though we did not assess regional provider screening patterns in our current study, growing evidence from national and institutional databases suggest decreased rates of PSA screening. Comparing National Health Interview Survey data from 2005, 2010, and 2013, Drazer and associates18 found PSA screening rates significantly declined in men aged 50 to 59 years old (33.2%–24.8%; P = .01), 60 to 74 years old (51.2%– 43.6%; P = .01), and 75 years and older (43.9%–37.1%; P 5 .03) between 2010 and 2013.18 Using the electronic data warehouse, Cohn and coworkers19 reported a significant decrease (adjusted odds ratio 0.89; P = .0001) in PSA screening frequency among men 40 to 79 years old evaluated by primary care physicians in the 6 months following the USPSTF grade D recommendation.19 Recently presented data from Oregon Health & Science University (Portland, OR) noted PSA testing among new patients seeing primary care providers decreased by 50% in the year following release of the USPSTF grade D recommendation. 16 These findings are congruent with the significant decline in evaluations for elevated PSA levels seen in our practice and are consistent with downstream biopsy trends.
Our prostate biopsy volume decreased 21.7% in the 2 years following release of the USPSTF draft recommendation against PSA-based prostate cancer screening. Our findings echo decreasing prostate biopsy rates identified in multispecialty and tertiary care settings. 21,22 Banerji and colleagues21 noted a 31% decrease in the absolute number of prostate biopsies performed at Virginia Mason Hospital (Seattle, WA) in the 30 months following the USPSTF grade D recommendation. At the University Health Network in Toronto, Ontario, Canada, Bhindi and associates22 reported the median number of prostate biopsies performed per month decreased from 58.0 to 35.5 in the 12 months following the USPSTF recommendation. Though beyond the scope of our prostate biopsy data, these authors reported increasing rates of D’Amico risk classification24,25 of intermediate22 and high-risk21,22 cancer among those men undergoing prostate biopsies following the USPSTF grade D recommendation.
Our study has the inherent limitations of all time series analyses and administrative level data. Aggregated annual and monthly data did not permit us to account for individual level covariates. As we conducted an early assessment of evaluations for elevated PSA levels and prostate biopsies, our total number of monthly data points was small (n = 36), limiting our analysis and comparison to later studies. Though we are not aware of other events in our region or practice contributing to the decreases in elevated PSA level evaluations or prostate biopsy volume we observed over our study period, other causes for the changes we observed may exist. Our use of administrative level data to define total practice visits, evaluations for elevated PSA level, and prostate biopsies performed is subject to possible coding and documentation errors; the presence or ramifications of any such errors upon our research is uncertain. Although we were able to select and include in our analysis only new patient evaluations for elevated PSA levels, we were unable to differentiate initial versus repeat prostate biopsies within our administrative level dataset. Although this biopsy differentiation is important, the possible effect of men within the practice undergoing repeat biopsies during our study period would increase our total number of biopsies performed, and bias our results toward the null. As a result, the significant decrease we identified in prostate biopsies performed by our group following the USPSTF grade D recommendation may underestimate the true effect. Finally, there may be unique characteristics of our geographic setting, referring providers, and 32 urologists, beyond the scope of our research to examine, which may limit the external validity of our findings.
Conclusions
Despite a 24.1% increase in total visits, urologists in our large urology group practice completed 16.4% fewer evaluations for elevated PSA levels and 21.4% fewer prostate biopsies in the 2 years following the USPSTF grade D draft recommendation against PSA-based prostate cancer screening. Patient-level data and longer follow-up are needed to understand the oncologic and public health ramifications of the USPSTF recommendation change.
Main Points.
On October 7, 2011, the United States Preventive Services Task Force (USPSTF) released their evidence statement and draft recommendation against prostate-specific antigen (PSA)-based prostate cancer screening, noting the benefits of PSA-based screening for prostate cancer do not outweigh the harms (Grade D recommendation).
We used an interrupted time series study design with October 2011 as our interruption point to understand the effect of the USPSTF grade D recommendation upon practice volume.
Despite a 24.1% increase in total visits, the 32 urologists in our large urology group practice completed 16.4% fewer evaluations for elevated PSA and 21.4% fewer prostate biopsies in the 2 years following the USPSTF recommendation against PSA-based prostate cancer screening.
Our understanding of the effects of the USPSTF grade D recommendation is in its infancy. Further longitudinal data, including monitoring of Gleason grade and D’Amico risk classification at the time of diagnosis, may help guide future recommendations for prostate cancer screening.
Footnotes
The authors thank JoAnne Sowney for her assistance with data collection.
The authors report no real or apparent conflicts of interest.
References
- 1.Surveillance Epidemiology, and End Results Program. SEER Stat Fact Sheets: Prostate Cancer. National Cancer Institute website. [Accessed September 30, 2015].
- 2.Prostate-Specific Antigen (PSA) Test, authors. National Cancer Institute website. [Accessed September 30, 2015].
- 3.Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 4.Andriole GL, Crawford ED, Grubb RL, 3rd PLCO Project Team, authors. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ ERSPC Investigators, authors. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 7.AUA Health Policy Brief: November 2011, authors. AUA Response to USPSTF PSA Recommendations. American Urological Association website. [Accessed September 30, 2015].
- 8.Catalona WJ, D’Amico AV, Fitzgibbons WF, et al. What the U.S. Preventive Services Task Force missed in its prostate cancer screening recommendation. Ann Intern Med. 2012;157:137–138. doi: 10.7326/0003-4819-157-2-201207170-00463. [DOI] [PubMed] [Google Scholar]
- 9.Messing EM, Albertsen P, Andriole GL, et al. SUO’s Response to the USPSTF. Society of Urologic Oncology website. [Accessed September 30, 2015].
- 10.Moyer VA U.S. Preventive Services Task Force, authors. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 11.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Early Detection. National Comprehensive Cancer Network website. [Accessed September 30, 2015]. [DOI] [PubMed]
- 14.Tasian GE, Cooperberg MR, Potter MB, et al. PSA screening: determinants of primary-care physician practice patterns. Prostate Cancer Prostatic Dis. 2012;15:189–194. doi: 10.1038/pcan.2011.59. [DOI] [PubMed] [Google Scholar]
- 15.Pollack CE, Noronha G, Green GE, et al. Primary care providers’ response to the US Preventive Services Task Force draft recommendations on screening for prostate cancer. Arch Intern Med. 2012;172:668–670. doi: 10.1001/archinternmed.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werntz R, Acevedo AM, Conlin M, Amling C. Trends in PSA utilization by primary care physicians: impact of the USPSTF recommendation. J Urol. 2015;193(suppl):e897. Abstract PD 44-02. [Google Scholar]
- 17.Aslani A, Minnillo BJ, Johnson B, et al. The impact of recent screening recommendations on prostate cancer screening in a large health care system. J Urol. 2014;191:1737–1742. doi: 10.1016/j.juro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Drazer MW, Huo D, Eggener SE. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;33:2416–2423. doi: 10.1200/JCO.2015.61.6532. [DOI] [PubMed] [Google Scholar]
- 19.Cohn JA, Wang CE, Lakeman JC, et al. Primary care physician PSA screening practices before and after the final U.S. Preventive Services Task Force recommendation. Urol Oncol. 2014;32:41.e23–41.e30. doi: 10.1016/j.urolonc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Perez TY, Danzig MR, Ghandour RA, et al. Impact of the 2012 United States Preventive Services Task Force statement on prostate-specific antigen screening: analysis of urologic and primary care practices. Urology. 2015;85:85–89. doi: 10.1016/j.urology.2014.07.072. [DOI] [PubMed] [Google Scholar]
- 21.Banerji JS, Wolff EM, Massman JD, 3rd, et al. Prostate needle biopsy outcomes in the era of the U.S. Preventive Services Task Force recommendation against PSA-based screening [published online ahead of print August 5, 2015] J Urol. doi: 10.1016/j.juro.2015.07.099. doi: 10.1016/j.juro.2015.07.099. [DOI] [PubMed] [Google Scholar]
- 22.Bhindi B, Mamdani M, Kulkarni GS, et al. Impact of the U.S. Preventive Services Task Force recommendations against prostate specific antigen screening on prostate biopsy and cancer detection rates. J Urol. 2015;193:1519–1524. doi: 10.1016/j.juro.2014.11.096. [DOI] [PubMed] [Google Scholar]
- 23.State and County Quick Facts. United States Census Bureau website. [Accessed September 30, 2015].
- 24.D’Amico AV, Whittington R, Malkowicz SB, et al. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998;160(6 Pt 1):2096–2101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]
- 25.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]