Abstract
Transition metal oxides offer functional properties beyond conventional semiconductors. Bridging the gap between the fundamental research frontier in oxide electronics and their realization in commercial devices demands a wafer-scale growth approach for high-quality transition metal oxide thin films. Such a method requires excellent control over the transition metal valence state to avoid performance deterioration, which has been proved challenging. Here we present a scalable growth approach that enables a precise valence state control. By creating an oxygen activity gradient across the wafer, a continuous valence state library is established to directly identify the optimal growth condition. Single-crystalline VO2 thin films have been grown on wafer scale, exhibiting more than four orders of magnitude change in resistivity across the metal-to-insulator transition. It is demonstrated that ‘electronic grade' transition metal oxide films can be realized on a large scale using a combinatorial growth approach, which can be extended to other multivalent oxide systems.
 Precise valence state control to avoid performance deterioration in transition metal oxide films has proved challenging. Here, the authors establish a combinatorial approach to create a valence state library of VO2, allowing for the growth of wafer size VO2 thin films.
Precise valence state control to avoid performance deterioration in transition metal oxide films has proved challenging. Here, the authors establish a combinatorial approach to create a valence state library of VO2, allowing for the growth of wafer size VO2 thin films.
Multivalent transition metal oxides exhibit a wealth of interesting and useful properties that are beyond conventional semiconductors commonly employed in electronic and optoelectronic devices1,2,3,4. The energetically preferred ground state in this class of material is dominated by the valence state of the transition metal cations, giving rise to different types of order in the spin, charge or orbital degree of freedom5,6,7. Utilizing these functionalities to realize a new generation of electronic and optoelectronic devices requires manufacturing of transition metal oxide films with superior quality on a wafer scale. However, this has been proved challenging because film properties are highly sensitive to valence state variations, which can easily get compromised if the synthesis approach is plagued by an inferior valence state control5,6,8,9,10.
The commonly employed strategy towards stoichiometric multivalent oxide thin films involves extensive calibration series, whereby relevant growth parameters are systematically varied to identify optimal growth condition. In most cases, this is a daunting task, involving tedious and costly calibration series, which sometimes become necessary even in the favourable case of self-regulated growth of ternary transition metal thin films11,12,13, since it only addresses cation stoichiometry, leaving the determination of the demanded cation-to-anion ratio unaddressed.
The challenge is also present in multivalent binary oxides. VO2 has recently drawn much interest due to its electronic phase transformation from a metal to an insulator near room temperature14,15, making it attractive for next-generation transistors16, non-Boolean computing17 and memory metamaterials18. Recently, metamaterial electric circuits have also been demonstrated based on high-quality VO2 film grown on silicon wafer, which has potential application in adaptive matching resistor networks19. Although phase pure epitaxial VO2 films have been grown by pulsed laser deposition (PLD)20,21,22, sputtering23,24,25, molecular beam epitaxy (MBE)26,27 and chemical solution deposition technique28, the growth of high-quality VO2 thin films has been found very demanding21,26, attributed to the complex and rich structural phase diagram of vanadium oxide5. Vanadium oxide phases with lower or higher oxidation states than the targeted VO2 phase, namely, VnO2n−1 (Magnéli phases)29 or VnO2n+1 (Wadsley phases)5, can easily form if the vanadium to oxygen ratio is not precisely controlled throughout the growth21,26. The lack of valence state control directly compromises the metal-to-insulator transition (MIT), significantly reducing the resistivity change across the MIT8,30.
One possible strategy to address the valence state control during growth is the application of a combinatorial approach31,32,33,34,35,36,37, where a flux gradient across the sample is intentionally introduced during film growth to generate various growth conditions simultaneously at different sample locations. Film properties within the composition library are then characterized and related to the flux ratio calculated from the geometrical arrangement of sources and the sample. Such a combinatorial strategy requires (1) precise and direct control of a spatially varying fluxes; (2) two or more sources to independently supply the elements of interest; (3) a favourable deposition geometry, that is, a sufficiently large substrate compared with the flux density profile at sample position to create a sufficiently steep flux gradient; (4) large inelastic mean free path to avoid unintentional gas phase reactions. This approach has been successfully utilized to optimize the bandgap of III–V semiconductor nanowires38, discover new luminescent materials33, study the composition variance of metallic glasses34, increasing the Curie temperature of the ferromagnetic semiconductor GaMnAs37 and exploring the effect of LaAlO3 cation stoichiometry on electrical properties of the two-dimensional electron gas at the LaAlO3/SrTiO3 interface35.
Extending the combinatorial approach to binary oxides requires the supply of oxygen in a spatially varying fashion, which seems impractical in PLD, sputtering or MBE, where oxygen is supplied as a gas. Considering the large oxygen pressures (∼10 mtorr)21 required to stabilize stoichiometric VO2, the small inelastic mean free path of <1 cm severely limits the magnitude of oxygen concentration gradients across the wafer, rendering the combinatorial approach inapplicable using traditional growth techniques. We have circumvented this difficulty by supplying oxygen in a form pre-bonded to vanadium, which can be used to generate an oxygen concentration gradient across the wafer, thus locally varying the oxygen activity. For the oxygen-containing precursor molecule vanadium oxytriisopropoxide (VTIP), each vanadium atom is in the valence state 5+, forming three single bonds and one double bond to oxygen. Purely supplying VTIP with a V:O ratio of 1:4 resulted in an oxygen surplus in the film, which was compensated by independently supplying vanadium metal. Using this approach, a VO2 valence state library was deposited on a 3-inch r plane sapphire wafer and the optimal growth condition was identified within a single film growth. Under the optimized growth condition, single-crystalline VO2 films of superior quality were grown epitaxially on wafer-scale areas with excellent uniformity, exhibiting more than four orders of magnitude resistivity change across the MIT, being among the highest values reported for thin films.
Results
Combinatorial growth of VO2 thin films
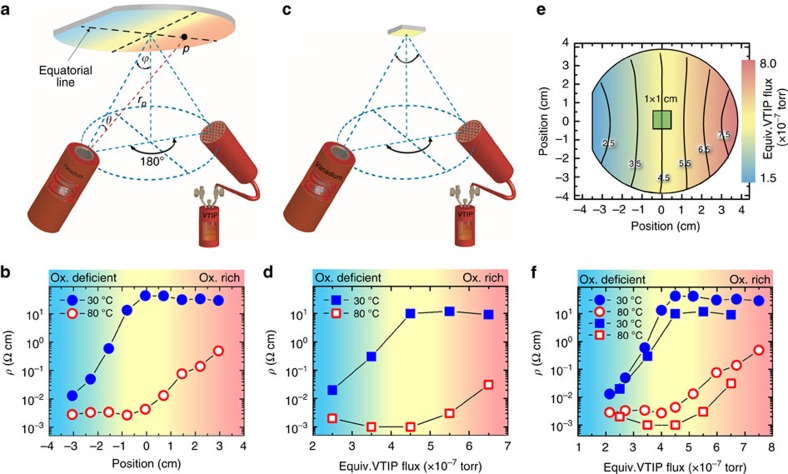
Figure 1a shows the schematic set-up in the growth chamber. A V-metal source and a VTIP source were mounted on diametral flanges, aligning the flux gradient of V and VTIP in opposite direction to increase their flux ratio gradient. The flux density at position p on the sample is given by:39
Figure 1. Combinatorial growth method.
(a) Schematic of V-effusion cell, VTIP gas injector and a 3-inch wafer arrangement to create a gradient of VTIP:V flux ratios along the equatorial line across the wafer. (b) Resistivity at 30 (insulating state) and 80 °C (metallic state) measured from nine devices of VO2 on r-Al2O3 located at different positions on the equatorial line. (c) Schematic of V-effusion cell, VTIP gas injector and 1 × 1 cm substrates arrangement for the growth of VO2 films with varying equivalent VTIP fluxes from sample to sample. (d) Resistivity at 30 (insulating state) and 80 °C (metallic state) of five 1 × 1 cm samples grown using the set-up in c. (e) Calculated equivalent VTIP flux distribution across a 3-inch wafer with 4.5 × 10−7 torr VTIP ion gauge pressure. For size comparison, the green square at the center represents a 1 × 1-cm sample. The colour key provided in e also applies to b,d and f. (f) Superposition of the resistivity data shown in b and d. The position axis in b was converted to equivalent VTIP flux using the flux profile calculated from equation (1) that is shown in e.
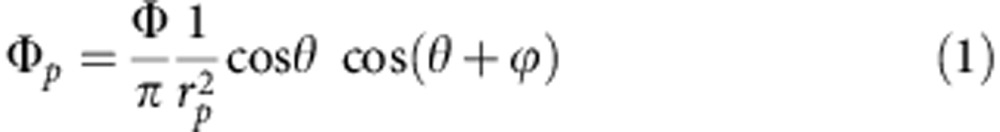 |
with Φ the flux density effusing from the source, rp the distance between the centre of the crucible orifice and the point p on the substrate. Angle θ and ϕ are the effusing angle and the angle of effusion cell with respect to the substrate normal at the centre. V and VTIP sources each generated a flux gradient across the substrate. The flux variation for both sources was calculated as +18 and –16% at the sample positions 3.8 cm off centred towards and away from the source on the equatorial line. Therefore, the total change of the flux ratio 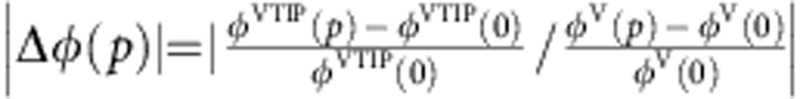 ranged from 140 to 71%. For simplicity, an equivalent VTIP flux
ranged from 140 to 71%. For simplicity, an equivalent VTIP flux 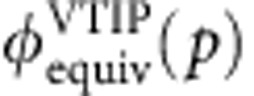 was defined at position p on the wafer to represent the VTIP:V flux ratio. At the centre of the substrate,
was defined at position p on the wafer to represent the VTIP:V flux ratio. At the centre of the substrate, 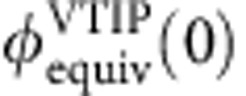 was set to be equal to the VTIP ion gauge pressure measured at the centre of sample position before growth, while the equivalent VTIP flux at position p was defined as
was set to be equal to the VTIP ion gauge pressure measured at the centre of sample position before growth, while the equivalent VTIP flux at position p was defined as 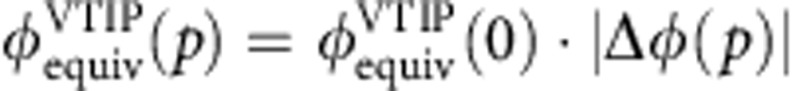 .
.
To create a VO2 valence state library on a 3-inch r plane sapphire substrate, the wafer was not rotated during growth. For the supply of VTIP, the flux was set to an beam equivalent pressure of 4.5 × 10−7 torr determined by a beam flux ion gauge at the sample position, while V-flux was fixed as 4 × 1012 cm−2 s−1, measured by a quartz crystal microbalance. Resistivity measurements at two different temperatures, 30 (insulating state) and 80 °C (metallic state) were taken on devices every 7.5 mm along the equatorial line of the wafer and are plotted in Fig. 1b. For the position on the wafer closest to the V effusion cell (−3 cm), a small resistivity ratio Δρ=(ρ30 °C−ρ80 °C)/ρ80 °C of only 4.7 was measured. The resistivity ratio progressively increased towards the centre of the wafer. While the resistivity of the metallic state at 80 °C remained almost constant, the resistivity of the insulating phase at 30 °C increased exponentially. At wafer positions closer to the V-cell, the low resistivity of the insulating phase was attributed to a pronounced oxygen deficiency in the film, which increased the unintentional carrier concentration via oxygen vacancy formation21,30. A similar trend in the Δρ was also observed for wafer positions closer to the VTIP gas injector (3 cm). In contrast, here the resistivity of the metallic state at 80 °C sharply increased with distance away from the wafer centre, attributed to enhanced scattering from secondary oxygen-rich phases8. The highest quality film, indicated by the largest resistivity ratio Δρ=1.1. × 104 was found at x=0 cm. X-ray photoelectron spectroscopy (XPS) measurements performed along the equatorial line confirmed the existence of an oxygen activity gradient during growth and its direct effect on the valence state of vanadium in the film (details in the Supplementary Note 1 and Supplementary Fig. 1).
To further confirm the validity of the combinatorial growth approach and the calculated flux gradient, five 30-nm-thick VO2 films were grown on 1 × 1 cm r plane sapphire substrate mounted at the centre of the substrate holder and were rotated at 3 r.p.m. to ensure good uniformity. Throughout this series, the V-flux was kept as 4 × 1012 cm−2 s−1 while the VTIP flux, determined as beam equivalent pressure at the sample position, was varied from 2.5 × 10−7 to 6.5 × 10−7 torr, see Fig. 1c. A similar resistivity trend as function of equivalent VTIP fluxes was reproduced and is shown in Fig. 1d. The resistivity results from both experiments (Fig. 1b,d) are shown in Fig. 1f for direct comparison. Using the calculated flux gradient in Fig. 1e, the positions along the equatorial line in Fig. 1b were converted into equivalent VTIP fluxes. The trends in resistivity with changes in the equivalent VTIP flux are well matched, evidencing the feasibility of the combinatorial approach to optimize cation-to-anion ratios in VO2 films.
Structural and physical properties of 3-inch VO2 films
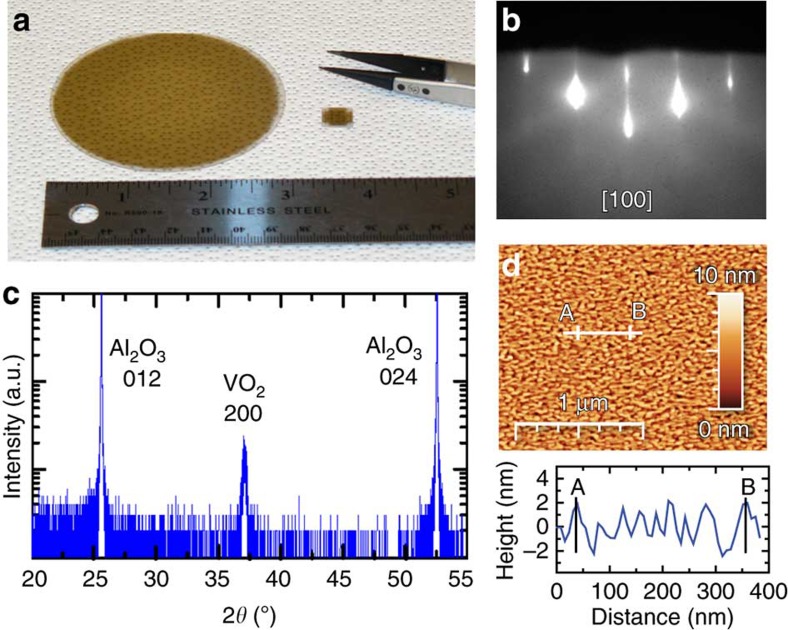
The approach to co-supply V and VTIP was used to demonstrate that a scale up of highly uniform VO2 films can be achieved when the substrate is rotated. A 30-nm-thick film was deposited on a 3-inch sapphire at 350 °C and rotated at 3 r.p.m., while supplying a VTIP flux with beam equivalent pressure of 4.5 × 10−7 torr, and a V-flux of 4 × 1012 cm−2 s−1. Figure 2a shows a direct comparison of the VO2 film grown on a 3-inch wafer to the film grown on a 1 × 1-cm substrate, both exhibiting good uniformity. Reflection high-energy electron diffraction pattern (RHEED) taken during and after the growth remained streaky, indicating an epitaxial film with very good surface roughness and crystallinity, which did not change when scanning the RHEED beam spot across the wafer, see Fig. 2b. X-ray diffraction 2θ−ω scans taken at the wafer centre revealed the absence of any other vanadium oxide phase but VO2 with an out-of-plane orientation VO2 (100)||Al2O3 (012) (refs 40, 41), see Fig. 2c. In plane X-ray diffraction Φ scans of the VO2 220 peak were carried out with 2θ=55.45° and ψ=46.86°, demonstrating a good in plane epitaxy with twofold symmetry, similar to previous report of epitaxial VO2 film on r plane sapphire22, see Supplementary Fig. 2. The film surface was found to be smooth, yet not atomically flat, with a root mean square roughness of 1.1 nm. The surface morphology shown in Fig. 2d was dominated by small irregularly shaped islands separated by corrugations of typically 1–2 nm, commonly observed for VO2 films grown on Al2O3 (ref. 21).
Figure 2. Characterization of VO2 films grown on a 3-inch r plane sapphire substrate.
(a) Comparison of substrate sizes used for the VO2 thin-film growth. (b) Streaky RHEED pattern of VO2 films observed along [100] azimuth after growth. (c) Wide range 2θ−ω X-ray diffraction scans of VO2 grown on a r planeAl2O3 substrate. (d) AFM scan of the film surface morphology and a line scan along AB.
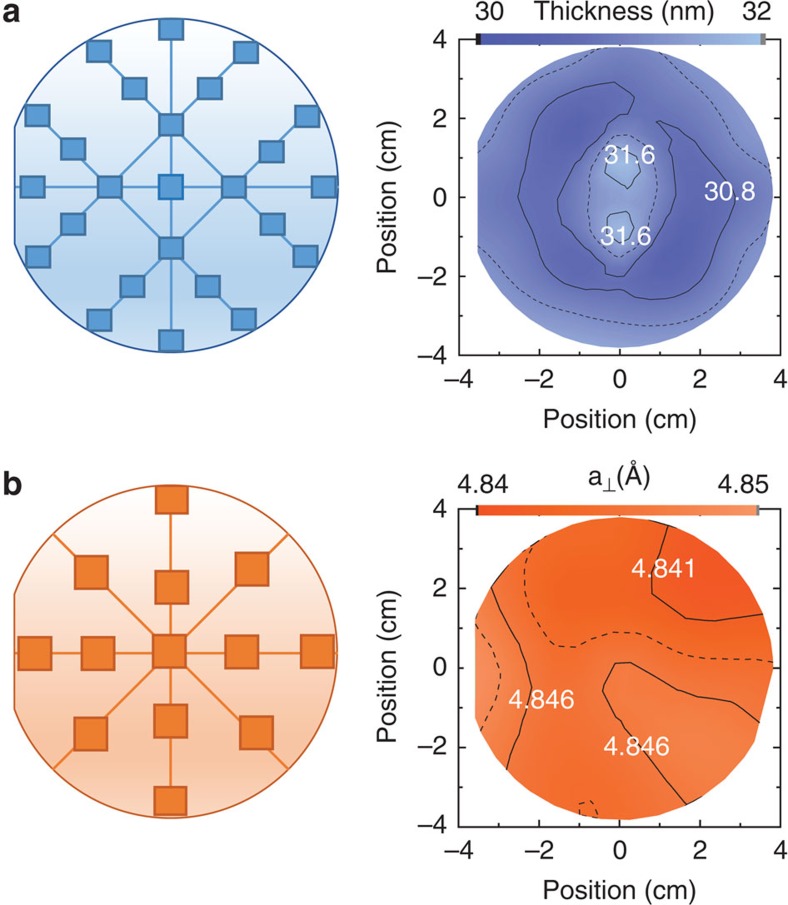
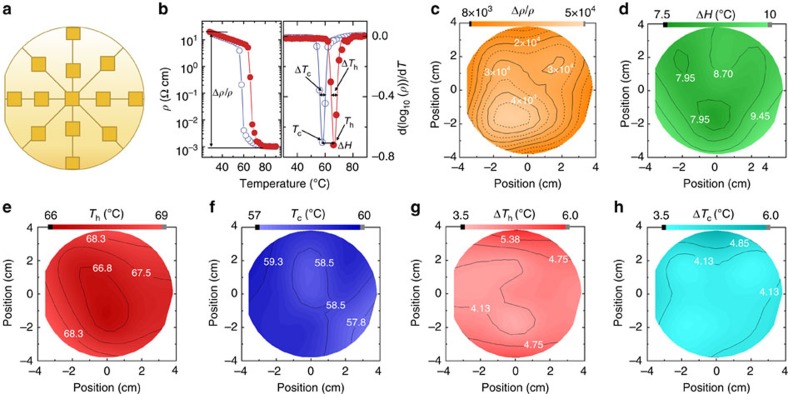
Figure 3 shows the wafer-scale metrology of the 3-inch VO2 thin film, where the film was characterized at multiple positions across the sample using spectroscopic ellipsometry (SE) and X-ray diffraction measurements. The film thickness profile as a function of position was extracted from SE measurement and is shown in Fig. 3a. Overall, the film thickness was uniform, with a s.d. of 0.37 nm (1.2%), which is well within the technical specifications of the MBE system. The thickness was maximum at the wafer centre, and continuously decreased towards the wafer edges. X-ray diffraction was used to map out the VO2 film out-of-plane lattice parameter, as shown in Fig. 3b. In contrast to the thickness variation, the lattice parameter remained virtually unaffected with a s.d. of 0.002 Å, limited by the precision of the X-ray diffraction measurement. Good uniformity of the MIT properties (resistivity ratio, transition point, sharpness and width) was confirmed by testing devices across the wafer. Figure 4a shows the locations and Fig. 4b the definitions of the MIT properties analysed. The resistivity ratio across the MIT is shown in Fig. 4c. More than 71% of the wafer area had resistivity ratio Δρ >2 × 104, a remarkable combination of high yield, uniformity and quality of wafer-scale VO2 films. In addition to the resistivity ratio, other MIT properties were also characterized across the 3-inch film, see Fig. 4 d–h. The derivative of log10 (ρ) as a function of temperature was plotted and fitted with a Gaussian function. The transition points of the heating and cooling cycle, Th and Tc, were defined at the respective peak position of the Gaussian and the transition width ΔH=Th−Tc was directly calculated. The transition sharpness ΔTh and ΔTc were determined from the full-width at half-maximum of the Gaussian peak. The properties were proved uniform, with an s.d. of 0.64 and 0.47 °C for Th and Tc, 0.35 and 0.41 °C for ΔTh and ΔTc, and 0.61 °C for ΔH.
Figure 3. Wafer-scale metrology of VO2 grown on a 3-inch r plane sapphire substrate.
The squares in the schematic on the left represent the probing locations on the wafer. (a) Film thickness uniformity extracted from spectroscopic ellipsometry measurements. (b) Map of the out-of-plane lattice parameter determined from X-ray diffraction scans.
Figure 4. Wafer-scale mapping of MIT properties of 3-inch VO2.
(a) Schematic with squares showing the probing locations on the wafer. (b) Definition of the MIT properties: resistivity ratio (Δρ/ρ), transition point (Th and Tc), transition sharpness (ΔTh and ΔTc) and transition width (ΔH). The subscripts h and c indicate the cooling and heating cycle, respectively. Closed red and open blue circles represent heating and cooling cycles, respectively. (c) Map of VO2 resistivity ratio Δρ/ρ=(ρ30 °C−ρ80 °C)/ρ80 °C obtained from four-point–probe measurements of resistivity ρ30 °C at 30 °C (VO2 insulating state) and ρ80 °C at 80 °C (VO2 metallic state). Wafer-scale mapping of MIT properties defined in b across the 3-inch VO2 film: (d) ΔH, (e) Th, (f) Tc, (g) ΔTh, (h) ΔTc.
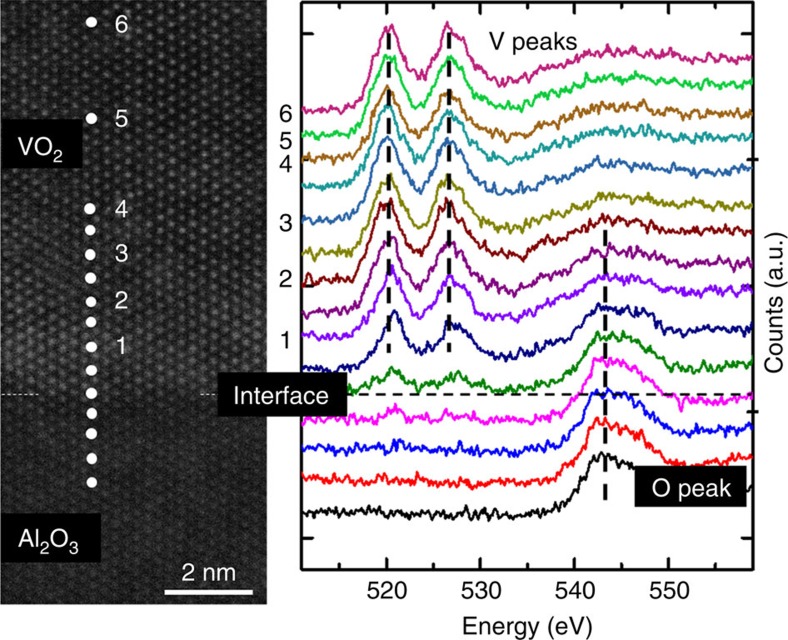
To expand the characterization of the film quality to other film properties and to provide film characterization across the wafer but with mesoscale resolution, Kelvin probe force microscopy (KPFM) measurements were performed at various positions, to extract the VO2 work function at room temperature as well as its uniformity across the 3-inch wafer. The work function was found to be uniform across the wafer with an average value of 4.85±0.10 eV, agrees with previous reports of VO2 work function measured by KPFM25,42 and ultraviolet photoelectron spectroscopy43 (details in the Supplementary Note 2 and Supplementary Fig. 3). To characterize the film structural quality and the vanadium valence state of the VO2 film at the atomic scale, high-angle annular dark-field scanning transmission electron microscopy and electron energy loss spectroscopy (EELS) measurements were performed across the VO2/Al2O3 interface. Figure 5 shows an annular dark-field scanning transmission electron microscopy image of an epitaxial sharp interface between VO2 and Al2O3. Line scan EELS spectra were taken with 0.5 nm step size starting from the substrate into the film to probe the evolution of the valence state of vanadium. The low-energy loss peak determined for the film was around 520 eV, which did not shift with scan position and remained between 519 and 521.5 eV measured for the V2O3 and V2O5 calibration samples, respectively (details in the Supplementary Fig. 4). The shift of EELS peaks between V2O3, VO2 and V2O5 observed here agrees with the trend previously reported44, showing dominant valence state of V to be 4+ state in the film and at the vicinity of the interface. In particular, the absence of a wider transition layer, which was previously observed for VO2 films grown on c plane sapphire and attributed to the formation of oxygen-deficient Magnéli phases VnO2n−1 (ref. 45), demonstrates the excellent control over the V-oxidation state right from the film growth initialization.
Figure 5. STEM and EELS characterization.
High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of the VO2/Al2O3 interface. Since Vanadium has a higher atomic number compared with Aluminum (23 versus 13), the film can be distinguished compared with the substrate by the higher intensity vanadium atoms. Electron energy loss spectroscopy (EELS) to determine the vanadium valence across the interface is shown on the right. EELS spectra were taken at the position indicated in the STEM image. The EELS data were collected from a beam spot size with ∼1-Å diameter.
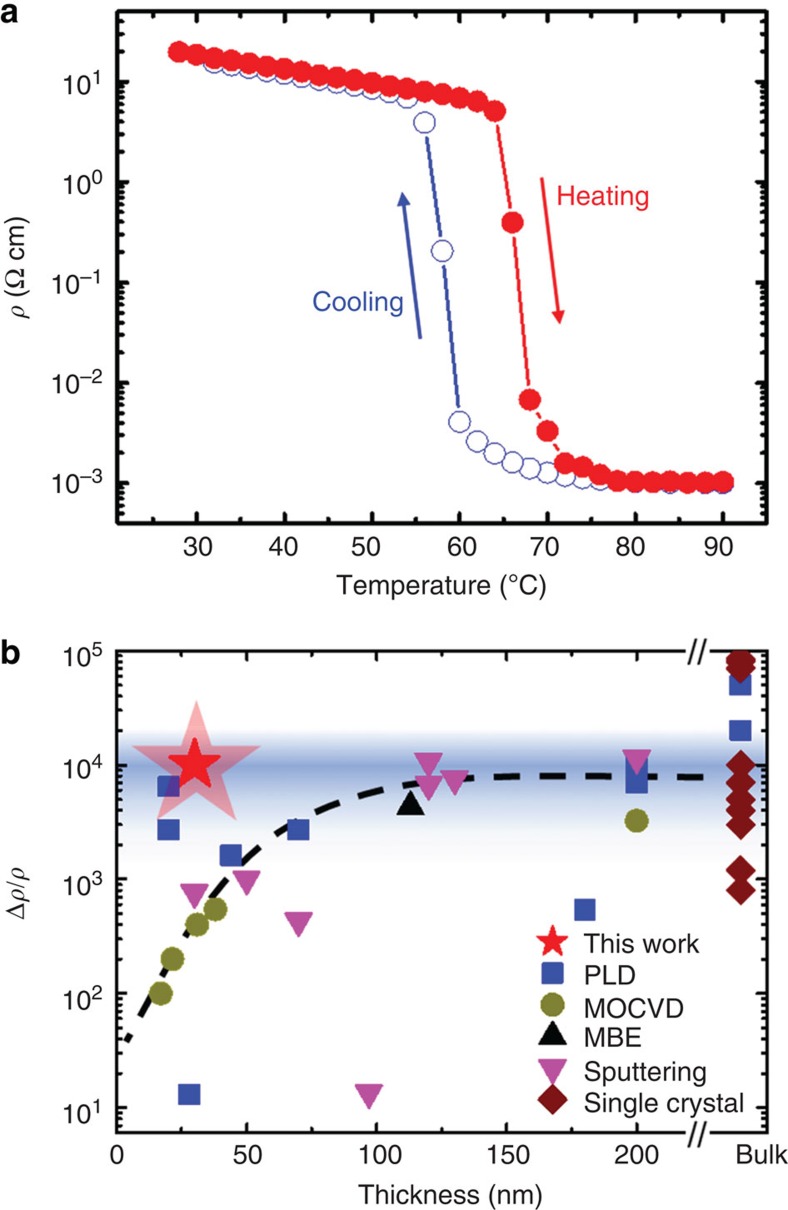
To benchmark the quality of VO2 thin film, temperature-dependent resistivity has been measured for a device at the wafer center and is shown in Fig. 6a. A representative set of data was compiled and is shown in Fig. 6b to allow for a direct comparison with bulk single crystals46,47,48,49,50,51,52 as well as VO2 thin films grown by deposition techniques such as PLD21,41,53,54,55,56, metalorganic chemical vapour deposition57,58, MBE27 and sputtering8,23,25,59,60,61,62. The selection criteria were either single crystals in case of bulk material, or completely relaxed VO2 films grown on sapphire samples, that is, with thicknesses exceeding the critical film thickness of 20 nm (ref. 21). Here the change of resistivity Δρ/ρ across the MIT was defined as Δρ/ρ=(ρ50 °C−ρ80 °C)/ρ80 °C≈ρ50 °C/ρ80 °C, which serves as a benchmark to compare material quality. For the best bulk single crystal, a resistivity ratio of Δρ/ρ=9 × 104 was reported46,47, while the majority of resistivity ratios achieved in bulk single crystals were much lower than that with an average of ∼5 × 103 and not exceeding 1 × 104 (refs 47, 48, 49, 51, 52). Resistivity ratios for VO2 thin films varied significantly with the highest values Δρ/ρ being comparable to the majority of those reported for bulk single crystals (shaded area in Fig. 6b). Notably, a reduction in Δρ/ρ was found to occur with decreasing film thickness, likely due to the formation of an interfacial transition layer45, which can occur if the cation-to-anion stoichiometry was not maintained throughout the entire growth. If this nonstoichiometric layer amounts to a significant fraction of the film, the resistivity ratio is ultimately compromised. However, the 30-nm-thick VO2 film grown here only showed an ultrathin transition layer at the vicinity of the VO2/Al2O3 interface (Fig. 5) and revealed a four orders of magnitude change in resistivity across the MIT, similar to the largest value reported by Jeong et al.21 for ultrathin VO2 films grown by PLD on sapphire and on par with the majority of bulk single-crystal values47,48,49,51,52. To demonstrate the reproducibility of this technique and its versatile applicability to challenging templates, similar optimization processes were repeated on a 3-inch c plane sapphire wafer and on the technologically interesting, but more challenging silicon substrate. Details are given in the Supplementary Notes 3 and 4 and Supplementary Figs 5 and 6. While a similarly high-resistivity ratio with four orders of magnitude change was obtained on c plane sapphire along the equatorial line of the wafer, the resistivity ratio of VO2 on silicon was reduced, which was attributed to the polycrystallinity of the film induced by the amorphous substrate that resulted in a high surface roughness and high grain boundary density63.
Figure 6. Benchmark of VO2 thin films.
(a) Temperature-dependent resistivity of VO2 films grown under optimized condition on a 3-inch r plane Al2O3. Measurement was taken at the wafer center. (b) Benchmark of resistivity ratios Δρ/ρ=(ρ50 °C−ρ80 °C)/ρ80 °C for VO2 across the metal-to-insulator transition. Note that the low temperature resistivity was taken at 50 °C. For a direct comparison, all films were grown on Al2O3 substrates above the critical films thickness. The blue-shaded area indicates the highest values reported for VO2 films on sapphire and typical values obtained in bulk single crystals. A dotted line was added as guide to the eye. Note the two PLD samples with resistivity ratios higher than Δρ/ρ=103 at film thicknesses <25 nm, from ref. 21. A thickness much >200 nm was estimated for the VO2 films grown by PLD in ref. 57 and the data were added as ‘bulk' value. Data for bulk VO2 were taken from refs 47, 48, 49, 50, 51, 52, 53. Properties of VO2 films on Al2O3 substrate grown by PLD were taken from refs 21, 42, 52, 53, 54, 55, 56, 57, MOCVD from refs 58, 59, MBE from ref. 27 and sputtering from refs 8, 23, 25, 60, 61, 62, 63.
Discussion
It is demonstrated that the highly desirable combination of superior material quality in VO2 thin films, with resistivity ratios rivalling the highest values reported for thin films and typical values obtained for bulk VO2 single crystals, excellent uniformity across a 3-inch wafer, and high yield is possible. The precise valence state control was achieved by a highly efficient combinatorial growth strategy, where a continuous vanadium valence state library was established across the wafer by supplying oxygen in a pre-bonded form. The ability to grow VO2 thin films with excellent reproducibility and minimal calibration time on wafer scale overcomes a critical roadblock, making it feasible to transfer VO2-based functionalities into viable technologies and directly impacting different application spaces, such as thermochromics64 or memory metamaterials18. This approach is not limited to VO2, but can be further extended to other oxide systems containing multivalent cations, therefore providing a new gateway to grow large scale multivalent oxides with superior valence state control.
Methods
Combinatorial growth of VO2 thin films
Growth was carried out in DCA M600 MBE growth chamber with a base pressure of 5 × 10−10 torr. VO2 thin films were grown on a 3-inch (Precision Micro-Optics LLC) and 1 × 1-cm (MTI Corporation) r plane sapphire substrates via co-supply of VTIP (vacuum distilled, trace metal impurity 4N, MULTIVALENT Laboratory) and vanadium metal (4N, Ames Laboratory). VTIP was kept in a bubbler (heated to 50 °C), connected to the MBE growth chamber through a heated gas inlet. A low-conductance gas injector was used to supply VTIP to the substrate. A proportional-integral-derivative (PID)-controlled adjustable leak valve and capacitance manometer were used to maintain a constant VTIP gas inlet pressure. An ion gauge was used to monitor VTIP flux, which was positioned at the center of the sample position before growth. Vanadium metal was evaporated from a high temperature effusion cell. V-flux calibration was performed using a quartz crystal microbalance at growth position. Substrates were cleaned in ultrasonic bath of acetone and then isopropanol before loading them into the MBE system and baked at 150 °C for 2 h in the load lock chamber. The substrates were transferred into the MBE growth chamber and heated to the film growth temperature of 350 °C. Before deposition substrates were exposed for 20 min in oxygen plasma (250 W, 9 × 10−7 torr background pressure). The growth was carried out by co-supplying V (4 × 1012 cm−2 s−1) and VTIP in the presence of an oxygen plasma. Film growth rate was 5 Å min−1. After growth the films were cooled in the presence of oxygen plasma.
VO2 thin-film characterization
Film growth was monitored in situ using RHEED. XPS measurement was performed on PHI VersaProbe with 200 μm spot size. Aluminum Kα radiation was used with a pass energy of 29.32 eV. Fitting was performed using CasaXPS software (Supplementary Note 1). Surface morphology of the VO2 thin film was characterized using a Bruker Dimension Icon Atomic Force Microscope operated in peak force tapping mode. KPFM measurements were carried out with the same set-up as atomic force microscope using conductive Pt-coated Si cantilevers. To reduce the effect of surface water absorption, samples were measured immediately after growth. Highly ordered pyrolytic graphite was used as a reference sample to calibrate the cantilever potential. During the measurement, an a.c. voltage with the amplitude of 3 V and frequency of 60 kHz was applied to the cantilever. X-ray diffraction was carried out by a Phillips X'Pert Panalytical MRD Pro thin-film diffractometer equipped with a duMond–Hart–Partels Ge (440) incident beam monochromator using Cu Kα1 radiation. The uniformity of VO2 film thickness was characterized ex situ using room temperature SE (M-2000U, J. A. Woollam) in the spectral range from 1.125 to 5.175 eV. Data were fitted using a model comprised of two layers: VO2 thin film and r plane sapphire substrate. The sapphire substrate was approximated using a Cauchy model
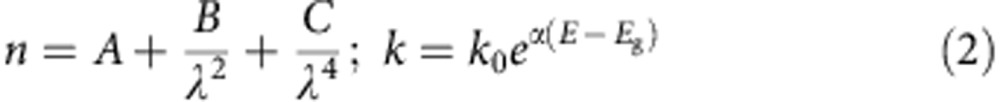 |
with Eg=8.8 eV. The VO2 thin films were modelled using three Tauc–Lorentz oscillators
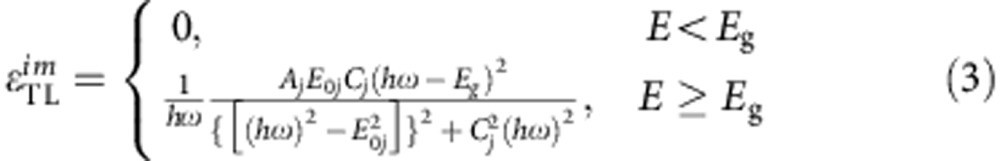 |
with parameters 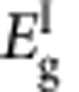 =0.541 eV,
=0.541 eV, 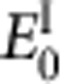 =1.144 eV,
=1.144 eV, 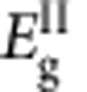 =1.857 eV,
=1.857 eV, 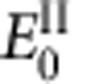 =3.469 eV,
=3.469 eV, 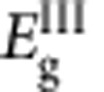 =3.568 eV and
=3.568 eV and 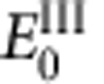 =4.807 eV, corresponding to optical interband transitions:
=4.807 eV, corresponding to optical interband transitions: 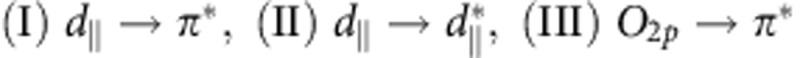 (refs 65, 66). Very good agreement between model and experiment was obtained with a root mean square error of ∼2. Temperature-dependent resistivity measurements were performed in the van der Pauw geometry on a Cascade probe station, where 5 × 5 mm pieces were cut from the wafer for resistivity measurement. Contour figures were plotted using Origin software with triangulation and linear interpolation method between the data points. High-angle annular dark-field scanning transmission electron microscopy images and EELS spectra were collected using the FEI Titan3 80–300 microscope operating at 200 kV in the STEM mode. The EELS spectra were collected using dual 2048 channel Gatan CCD detectors at a semi-angle of 21.4 mrad with an energy dispersion of 0.1 eV per channel. The L2,3 edge peak position in the vanadium films were compared with V2O3 and V2O5 powder reference samples, obtained from Sigma-Aldrich.
(refs 65, 66). Very good agreement between model and experiment was obtained with a root mean square error of ∼2. Temperature-dependent resistivity measurements were performed in the van der Pauw geometry on a Cascade probe station, where 5 × 5 mm pieces were cut from the wafer for resistivity measurement. Contour figures were plotted using Origin software with triangulation and linear interpolation method between the data points. High-angle annular dark-field scanning transmission electron microscopy images and EELS spectra were collected using the FEI Titan3 80–300 microscope operating at 200 kV in the STEM mode. The EELS spectra were collected using dual 2048 channel Gatan CCD detectors at a semi-angle of 21.4 mrad with an energy dispersion of 0.1 eV per channel. The L2,3 edge peak position in the vanadium films were compared with V2O3 and V2O5 powder reference samples, obtained from Sigma-Aldrich.
Additional information
How to cite this article: Zhang, H.-T. et al. Wafer-scale growth of VO2 thin films using a combinatorial approach. Nat. Commun. 6:8475 doi: 10.1038/ncomms9475 (2015).
Supplementary Material
Supplementary Figures 1-6, Supplementary Notes 1-4 and Supplementary References
Acknowledgments
The financial support from the National Science Foundation through Grant No. DMR-1352502 and the Penn State MRSEC program DMR-1420620 is gratefully acknowledged. We thank Y.-C. Lin for the help in KPFM measurements, G. Barber and the Materials Characterization Lab (MCL) at Penn State for the help in XPS measurements, as well as B. Jalan, M. Brahlek and C. Eaton for helpful discussions.
Footnotes
Author contributions H.-T.Z. and R.E.-H. conceived the experiments. H.-T.Z. performed film growth, KPFM measurements, XPS analysis, structural and electrical characterizations. L.Z. assisted in the X-ray diffraction measurement and flux calculation. D.M. performed STEM and EELS experiments and analysed the data with N.A., Y.-X.Z. performed SE measurements and analysed the data. R.C.H. helped with the flux calibration measurements. All authors contributed to the discussion, R.E.-H. and H.-T.Z. co-wrote the paper.
References
- Jany R. et al. Monolithically integrated circuits from functional oxides. Adv. Mater. Interfaces 1, 1–7 (2014). [Google Scholar]
- Mathews S., Ramesh R., Venkatesan T. & Benedetto J. Ferroelectric field effect transistor based on epitaxial perovskite heterostructures. Science 276, 238–240 (1997). [DOI] [PubMed] [Google Scholar]
- Takagi H. & Hwang H. Y. An emergent change of phase for electronics. Science 327, 1601–1602 (2010). [DOI] [PubMed] [Google Scholar]
- Assmann E. et al. Oxide heterostructures for efficient solar cells. Phys. Rev. Lett. 110, 078701 (2013). [DOI] [PubMed] [Google Scholar]
- Katzke H., Tolédano P. & Depmeier W. Theory of morphotropic transformations in vanadium oxides. Phys. Rev. B 68, 024109 (2003). [Google Scholar]
- Chambers S. A. Epitaxial growth and properties of thin film oxides. Surf. Sci. Rep. 39, 105–180 (2000). [Google Scholar]
- Imada M., Fujimori A. & Tokura Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039–1263 (1998). [Google Scholar]
- Griffiths C. H. Influence of stoichiometry on the metal-semiconductor transition in vanadium dioxide. J. Appl. Phys. 45, 2201 (1974). [Google Scholar]
- Abdelmoula N., Guidara K., Cheikh–Rouhou A., Dhahri E. & Joubert J. C. Effects of the oxygen nonstoichiometry on the physical properties of La0.7Sr0.3MnO3−δ manganites (0≤δ≤0.15). J. Solid State Chem. 151, 139–144 (2000). [Google Scholar]
- Li J., Ong C. K., Liu J.-M., Huang Q. & Wang S. J. Oxygen-deficiency-activated charge ordering in La2/3Sr1/3MnO3−δ thin films. Appl. Phys. Lett. 76, 1051 (2000). [Google Scholar]
- Ihlefeld J. F. et al. Adsorption-controlled molecular-beam epitaxial growth of BiFeO3. Appl. Phys. Lett. 91, 071922 (2007). [Google Scholar]
- Theis C. D., Yeh J., Schlom D. G., Hawley M. E. & Brown G. W. Adsorption-controlled growth of PbTiO3 by reactive molecular beam epitaxy. Thin Solid Films 325, 107–114 (1998). [Google Scholar]
- Jalan B., Moetakef P. & Stemmer S. Molecular beam epitaxy of SrTiO3 with a growth window. Appl. Phys. Lett. 95, 032906 (2009). [Google Scholar]
- Yang Z., Ko C. & Ramanathan S. Oxide electronics utilizing ultrafast metal-insulator transitions. Annu. Rev. Mater. Res. 41, 337–367 (2011). [Google Scholar]
- Yang Z., Zhou Y. & Ramanathan S. Studies on room-temperature electric-field effect in ionic-liquid gated VO2 three-terminal devices. J. Appl. Phys. 111, 014506 (2012). [Google Scholar]
- Nakano M. et al. Collective bulk carrier delocalization driven by electrostatic surface charge accumulation. Nature 487, 459–462 (2012). [DOI] [PubMed] [Google Scholar]
- Shukla N. et al. Synchronized charge oscillations in correlated electron systems. Sci. Rep. 4, doi: 10.1038/srep04964 (2014). [DOI] [Google Scholar]
- Driscoll T. et al. Memory metamaterials. Science 325, 1518–1521 (2009). [DOI] [PubMed] [Google Scholar]
- Savo S. et al. Reconfigurable anisotropy and functional transformations with VO2-based metamaterial electric circuits. Phys. Rev. B 91, 134105 (2015). [Google Scholar]
- Martens K., Aetukuri N., Jeong J., Samant M. G. & Parkin S. S. P. Improved metal-insulator-transition characteristics of ultrathin VO2 epitaxial films by optimized surface preparation of rutile TiO2 substrates. Appl. Phys. Lett. 104, 081918 (2014). [Google Scholar]
- Jeong J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402–1405 (2013). [DOI] [PubMed] [Google Scholar]
- Bayati M. R. et al. Correlation between structure and semiconductor-to-metal transition characteristics of VO2/TiO2/sapphire thin film heterostructures. Acta Mater. 61, 7805–7815 (2013). [Google Scholar]
- Ruzmetov D., Zawilski K. T., Narayanamurti V. & Ramanathan S. Structure-functional property relationships in rf-sputtered vanadium dioxide thin films. J. Appl. Phys. 102, 113715 (2007). [Google Scholar]
- Wong F. J., Zhou Y. & Ramanathan S. Epitaxial variants of VO2 thin films on complex oxide single crystal substrates with 3m surface symmetry. J. Cryst. Growth 364, 74–80 (2013). [Google Scholar]
- Ko C., Yang Z. & Ramanathan S. Work function of vanadium dioxide thin films across the metal-insulator transition and the role of surface nonstoichiometry. ACS Appl. Mater. Interfaces 3, 3396–3401 (2011). [DOI] [PubMed] [Google Scholar]
- Tashman J. W. et al. Epitaxial growth of VO2 by periodic annealing. Appl. Phys. Lett. 104, 063104 (2014). [Google Scholar]
- Fan L. L. et al. Growth and phase transition characteristics of pure M-phase VO2 epitaxial film prepared by oxide molecular beam epitaxy. Appl. Phys. Lett. 103, 131914 (2013). [Google Scholar]
- Ji Y. D. et al. Epitaxial growth and metal-insulator transition of vanadium oxide thin films with controllable phases. Appl. Phys. Lett. 101, 071902 (2012). [Google Scholar]
- Schwingenschlögl U. & Eyert V. The vanadium Magnéli phases VnO2n-1. Ann. Phys. 13, 475–510 (2004). [Google Scholar]
- Liu S.-J., Su Y.-T. & Hsieh J.-H. Effects of postdeposition annealing on the metal–insulator transition of VO2−x thin films prepared by RF magnetron sputtering. Jpn. J. Appl. Phys. 53, 33201 (2014). [Google Scholar]
- Kennedy K., Stefansky T., Davy G., Zackay V. F. & Parker E. R. Rapid method for determining ternary-alloy phase diagrams. J. Appl. Phys. 36, 3808 (1965). [Google Scholar]
- Xiang X.-D. et al. A combinatorial approach to materials discovery. Science 268, 1738–1740 (1995). [DOI] [PubMed] [Google Scholar]
- Danielson E. et al. A combinatorial approach to the discovery and optimization of luminescent materials. Nature 389, 944–948 (1997). [Google Scholar]
- Ding S. et al. Combinatorial development of bulk metallic glasses. Nat. Mater. 13, 494–500 (2014). [DOI] [PubMed] [Google Scholar]
- Warusawithana M. P. et al. LaAlO3 stoichiometry is key to electron liquid formation at LaAlO3/SrTiO3 interfaces. Nat. Commun. 4, 2351 (2013). [DOI] [PubMed] [Google Scholar]
- Dover R. B., Van, Schneemeyer L. F. & Fleming R. M. Discovery of a useful thin-film dielectric using a composition-spread approach. Nature 392, 162–164 (1998). [Google Scholar]
- Mack S., Myers R. C., Heron J. T., Gossard A. C. & Awschalom D. D. Stoichiometric growth of high Curie temperature heavily alloyed GaMnAs. Appl. Phys. Lett. 92, 192502 (2008). [Google Scholar]
- Kuykendall T., Ulrich P., Aloni S. & Yang P. Complete composition tunability of InGaN nanowires using a combinatorial approach. Nat. Mater. 6, 951–956 (2007). [DOI] [PubMed] [Google Scholar]
- Herman M. A. & Sitter H. Molecular Beam Epitaxy, Fundamentals and Current Status 7, Springer-Verlag (1989). [Google Scholar]
- Bialas H., Dillenz A., Downar H. & Ziemann P. Epitaxial relationships and electrical properties of vanadium oxide films on r-cut sapphire. Thin Solid Films 338, 60–69 (1999). [Google Scholar]
- Yang T.-H. et al. Semiconductor-metal transition characteristics of VO2 thin films grown on c- and r-sapphire substrates. J. Appl. Phys. 107, 053514 (2010). [Google Scholar]
- Sohn A. et al. Evolution of local work function in epitaxial VO2 thin films spanning the metal-insulator transition. Appl. Phys. Lett. 101, 191605 (2012). [Google Scholar]
- Wang Y. & Zhang Z. Synthesis and field emission property of VO2 nanorods with a body-centered-cubic structure. Phys. E 41, 548–551 (2009). [Google Scholar]
- Fitting Kourkoutis L., Hotta Y., Susaki T., Hwang H. & Muller D. Nanometer scale electronic reconstruction at the interface between LaVO3 and LaVO4. Phys. Rev. Lett. 97, 256803 (2006). [DOI] [PubMed] [Google Scholar]
- Zhou H., Chisholm M. F., Yang T.-H., Pennycook S. J. & Narayan J. Role of interfacial transition layers in VO2/Al2O3 heterostructures. J. Appl. Phys. 110, 073515 (2011). [Google Scholar]
- Bando Y., Nagasawa K., Kato Y. & Takada T. Growth of VO2 single crystals by chemical transport reaction. Jpn J. Appl. Phys. 8, 633 (1969). [Google Scholar]
- Berglund C. N. & Guggenheim H. J. Electronic properties of VO2 near the semiconductor-metal transition. Phys. Rev. 185, 1022–1033 (1969). [Google Scholar]
- Bongers P. F. Anisotropy of the electrical conductivity of VO2 single crystals. Solid State Commun. 3, 275–277 (1965). [Google Scholar]
- Kodaira K., Jogenji I., Shimada S. & Matsushita T. Synthesis and properties of vanadium dioxide single crystals under pressure. Mater. Res. Bull. 16, 369–372 (1981). [Google Scholar]
- Ladd L. A. & Paul W. Optical and transport properties of high quality crystals of V2O4 near the metallic transition temperature. Solid State Commun. 7, 425–428 (1969). [Google Scholar]
- Sasaki H. & Watanabe A. A new growing method for VO2 single crystals. J. Phys. Soc. Jpn 19, 1748 (1964). [Google Scholar]
- Wilke K.-T. Eigenschaften und Kristallzüchtung von Oxiden mit Halbleiter-Metall-Übergängen. Krist. und Tech. 8, 443–452 (1973). [Google Scholar]
- Koo H. et al. Effect of lattice misfit on the transition temperature of VO2 thin film. J. Mater. Sci. 47, 6397–6401 (2012). [Google Scholar]
- Chang Y. et al. Surface versus bulk characterizations of electronic inhomogeneity in a VO2 thin film. Phys. Rev. B 76, 075118 (2007). [Google Scholar]
- Garry G., Durand O. & Lordereau A. Structural, electrical and optical properties of pulsed laser deposited VO2 thin films on R- and C-sapphire planes. Thin Solid Films 453-454, 427–430 (2004). [Google Scholar]
- Kim D. H. & Kwok H. S. Pulsed laser deposition of VO2 thin films. Appl. Phys. Lett. 65, 3188–3190 (1994). [Google Scholar]
- Kim H. K. et al. Finite-size effect on the first-order metal-insulator transition in VO2 films grown by metal-organic chemical-vapor deposition. Phys. Rev. B 47, 12900–12907 (1993). [DOI] [PubMed] [Google Scholar]
- Maruyama T. & Ikuta Y. Vanadium dioxide thin films prepared by chemical vapour deposition from vanadium(III) acetylacetonate. J. Mater. Sci. 28, 5073–5078 (1993). [Google Scholar]
- Cui Y. & Ramanathan S. Substrate effects on metal-insulator transition characteristics of rf-sputtered epitaxial VO2 thin films. J. Vac. Sci. Technol. A 29, 041502 (2011). [Google Scholar]
- Zimmers A. et al. Role of thermal heating on the voltage induced insulator-metal transition in VO2. Phys. Rev. Lett. 110, 056601 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Structural, electrical, and terahertz transmission properties of VO2 thin films grown on c-, r-, and m-plane sapphire substrates. J. Appl. Phys. 111, 053533 (2012). [Google Scholar]
- Zhu N.-W., Hu M., Xia X.-X., Wei X.-Y. & Liang J.-R. Preparation and modification of VO2 thin film on R-sapphire substrate by rapid thermal process. Chinese Phys. B 23, 048108 (2014). [Google Scholar]
- Narayan J. & Bhosle V. M. Phase transition and critical issues in structure-property correlations of vanadium oxide. J. Appl. Phys. 100, 103524 (2006). [Google Scholar]
- Zhou J. et al. VO2 thermochromic smart window for energy savings and generation. Sci. Rep. 3, doi: 10.1038/srep03029 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari M. et al. Temperature dependence of the optical properties of VO2 deposited on sapphire with different orientations. Phys. Rev. B 87, 35142 (2013). [Google Scholar]
- Eyert V. VO2: a novel view from band theory. Phys. Rev. Lett. 107, 16401 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Notes 1-4 and Supplementary References