Abstract
Background. The characteristics, determinants, and potential contribution to transmission of asymptomatic cases of respiratory syncytial virus (RSV) infection have not been well described.
Methods. A cohort of 47 households (493 individuals) in coastal Kenya was recruited and followed for a 26-week period spanning a complete RSV season. Nasopharyngeal swab specimens were requested weekly, during the first 4 weeks, and twice weekly thereafter from all household members, regardless of illness status. The samples were screened for a range of respiratory viruses by multiplex real-time polymerase chain reaction.
Results. Tests on 16 928 samples yielded 205 RSV infection episodes in 179 individuals (37.1%) from 40 different households. Eighty-six episodes (42.0%) were asymptomatic. Factors independently associated with an increased risk of asymptomatic RSV infection episodes were higher age, shorter duration of infection, bigger household size, lower peak viral load, absence of concurrent RSV infections within the household, infection by RSV group B, and no prior human rhinovirus infections. The propensity of RSV spread in households was dependent on symptom status and amount (duration and load) of virus shed.
Conclusions. While asymptomatic RSV was less likely to spread, the high frequency of symptomless RSV infection episodes highlights a potentially important role of asymptomatic infections in the community transmission of RSV.
Keywords: respiratory syncytial virus (RSV), asymptomatic, transmission, households, Kenya
Determination of the incidence of infection is key in studying respiratory disease epidemiology and transmission patterns and, hence, in designing appropriate interventions. However, most studies rely on the clinical presentation of the illness to initiate diagnostic procedures [1]. Unrecognized asymptomatic infections are likely to form important links in transmission of respiratory viruses in the community. Few community studies have attempted diagnosis of respiratory syncytial virus (RSV) infection irrespective of symptoms [2–4]. Consequently, estimates of the proportion of RSV infections that are without symptoms are limited, and their contribution to transmission patterns at the community level has not been characterized. This may be especially important in older children and adults, in whom virus shedding (both amount and duration) is less than for younger individuals [5, 6]. These issues find focus in this article.
To effectively identify infections, as opposed to just disease, intensive surveillance is required. This involves collecting samples at intervals of the order of the infection generation time (ie, days for most respiratory viruses), regardless of illness status, and using highly sensitive diagnostic procedures. Additionally, the procedures must be acceptable to the local population, to maintain compliance over a sufficient period of study. The current work analyses data from a study conducted in rural coastal Kenya that conformed to these requirements. The primary objective was to identify who infects whom and, in particular, who infects young infants in rural households [7]. The current analysis aimed to estimate the prevalence of asymptomatic RSV infections, identify predictors of asymptomatic episodes, and assess the role of asymptomatic cases in the spread of RSV in households.
MATERIALS AND METHODS
The study was undertaken in rural coastal Kenya within the Kilifi Health and Demographic Surveillance System [8]. A cohort of 47 households with young infants and other members (n=493 individuals) were prospectively sampled, regardless of their illness status, weekly over the first 4 weeks and thereafter twice weekly over 1 complete RSV season (December 2009 through June 2010) that lasted 26 weeks, as has been described previously [5, 7]. A household was defined as a group of individuals living in the same compound and with communal cooking arrangements. The RSV season was identified from surveillance conducted by the local county hospital and was defined as a period in which at least 2 RSV cases were detected per week in each of the 2 contiguous weeks and continued as long as these conditions were met [9]. During every home visit, trained field assistants recorded the presence of respiratory symptoms (ie, cough, runny or blocked nose, or difficulty in breathing). The presence of 1 or more of these symptoms defined acute respiratory disease (ARD). Nasopharyngeal swab specimens were collected and screened for RSV (groups A and B), human rhinovirus (hRV), human coronavirus (hCoV-OC43, NL63, and 229E), and adenovirus (AdV), using multiplex real-time polymerase chain reaction (PCR) [10]. For each test target, a cycle threshold (Ct) value of ≤35 was considered positive for virus [11].
Statistical Analysis
All data analyses were done using Stata, version 11.2 (Stata, College Station, Texas). An RSV infection episode was assigned to an individual for whom there was 1 or more RSV-positive sample, with no 2 consecutive positive samples separated by >14 days. The episode period lasted from the day of the first positive sample through the last day within that episode.
The midpoint duration of shedding calculated in a previous publication [5], which assumed that RSV shedding begins midway between the time of the preceding PCR-negative sample and first PCR-positive sample and ends midway between last positive and the next negative specimen of the episode, was used in this study. Episodes whose first sample was positive for both RSV group A and B counted as 1 infection episode and were assigned to either RSV group in the final analysis, depending on the RSV group with the higher peak viral load or longer virus shedding duration. An asymptomatic case was defined as the absence of any ARD symptoms in the course of the RSV infection episode. The timing of the start of symptoms was defined for every episode. The first person(s) in the household to acquire RSV infection, based on the date of sample collection, was referred to as the index case(s). A household RSV outbreak was identified when at least 1 other infection occurred during or within 14 days from the end of the infection episode of an index case in the same household. The outbreaks were confirmed to be of the same strain by comparing partial G gene sequences of the selected virus-positive specimens in the household [7]. The prevalence of asymptomatic RSV episodes was estimated and stratified by a number of characteristics, including age, infecting RSV group, sex, minimum Ct value during the episode, detection of other respiratory viruses before and during the episode, and presence of concurrent RSV infection in the same household. The minimum Ct value over the RSV episode was used as a proxy measure for the peak viral load, on the assumption that Ct values are inversely proportional to the quantity of specific viral nucleic acid in the sample [12]. The Ct values were also converted to arbitrary units (AU), where 1 unit increment was equivalent to a change of 3.3 Ct units and estimated to be a 10-fold increase in viral load [13]. Multilevel mixed-effects logistic regression methods were used to identify predictors for asymptomatic RSV infections and for within-household spread of index infections. Participants were nested within households, defining the grouping structure in the final multilevel model. A P value of ≤.05 was considered statistically significant.
Ethical Considerations
The KEMRI Ethical Review Committee in Kenya and University of Warwick Biomedical Research Ethical Committee in the United Kingdom approved the study. Verbal assent was sought from participants aged <18 years, and written informed consent obtained from all participants or their parents/guardians.
RESULTS
Baseline Characteristics and Sampling
The median age of the 493 study participants was 11.0 years (range, 2 weeks to 92 years), with 11.4%, 28.4%, 62.1%, and 91.3% aged <1, <5, <15, and <40 years of age, respectively, at the start of the study. A total of 221 (44.8%) were male. The median size of the 47 households was 8 members (range, 4–37 members). Additional baseline characteristics are shown in Supplementary Table 1 and elsewhere [5, 7]. The study was active for 178 days, and the median duration of an individual's participation was 171 days (interquartile range [IQR], 163–176 days). Based on the frequency of nasopharyngeal swab specimen collection, we expected 20 868 specimens from the 493 individuals for the period they were under active follow-up. A total of 16 928 swab specimens were collected, representing a sampling compliance of 81.1%. Only 2% of individuals in the study (10 of 493) were never sampled. The missed sampling was mainly due to refusal of swabbing or when the individuals were away during home visits, as previously reported [5, 7]. Of the 483 individuals with at least 1 sample collected, the median number of nasopharyngeal swab specimens collected was 41 (IQR, 30–44).
RSV Infection Detections
Of the 16 928 samples tested, RSV was detected in 537 (3.2%), of whom 231 (43.0%) had infection with RSV group A alone, 287 (53.4%) had infection with group B alone, and 19 (3.5%) had coinfection with groups A and B. The 2 RSV groups cocirculated with peak occurrence in March through May 2010 [7]. Of the 537 RSV-positive specimens, 229 (42.6%) were collected when the individuals had concurrent symptoms of ARD, while of the 16 391 RSV-negative specimens, 3335 (20.3%) were from individuals with concurrent ARD symptoms (P <.0001, by the χ2 test). Of the 483 individuals sampled, 179 (37.1%) had at least 1 RSV infection episode, of these, 83 (46.4%) were male, and the median age at infection was 6.5 years (IQR, 2.4–14.5 years; Table 1). One or more RSV infection episodes were identified in 40 of 47 households (85.1%).
Table 1.
Prevalence of Asymptomatic Respiratory Syncytial Virus (RSV) Infection Episodes, by Various Characteristics Among 483 Individuals in a Prospective Household Study in Rural Kenya
| Characteristic | Evaluated Subjects, No. | Asymptomatic Episode, | P Valuesa,b |
|---|---|---|---|
| No. (%) | |||
| Age at infection, y | |||
| 0 to <1 | 33 | 3 (9.1) | <.0001b |
| 1 to <5 | 52 | 9 (17.3) | |
| 5 to <15 | 73 | 38 (52.1) | |
| 15 to <40 | 38 | 29 (76.3) | |
| ≥40 | 9 | 7 (77.8) | |
| Sex | |||
| Female | 116 | 58 (50.0) | .008 |
| Male | 89 | 28 (31.5) | |
| Detection of other respiratory viruses during the RSV infection episode | |||
| None | 124 | 62 (50.0) | Reference |
| Adenovirus | 23 | 10 (43.5) | .566 |
| Human coronavirus | 17 | 6 (35.3) | .255 |
| Human rhinovirus | 19 | 3 (15.8) | .005 |
| Mixed viruses (≥2) | 22 | 5 (22.7) | .018 |
| During an RSV outbreak in household | |||
| No | 42 | 26 (61.9) | .003 |
| Yes | 163 | 60 (36.8) | |
| Participant in school | |||
| No | 137 | 46 (33.6) | .001 |
| Yes | 68 | 40 (58.8) | |
| Order of the individual infection episode | |||
| First | 179 | 72 (40.2) | .188 |
| Secondc | 26 | 14 (53.9) | |
| Infecting RSV group(s) | |||
| Group A | 81 | 34 (42.0) | Reference |
| Group B | 110 | 50 (45.5) | .771 |
| Groups A and B | 14 | 2 (14.3) | .072 |
| Minimum Ct value during the infection episode | |||
| <20 | 40 | 4 (10.0) | <.0001b |
| 20–<25 | 76 | 24 (31.6) | |
| 25–<30 | 52 | 32 (61.5) | |
| 30–<35 | 37 | 26 (70.3) | |
Abbreviation: Ct, cycle threshold.
a For comparison of the percentages of asymptomatic episodes.
b By the nonparametric test for trend applies for age groups and minimum Ct value categories. The rest of the P values are from chi-square test.
c Includes the second and the third repeat RSV infections per individual.
RSV Infection Episodes
A total of 205 RSV infection episodes were determined in the 179 infected individuals: 155 individuals had 1 episode, 22 had 2 episodes, and 2 had 3 episodes. RSV group A was identified in 81 infection episodes and RSV group B in 110, while 14 episodes had both RSV group A and B. Concurrent ARD symptoms were observed in 119 infection episodes (58.0%). The main ARD symptoms observed were running or blocked nose (117 episodes [98.3%]) and cough (89 [74.8%]). Difficulty breathing was uncommon (6 episodes [5.0%]). Fever (reported or measured; defined as a axillary temperature of >38.0°C) was documented in 17 (14.3%) of the symptomatic episodes. Of those individuals with symptomatic episodes, 19 (16.0%) sought medical care at a health facility, but none was hospitalized.
Predictors of Asymptomatic RSV Infection Episodes
Of the 205 RSV infection episodes, 86 (42.0%) were asymptomatic. For the symptomatic episodes, 41 (34.5%) had symptom onset after virus was detected, with a median interval from virus detection to the start of symptoms (ie, at the next 2 sampling times) of 4 days (IQR, 3–6 days).
Age at infection, duration of infection episode, household size, peak viral load, prevalence of concurrent RSV infections within the household, infection by RSV group B, and prior hRV infections were independent predictors of asymptomatic RSV infection episodes (Table 2, Figures 1 and 2, and Supplementary Table 2). The association of age in years and odds of asymptomatic infections was nonlinear (quadratic), with an initial increase of 20% per year (adjusted odds ratio [aOR], 1.20; 95% confidence interval [CI], 1.09–1.33) but diminishing by 0.002% per age squared (aOR, 0.998; 95% CI, .996–.999). Individuals with RSV group B infections were 2 times more likely to be asymptomatic than those with group A infections (aOR, 2.40; 95% CI, .998–5.75). The odds of an asymptomatic episode declined by 35% for every 10-fold increase in the peak viral load (aOR, 0.65; 95% CI, .46–.91). RSV infection episodes linked to an hRV infection in the preceding 14 days were less likely to be asymptomatic than those with no prior detections of hRV infection (aOR, 0.31; 95% CI, .12–.83). Prior AdV or hCoV infections or any concurrent virus infection were not significant predictors of asymptomatic RSV infection episodes (Supplementary Table 2). For every 1% increase in the number of individuals with concurrent RSV infection in a household, the odds of asymptomatic infections declined by 2% (aOR, 0.98; 95% CI, .96–1.00), while for a unit increase in household size, the odds of asymptomatic episodes increased by 7% (aOR, 1.07; 95% CI, 1.02–1.13). The association with household size was not statistically significant after excluding the largest household (n = 37 members), whose 15 RSV group A–infected members (60% of the household total) were asymptomatic (Supplementary, Table 3). This household was atypical in composition and size (with 12 and 8 children aged <5 years and 5–15 years, respectively). The risk of clinical RSV infection did not differ by sex after adjustment for potential confounders. No interactions were identified.
Table 2.
Predictors of Asymptomatic Episodes of Respiratory Syncytial Virus (RSV) Infection From a Rural Kenyan Prospective Household Study
| Predictor | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Age | ||
| Per y | 1.20 (1.09–1.33) | <.001 |
| Per y squared | 0.998 (.996–.999) | .011 |
| Household size, per unit size | 1.07 (1.02–1.13) | .003 |
| Duration of RSV shedding, per d | 0.90 (.82–.98) | .010 |
| Virus load, per unit log10 AU | 0.65 (.46–.91) | .001 |
| Infecting RSV group | ||
| Group A | 1.00 (reference) | |
| Group B | 2.40 (.998–5.745) | .050 |
| Percentage of individuals in household infected, per 1% increase | 0.98 (.958–.999) | .042 |
| Detection of hRV during 14 d before RSV episode | ||
| No hRV | 1.00 (reference) | |
| hRV | 0.32 (.12–.83) | .020 |
Abbreviations: AU, arbitrary units; CI, confidence interval; hRV, human rhinovirus; OR, odds ratio.
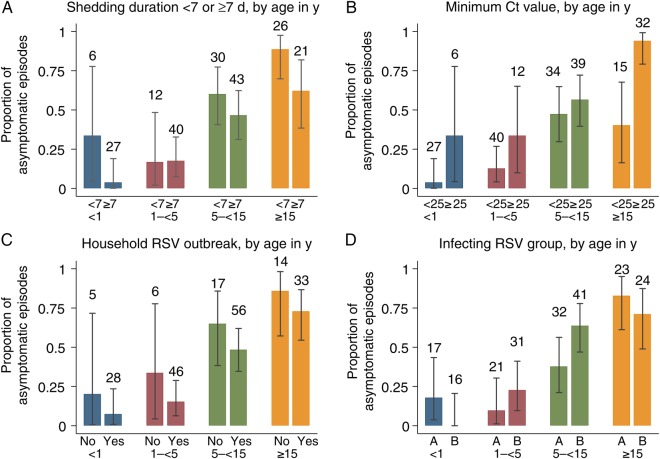
Figure 1.
Proportion of asymptomatic episodes of respiratory syncytial virus (RSV) infection in a household cohort study in Kenya, by age in years (A), shedding duration (B), minimum cycle threshold (Ct) values (C), and percentage of individuals with concurrent RSV infection in the household (D). Data are mean proportions (95% confidence intervals). Numbers of episodes per category appear above each bar.
Figure 2.
Proportion of asymptomatic respiratory syncytial virus (RSV) infection episodes in a household cohort study in Kenya, by age in years, stratified by shedding duration (A), minimum threshold cycle (Ct) value (B), presence of other individuals with concurrent RSV infection in the household (C), and infecting RSV group (D). Numbers of episodes per category appear above each bar.
Role of Symptomatic Status in Transmission of RSV Infections in Households
Of the 205 RSV infection episodes, 84 (41.0%) were index cases. Thirty-seven index cases (44.0%) were asymptomatic. Within-household spread of RSV infection occurred in 10 asymptomatic index cases (27.0%), compared with 29 symptomatic index cases (61.7%; P<.002, by the χ2 test). After accounting for differences in age and household size, the odds of RSV spread upon introduction into the household was about 5 times in the symptomatic index cases relative to asymptomatic cases (aOR, 4.73; 95% CI, 1.21–18.53; Table 3). The duration of RSV shedding was longer in symptomatic index cases relative to that in asymptomatic index cases (mean, 13.3 days [95% CI, 10.67–15.89 days] vs 6.8 days [95% CI, 5.3–8.4 days]; P = .0001, by the 2-sided t test; Figures 3 and 4 and Supplementary Figure 1). The corresponding comparison of mean peak viral load was log10 5.1 AU (9% CI, 4.7–5.5 AU) for the symptomatic index case, compared with log10 3.7 AU (95% CI, 3.3–4.2 AU) for the asymptomatic index cases (P<.0001, by the 2-sided t test). The risk of within-household spread was influenced by virus shedding duration and peak viral load (aOR, 1.21 [95% CI, 1.06–1.37] and 2.24 [95% CI, 1.21–4.2], respectively). No effect modification between shedding duration and load was observed (P = .9083 for interaction, by the likelihood ratio test) on the odds of within-household spread.
Table 3.
Multilevel Mixed Effects Logistic Regression Analysis of Respiratory Syncytial Virus Spread Within Households
| Predictor | Adjusted ORa (95% CI) | P Value |
|---|---|---|
| Clinical status | ||
| No ARD | 1 (reference) | |
| ARD | 4.73 (1.21–18.53) | .026 |
| Duration, per d | 1.21 (1.06–1.37) | .005 |
| Viral load, per unit log10 AU | 2.24 (1.21–4.17) | .011 |
Abbreviations: ARD, acute respiratory disease; AU, arbitrary unit; CI, confidence interval; OR, odds ratio.
a Values adjusted for age and household sizes.
Figure 3.
Association of respiratory syncytial virus (RSV) shedding duration (A) and peak load (B) with age in years among index cases in a household cohort study in Kenya, stratified by clinical status. Acute respiratory disease (ARD) presence is denoted by red, ARD absence is denoted by blue, and cases linked with virus spread are denoted by green. Abbreviation: AU, arbitrary units.
Figure 4.
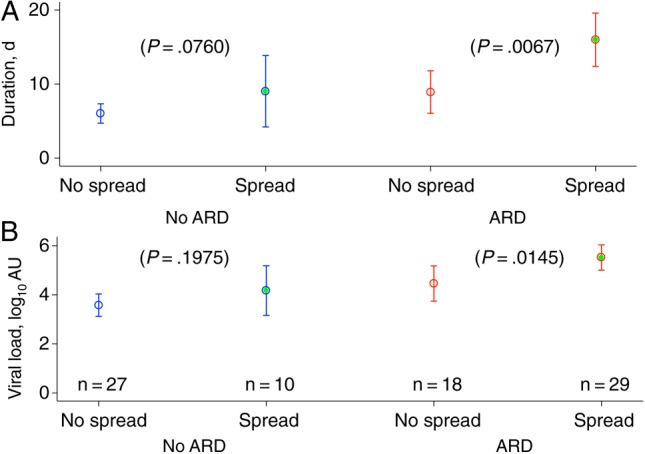
Duration (A) and peak load (B) of respiratory syncytial virus (RSV) shedding in a household cohort study in Kenya, by clinical and household transmission status. Acute respiratory disease (ARD) presence is denoted by red, ARD absence is denoted by blue, and cases linked with virus spread are denoted by green. Data are mean values (95% confidence intervals). P values were calculated by 2-sided t tests comparing the mean values in index cases linked with spread, stratified by clinical status. Abbreviation: AU, arbitrary units.
DISCUSSION
We report the prevalence of asymptomatic RSV infections from a study following 47 households with 493 individuals over the 26 weeks of an RSV epidemic. Nasopharyngeal swab specimens were collected twice weekly regardless of symptoms and screened for a range of respiratory viruses, including RSV. Over 40% of the 205 RSV infection episodes were asymptomatic. The probability of symptomless RSV infection increased with age, peaking at about 40 years, and declined in older ages (Supplementary Figure 2). This accords with published observations that the risk of severe disease from RSV infection decreases with age [14–17] and increases in elderly individuals [18]. Evidence from experimental challenge and epidemiological studies also support these observations and provide possible mechanisms [9, 16, 19, 20]. An experimental study using RSV A2 strain in young adult volunteers, together with infection being determined by virus isolation, reported a greater risk of infection in individuals administered a high RSV inoculum, compared with those administered a low dose (57% vs 36%), with asymptomatic infections occurring in 63% and 40% of the infections, respectively [19]; individuals who were infected had statistically lower serum neutralizing antibody titers than uninfected individuals. In another experimental infection study involving 13 adult volunteers, with low neutralizing antibody titers, 12 had detectable viral shedding and respiratory illness when administered a high dose of RSV A2 inoculum [19]. Even though these experiments used a prototype virus, rather than the wild-type virus as in the current study, their findings suggest that acquired immunity against RSV and physiological development of the airways could play an important role in reducing the severity of RSV-associated respiratory disease. Serological studies show that antibody produced during RSV infection in young children and elderly individuals is ineffective and associated with an inappropriate cytokine response and excess inflammation of the airways [21], partly explaining the increased disease severity in these age groups. RSV has been identified as an important pathogen in older ages [22].
In a US family study by Hall et al that used a similar intensive specimen collection regimen but detection by virus culture [2], the prevalence of asymptomatic infection was low in families exposed to RSV, which could be due to exposure to large inoculum from close contacts within the household. Only 5% of infected individuals (2 of 39) had asymptomatic infection in the US study. In a study of infants admitted with RSV to intensive care units, Crowcroft et al reported a prevalence of asymptomatic infection, as determined by PCR of pernasal swabs, of 48% and 49% in the siblings and parents, respectively [23]. This suggests that the study by Hall et al [2] might have missed subclinical infections, particularly in older children and adults, owing to reduced sensitivity of the viral culture in asymptomatic cases. Alternatively, it is possible that in the present study, virus shed from asymptomatic cases was not viable, resulting in an overestimation of subclinical infections. We show that infection episodes with low peak viral load or absence of concurrent RSV infections in the household were associated with a reduced risk of ARD. In general, it appears that asymptomatic RSV infections are likely in individuals with previous exposure to RSV (and with concomitant high levels of neutralizing antibodies), such as older children and young adults [19].
Our findings add to the debate on the differential virulence of RSV strains [24]. We found that group B RSV infections were associated with higher risk of asymptomatic infection than group A infections. This association had a stronger statistical significance when we excluded the atypical household (Supplementary Table 3). The consistency of this finding with results from published studies [25, 26] supports the hypothesis that group B RSV infections are less severe than group A RSV infections in the community. Walsh et al observed that hospitalized infants without underlying medical conditions who were infected with RSV group B rarely required respiratory support, compared with those infected with group A (1% vs 12%) [25]. The individual attack rates for groups A and B were similar (48.0% and 56.4%, respectively), and the preceding epidemic was predominated by group A (Nokes et al, unpublished data). This precludes that any differential effect of infection severity was due to dominance of one group in the community. However, other studies have reported conflicting results on the relationship of RSV groups to disease severity [27–31]. Most of these are hospital studies that used less sensitive virus detection methods (ie, viral culture and immunofluorescence techniques) or collected specimens upon report of respiratory illness.
We have previously reported an association of the presence of other respiratory viruses (hRV, AdV, and hCoV) just prior to RSV infection with reduced shedding duration and an increase in shedding duration if these respiratory viruses are codetected with RSV [5]. In the current study, hRV infection <14 days before the start of RSV infection was associated with a reduced risk of asymptomatic infection. However, no significant association was observed between the presence of other respiratory viruses (AdV and hCoV) before or during the RSV episode and the risk of symptomless RSV infection. This association between RSV and hRV infections is more likely to be mediated through nonspecific cross-reactivity of innate immunity, such as the physiological status of respiratory epithelia following virus infection. Additional investigation is required to explore these effects.
About one fourth (27%) of the asymptomatic index cases were linked with household spread, compared with 61% for the symptomatic index cases. We observed a strong positive correlation between the markers of RSV shedding (ie, duration and viral load) over the infection period (Figure 4) and the probability of spread. Longer RSV infection episodes or those with a higher peak viral load were more likely to be symptomatic than their counterparts. The presence of symptoms and correlates of the amount of virus shedding (ie, duration and load) were independently associated with the risk of within-household spread among the index cases, after adjustment for age and household size. These findings taken together indicate that symptomatic individuals were more infectious and, thus, better transmitters than those without symptoms. The correlation of ARD and the amount of virus shedding suggests that vaccines that decrease viral shedding severity upon exposure to RSV, even if they do not reduce the risk of infection itself, could diminish virus transmission in the population. However, 34% of the symptomatic RSV episodes had the illness appear on average 4 days after first detection of the virus. If individuals change their behavior as a result of symptoms, such as by reducing their contacts, the period of shedding before symptoms appear or the prevalence of asymptomatic infections might have an accentuated role in transmission of RSV in the population. Furthermore, the high frequency of asymptomatic RSV cases observed in this study, coupled with the nonnegligible infectivity of the asymptomatic state, suggests that these individuals could play an important role in the transmission of RSV in a population.
One limitation of the study relates to the use of a Ct value of ≤35 to define virus-positive samples. The detection of RNA does not necessarily correlate with the presence of infectious virus. In the future, the Ct value associated with the potential to infect must be established, and assessment of the viability (infectiousness) of the viruses detected in asymptomatic infections is warranted. However, one fourth of the index cases linked with household spread were without symptoms in the current study. Despite the intensive sampling regimen, RSV infections with low viral load or short duration of shedding (<3 days), especially the asymptomatic cases, might have been missed. Our estimate of the prevalence of asymptomatic infections might be an underestimate, even though use of sensitive PCR screening methods might have diminished the detection bias.
In conclusion, the high prevalence of asymptomatic RSV infection highlights the importance of detecting respiratory viruses, regardless of illness status, to gain a nearly full understanding on the virus epidemiology in the community. Our results show that a significant proportion of RSV infections are asymptomatic; thus, the amount of the virus circulating in the community would be underestimated if the contribution of asymptomatic infections is overlooked. We provide empirical data that will be useful in developing a realistic RSV transmission model for assessing the impact of RSV vaccines and other interventions on viral circulation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the Viral Epidemiology and Control group at KEMRI–Wellcome Trust Research Programme in Kilifi, Kenya, for their critique of an earlier version of the manuscript; and the study field workers and laboratory technicians, for their commitment in the sample collection and testing, respectively. The article was published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. This work was supported by the Wellcome Trust (grants 090853, 084633, 102975, and 077092).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Monto AS, Malosh RE, Petrie JG, Thompson MG, Ohmit SE. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over three surveillance seasons. J Infect Dis 2014; 210:1792– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR Jr. Respiratory syncytial virus infections within families. N Engl J Med 1976; 294:414–9. [DOI] [PubMed] [Google Scholar]
- 3.Cowling BJ, Muller MP, Wong IO et al. Alternative methods of estimating an incubation distribution: examples from severe acute respiratory syndrome. Epidemiology 2007; 18:253–9. [DOI] [PubMed] [Google Scholar]
- 4.Cowling BJ, Chan KH, Fang VJ et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med 2010; 362:2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munywoki PK, Koech DC, Agoti CN et al. Influence of age, severity of infection, and co-infection on the duration of respiratory syncytial virus (RSV) shedding. Epidemiol Infect 2014:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casiano-Colon AE, Hulbert BB, Mayer TK, Walsh EE, Falsey AR. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol 2003; 28:169–74. [DOI] [PubMed] [Google Scholar]
- 7.Munywoki PK, Koech DC, Agoti CN et al. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis 2014; 209:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott JA, Bauni E, Moisi JC et al. Profile: the Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol 2012; 41:650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nokes DJ, Ngama M, Bett A et al. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis 2009; 49:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammitt LL, Kazungu S, Morpeth SC et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis 2012; 54(suppl 2):S190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed A, Engelberts MF, Boer KR, Ahmed N, Hartskeerl RA. Development and validation of a real-time PCR for detection of pathogenic Leptospira species in clinical materials. PLoS One 2009; 4:e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bustin SA, Benes V, Nolan T, Pfaffl MW. Quantitative real-time RT-PCR--a perspective. J Mol Endocrinol 2005; 34:597–601. [DOI] [PubMed] [Google Scholar]
- 13.Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc 2006; 1:1559–82. [DOI] [PubMed] [Google Scholar]
- 14.Henderson F, Collier A, Clyde WJ, Denny F. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med 1979; 300:530–4. [DOI] [PubMed] [Google Scholar]
- 15.Nokes DJ, Okiro EA, Ngama M et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis 2008; 46:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
- 17.Ohuma EO, Okiro EA, Ochola R et al. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol 2012; 176:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000; 13:371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Res 2004; 63:191–6. [DOI] [PubMed] [Google Scholar]
- 20.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 21.Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:211–31. [DOI] [PubMed] [Google Scholar]
- 22.Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets 2012; 12:98–102. [DOI] [PubMed] [Google Scholar]
- 23.Crowcroft NS, Zambon M, Harrison TG, Mok Q, Heath P, Miller E. Respiratory syncytial virus infection in infants admitted to paediatric intensive care units in London, and in their families. Eur J Pediatr 2008; 167:395–9. [DOI] [PubMed] [Google Scholar]
- 24.Kneyber MC, Brandenburg AH, Rothbarth PH, de Groot R, Ott A, van Steensel-Moll HA. Relationship between clinical severity of respiratory syncytial virus infection and subtype. Arch Dis Child 1996; 75:137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis 1997; 175:814–20. [DOI] [PubMed] [Google Scholar]
- 26.McConnochie KM, Hall CB, Walsh EE, Roghmann KJ. Variation in severity of respiratory syncytial virus infections with subtype. J Pediatr 1990; 117:52–62. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh ED, De Silva LM, Oates RK. Clinical severity of respiratory syncytial virus group A and B infection in Sydney, Australia. Pediatr Infect Dis J 1993; 12:815–9. [DOI] [PubMed] [Google Scholar]
- 28.Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995; 126:212–9. [DOI] [PubMed] [Google Scholar]
- 29.Hendry RM, Talis AL, Godfrey E, Anderson LJ, Fernie BF, McIntosh K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J Infect Dis 1986; 153:291–7. [DOI] [PubMed] [Google Scholar]
- 30.Monto AS, Ohmit S. Respiratory syncytial virus in a community population: circulation of subgroups A and B since 1965. J Infect Dis 1990; 161:781–3. [DOI] [PubMed] [Google Scholar]
- 31.Tsutsumi H, Onuma M, Nagai K, Yamazaki H, Chiba S. Clinical characteristics of respiratory syncytial virus (RSV) subgroup infections in Japan. Scand J Infect Dis 1991; 23:671–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.