Abstract
Background. Hemoglobin C trait, like hemoglobin S trait, protects against severe malaria in children, but it is unclear whether hemoglobin C trait also protects against uncomplicated malaria. We hypothesized that Malian children with hemoglobin C trait would have a lower risk of clinical malaria than children with hemoglobin AA.
Methods. Three hundred children aged 0–6 years were enrolled in a cohort study of malaria incidence in Bandiagara, Mali, with continuous passive and monthly active follow-up from June 2009 to June 2010.
Results. Compared to hemoglobin AA children (n = 242), hemoglobin AC children (n = 39) had a longer time to first clinical malaria episode (hazard ratio [HR], 0.19; P = .001; 364 median malaria-free days vs 181 days), fewer episodes of clinical malaria, and a lower cumulative parasite burden. Similarly, hemoglobin AS children (n = 14) had a longer time to first clinical malaria episode than hemoglobin AA children (HR, 0.15; P = .015; 364 median malaria-free days vs 181 days), but experienced the most asymptomatic malaria infections of any group.
Conclusions. Both hemoglobin C and S traits exerted a protective effect against clinical malaria episodes, but appeared to do so by mechanisms that differentially affect the response to infecting malaria parasites.
Keywords: cohort study, hemoglobin C, hemoglobin S, malaria, Mali, Plasmodium falciparum
Sickle hemoglobin trait protects against severe and uncomplicated Plasmodium falciparum malaria [1–3], but at a cost, in that hemoglobin (Hb) SS disease is associated with significant morbidity and mortality [4]. In contrast, hemoglobin C trait, which is primarily found in West Africa [5], appears to offer protection against severe malaria [6], but hemoglobin CC disease does not have the degree of morbidity and mortality associated with hemoglobin SS disease [7]. Early work demonstrating the protective effects of hemoglobin C trait against severe malaria involved studies of the predominantly Dogon population in east-central Mali [8]. Subsequently, a case-control study in Burkina Faso found that hemoglobin C trait may be protective against both severe and uncomplicated forms of malaria in children [9]. In contrast, a recent longitudinal study of children less than 2 years of age in Ghana found that hemoglobin S trait protected against uncomplicated malaria and anemia (hemoglobin <7.5 g/dL), while hemoglobin C trait did not protect against either [10]. A study in a non-Dogon population in Mali has indicated that hemoglobin S trait is associated with longer time to first clinical malaria episode and fewer clinical malaria episodes; no such association was found for hemoglobin C trait, which is infrequent in non-Dogon ethnic groups in Mali [11].
The exact mechanism of how hemoglobin C and S confer protection against clinical malaria is unclear, but a leading hypothesis is that both traits alter the display of Plasmodium falciparum erythrocyte membrane protein-1 (PfEMP1), a family of parasite proteins expressed on the erythrocyte surface that mediate binding to endothelial receptors. Parasitized erythrocytes from individuals with hemoglobin C and hemoglobin S traits have a reduced ability to attach to host endothelial receptors, potentially limiting the ability of these cells to sequester in host tissues and evade the immune system [12, 13].
We conducted a longitudinal study of malaria incidence in a predominantly Dogon population of Bandiagara, Mali, with 16% prevalence of hemoglobin C trait and 3% prevalence of hemoglobin S trait [8, 14, 15]. We hypothesized that there would be a longer time to first clinical malaria episode in subjects with hemoglobin C or S trait compared to subjects with no hemoglobinopathies (hemoglobin AA). We also hypothesized that subjects with hemoglobin C or S trait would experience both fewer episodes of parasitemia and lower cumulative parasite burden than the control group, but have a higher incidence of anemia, supposing accelerated senescence of HbAC and HbAS erythrocytes compared to HbAA erythrocytes with falciparum infection.
METHODS
Study Setting
The study was conducted at the Bandiagara Malaria Project research clinic, adjacent to the district hospital in Bandiagara, a town of 13 634 inhabitants (2002 census) in the Dogon Country in east-central Mali. Bandiagara is relatively dry, with a mean annual rainfall of 600 mm. Anopheles gambiae is the principal malaria vector. Malaria transmission is highly seasonal, with minimal transmission at the height of the dry season in March; less than 1 infected mosquito bite per person per month as the transmission season starts and ends in June and December, respectively; and a peak of up to 60 infected bites per person per month [16, 17]. P. falciparum represents 97% of malaria infections with 3% due to P. malariae and rare infections with P. ovale. The malaria burden is heavy: children average 1.3 to 2.0 clinical malaria episodes per year [18], and severe malaria afflicts 1 in 50 children aged less than 6 years each year [17]. Older children and adults are relatively protected against malaria disease, but remain susceptible to infection.
Participants
Three hundred children aged 0–6 years from all 9 defined Bandiagara neighborhoods (quartiers) were enrolled in a prospective, longitudinal cohort study of malaria infection from June 2009 to June 2010 on a first-come, first-served basis after obtaining community consent and releasing a radio announcement [18]. The age-specific incidence of malaria disease was measured by continuous passive and monthly active parasitological and clinical surveillance. Uncomplicated malaria was treated with artemisinin-based combination treatment (artesunate/amodiaquine or artemether/lumefantrine) following Mali National Malaria Control Program guidelines. Outcomes measured included malaria disease episodes resulting in clinic visits and/or hospitalizations, and asymptomatic malaria infections. Monthly malaria smears were read retrospectively to diagnose asymptomatic malaria episodes.
The protocol was approved by institutional review boards of the Faculty of Medicine, Pharmacy and Dentistry, Bamako, Mali, and the University of Maryland, Baltimore. Written informed consent was obtained for screening and enrollment in the study, including separate informed consent obtained after enrollment for α-thalassemia testing.
Malaria Case Definitions
A clinical episode of malaria was defined as the presence of fever (axillary temperature of 37.5°C or higher) with an asexual P. falciparum density of at least 2500 parasites per cubic millimeter of blood. Analyses were repeated using less strict definitions of malaria: without a minimum parasitemia threshold and also without a fever (but with the presence of malaria symptoms such as body aches, headache, or abdominal pain and treatment-seeking).
Each individual malaria episode, either symptomatic or asymptomatic, that was detected fewer than 7 days after another episode of either type was excluded from the analysis. Parasitemia counts measured at these excluded points were, however, included in cumulative estimates of parasite burden.
Laboratory Procedures
Hemoglobin type was determined by high-performance liquid chromatography (D-10 instrument; Bio-Rad). Restriction fragment-length polymorphism analysis was used to identify the (A-) allele of the glucose-6-phosphate dehydrogenase (G6PD) deficiency, as previously described [19]. Parasitemia was determined from Giemsa-stained thick smears by counting parasites against 300 leukocytes for parasite count/µL, using a conversion factor of 25 based on an assumed leukocyte count of 7500/µL. Hemoglobin determinations were made using HemoCue Hb 201+ hemoglobin analyzers (HemoCue Inc, Cypress, California).
Typing for the 3.7-kb deletional determinant of α-thalassemia was determined by nested polymerase chain reaction (PCR) [11] modified for dried-blood spots. DNA was extracted from dried-blood spots using the Qiagen QIAamp 96 DNA Blood Kit (Valencia, California). The primary PCR was performed using TaKaRa's LA Taq Polymerase (Shiga, Japan) following the manufacturer's directions. Each 25 µL primary PCR reaction mixture contained 1 µL extracted DNA, 2.5 µL of 10× LA PCR Buffer II, 0.05 µM of each primer, 0.4 µM deoxynucleotide triphosphates, and 5 U Taq Polymerase. In contrast to [11], the primary PCR was split into 2 singleplex reactions: one detecting the deletion, the other detecting the wild-type. The secondary reaction mixture differed from the primary in that 1 µL of primary PCR product (without any dilution) was added instead of DNA template. The cycling conditions were 95°C for 5 minutes followed by 35 cycles for the primary and 40 seconds for the secondary of 97°C for 45 seconds, 60°C for 75 seconds, and 72°C for 150 seconds. The final elongation step was 72°C for 5 minutes. The product was resolved on a 0.8% agarose gel and viewed by ethidium bromide staining.
Statistical Analysis
Time to First Malaria Episode
We estimated the hazard ratio (HR) for the first/only episode of malaria in each group (log-rank test to compare hemoglobin AA genotype vs AS vs AC). HRs were estimated using a standard Cox regression model initially unadjusted for other covariates. If significant differences were found, the Cox regression was also calculated, taking into account the effects of subject G6PD status, α-thalassemia status, age, sex, ethnic group, and neighborhood.
Total Number of Malaria and Anemia Episodes
Differences between hemoglobin groups in malaria episode counts were analyzed with a Poisson regression model, with incidence rate ratios (IRRs) reported. Group differences were detected with a Kruskal–Wallis test. Pairwise comparisons used a generalized estimating equation model to account for within-subject correlations. Significance was first calculated considering only hemoglobin group differences (“unadjusted,” with crude IRR reported). When unadjusted differences were significant, hemoglobin group differences were also calculated taking into account the effects of subject G6PD status, α-thalassemia status, age, sex, ethnic group, and neighborhood (reported as “adjusted”).
Differences in parasitemia between hemoglobin groups (Table 5) were calculated with a generalized estimating equation to account for within-subject correlations in parasitemia measurements.
Table 5.
Malaria Episode Counts and Median Parasitemia Per Episode
| Symptoms, Parasitemia >2500/µL, Fever | Median Parasitemia (parasites/µL) | Symptoms, Any Parasitemia, Fever | Median Parasitemia (parasites/µL) | Symptoms, Any Parasitemia | Median Parasitemia (parasites/µL) | Asymptomatic | Median Parasitemia (parasites/µL) | |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin AA (n = 242) | 120 | 27 975 (11 807–66 882) | 129 | 25 650 (10 875–63 000) | 152 | 20 713 (6982–59 332) | 155 | 11 683 (846–39 088) |
| Hemoglobin AC (n = 39) | 8 | 22 669 (9150–53 825) | 14 | 7225a (875–28 725) | 18 | 7225 (819–28 725) | 20 | 2700 (522–16 957) |
| Hemoglobin AS (n = 14) | 2 | 9532 (3613–15 450) | 3 | 3613 (1200–15 450) | 7 | 1050b,c (700–3613) | 5 | 1050 (988–3613) |
Parasitemia reported as median (lower quartile – upper quartile).
a Significantly different from hemoglobin AA in the generalized estimating equation analysis (unadjusted P = .044; P = .018 adjusted).
b Significantly different from hemoglobin AA in the generalized estimating equation analysis (unadjusted P = .011; P = .005 adjusted).
c Significantly different from hemoglobin AC in the generalized estimating equation analysis (unadjusted P = .036; P = .019 adjusted).
Cumulative Parasite Burden
A cumulative total area-under-the-curve (AUC) of P. falciparum parasite burden was calculated for each hemoglobin group. Cumulative parasite density was estimated for each individual by measuring total AUC for the entire study period, as described previously [20] using smear results from monthly clinic visits and sick clinic visits. The results for each subject group were divided by the total number of subjects to adjust for differences in group sizes. All recorded episodes of parasitemia were included irrespective of presence or absence of symptoms of malaria; parasite density was assumed to decline linearly to zero 3 days after a treated malaria episode [20] unless there was a malaria smear reading performed that indicated otherwise. AUC was compared between hemoglobin groups using a continuity-adjusted Wilcoxon–Mann–Whitney test.
All P values presented are 2-sided. Statistical analyses were primarily conducted using SAS 9.2.
RESULTS
Clinical Characteristics
Demographic analysis of the 300 enrolled children found that they derived from 10 different ethnic groups; 75.3% were Dogon. There were 242 hemoglobin AA children, 39 hemoglobin AC children, 14 hemoglobin AS children, 3 hemoglobin SC children, and 2 hemoglobin CC children (Table 1). Thirty-five children had the G6PD*A- genotype, including 10.7% of Hb AA children, 10.3% of Hb AC children, and 35.7% of Hb AS children. Alpha-thalassemia testing consent was obtained for 250 children; of these, samples from 240 achieved results. A minority of children was heterozygous (−α/αα) for α-thalassemia: 25% of Hb AA children, 38.7% of Hb AC children, and 27.3% of Hb AS children. Only 5 children were homozygous (−α/–α) for α-thalassemia. Among Dogons, 14.6% had hemoglobin AC, while 8.1% of non-Dogons had hemoglobin AC.
Table 1.
Analysis of Baseline Characteristics of Subjects Stratified by Hemoglobin Type
| Hb SC (n = 3) | Hb CC (n = 2) | Hb AA (n = 242) | Hb AC (n = 39) | Hb AS (n = 14) | |
|---|---|---|---|---|---|
| Number male (%) | 1 (33.3) | 1 (50.0) | 113 (46.7) | 20 (51.2) | 6 (42.9) |
| Mean age at enrollment ± SD (months) | 44.3 ± 31.5 | 51.7 ± 21.7 | 43.8 ± 21.6 | 44.6 ± 22.2 | 48.7 ± 25.9 |
| G6PD A- | 0 | 0 | 26 (10.7%) | 4 (10.3%) | 5 (35.7%) |
| α-thalassemiaa | |||||
| Wild-type (αα/αα) | 1 (50%) | 1 (50%) | 134 (72.8%) | 19 (61.3%) | 7 (63.6%) |
| Heterozygous (−α/αα) | 1 (50%) | 46 (25%) | 12 (38.7%) | 3 (27.3%) | |
| Homozygous (−α/−α) | 1 (50%) | 4 (2.2%) | 0 | 1 (9.1%) | |
| Ethnicity | |||||
| Dogon | 1 (33.3%) | 2 (100.0%) | 182 (75.2%) | 33 (84.6%) | 8 (57.1%) |
| Non-Dogon | 2 (66.7%) | 0 | 60 (24.8%) | 6 (15.4%) | 6 (42.9%) |
| Dropped out/lost to follow-up | 0 | 0 | 17 | 3 | 1 |
Abbreviations: G6PD, glucose-6-phosphate-dehydrogenase; Hb, hemoglobin.
a α-thalassemia testing consent was obtained for 250 children; of these, samples from 240 achieved results.
Twenty-one subjects dropped out or were lost to follow-up (Table 1), including 1 Hb AA subject who died from cerebral malaria. Data collected until the point of drop-out for each subject were included in the analysis.
Time to First Malaria Episode
Children in the 3 hemoglobin groups differed in time to first clinical malaria episode (log-rank test, degrees of freedom [DF] = 2; P < .001). Children with hemoglobin AC had a longer time to first clinical malaria episode than children with hemoglobin AA (Table 2; Figure 1A). Similarly, children with hemoglobin AS had a longer time to first clinical malaria episode than children with hemoglobin AA. Children with hemoglobin AS and AC did not differ in this regard (HR: 0.73, 95% confidence interval [CI], .16–1.24; P = .69 unadjusted), but there may have been insufficient power to detect this, given the low number of children with hemoglobin AS.
Table 2.
Time to First Malaria Episode
| Clinical Malaria Episode |
Clinical Episode With Any Parasitemia |
Clinical Episode With Any Parasitemia, no Fever Criteria |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (Unadjusted) | HR (Adjusted) | Median Malaria-Free Days | HR (Unadjusted) | HR (Adjusted) | Median Malaria-Free Days | HR (Unadjusted) | HR (Adjusted) | Median Malaria-Free Days | |
| Hb AA (n = 242) | 1 | 1 | 181 | 1 | 1 | 168 | 1 | 1 | 142 |
| 0.32 | 0.19 | 0.55 | 0.32 | 0.60 | 0.41 | ||||
| Hb AC (n = 39) | (0.15, 0.65) | (0.07, 0.49) | 364 | (0.32, 0.96) | (0.15, 0.67) | 364 | (0.37, 0.98) | (0.21, 0.77) | 362 |
| (P = .002) | (P = .001) | (P = .035) | (P = .003) | (P = .043) | (P = .006) | ||||
| 0.23 | 0.15 | 0.31 | 0.25 | 0.75 | |||||
| Hb AS (n = 14) | (0.06, 0.92) | (0.03, 0.69) | 364 | (0.10, 0.98) | (0.07, 0.88) | 364 | (0.35, 1.60) | … | 157.5 |
| (P = .037) | (P = .015) | (P = .046) | (P = .031) | (P = .46) | |||||
Hazard ratios are reported with 95% confidence intervals.
Abbreviations: Hb, hemoglobin; HR, hazard ratio.
Figure 1.
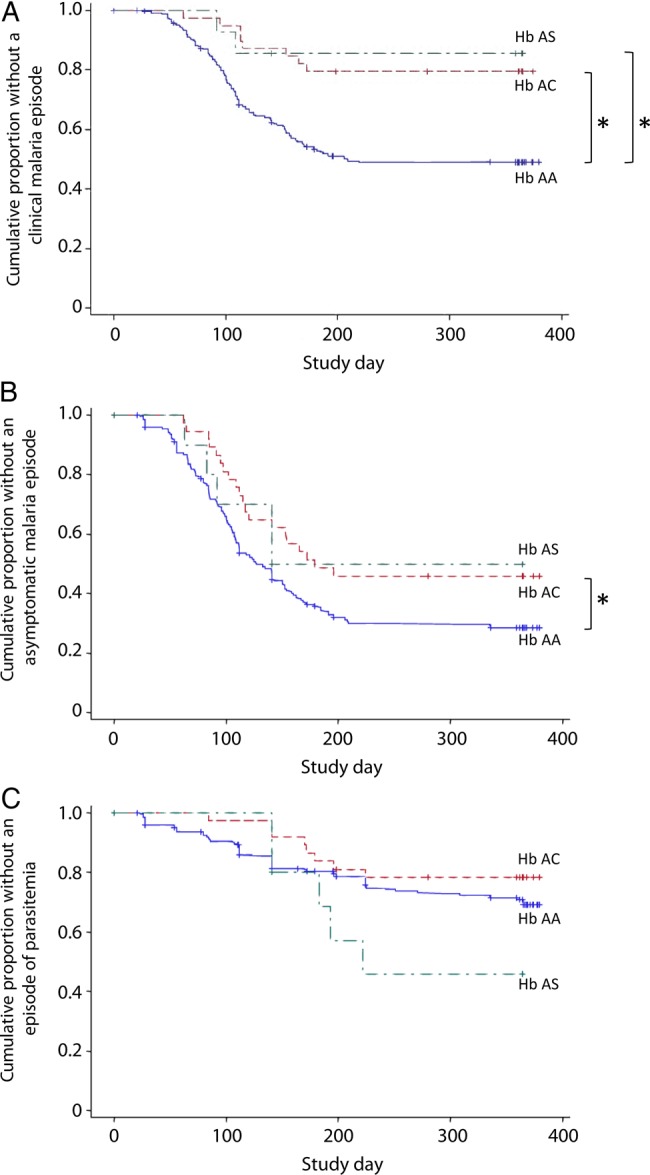
Time to first episode of malaria stratified for hemoglobin genotype. Kaplan–Meier estimates of the fraction of children in each hemoglobin group over time for first clinical malaria episode (A), first asymptomatic malaria episode (B), and first episode of parasitemia, including both symptomatic and asymptomatic malaria episodes (C). Blue line represents hemoglobin AA; green, hemoglobin AS; and red, hemoglobin AC. Asterisks indicate significant differences using a standard Cox regression model. Abbreviation: Hb, hemoglobin.
When a less specific definition of clinical malaria was employed, these group differences persisted (log-rank test, DF = 2; P = .013). Defining a clinical malaria episode as the presence of any symptom, fever, and any level of parasitemia at presentation, children with hemoglobin AC had a longer time to first clinical malaria episode than children with hemoglobin AA, as did children with hemoglobin AS (Table 2). There was no difference in time to such a malaria episode between children with hemoglobin AS and AC (HR: 0.57, 95% CI, .16–1.98; P = .38 unadjusted).
When an episode was defined as the presence of symptoms and “any” parasitemia, regardless of temperature at presentation, group differences in time to such an episode decreased (log-rank test, DF = 2; P = .10). Children with hemoglobin AC still had a longer time than children with hemoglobin AA to such a clinical malaria episode (Table 2). However, children with hemoglobin AS did not differ in time to first clinical malaria episode with children with hemoglobin AA or with hemoglobin AC (HR: 1.26, 95% CI, .52–3.01; P = .61 unadjusted).
There were group differences in time to first asymptomatic malaria episode (log-rank test, DF = 2; P = .060). Children with hemoglobin AC had a longer time to first asymptomatic malaria episode than children with hemoglobin AA (Figure 1B; Table 3). Children with hemoglobin AS did not differ in time to first asymptomatic malaria episode with children with hemoglobin AA or with hemoglobin AC (HR: 1.04, 95% CI, .39–2.77; P = .94 unadjusted).
Table 3.
Time to First Asymptomatic Malaria Episode and to Any Parasitemia Episode
| Asymptomatic Malaria Episode |
Episode With Any Parasitemia |
|||||
|---|---|---|---|---|---|---|
| HR (Unadjusted) | HR (Adjusted) | Median Malaria-Free Days | HR (Unadjusted) | HR (Adjusted) | Median Malaria-Free Days | |
| Hemoglobin AA (n = 242) | 1 | 1 | 364 | 1 | 1 | 112 |
| 0.60 | 0.49 | 0.69 | ||||
| Hemoglobin AC (n = 39) | (0.38, 0.96) | (0.27, 0.88) | 364 | (0.33, 1.44) | … | 172 |
| (P = .032) | (P = .016) | (P = .32) | ||||
| 0.62 | 1.90 | |||||
| Hemoglobin AS (n = 14) | (0.25, 1.50) | … | 162 | (0.76, 4.74) | … | 116.5 |
| (P = .29) | (P = .17) | |||||
Hazard ratios are reported with 95% confidence intervals.
Abbreviation: HR, hazard ratio.
Interestingly, there were no group differences in time to first episode of any parasitemia (including both symptomatic and asymptomatic episodes) (log-rank test, DF = 2; P = .19; Table 3; Figure 1C). Differences were not significant in time to first episode of any parasitemia for children with hemoglobin AS versus hemoglobin AA or versus hemoglobin AC (HR: 2.84, 95% CI, .92–8.75; P = .068 unadjusted).
Total Number of Malaria Episodes
Children in the 3 hemoglobin groups differed in the number of parasitemic episodes (Kruskal–Wallis test, DF = 2; P = .047), clinical malaria episodes (Kruskal–Wallis test, DF = 2; P = .060), and asymptomatic malaria episodes (Kruskal–Wallis test, DF = 2; P = .025). Children with hemoglobin AC had fewer episodes of parasitemia than children with hemoglobin AA (Table 4), including fewer clinical malaria episodes. The 2 groups did not differ in number of asymptomatic parasitemia episodes after adjusting for covariates.
Table 4.
Numbers of Episodes of Malaria and Anemia Per Child, Including Incidence Rate Ratios
| Parasitemic Episodes |
Asymptomatic Malaria Episodes |
Clinical Malaria Episodes |
Anemia Episodes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Number | IRR (Unadjusted) | IRR (Adjusted) | Total Number | IRR (Unadjusted) | IRR (Adjusted) | Total Number | IRR (Unadjusted) | IRR (Adjusted) | Total Number | |
| Hemoglobin AA (n = 242) | 1 (0–3) | 1 | … | 0 (0–1) | 1 | … | 1 (0–2) | 1 | … | 2 (1–4) |
| 0.48 | 0.62 | 0.51 | 0.80 | 0.45 | 0.52 | |||||
| Hemoglobin AC (n = 39) | 1 (0–2) | (0.34, 0.68) | (0.43, 0.91) | 0 (0–1) | (0.30, 0.84) | (0.46, 1.39) | 0 (0–1) | (0.28, 0.74) | (0.31, 0.88) | 2 (1–4) |
| (P < .001) | (P = .013) | (P = .009) | (P = .44) | (P = .002) | (P = .014) | |||||
| 1.35 | 1.75 | 1.71 | 0.95 | |||||||
| Hemoglobin AS (n = 14) | 3 (0–4) | (0.92, 1.97) | … | 1 (0–2) | (1.07, 2.86) | (1.04, 2.80) | 0.5 (0–2) | (0.51, 1.76) | … | 1 (1–3) |
| (P = .12) | (P = .027) | (P = .035) | (P = .86) | |||||||
Episode counts are reported as median (lower quartile – upper quartile).
Abbreviation: IRR, incidence rate ratio.
Children with hemoglobin AS had similar numbers of episodes of parasitemia as children with hemoglobin AA (Table 4), including similar numbers of clinical malaria episodes. In contrast, children with hemoglobin AS had more asymptomatic parasitemia episodes than children with hemoglobin AA.
Children with hemoglobin AS had more episodes of parasitemia than children with hemoglobin AC, but this difference was not significant in the adjusted analysis (Table 4; unadjusted IRR = 2.82, 95% CI, 1.74–4.57; P < .001; adjusted IRR = 1.75, 95% CI, .81–3.76; P = .15). While these 2 groups had similar numbers of clinical malaria episodes (unadjusted IRR = 1.99, 95% CI, .95–4.17; P = .07), children with hemoglobin AS had more episodes of asymptomatic parasitemia than children with hemoglobin AC, but this difference was not significant in the adjusted analysis (unadjusted IRR = 3.70, 95% CI, 1.93–7.09; P < .001; adjusted IRR = 1.80, 95% CI, .59–5.40; P = .30).
Episode counts for each malaria case definition are listed in Table 5 along with parasitemias. Children with hemoglobin AS had the lowest parasitemias of the 3 hemoglobinopathy groups for both clinical and asymptomatic episodes, although these differences were significant only for clinical episodes defined as the presence of any symptoms and parasitemia, with or without fever (Table 5).
Total Number of Anemia Episodes
With regard to number of anemia episodes (hemoglobin <8.4 g/dL) experienced, children with hemoglobin AA did not differ from children with hemoglobin AC (Table 4; unadjusted IRR = 0.99, 95% CI, .80–1.22; P = .92) or children with hemoglobin AS (unadjusted IRR = 0.74, 95% CI, .50–1.09; P = .39).
Cumulative Analysis of Parasite Burden
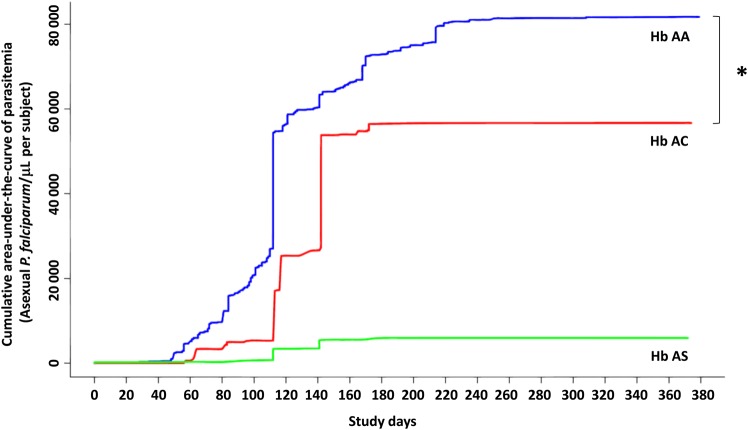
Hemoglobin AC children had a lower cumulative parasite density than hemoglobin AA children (Figure 2; P < .01). Children with hemoglobin AS did not differ in cumulative parasite density with children with hemoglobin AA (P = .12) or AC (P = .57), but there may have been insufficient power to detect such a difference, given the low number of children with hemoglobin AS.
Figure 2.
Cumulative mean area-under-the-curve of parasite burden for children with hemoglobin AA, AC, and AS. Blue line represents hemoglobin AA; green, hemoglobin AS; and red, hemoglobin AC. Asterisks indicate significant differences using a continuity-adjusted Wilcoxon–Mann–Whitney test. Abbreviation: Hb, hemoglobin.
DISCUSSION
Young children with hemoglobin C trait had a lower incidence of clinical malaria compared to those with hemoglobin AA. Children with hemoglobin AC or with AS had a similar number of clinical malaria episodes and had a delay in time to first clinical malaria episode compared to children with hemoglobin AA. Our study indicates that the protection offered by hemoglobin C trait against severe malaria in this population [8] extends to all clinical malaria episodes. Given the lower morbidity and mortality associated with hemoglobin C disease versus hemoglobin S disease, this advantage may contribute to the high frequency of hemoglobin C seen in certain ethnic groups in West Africa.
These results are consistent with those of a case-control study in Burkina Faso that found that hemoglobin C trait protected against uncomplicated malaria [9]. A family-based longitudinal analysis of malaria incidence in Burkina Faso similarly showed that hemoglobin C was associated with decreased parasitemia and a lower risk of clinical malaria episodes throughout childhood [21]. Our findings differ, however, from results of several other studies examining the clinical significance of Hemoglobin C trait. Work in a non-Dogon cohort of Malian children found that while hemoglobin S trait delayed time to first clinical malaria episode, hemoglobin C trait did not [11]. This study cohort consisted of 176 children aged 2–10 years followed over the course of 8 months, whereas the work presented here reflects malaria incidence in a 0–6-year-old pediatric cohort followed for a year. The degree of protection against uncomplicated malaria conferred by hemoglobin AS may vary with age [3]. If this is also true of hemoglobin AC, with protective effects greatest in early childhood, the greater age span of that study's cohort may have diluted the power to detect a protective effect of hemoglobin C trait. Moreover, Dogon children with hemoglobin C trait may have a unique environmental or genetic characteristic that also contributes to a decreased risk of symptomatic malaria. A longitudinal cohort study of children aged 0–2 years in Ghana found no difference in numbers of symptomatic malaria episodes between children with hemoglobin AA and hemoglobin AC, while children with hemoglobin AS had fewer such episodes [10]. Our cohort's predominant Dogon composition and particular age span may thus have allowed us to detect a difference in symptomatic malaria episodes. Finally, a previous case-control study in the same predominantly Dogon population demonstrated that hemoglobin C conferred protection against severe malaria, but did not appear to affect vulnerability to uncomplicated malaria [8]. However, this case-control study was not optimally designed to evaluate uncomplicated malaria risk, which is more suitably addressed in a prospective cohort study.
Unlike previous work, our study recorded asymptomatic malaria episodes and measured cumulative parasite density, outcomes that may illuminate differences in mechanisms of protection against symptomatic malaria for hemoglobin S versus hemoglobin C trait. Children with hemoglobin AC had lower cumulative parasite density than children with hemoglobin AA, while children with hemoglobin AS had more asymptomatic malaria episodes than children with either hemoglobin AA or AC. This result contrasts with findings from a longitudinal Kenyan study that found no difference in asymptomatic malaria prevalence between children with hemoglobin AA and AS [2], and of an older cross-sectional study in Ghana that found that children with hemoglobin AS actually had a lower prevalence of asymptomatic infections and lower parasite densities than children with hemoglobin AA [22, 23]. Our findings suggest that protection against malaria may be mediated by different mechanisms in children with hemoglobin AS versus hemoglobin AC. Unlike hemoglobin AS, which reduces the risk of clinical manifestations of malaria without obviously inhibiting parasite growth (presumably by interfering with the ability of infected erythrocytes to adhere to endothelial receptors), hemoglobin AC appears to lessen the risk of both asymptomatic parasitemia and clinical disease, suggesting that it may directly attenuate parasite replication in erythrocytes as well as curtail cytoadherence. In culture, hemoglobin CC erythrocytes have lower parasite replication rates than hemoglobin AA erythrocytes [24]; this same phenomenon may be occurring to a more limited extent in hemoglobin AC erythrocytes. Our results do not differentiate between hypotheses of a particular mechanism underlying hemoglobin C protection, such as a decreased ability of parasites to invade erythrocytes or altered PfEMP1 display. In addition, relatively few children with hemoglobin AS were enrolled, limiting the study's power to measure associations between hemoglobin AS and risk of malaria infection and disease, including detecting potential effects on parasite density.
These results have implications for interventional studies, particularly in areas of high hemoglobin C prevalence. Time to first clinical malaria episode is a common primary endpoint of vaccine studies. As with a previous study in Mali, children with hemoglobin AS had a delayed time to first malaria episode compared to children with hemoglobin AA [11], but in addition, and contrasting with this previous study, children with hemoglobin C trait also had a delayed time to first malaria episode. Thus, field vaccine studies should ideally compare the prevalence of hemoglobinopathies in study groups to rule out potential confounding effects on study outcomes, particularly for multisite studies involving populations with differing prevalences of hemoglobin traits.
Children with hemoglobin AC did not experience increased anemia episodes compared to children with hemoglobin AA. This confirms previous work done at this site that showed no difference in hemoglobin levels between these 2 groups [25]. However, a study of children aged 0–2 years found that hemoglobin S trait protected against anemia episodes, while hemoglobin C trait did not, supporting the notion that protection against malaria afforded by hemoglobinopathies may be somewhat different in children of different ages [10].
We found that hemoglobin C trait provides protection from clinical falciparum malaria. Protection against clinical malaria may be mediated by different mechanisms in hemoglobin AC and AS groups. Future work investigating the basis of such protection will improve our understanding of differences in vulnerability to malaria across diverse populations in Africa and Asia.
Notes
Acknowledgments. We thank Danzele Coulibaly, Sekouba Mariko, Moctar Traore, Nicole Eddington, and Carey Martin for administrative support; the team of the Bandiagara Malaria Project in Bandiagara for their dedication; and the community of Bandiagara, Mali.
Financial support. This work was supported by a cooperative agreement (U19AI065683) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health; a training grant (D43TW001589) from the Fogarty International Center of the National Institutes of Health; and awards to C. V. P. from the Doris Duke Charitable Foundation and the Howard Hughes Medical Institute. M. A. Travassos was supported by a Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hill AV, Allsopp CE, Kwiatkowski D et al. Common west African HLA antigens are associated with protection from severe malaria. Nature 1991; 352:595–600. [DOI] [PubMed] [Google Scholar]
- 2.Williams TN, Mwangi TW, Wambua S et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis 2005; 192:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams TN, Mwangi TW, Roberts DJ et al. An immune basis for malaria protection by the sickle cell trait. PLOS Med 2005; 2:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010; 376:2018–31. [DOI] [PubMed] [Google Scholar]
- 5.Allison AC. Genetic factors in resistance to malaria. Ann NY Acad Sci 1961; 91:710–29. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel RL, Steinberg MH. Hemoglobin SC disease and HbC disorders. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, eds. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. 1st ed. Cambridge: Cambridge University Press, 2001:756–85. [Google Scholar]
- 8.Agarwal A, Guindo A, Cissoko Y et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood 2000; 96:2358–63. [PubMed] [Google Scholar]
- 9.Modiano D, Luoni G, Sirima BS et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 2001; 414:305–8. [DOI] [PubMed] [Google Scholar]
- 10.Kreuels B, Kreuzberg C, Kobbe R et al. Differing effects of HbS and HbC traits on uncomplicated falciparum malaria, anemia, and child growth. Blood 2010; 115:4551–8. [DOI] [PubMed] [Google Scholar]
- 11.Crompton PD, Traore B, Kayentao K et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis 2008; 198:1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cholera R, Brittain NJ, Gillrie MR et al. Impaired cytoadherence of Plasmodium falciparum–infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci USA 2008; 105:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairhurst RM, Baruch DI, Brittain NJ et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 2005; 435:1117–21. [DOI] [PubMed] [Google Scholar]
- 14.Diallo D, Traore AK, Baby M, Rhaly AA, Bellis G, Chaventre A. Haemoglobinopathies C and S in the Dogons. Nouv Rev Fr Hematol 1994; 35:551–4. [PubMed] [Google Scholar]
- 15.Ducrocq R, Bennani M, Bellis G et al. Hemoglobinopathies in the Dogon Country: presence of beta S, beta C, and delta A’ genes. Am J Hematol 1994; 46:245–7. [DOI] [PubMed] [Google Scholar]
- 16.Coulibaly D, Diallo DA, Thera MA et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg 2002; 67:604–10. [DOI] [PubMed] [Google Scholar]
- 17.Lyke KE, Dicko A, Kone A et al. Incidence of severe Plasmodium falciparum malaria as a primary endpoint for vaccine efficacy trials in Bandiagara, Mali. Vaccine 2004; 22:3169–74. [DOI] [PubMed] [Google Scholar]
- 18.Coulibaly D, Travassos MA, Kone AK et al. Stable malaria incidence despite scaling up control strategies in a malaria vaccine-testing site in Mali. Malar J 2014; 13:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guindo A, Fairhurst RM, Doumbo OK, Wellems TE, Diallo DA. X-linked G6PD deficiency protects hemizygous males but not heterozygous females against severe malaria. PLOS Med 2007; 4:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thera MA, Doumbo OK, Coulibaly D et al. A field trial to assess a blood-stage malaria vaccine. N Engl J Med 2011; 365:1004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rihet P, Flori L, Tall F, Traore AS, Fumoux F. Hemoglobin C is associated with reduced Plasmodium falciparum parasitemia and low risk of mild malaria attack. Hum Mol Genet 2004; 13:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Thompson GR. Significance of haemoglobins S and C in Ghana. Br Med J 1962; 1:682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson GR. Malaria and stress in relation to Haemoglobins S and C. Br Med J 1963; 2:976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fairhurst RM, Fujioka H, Hayton K, Collins KF, Wellems TE. Aberrant development of Plasmodium falciparum in hemoglobin CC red cells: implications for the malaria protective effect of the homozygous state. Blood 2003; 101:3309–15. [DOI] [PubMed] [Google Scholar]
- 25.Diallo DA, Doumbo OK, Dicko A et al. A comparison of anemia in hemoglobin C and normal hemoglobin A children with Plasmodium falciparum malaria. Acta Trop 2004; 90:295–9. [DOI] [PubMed] [Google Scholar]