Abstract
Improved biomarkers are needed for tuberculosis. To develop tests based on products secreted by tubercle bacilli that are strictly associated with viability, we evaluated 3 bacterial-derived, species-specific, small molecules as biomarkers: 2 mycobactin siderophores and tuberculosinyladenosine. Using liquid chromatography–tandem mass spectrometry, we demonstrated the presence of 1 or both mycobactins and/or tuberculosinyladenosine in serum and whole lung tissues from infected mice and sputum, cerebrospinal fluid (CSF), or lymph nodes from infected patients but not uninfected controls. Detection of the target molecules distinguished host infection status in 100% of mice with both serum and lung as the target sample. In human subjects, we evaluated detection of the bacterial small molecules (BSMs) in multiple body compartments in 3 patient cohorts corresponding to different forms of tuberculosis. We detected at least 1 of the 3 molecules in 90%, 71%, and 40% of tuberculosis patients' sputum, CSF, and lymph node samples, respectively. In paucibacillary forms of human tuberculosis, which are difficult to diagnose even with culture, detection of 1 or more BSM was rapid and compared favorably to polymerase chain reaction–based detection. Secreted BSMs, detectable in serum, warrant further investigation as a means for diagnosis and therapeutic monitoring in patients with tuberculosis.
Keywords: biomarker, diagnostic, mycobactin, small molecule, tuberculosinyladenosine, tuberculosis
Tuberculosis remains a leading cause of mortality and morbidity worldwide, resulting in 1.3 million deaths annually and infecting approximately one-third of the global human population. The recent emergence of drug-resistant forms of tuberculosis underscores the inadequacies of current public health-control efforts. Major factors in the poor state of global tuberculosis control are the lack of rapid and specific point-of-care diagnostics and new drugs to treat the newly resistant forms of tuberculosis. Many experts regard the lack of validated markers as a major obstacle to conducting the clinical trials required to bring a new drug to market in a cost-effective manner [1]. Consequently, there is an urgent need for effective new tuberculosis biomarkers both for diagnosing disease and monitoring its response to treatment.
While there has been relative success in developing blood-based diagnostics for latent tuberculosis based on host immune responses with interferon-γ release assays, the majority of current diagnostics for active tuberculosis are sputum-based. This limits their utility both in children, who do not reliably produce sputum [2], and as treatment response markers because coughing and sputum production resolve many months before cure in most active tuberculosis patients. Moreover, since human immunodeficiency virus (HIV) coinfection and other forms of immunocompromise are common risk factors for tuberculosis, diagnostics targeting host antibody or T-cell responses may have limitations in this significant sector of at-risk individuals [3]. For these reasons, we focused our studies on secreted, bacterial-derived small molecules, which may enter compartments outside of the lung such as blood or urine.
After phagocytosis by alveolar macrophages, Mycobacterium tuberculosis depends on a number of strategies for surviving intracellularly; these include mechanisms for harvesting host iron stores and for preventing phagosome maturation. M. tuberculosis acquires iron through the secretion and subsequent reinternalization of siderophores mycobactin T (MBT) and carboxymycobactin (cMBT) [4]. Species-specific differences in mycobactins T, S, and J, from M. tuberculosis, M. smegmatis, and M. paratuberculosis, respectively, have been identified [5]. M. avium, M. tuberculosis, M. smegmatis, and M. bovis all secrete cMBT. Disruption of siderophore production or secretion significantly attenuates mycobacterial growth and virulence [6]. Additionally, M. tuberculosis produces and sheds the terpene nucleoside 1-tuberculosinyladenosine (1-TbAd), which likely regulates macrophage phagosomal maturation [7, 8]. The genes encoding its biosynthetic enzymes (Rv3377c and Rv3378c) are only found within the M. tuberculosis complex. However, the locus contains loss-of-function mutations in M. bovis and M. bovis bacillus Calmette-Guérin (BCG), and it appears that M. tuberculosis may be the only complex member that produces 1-TbAd [8, 9]. These molecules—MBT, cMBT, and TbAd—have shared properties that are attractive as diagnostic targets, including their abundant biosynthesis, export to the surface for shedding, and their promotion of M. tuberculosis virulence, which suggests continuous expression in vivo. Also, their similar polarity and mass profiles facilitated the development of a single liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based method for their detection.
RESULTS AND DISCUSSION
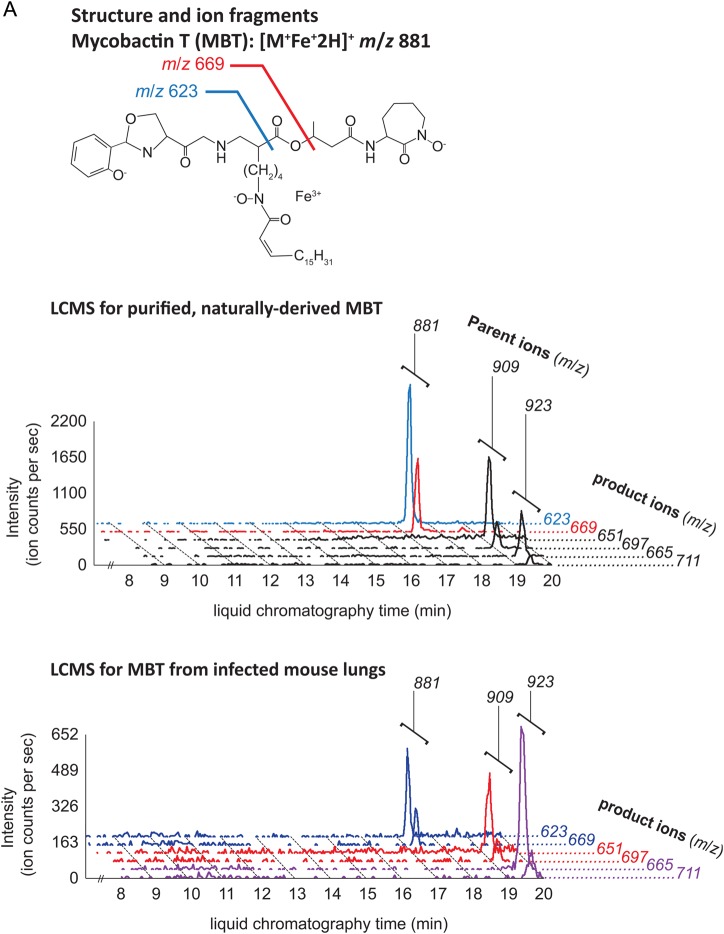
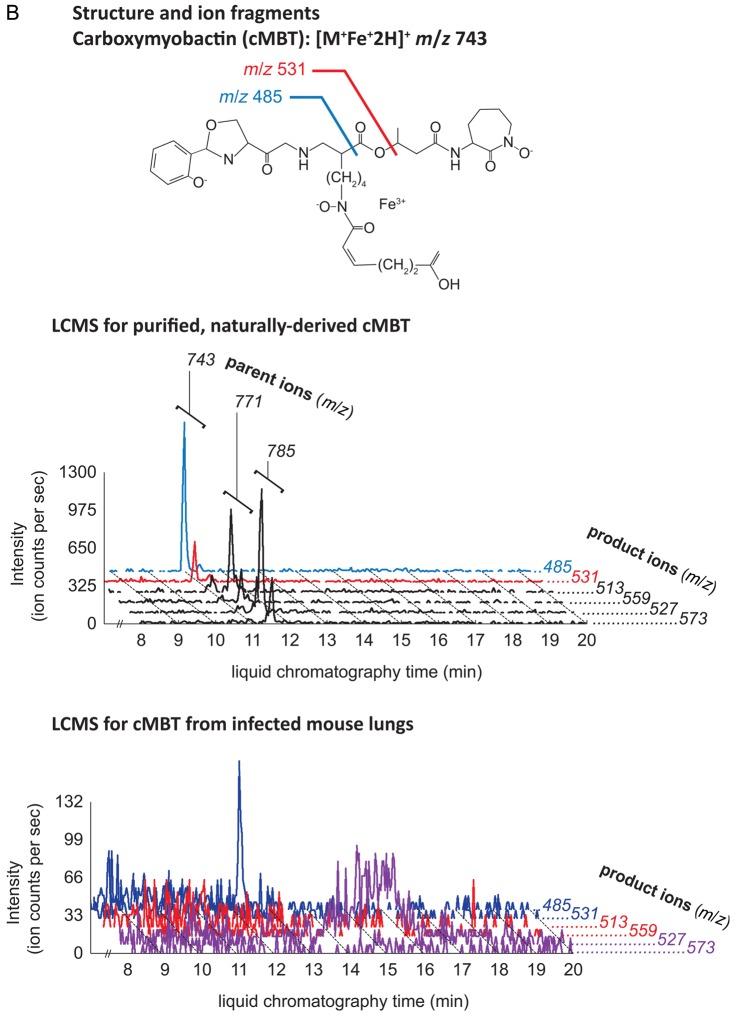
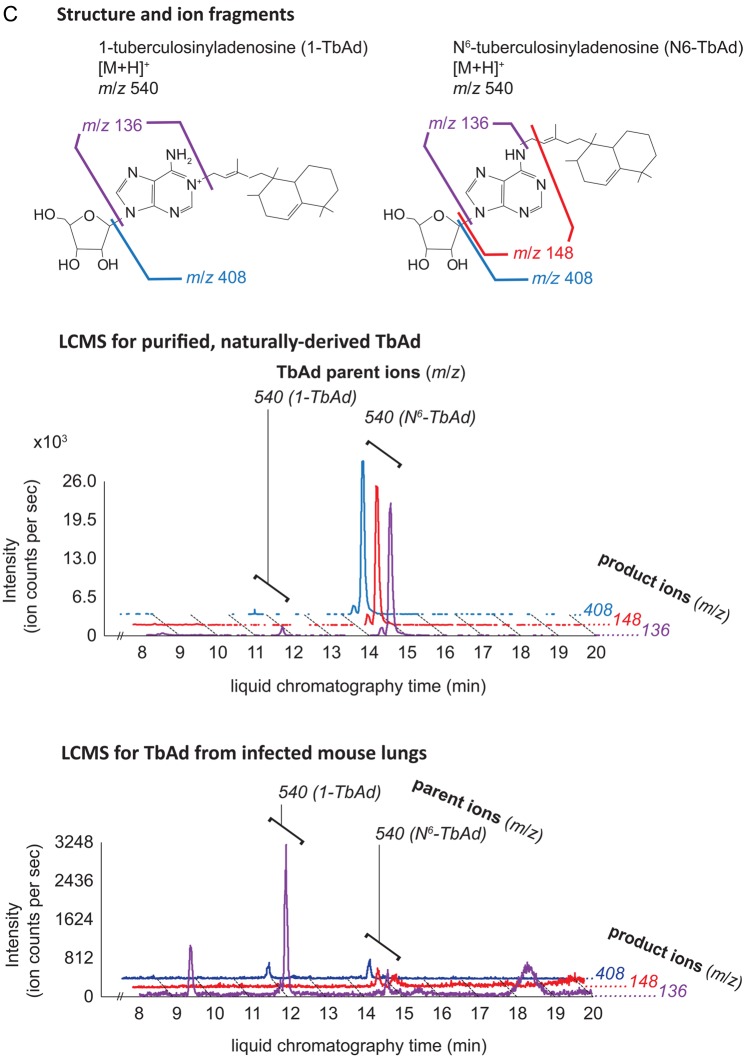
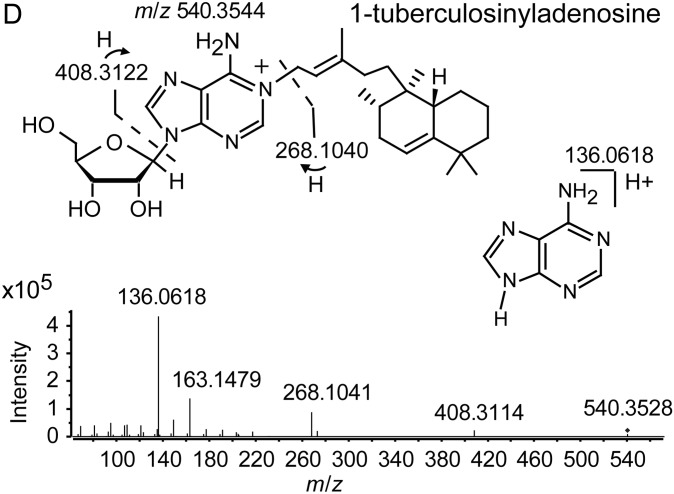
MBT and cMBT were purified from sterilized lipid extracts of M. tuberculosis H37Rv cultured in iron-deficient media to induce siderophore production. We confirmed detection of the molecules using a collision-induced mass spectrometry (CID-MS) protocol. For both MBT and cMBT, the method revealed 3 major molecular variants (Figure 1A and 1B). 1-TbAd and an isomer recently identified as N6-TbAd were separately purified (see Supplementary Methods), and their CID-MS profiles characterized (Figure 1C) [9]. The isomer fragments similarly to 1-TbAd, but with 1 additional major fragment ion at m/z 148 (Figure 1C). The structure of 1-TbAd used in this study was confirmed by nuclear magnetic resonance (Figure 1D). Beyond these studies of purified, naturally derived mycobacterial lipids, we confirmed that the method successfully detected them in uninfected mouse lung tissue spiked with these small molecules. For positivity of a given molecule, we required detection of at least 1 parent ion and associated transition pair with a signal-to-noise (S/N) ratio ≥3:1. For the purposes of analysis, we combined the 2 TbAd isomers into 1 category and did not differentiate between individual molecular variants of MBT or cMBT.
Figure 1.
Structures and ion fragments (top), representative ion chromatograms of purified, naturally derived, tuberculosis-specific bacterial small molecules (BSMs), illustrating the characteristic ion intensity peaks with collision-induced liquid chromatography mass spectrometry (LCMS) in positive ion mode at concentrations of 10 nM (middle), and characteristic ion chromatograms of the product ions detected by LCMS analysis from infected mouse lungs (bottom) for mycobactin T (A), carboxymycobactin (B), and tuberculosinyladenosine in its 2 forms, 1-TbAd and the isomeric N6-TbAd (C). For mycobactin T, the 15 methylene aliphatic side chain as shown gives a parent ion m/z of 881, but 17- (m/z 909) and 18-methylene (m/z 923) forms are also produced. For carboxymycobactin, the aliphatic link as shown gives a parent ion m/z of 743 but the 2-methylene aliphatic chain shown may extend to 4 (m/z 771) or 5 methylenes (m/z 785) in length. For mouse infections, LCMS analyses are shown from 1 processed lung tissue sample from a BALB/c mouse sacrificed 21 days after aerosol infection with M. tuberculosis H37Rv. Infected mouse lungs showed characteristic peaks for mycobactin T (A, bottom panel) and 1-TbAd (C, bottom panel), but carboxymycobactin was less abundant with only 1 ion transition observed above the detection limit (B, bottom panel). Trace levels of N6-TbAd were observed eluting later than 1-TbAd in the infected mouse lung (C, bottom panel). The 1-TbAd used as standard material in this study was fully characterized by mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy. This included accurate mass assignments (elemental composition), collisionally induced dissociation fragment analysis, and 2-dimensional NMR, allowing characterization of the 1-TbAd structure (D). Abbreviation: TbAd, tuberculosinyladenosine.
To evaluate target molecule detection in samples from M. tuberculosis–infected hosts, we infected BALB/c mice with a high dose of the virulent M. tuberculosis H37Rv. Mice were sacrificed at postinfection day 1 for determination of the initial bacterial implantation, and at day 21 for evaluation of the final bacterial burden and presence of the 3 targeted bacterial small molecules (BSMs). The implantation and final bacterial burdens were found to be typical for a high-dose aerosol infection (Supplementary Figure 1A), and we observed discrete pulmonary lesions in the lungs of mice 21 days postinfection (Supplementary Figure 1B).
LC-MS/MS evaluation revealed MBT in lung tissue and serum of 100% (7/7) M. tuberculosis–infected mice (Table 1a and Figure 1A–C). TbAd was detected in lung tissue of 100% (7/7) of infected mice, but in serum samples of only 43% (3/7). By contrast, we detected cMBT in the serum of only 1 infected mouse and in no lung samples. The sensitivity for any BSMs being positive in either mouse serum or lung tissue was 100%. Lung and serum samples from 100% (6/6) of the uninfected mice were negative for all 3 molecules of interest, thus the specificity for any BSMs being positive in mouse serum or lung tissue was also 100%. Because TbAd is not produced by M. bovis BCG [7], we tested lungs of BCG-infected mice and showed that TbAd was not detected despite the presence of 1.1 × 104 colony forming units of BCG in the animal lungs at 3 weeks postinfection.
Table 1.
Detection of Bacterial Small Molecules in Mouse and Human Samples
| Sample | Molecule Targeted | Detection Among Mice, by Tuberculosis Disease Status (%) |
||
|---|---|---|---|---|
| Infected | Uninfected | P Value | ||
| (a) | ||||
| Serum | Mycobactin T | 7/7 (100) | 0/6 (0) | <.001 |
| Carboxymycobactin | 1/7 (0) | 0/6 (0) | 1.00 | |
| TbAd | 3/7 (43) | 0/6 (0) | .192 | |
| Lung | Mycobactin T | 7/7 (100) | 0/6 (0) | <.001 |
| Carboxymycobactin | 0/7 (14) | 0/6 (0) | 1.00 | |
| TbAd | 7/7 (100) | 0/6 (0) | <.001 | |
| Sample | Molecule Targeted | Detection in Patient Sputum Samples, by Tuberculosis Disease Status (%) |
||
| Infected | Uninfected | P Value | ||
| (b) | ||||
| Sputum | Mycobactin T | 9/10 (90) | 0/10 (0) | .00058 |
| Carboxymycobactin | 0/10 (0) | 0/10 (0) | 1.00 | |
| TbAd | 6/10 (60) | 0/10 (0) | .011 | |
| Correlation Coefficient | P Value | |||
| (c) | ||||
| Mycobactin T | ||||
| AFB smear microscopy score | 0.8739 | .0024 | ||
| MGIT TTP time | −0.2970 | .4069 | ||
| GeneXpert grade | 0.5919 | .0952 | ||
| TbAd | ||||
| AFB smear microscopy score | 0.8587 | .0095 | ||
| MGIT TTP time | −0.5253 | .0989 | ||
| GeneXpert grade | 0.8980 | .0079 | ||
| Detection in Patient Cerebrospinal Fluid Samples, by Culture-Confirmed Meningitis Diagnosis (%) |
||||
| Molecule Targeted | Tuberculosis | Cryptococcal | P Value | |
| (d) | ||||
| Mycobactin T | 10/14 (53) | 0/12 (0) | .0002 | |
| TbAd | 1/14 (7) | 0/12 (0) | 1.00 | |
| Sample | Detection of M. tuberculosis in Patient Lymph Node Samples, Fraction Positive (%) |
P Value | ||
| Detection Method | Infected | Uninfected | ||
| (e) | ||||
| Lymph node | Any BSMs | 2/5 (40) | 0/5 (0) | .44 |
| AFB smear | 1/5 (20) | 0/5 (0) | 1.00 | |
| GeneXpert | 5/5 (100) | 0/5 (0) | .008 | |
| Culture | 5/5 (100) | 0/5 (0) | .008 | |
| Blood | Any BSMs | 1/5 (20) | 0/5 (0) | 1.00 |
| GeneXpert | 1/5 (20) | 0/5 (0) | 1.00 | |
| Culture | 0/5 (0) | 0/5 (0) | 1.00 | |
Abbreviations: AFB, acid-fast bacilli; BSMs, bacterial small molecules; MGIT, mycobacterial growth indicator tube; TbAd, tuberculosinyladenosine; TTP, time to positivity.
To test the utility of these BSMs as markers of tuberculosis disease in humans, we analyzed sputum samples from pulmonary tuberculosis patients and culture-negative, healthy controls. All tuberculosis patients were positive for tuberculosis by acid-fast bacilli (AFB) sputum smear, culture, and GeneXpert testing. As shown in Table 1b, we detected MBT in 90% (9/10) of sputum samples from infected individuals and TbAd in 60% (6/10), and in none of 10 uninfected control sputum samples (90% sensitivity, 100% specificity for any BSMs being positive). The 1 sputum sample negative for MBT had a low bacterial burden as indicated by GeneXpert and AFB smear. The S/N ratio of cMBT was below the detection threshold in this and subsequent analyses.
To determine whether MBT and TbAd levels may correlate with bacterial loads, we compared signal intensities of these small molecules with other quantitative markers of M. tuberculosis abundance in the above sputum study. We correlated the ion counts per second of the molecular ions for MBT and TbAd in each positive sputum sample with the AFB smear microscopy scores, mycobacterial growth indicator tube (MGIT) time-to-positivity, and GeneXpert grade for each respective sputum sample. As may be seen in Table 1c, MBT levels showed a significant correlation with AFB smear microscopy and approached significance in its correlation with GeneXpert grade. TbAd levels showed significant correlations with AFB smear microscopy and GeneXpert grades, and approached significance in its correlation with MGIT time-to-positivity. These results suggest that the LC-MS/MS intensities of these 2 small molecule biomarkers track with other commonly used measures of bacterial abundance.
Next, we analyzed cerebrospinal fluid (CSF) from patients with culture-confirmed tuberculous meningitis, comparing them to control samples from patients with culture-confirmed cryptococcal meningitis. We were able to detect MBT in CSF from 71% (10/14) of tuberculous meningitis cases and TbAd in 7% (1/14) of cases. There was no detectable MBT or TbAd in any of the 12 M. tuberculosis culture-negative, Cryptococcus neoformans culture-positive CSF patient samples tested (Table 1d) (71% sensitivity, 100% specificity for any BSMs being positive). In 11 subjects with culture-confirmed tuberculous meningitis who also had AFB smear performed on their CSF, 9/11 were positive for any BSMs and 7/11 were AFB smear-positive (sensitivity of any BSMs being positive 82%, sensitivity of AFB smear 64%).
Last, we tested the BSMs in another paucibacillary form of tuberculosis, tuberculous lymphadenitis. We obtained cervical lymph nodes from 10 South African patients with lymphadenitis who underwent diagnostic excisions. In addition to histopathology and mycobacterial culture, all samples were evaluated by AFB smear, GeneXpert, and LC-MS/MS. Five patients had lymph nodes which were M. tuberculosis culture-positive, while the other 5 had histology consistent with malignancy or reactive lymphadenitis with no evidence of caseation. We detected MBT in 40% (2/5) of the M. tuberculosis–positive lymph nodes and TbAd in 20% (1/5) as shown in Table 1e. All M. tuberculosis–negative samples also tested negative for the target molecules (sensitivity 40%, specificity 100% for any BSMs being positive). AFB smear was positive in 20% (1/5) of the tuberculous lymph nodes, while GeneXpert was positive in 100% (5/5). Interestingly, 1 of the patients with tuberculous lymphadenitis also provided a blood sample that was GeneXpert positive and was BSM-positive (MBT positive/TbAd negative); however, the blood sample was culture-negative for M. tuberculosis. None of the other 9 patients had evidence of mycobacteremia by any of the 3 tests.
Our studies demonstrate that 2 types of BSMs released by M. tuberculosis, MBT and TbAd, may be found in blood, sputum, CSF, lung, and lymph node samples from mice and human infected with M. tuberculosis. Evidence suggests that these 2 molecules play a role in tuberculosis virulence—MBT in nutrient acquisition and survival inside host cells [6] and TbAd in resistance against macrophage killing [8]. So it is not surprising that they are actively produced during infection. Not only were these molecules found in infected mice and in sputum samples from infected human patients, we were also able to detect them in human CSF and lymph node tissue in which the bacterial burden is low. The potential use of these BSMs in monitoring response to therapy in pulmonary tuberculosis patients warrants further investigation.
Our work has some limitations. To establish proof-of-principle for the presence of the target BSMs in multiple forms of tuberculosis, we studied relatively small numbers of patients. Hence, the current data cannot predict the population sensitivity and specificity of this test in the clinical setting. Moreover, all sputum samples from tuberculosis patients in South Africa were smear-positive, whereas the control samples were obtained from the United States. The fact that no sputum samples from smear-negative tuberculosis patients were used and that the positive and control samples came from different countries might affect test accuracy. Additionally, our detection method, LC-MS/MS, may not at present be amenable to point-of-care diagnosis. While LC-MS/MS–based biomarker monitoring could be valuable for reducing the cost of tuberculosis clinical trials, simpler and less expensive methods of detection would be required for use in routine clinical settings. Although specific antibodies are not currently available, both molecules are readily targetable with enzyme-linked immunoassays.
Our study is unique in focusing on bacterial-derived, secreted, small molecules. Previous studies of bacterial antigens have focused on nonsecreted macromolecules, which may be shed less readily into other body compartments. These include proteins [10], lipoarabinomannan (LAM) [11], dimycocerosates [12], and mycolic acids [13]. LAM, an amphipathic bacterial macromolecule that appears in urine and blood, has been tested in several clinical trials as a tuberculosis biomarker. While in general LAM antigen detection assays have been inferior to traditional tuberculosis diagnostics, some studies have demonstrated good sensitivity in HIV-infected individuals, suggesting that the test may be valuable in certain subpopulations [14]. The introduction of nucleic acid amplification tests for tuberculosis has been an important advance during the past 2 decades. In particular, the GeneXpert platform, which may deliver results in less than 2 hours, has proven useful in tuberculosis control programs [15]. This and other nucleic-acid amplification tests are limited in their ability to detect tuberculosis using blood and urine and also to quantify disease burden over time because the DNA of killed bacteria remains detectable for many days despite effective therapy. Hence, there remains an ongoing need for tuberculosis biomarkers, similar to HIV viral load testing, which quantify disease progression or regression using readily available patient samples such as blood or urine. In this study, we present proof-of-principle evidence that bacterial-derived, pathogen-specific, small molecules—whose presence is independent of host immunity—may prove valuable as novel biomarkers for tuberculosis. Such markers would be valuable supplements to the currently available platform of tools.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Colin Ratledge of the University of Hull in the United Kingdom for providing advice and sample mycobactin extracts that aided our initial method development. We thank Shaaretha Pelly for critical review of the manuscript.
Financial support. This work was supported by the US National Institutes of Health award AI097138 (W. B.), the Howard Hughes Medical Institute (W. B. and T. N.), the South African Research Chairs Initiative (T. N.), and the Victor Daitz Foundation (T. N.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Wallis RS, Kim P, Cole S et al. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis 2013; 13:362–72. [DOI] [PubMed] [Google Scholar]
- 2.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis 2010; 50(suppl 3):S184–94. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Zhang R, Wang J et al. Interferon-gamma release assays for the diagnosis of active tuberculosis in HIV-infected patients: a systematic review and meta-analysis. PLOS One 2011; 6:e26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratledge C. Iron, mycobacteria and tuberculosis. Tuberculosis (Edinb) 2004; 84:110–30. [DOI] [PubMed] [Google Scholar]
- 5.Snow GA. Mycobactins: iron-chelating growth factors from mycobacteria. Bacteriol Rev 1970; 34:99–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy PV, Puri RV, Chauhan P et al. Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J Infect Dis 2013; 208:1255–65. [DOI] [PubMed] [Google Scholar]
- 7.Layre E, Lee HJ, Young DC et al. Molecular profiling of Mycobacterium tuberculosis identifies tuberculosinyl nucleoside products of the virulence-associated enzyme Rv3378c. Proc Natl Acad Sci USA 2014; 111:2978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pethe K, Swenson DL, Alonso S, Anderson J, Wang C, Russell DG. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc Natl Acad Sci USA 2004; 101:13642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young DC, Layre E, Pan SJ et al. Terpene nucleosides are transformed in vivo and serve as specific chemical markers for M. tuberculosis infection. Chem Biol 2015; 22:515–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young BL, Mlamla Z, Gqamana PP et al. The identification of tuberculosis biomarkers in human urine samples. Eur Resp J 2014; 43:1719–29. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee D, Bozic CM, McNeil M, Brennan PJ. Structural features of the arabinan component of the lipoarabinomannan of Mycobacterium tuberculosis. J Biol Chem 1991; 266:9652–60. [PubMed] [Google Scholar]
- 12.O'Sullivan DM, Nicoara SC, Mutetwa R et al. Detection of Mycobacterium tuberculosis in sputum by gas chromatography-mass spectrometry of methyl mycocerosates released by thermochemolysis. PLOS One 2012; 7:e32836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shui G, Bendt AK, Jappar IA et al. Mycolic acids as diagnostic markers for tuberculosis case detection in humans and drug efficacy in mice. EMBO Mol Med 2012; 4:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gounder CR, Kufa T, Wada NI et al. Diagnostic accuracy of a urine lipoarabinomannan enzyme-linked immunosorbent assay for screening ambulatory HIV-infected persons for tuberculosis. J Acquir Immune Defic Syndr 2011; 58:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. New Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.